Abstract
Free full text

Recent Trends in Research with Human Pluripotent Stem Cells: Impact of Research and Use of Cell Lines in Experimental Research and Clinical Trials
Associated Data
Summary
The human pluripotent stem cell (hPSC) research landscape is rapidly evolving. To assess possible novel trends in hPSC usage, we analyzed experimental hPSC research published from 2014 to 2016 and compared our data with those of earlier periods. The number of papers describing experimental work involving hPSCs increased further with clear differences in the scientific impact of publications from different countries. Our results confirm the leading position of US-based hPSC research, although to a lesser degree than observed previously. Our data reveal that research into human induced pluripotent stem cells alone surpassed human embryonic stem cell (hESC) research by 2015 and rapidly grew after that. We also report on continuing and even slightly growing research activities in the hESC field as well as on a generally declining rate of the generation of new hESC lines. An increasing portion of new hESC lines represents disease-specific and clinical-grade cell lines. The previously noted usage of only a few early established hESC lines in the vast majority of scientific work is sustained. We also provide a comprehensive overview on clinical trials on the basis of hPSCs. We find that the vast majority of those trials are based on hESC-derived cell products that were generated from an only limited number of relatively old cell lines.
Introduction
Since the first derivation of human embryonic stem cells (hESCs) in 1998 (Thomson et al., 1998), research has focused on a better understanding of the unique characteristics of these cells and on a future use of hESC derivatives for cell replacement therapies. However, derivation and use of hESCs has been controversial due to the origin of these cells from human extracorporeal embryos, which are usually destroyed in the process of hESC derivation. With the first establishment of human induced pluripotent stem cells (hiPSCs) in 2007 (Takahashi et al., 2007, Yu et al., 2007) the debate has been raised again. hiPSCs share fundamental characteristics of hESCs but are not burdened with the embryonic origin of hESCs. Since hiPSCs are derived from somatic cells, they can be easily used to establish any disease-specific cell line for analysis of cellular processes during pathogenesis as well as for development of novel agents and drugs. Moreover, because of the option to produce hiPSCs for any diseased individual, they are expected to have advantages over hESCs in future cell and tissue replacement therapies with respect to immune rejection. Some initial disadvantages of hiPSCs, such as dependence on use of retroviruses for reprogramming, have meanwhile been overcome, while others, such as high variability in differentiation potential and genetic stability, still remain subjects of intense research. However, faced with an alternative source for human pluripotent stem cells (hPSCs), it was widely discussed whether hiPSCs would replace hESCs in research and whether hESCs would be exclusively needed to verify the pluripotency of hiPSCs only for a transition period (Holm, 2008, Hyun et al., 2007, Power and Rasko, 2011, Sipp, 2009).
We and others have studied research trends in the field of human pluripotent stem cells over the past 12 years (Guhr et al., 2006, Löser et al., 2010, Negoro et al., 2017, Owen-Smith and McCormick, 2006). Recently, we reported that research with hESCs did not decline but rather broadened from 2008 to 2013 despite the availability of hiPSCs (Kobold et al., 2015). Moreover, we observed a diversification of the research fields in which hESCs and hiPSCs were used to answer partially different research questions. In the current report, we extended this analysis to cover more recent developments. By evaluating all original research papers, which report results of experimental use of hPSCs, we show that there is a considerable increase in hiPSC research while research with hESCs grows only slightly. Furthermore, there is an only limited overlap of both research fields. We also present data on hESC line usage in experimental research and found that the increase in hESC research over the past 10 years is not linked to a considerable rise in the derivation of novel hESC lines. We also present an overview on current clinical trials involving hPSC-derived cell products and investigate the cell line usage in these trials.
Results
Data Acquisition
Over the past years we have established a vast validated database on publicly known hESC lines and on original scientific papers reporting experimental use of hPSCs. This manually validated database was established by annual searches of the PubMed database for relevant literature and now contains data on more than 1,500 hESC lines published in peer-reviewed papers. In addition, we have documented more than 3,300 and about 1,400 papers that were published by end of 2013 and reported on experimental use of hESCs or hiPSCs, respectively. Using the search routines described earlier, we intended to identify hPSC research papers that were published in the more recent past, namely from 2014 to 2016. Our search resulted in 7,597 primary hits for hESC-related studies and 6,359 primary hits for hiPSC-related studies. Of those, we excluded papers that were categorized by PubMed as non-experimental research (Comments, Editorials, Reviews, etc.). We also excluded studies that appeared in journals that usually do not report original research. We then manually inspected the abstracts or/and full texts of the remaining 4,118 hESC-related and 3,370 hiPSC-related papers and identified 1,799 and 2,162 original research papers that report on the experimental use of hESCs or hiPSCs, respectively. Since 676 papers reported experimental use of both hESCs and hiPSCs (“overlap”), a total of 3,285 original publications were examined in the analyses described below. In addition, we found that in 43 or 171 studies (usually commercially available) cell derivatives of hESCs or hiPSC were used, respectively. These studies were not included in the subsequent analyses for reasons detailed in Kobold et al. (2015).
Number and Origin of hPSC Research Papers
We first intended to determine the number and origin of research papers that involved experimental use of hESCs and hiPSCs. Figure 1A shows the results of the respective analysis for the past 3 years (2014–2016). In 2014, the number of research papers reporting on hiPSCs equaled the number of hESC studies for the first time. In the following 2 years, the hiPSC paper count clearly surpassed that of hESC papers. Whereas the number of research papers involving hESCs raised only slightly by about 7% from 2014 to 2016, the count of published hiPSC studies increased by more than 55% during the same period. When these numbers were compared to those of the preceding 3-year periods (2008–2010 and 2011 to 2013, respectively), an increase in the number of both hESC and hiPSC research papers by 51.0% and 70.9%, respectively, was noted (Figure 1B). The strong increase in hiPSC research papers clearly indicates the enormous interest in these cells but is also a consequence of the relative novelty of this research field, in which only few publications were produced from 2008 to 2010. We also noted that there is a considerable number of studies in which both hESCs and hiPSCs were used (light gray and gray/white striped portions of the bars in Figure 1B). Closer inspection of stem cell usage in these papers revealed that the number of papers in which hESCs were used for mere comparison to verify certain characteristics of hiPSCs (“"gold standard” usage, gray/white striped portions of the bars in Figure 1B) markedly decreased during the last years indicating that, despite the growth of the hiPSC field, hESCs are still an independent research object. In addition, nine of the hESC studies identified here also report on derivation/experimental use of pluripotent stem cells derived from embryos produced by somatic cell nuclear transfer.
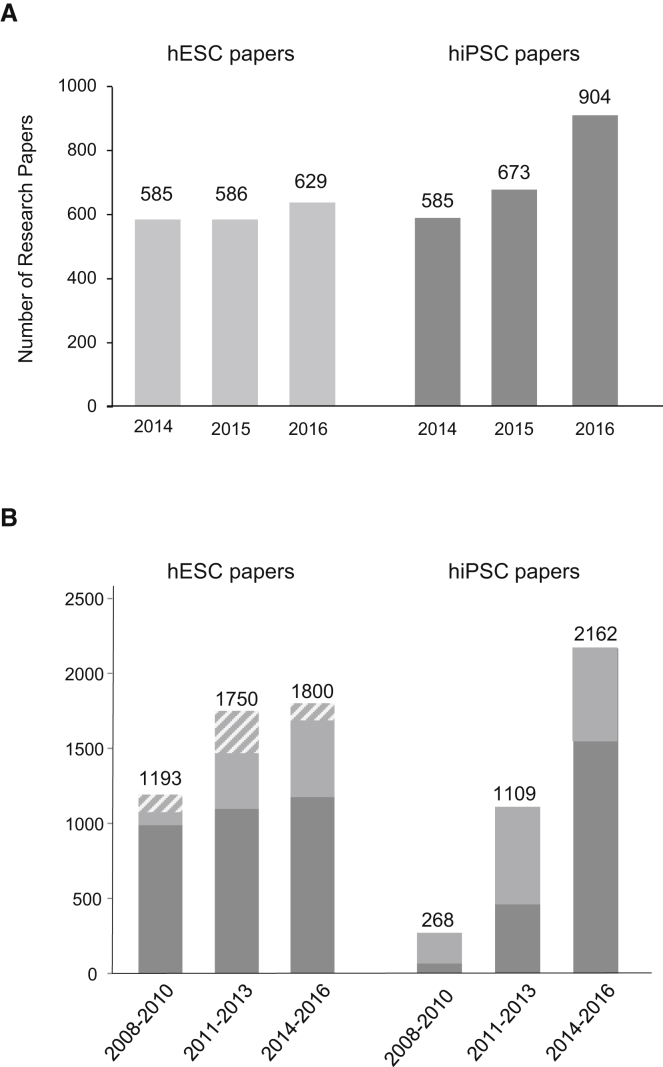
Worldwide Research in Human Pluripotent Stem Cells
(A) Number of original research papers published from 2014 to 2016. Included are all studies in which experimental use of hESCs (left) or/and hiPSCs (right) is reported.
(B) Number of research papers published on experimental use of hESCs (left) or/and hiPSCs (right) in the 3-year periods indicated. The number of papers in which both hESCs and hiPSCs were used in the same study is shown in lighter gray. Mere gold standard usage of hESCs is indicated by gray-white striped lines.
We next wished to examine the origin of research papers in the hPSC field. About 70% of papers in both the hESC and hiPSC fields were authored by scientists from only one country, while in about 30% authors from laboratories based in at least two countries contributed to the publication. As in our earlier studies, we assigned the latter papers to the country of the corresponding author and excluded papers from the hESC paper pool where hESCs were used for mere comparison with hiPSCs (“gold standard” use of hESCs; for example, when novel hiPSC lines were generated and investigated for their pluripotency in comparison with hESCs, 113 papers for the 2014 to 2016 period). From 2014 to 2016, research groups from 40 nations published results of hESC research, while hiPSC research papers came from groups based in 37 countries. Most papers published in both research fields came from US-based groups, followed by groups from China, the UK, Japan, and Korea (hESC field) and by Japan, China, Germany, and the UK (hiPSC field), respectively. To reveal longer-term trends in the contribution of groups from specific nations to hPSC research, we combined our new data for the 2014 to 2016 period with data collected for the years 2008 to 2013 (Kobold et al., 2015) (Figure 2). This analysis confirmed that most of the 4,744 hESC research papers published during the whole period came from research groups based in the United States (40.5% of all research papers worldwide), followed by groups from China (9.2%), the United Kingdom (7.6%), Japan (4.7%), and South Korea (4.1%, Figure 2A). It should be noted that the US contribution to worldwide hESC research decreased by 15.8% (from 44.3% in the 2008 to 2010 period to 37.3% in the 2014 to 2016 period), whereas the contribution of scientific groups from China increased by 58.6% in the same period (from less than 7%–11.1%). With respect to the 3,544 hiPSC papers published during the whole period, we also noted a sustained leading position of US-based research, which contributed with 41.9% to worldwide published hiPSC research, followed by Japan (14.6%), China (8.3%), Germany (6.3%), and the United Kingdom (4.4%, Figure 2B). As in the hESC field, a relative decrease by nearly 30% in the contribution of US research groups was noted (from 53% in the 2008 to 2010 period to about 37.3% in the 2014 to 2016 period), whereas the relative contribution of groups from China and Germany markedly increased.
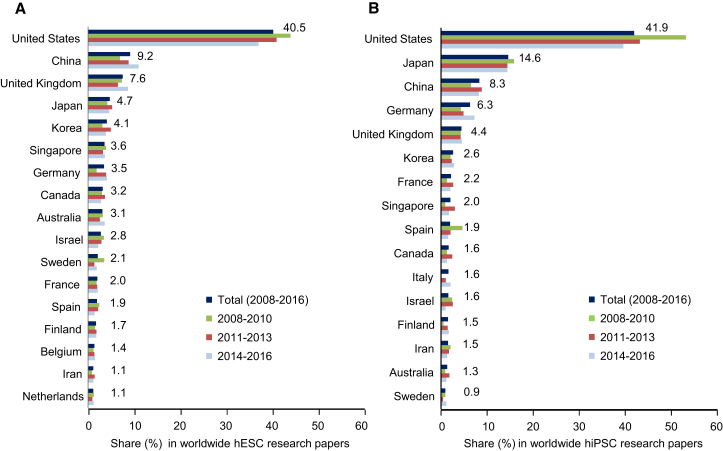
Allocation of hPSC Research to Specific Countries
Share (percentage) of papers from a given country in relation to the total number of hESC research papers (A) and hiPSC research papers (B) for the indicated periods. Only results for countries with more than 50 or 30 original publications in the hESC or hiPSC field, respectively, were included. The numbers to the right of the upper bars relate to the total 9-year period (2008–2016).
Impact of hPSC Research Papers
Next we assessed the scientific impact of published hPSC research from specific countries. Since reliable and comparable citation frequencies are not yet available for papers published in 2016, those papers were not included in the analysis. Instead, we included in our analysis papers published in 2013 since the impact of these papers has not been assessed so far. Thus, the period from 2013 through 2015 was assessed here.
We first determined the average journal impact factor provided by the Institute for Science Information (ISI) for journals that published hESC and hiPSC studies from 2013 to 2015. The weighted overall average 2016 5-year impact factor for journals that had published experimental hESC work was 7.749, while journals that published hiPSC research had an average impact factor of 8.223, indicating a sustained scientific interest in hPSC research. The average impact of papers from specific countries is summarized in Table 1. As already reported for earlier research periods, hPSC research from countries such as Canada, the Netherlands, Israel, or the United States was published in more influential journals than studies published by Chinese, Japanese, or Korean groups.
Table 1
Impact of Research in Human hPSC Published from 2013 to 2015
| hESC Papers (2013–2015) | hiPSC Papers (2013–2015) | ||
|---|---|---|---|
| Country | Average Journal Impact Factor | Country | Average Journal Impact Factor |
| Canada | 12.377 | Canada | 15.503 |
| Netherlands | 9.885 | Israel | 12.709 |
| United States | 9.422 | Netherlands | 11.605 |
| Israel | 9.389 | United States | 10.170 |
| France | 8.943 | Spain | 9.695 |
| Total average | 7.749 | France | 8.650 |
| Singapore | 7.594 | United Kingdom | 8.522 |
| United Kingdom | 7.585 | Total average | 8.223 |
| Germany | 7.345 | Australia | 7.482 |
| Japan | 6.992 | Italy | 7.445 |
| Sweden | 6.647 | Korea | 6.840 |
| Spain | 6.381 | Germany | 6.799 |
| Korea | 6.055 | Singapore | 6.174 |
| Belgium | 6.054 | Sweden | 5.820 |
| China | 4,961 | Japan | 5.794 |
| Australia | 4.892 | China | 5.247 |
| Finland | 4.068 | Finland | 4.244 |
| Iran | 2.836 | Iran | 2.544 |
The 2016 5-years impact factors of the journals that published experimental hESC or hiPSC research papers, respectively, from the countries indicated were summed and divided by the number of research papers from the respective country. In case of hESC papers, work in which hESCs were only used as gold standard for hiPSC research was omitted. Only research from countries with at least 20 hESC and 15 hiPSC research papers, respectively, was included.
However, the impact factor of a journal does not necessarily mirror the actual citation numbers of individual papers published in the respective journal. Therefore, we analyzed, as a more reliable measure for the impact of research, the average frequencies at which hESC and hiPSC research papers were cited through the end of 2017. For this purpose, the citation numbers for each paper were determined using the Web of Science database and weighted according to the date of publication. Average citation frequencies per year of papers from selected countries are shown in Table 2. Although there are minor differences in comparison with the data shown in Table 1, the results in principle confirm the high influence of research published by groups from Canada, the Netherlands, the United States, and Israel in both research fields. Unexpectedly, hESC research from Japan and Germany over-performed with respect to actual citation frequencies, while the impact of hiPSC research from both countries is lower than average. This is surprising, because hiPSC research is highly supported in these countries, while use of hESC is strictly regulated, especially in Germany. To ascertain that the diversity in average citation frequencies among papers from several countries is not caused by only a few popular and extremely highly cited papers, we grouped studies according to their citation frequency per year (Tables S1 and S2). While the percentage of all papers in the hESC and hiPSC field that were cited more than 15 times per year was less than 15%, the proportion of papers from Canada, the Netherlands, and the United States that were cited at high rates was clearly higher. Therefore, a rather broad range of hPSC papers contributes to the high citation frequency of research from these countries.
Table 2
Average Citation Frequencies per Year of hPSC Research Papers
| hESC Papers (2013–2015) | hiPSC Papers (2013–2015) | ||
|---|---|---|---|
| Country | Average Citation Numbers per Year | Country | Average Citation Numbers per Year |
| Netherlands | 13.6 | Canada | 13.8 |
| United States | 12.2 | United States | 13.8 |
| Canada | 11.6 | Israel | 13.3 |
| Germany | 10.1 | United Kingdom | 12.0 |
| Japan | 9.7 | Australia | 11.9 |
| United Kingdom | 9.5 | Netherlands | 11.2 |
| Total average | 9.2 | Total average | 10.7 |
| Israel | 8.8 | Spain | 9.3 |
| France | 7.6 | Germany | 8.9 |
| Singapore | 7.3 | Japan | 8.6 |
| Korea | 6.9 | Italy | 7.8 |
| Sweden | 6.3 | Sweden | 7.3 |
| Spain | 5.1 | Singapore | 6.4 |
| Australia | 5.0 | Korea | 6.3 |
| Belgium | 4.9 | France | 6.3 |
| China | 4.9 | China | 6.1 |
| Finland | 4.3 | Finland | 5.1 |
| Iran | 2.9 | Iran | 3.4 |
Citation numbers were determined using the Web of Science database, and data were normalized as described in the Experimental Procedures section. In the case of hESC papers, work in which hESCs were only used as gold standard for hiPSC research was omitted. Only research from countries with at least 20 hESC and 15 hiPSC research papers, respectively, was included.
Application of hESC Lines in Research
Over the past decade, we and others reported the predominant use of only a few hESC lines in experimental research, which were derived early in the field (Guhr et al., 2006, Löser et al., 2010, McCormick et al., 2009, Scott et al., 2009). However, while some supposed that this phenomenon was caused by a long-lasting and trans-national effect of the research policy of the Bush administration (Scott et al., 2009), we demonstrated more recently that it can be better explained on the basis of power laws (Schuldt et al., 2013). To elucidate whether there are novel trends in the application of certain established hESCs, we analyzed the use of hESC lines in experimental research published from 2008 to 2016. Inspection of hESC research papers revealed that application and/or derivation of a total of 1,419 different hESC lines was reported from 2008 to 2016. Table 3 shows those 21 hESC lines that were used most frequently in these studies. There was no fundamental change in the usage of hESC lines compared with our earlier studies (Guhr et al., 2006, Löser et al., 2010, Schuldt et al., 2013), as expected on the basis of our power law model. Again, three of the five oldest hESC lines (WiCell H1 [WAe001-A], H7 [WAe007-A], and H9 [WAe009-A]), already published in 1998, were most commonly used. These three hESC lines were used in more than 74% of countries that contributed to hESC research from 2008 to 2016 (32 of 43 countries). However, research from countries in which none of these lines were applied accounted for only 1.5% of total papers. The cell line H9 was used in more than 2,200 studies published from 2008 to 2016 (46.4% of all published original research papers involving experimental use of hESCs), followed by hESC lines H1 (23.5%), H7 (7.4%), HES-3 (ESIBIe003-A, 6.0%), HUES9 (HVRDe009-A, 3.8%), and BG01 (VIACe001-A, 3.6%). With the notable exception of HUES9, which was only published in 2004, these hESC lines as well as five other lines among these top 21 ( H14 [WAe014-A], HES-2 [ESIBIe002-A], HSF-1 [UCSFe003-A] and HSF-6 [UCSFe002-A], and H13 [WAe013-A]) were already available to NIH-funded US researchers before the change in the US stem cell policy under the Obama administration, supporting our earlier notion that this political shift had little impact on established stem cell usage patterns.
Table 3
Most Frequently Used hESC Lines
| hESC Line | hPSCreg Nomenclature | 2008–2010 | 2011–2013 | 2014–2016 | Total (2008–2016) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Papers | % of Papers | Number of Papers | % of Papers | Number of Papers | % of Papers | Number of Papers | % of Papers | ||
H9 | WAe009-A | 495 | 41.5 | 847 | 48.4 | 860 | 47.8 | 2202 | 46.4 |
H1 | WAe001-A | 289 | 26.9 | 411 | 23.5 | 414 | 23.0 | 1114 | 23.5 |
H7 | WAe007-A | 93 | 8.7 | 136 | 7.8 | 122 | 6.8 | 351 | 7.4 |
HES-3 | ESIBIe003-A | 80 | 7.4 | 104 | 5.9 | 103 | 5.7 | 287 | 6.0 |
| HUES9 | HVRDe009-A | 60 | 5.6 | 64 | 3.7 | 54 | 3.0 | 178 | 3.8 |
BG01 | VIACe001-A | 81 | 7.5 | 60 | 3.4 | 31 | 1.7 | 172 | 3.6 |
HES-2 | ESIBIe002-A | 60 | 5.6 | 67 | 3.8 | 45 | 2.5 | 172 | 3.6 |
| KhES-1 | KUIMSe001-A | 37 | 3.4 | 52 | 3.0 | 45 | 2.5 | 134 | 2.8 |
| HUES7 | HVRDe007-A | 31 | 2.9 | 35 | 2.0 | 34 | 1.9 | 100 | 2.1 |
| KhES-3 | KUIMSe003-A | 24 | 2.2 | 47 | 2.7 | 25 | 1.4 | 96 | 2.0 |
HSF-6 | UCSFe002-A | 44 | 4.1 | 31 | 1.8 | 17 | 0.9 | 92 | 1.9 |
H14 | WAe014-A | 27 | 2.5 | 35 | 2.0 | 23 | 1.3 | 85 | 1.8 |
| HUES6 | HVRDe006-A | 16 | 1.5 | 33 | 1.9 | 26 | 1.4 | 75 | 1.6 |
| HUES1 | HVRDe001-A | 28 | 2.6 | 22 | 1.3 | 17 | 0.9 | 67 | 1.4 |
| HUES3 | HVRDe003-A | 23 | 2.1 | 26 | 1.5 | 17 | 0.9 | 66 | 1.4 |
| HUES8 | HVRDe008-A | 17 | 1.6 | 20 | 1.1 | 28 | 1.6 | 65 | 1.4 |
| HS181 | KIe001-A | 29 | 2.7 | 20 | 1.1 | 12 | 0.7 | 61 | 1.3 |
HSF-1 | UCSFe003-A | 25 | 2.3 | 23 | 1.3 | 8 | 0.4 | 56 | 1.2 |
| MEL-1 | SCSe001-A | 12 | 1.1 | 24 | 1.4 | 18 | 1.0 | 54 | 1.1 |
| CA1 | MSHRIe001-A | 16 | 1.5 | 24 | 1.4 | 10 | 0.6 | 50 | 1.1 |
H13 | WAe013-A | 17 | 1.6 | 23 | 1.3 | 8 | 0.4 | 48 | 1.0 |
Shown are the numbers of papers that report on experimental use of the particular hESC line. The percentage values present the share of publications in the total number of hESC research papers in the given period. Only results for cell lines that were used in at least 1% of papers published from 2008 to 2016 are shown. Please note that in many studies more than one hESC line was used. Sublines and (genetically modified) derivatives were allocated to the respective parental hESC line. hESC lines that were derived before August 22nd 2001 are marked with asterisks. hPSCreg, Human Pluripotent Stem Cell Registry of the European Union.
To examine whether the predominant use of only a few well-characterized hESC lines is mirrored by a possible long-lasting decrease in the derivation of novel hESC lines, we next determined the number of hESC lines derived from IVF embryos and published during the 9-year period from 2008 to 2016. hESC lines that were produced from nuclear transfer embryos or entities derived by parthenogenetic activation of human oocytes were not included in our analyses. By November 2009, 1,071 such original hESC lines were publicly known, and nearly 694 (64.8%) of them were published in peer-reviewed English-language journals listed in the PubMed database (Löser et al., 2010). By 2016, at least 2,168 original hESC lines were publicly known, 1,544 (71.2%) of which were published in peer-reviewed journals. To unveil possible trends in the time course of hESC derivation, we inspected hESC research papers published from 2008 to 2016 for reports on derivation of novel hESC lines or on the use of (novel) hESC lines that were not used in previously published research, respectively. The results of the analysis are shown in Figure 3. Although 566 novel hESC lines were reported in the scientific literature published from 2008 to 2010, this number decreased to 301 and 220 for the following 3-year periods 2011 to 2013 and 2014 to 2016, respectively (Figure 3A). Notably, an increasing percentage of the novel lines were either derived from pre-implantation genetic diagnosis embryos to model genetically inherited diseases (disease-specific hESC lines) or were produced for future clinical applications (clinical-grade hESC lines; Figure 3B). Thus, the availability of well-characterized hESC lines that are accepted and broadly used by the scientific community seems to cause a decreasing interest in deriving novel research hESC lines, and obviously new hESC lines are produced increasingly for specific scientific and future clinical applications.
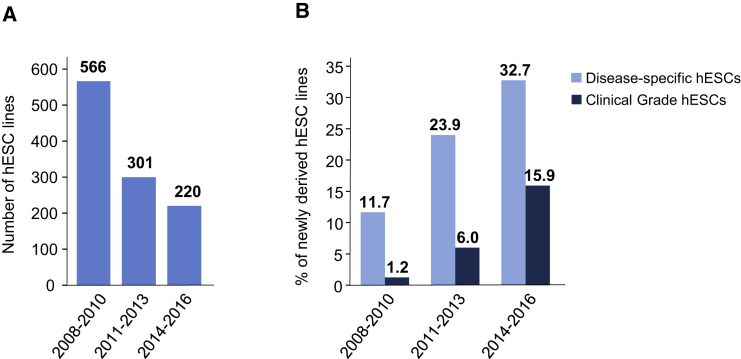
Derivation of New hESC Lines (2008–2016)
(A) Number of hESC lines that were reported for the first time in original research papers during the time periods indicated.
(B) Percentage of disease-specific (light blue) and clinical-grade (dark blue) hESC lines in total new cell lines reported in original research papers during the time periods indicated.
Application of hESC Lines in Clinical Trials
Next we were interested to determine which particular pluripotent stem cell lines had been used in clinical trials performed so far that involved hPSC-derived cell products. Since research in hPSCs has resulted in considerable progress, clinical trials based on such cell products have been initiated roughly over the past 5 years to test novel cell-based therapies for the treatment of different pathologic conditions, such as macular degeneration, diabetes mellitus, or spinal cord injury in humans (Trounson and DeWitt, 2016, Trounson and McDonald, 2015). An overview on clinical studies so far approved based on hPSC-derived cell products is given in Table 4. Currently (March 2018), there is public information on 29 trials involving hESC-derived stem cell products, while three trials that involve hiPSC derivatives have been approved so far. Most of these studies are aiming at testing of safety and tolerability of the respective stem cell products in a small patient cohort. Five of the 29 hESC-based trials are follow-up studies and two additional already-approved trials were surrendered to modify the study design. In addition, there is one clinical trial that is based on pluripotent stem cells derived from a parthenote. A closer analysis of information publicly provided on hESC-based studies revealed that the stem cell materials used in these clinical trials are derived from only a few hESC lines (Table S3). To our knowledge, of the cell lines listed in Table S3, only HADC102 was established as a clinical-grade cell line from the start, while the other lines were originally established as research-grade lines and only later adapted to cGMP conditions for clinical use. With respect to hiPSCs, it is notable that only one of the three trials approved so far was planned to be performed with autologous cell products derived from the patient's own hiPSCs.
Table 4
Clinical Trials Based on Human Pluripotent Stem Cells
| Sponsor | Disease(s) (as Indicated) | Study ID | Country |
|---|---|---|---|
| Clinical Trials Based on hESCs | |||
| Assistance Publique – Hôpitaux de Paris | ischemic heart disease | NCT02057900 | France |
| Astellas Pharma | Stargardt macular dystrophy | NCT01345006 | United States |
| Astellas Pharma | advanced dry AMD | NCT02463344 | United States |
| Astellas Pharma | AMD | NCT03178149 | not specified |
| Astellas Pharma | AMD | NCT01344993 | United States |
| Astellas Pharma | Stargardt macular dystrophy | NCT02941991 | UK |
| Astellas Pharma | Stargardt macular dystrophy | NCT01469832 | UK |
| Astellas Pharma | Stargardt macular dystrophy | NCT02445612 | United States |
| Astellas Pharma | macular degenerative disease | NCT03167203 | not specified |
| Asterias Biotherapeutics | spinal cord injury | NCT02302157 | United States |
| Asterias Biotherapeutics | spinal cord injury | NCT01217008 | United States |
| Cell Cure Neurosciences | AMD | NCT02286089 | Israel, United States |
| CHA Biotech | dry AMD | NCT01674829 | Korea |
| CHA Biotech | Stargardt macular dystrophy | NCT01625559 | Korea |
| Chinese Academy of Sciences | dry AMD | NCT03046407 | China |
| Chinese Academy of Sciences | nonexudative AMD | NCT02755428 | China |
| Chinese Academy of Sciences | Parkinson's disease | NCT03119636 | China |
| Chinese Academy of Sciences, Institute of Zoology | dry AMD | ChiCTR-OCB-15007054 | China |
| Chinese Academy of Sciences, Institute of Zoology | retinitis pigmentosa | ChiCTR-OCB-15007055 | China |
| Eye Institute of Xiamen University | severe ocular surface diseases | ChiCTR-OCB-15005968 | China |
| Federal University of São Paulo | AMD, Stargardt disease, exudative AMD | NCT02903576 | Brazil |
| Pfizer | AMD | NCT03102138 | UK |
| Pfizer | AMD | NCT01691261 | UK |
| Regenerative Patch Technologies | dry AMD | NCT02590692 | United States |
| Southwest Hospital, China | macular degeneration diseases, not specified | NCT02749734 | China |
| Viacyte | diabetes mellitus type 1 | NCT03162926 | Canada |
| Viacyte | diabetes mellitus type 1 | NCT02239354 | United States, Canada |
| Viacyte | diabetes mellitus type 1 | NCT02939118 | United States, Canada |
| Viacyte | diabetes mellitus type 1 with hypoglycemia | NCT03163511 | United States |
| Clinical Trials Based on hiPSCs | |||
| RIKEN | exudative AMD | UMIN000011929 (based on autologous iPSCs) | Japan |
| Cynata Therapeutics | graft-versus-host disease | NCT02923375 (based on allogenic iPSCs) | Australia, UK |
| Kobe City Medical Center General Hospital | neovascular AMD | UMIN000026003 (based on allogenic iPSCs) | Japan |
Follow-up studies among hPSC-based trials are highlighted with a blue background. Withdrawn studies were not included. Studies that only aim at the derivation of patient-specific hiPSC lines and therapeutic cells thereof (but not at the treatment of patients with these hiPSC-derived therapeutic cells) were not included either. iPSCs, induced pluripotent stem cells.
Discussion
Nearly 20 years after the first publication of the successful derivation of hPSCs, we report a sustained interest in hESCs and strongly increasing research activities with hiPSCs. Our findings are based on a manually curated database of experimental hPSC papers that mirrors the genuine research in this field. The manual curation avoids the risk of the presence of non-relevant papers usually found in data pools based on mere and partially automatized web searches, which may result in over-estimation of real research activities (Kobold et al., 2015).
Our analysis of the hPSC landscape shows that research involving hESCs and/or hiPSCs is still increasing, albeit with a much faster increase for the hiPSC field. While this trend can be easily anticipated for hiPSCs, a continuing interest in hESC research cannot necessarily be expected. hESCs are more difficult to derive than hiPSCs and are not as easily accessible as hiPSCs for researchers. Consequently, only comparatively few laboratories have their own hESC lines. Moreover, research in hESCs is controversial for ethical reasons and legally tightly regulated in many countries. For example, in Germany, researchers who plan to use hESCs have to show that hESCs cannot be replaced by hiPSCs in their research project before hESC usage will be approved. Despite this, our data consolidate the previous finding, that research in hESCs and hiPSCs still existed independently and only partially overlapped in recent years. Moreover, use of hESC as a mere “gold standard” control for hiPSC research (which was speculated to be the main area of application for hESCs after emergence of hiPSCs) remains a rather small area.
We also detected some changes in regional distribution of hPSC research. Although US-based scientists considerably contributed to hPSC work published in recent years, the share of US-based research in worldwide research is decreasing. In contrast, especially Chinese groups increasingly published in the hESC field, while scientific groups from Germany increasingly contributed to worldwide hiPSC research. The growing performance of groups from these two countries in hESC and hiPSC research, respectively, may be an immediate consequence of extensive funding programs and strong political support of these particular types of research.
A general look at the impact of hESC research reveals a gradual growth of the overall average impact factor from 6.030 in 2005 (Guhr et al., 2006) to 7.422 in 2009 (Löser et al., 2010) and 7.749 in the present study. In recent years, research with the highest impact was published by researchers from Canada, the Netherlands, the United States, and Israel, confirming their position from previous years, while research from Korea, Singapore, and China underperformed. In addition to real quality differences of research, a possible publication bias in English-language journals, especially for groups from Asian countries, may explain this observation.
Also confirming earlier findings is the high divergence of the average citation numbers of papers from different countries, which is a more reliable measure of the actual relevance of a given publication. Again, we found a predominance of papers from countries such as Canada, the US, the Netherlands, the UK, and Israel with respect to their average citation numbers, while the above-average position of hESC papers from Germany is a rather unexpected result since Germany has relatively restrictive hESC legislation. Interestingly, we noted an average underperformance of research from Japanese and German groups with respect to impact in the hiPSC field. This is difficult to explain, especially for Japan. For this reason, we repeated the citation analysis using the Scopus database (Elsevier), which confirmed our results in general (data not shown). One possible explanation for this phenomenon could be the comparatively broad and generous funding policy with respect to hiPSC (but not to hESC) research in both countries, which might also promote less competitive research performed by less experienced scientists. This could possibly reduce the average quality of research, resulting in a diminished average impact of publications. However, such a broad funding policy will most likely open hiPSC research to scientists new in the field who may contribute substantially to the scientific performance of these countries in the future.
When looking at the hESC lines actually used in published research, our data confirm former results with respect to hESC usage patterns of only a handful of established lines as predicted by our power law model (Schuldt et al., 2013). Overall, although research with hESC is not decreasing, the derivation of new lines is regressive. This is likely due to the existence and widespread use of well-characterized established lines such as H1, H7, and H9, and to the emergence of hiPSCs. The use of only few lines in a large percentage of hESC studies may be of advantage with respect to the comparability and reproducibility of results. On the other hand, the dominance of only a few hESC lines in research is accompanied by a lack of universal applicability of results as well as by genetic under-representation and dependence on only a few suppliers.
Additionally, our findings are in good agreement with data obtained from the hPSC registry of the European Union (hPSCreg, https://hpscreg.eu) (Seltmann et al., 2016). According to the data in hPSCreg, the generation of new hESC lines increased and peaked in the 2005 to 2007 period, after which the numbers of newly derived lines started to decrease. We found that a large portion of hESC lines published in the past decade were generated for specific purposes such as to provide disease models or to generate clinical-grade lines. We are aware that the actual number of hESC lines cannot be determined since only limited information is publicly available. In this study, we therefore focused on hESC lines published in the peer-reviewed scientific literature since we consider this a reliable measure for quality and accessibility of hESC lines. It should also be noted that there is frequently a gap between derivation and publication dates of hESC lines. Consequently, our data reflect the time points of availability of new hESC lines to the scientific community rather than the dates of their derivation.
A quantitative assessment of trends in hiPSC derivation is almost impossible. We noticed that the increasing convenience of hiPSC derivation has caused many laboratories to establish their own hiPSCs. However, in many cases, only limited information on the exact number and the degree of characterization of derived hiPSC lines is provided in published research. In addition, the naming of lines is frequently ambiguous and, in a range of cases, well-characterized lines and picked clones cannot be discriminated from the data presented in the respective papers. A recently proposed standard nomenclature for hPSC lines would be required to trace the cells and their data (Kurtz et al., 2018). However, based on our analysis of more than 3,500 hiPSC research papers, we estimate that the number of hiPSC lines published in the literature up to now exceeds 10,000 by far, many of them derived from patients suffering from genetic diseases. In addition, there are commercial and academic hiPSC banks that host thousands of hiPSC lines, although the degree of characterization of specific cell lines is not clear in many cases (De Sousa et al., 2017). hPSCreg currently registers approximately five times more hiPSC than hESC lines, reflecting the ratio and an increasing number of hiPSC lines versus a rather stable number of registered hESC lines (S.S. et al., unpublished data).
One of the major promises of hPSC is their clinical application in regenerative therapies. We provide a comprehensive overview of clinical trials that have been performed so far based on hESC- and hiPSC-derived cell products. Our data are based on an intensive long-term observation of the field and collection of publicly available information over the past 7 years. However, it should be noted that some of the clinical trials listed in Table 4 are performed under the clinical research study pathway, which may not directly lead to a new medical product marketing registration. These and other studies, which are performed in the frame of specific “regenerative medicine” regulatory frameworks, have the potential to speed up the clinical translation of hPSC research in an academic setting but may not directly result in applicable cell products. This is different for the often commercially driven clinical trials that are performed under the formal "clinical trial" pathway supervised by the national drug regulatory agencies. It should also be noted that simply searching clinical trial registries for the term “hiPSC” usually results in many hits. However, an analysis of the respective trials reveals that most of them only refer to the production of clinical-grade hiPSC lines from specific patients but not to a treatment of patients with hiPSC-derived therapeutic cells. These studies were therefore not included in our results, but it shows that a dedicated clinical trial registry for hPSC-based studies is highly desirable for ethical reasons to provide transparency and avoid replication (Fortunato et al., 2018). Our data show that most studies performed so far use hESCs, most likely because these cells have been available for a longer period, and their derivation is not complicated by additional reprogramming manipulations. The hESC-based clinical studies are performed with very few, mostly older lines. However, we also found publicly available information on at least 11 additional planned hPSC-based studies, four of them using hESCs. Since newer, clinical-grade hESC lines are available now, it will be interesting to see whether hESC usage patterns will be sustained in clinical studies as well, or whether these new lines will replace older lines quickly.
One of the major advantages of hiPSCs is the potential for autologous application. However, the only study using autologous hiPSC lines was put on hold because of mutations of unknown potential risk in the hiPSC source material (Garber, 2015). This study was continued with allogeneic, HLA-matched hiPSCs. Whether autologous, personalized hiPSCs, or HLA-haplobanks of hiPSC lines, will become an affordable option for personalized medicine remains disputable (Blair and Barker, 2016). Since HLA matching will not be perfect in the majority of cases, lifelong immunosuppression may be unavoidable. Unless affordable technologies for autologous clinical hiPSC use will be developed, their allogenic use will most likely diminish the effects of the biggest advantage of hiPSCs over hESCs for cell therapy applications.
In conclusion, we have shown a continuing parallel research at high level and impact with hESCs and hiPSCs, while the increase in published research with hiPSCs accelerates faster than that of hESC research, surpassing those by 2015. While hESC usage patterns remained unchanged over the past decade, the derivation of new hESC lines for disease modeling and clinical application may foreshadow a similar trend for hiPSC research, although data to assess these tendencies are difficult to obtain and would need centralized resources, common standards for characterization, and traceable nomenclature. However, we expect that the hiPSC usage patterns will be less pronounced than that observed for hESC lines in research since hiPSCs are much easier to obtain. As the field is consolidating, we predict a trend toward a more diverse source of hESC and hiPSC lines required for tailored applications; e.g., to reflect human and disease diversity, or to improve safety by using dedicated clinical-grade lines in clinical trials.
Experimental Procedures
Paper Selection
The publication repositories for experimental work involving hESCs and/or hiPSCs used for former analyses were described in Kobold et al. (2015). We extended these repositories to papers published from 2014 to 2016 by searches of the PubMed database accessible through the NIH National Library of Medicine using the search strings described earlier (Guhr et al., 2006, Muller et al., 2010) with slight modifications of the search string to identify papers involving hiPSCs. All papers were inspected manually for use of hESCs and hiPSCs, respectively, before they were added to the repositories, and studies that exclusively used pluripotent stem cells from species other than human (e.g., mouse, primates) were removed. Our hPSC paper repositories therefore only contain original research papers in which hESCs and/or hiPSCs were used experimentally. Review papers, news, comments, and editorials, as well as studies on legal and ethical aspects of research in human pluripotent stem cells, were not included. The paper repository contains neither methodical reviews and previously published protocols nor papers that describe experimental work that exclusively used stem cells derived from embryos produced by nuclear transfer or from entities produced by parthenogenetic activation of human oocytes. Studies in which pluripotent stem cell-derived material (such as hESC RNA) or cells (such as hiPSC-derived cardiomyocytes) were not included either. The same applies to studies in which data obtained in previous research (such as expression data available from the GEO database) were used. Papers that were only pre-published in 2016 (but appeared in print in 2017 or later) were not considered. The decision on the assignment of hESC use to the category “gold standard” application was made by using the criteria described before (Kobold et al., 2015). Allocation of a paper to a country was done according to the corresponding author's affiliation. Detailed search strings are available on request.
Determination of Weighted Average Impact Factors and Citation Numbers
Determination of weighted average impact factors was performed using the 2016 5-year impact factors published by the Journal Citation Reports (Clarivate Analytics). Of 828 journals that published experimental work on hPSCs from 2014 to 2016, 95 (11.4%) had not been judged by the ISI, affecting 103 papers extracted by our search method (3.1%). These papers were not included in the analysis. The 5-year impact factor for each journal was multiplied by the number of papers that were published in the respective journal. The results were summed and divided by the total paper numbers to obtain the average 5-year impact factor.
Citation numbers were determined by using the Web of Science database (Clarivate Analytics). Average annual citation frequencies of papers published in journals with an impact factor were calculated by dividing the citation number by the number of years after the study was published (e.g., for a study that was published in 2013, number of citations from 2014 to 2017 was summed and divided by 4).
Determination of hESC Line Numbers and Usage
Information on hESC lines reported by the end of 2009 is based on data published previously (Löser et al., 2010). Information on novel hESC lines was collected from the scientific papers identified in this study and by Kobold et al. (2015). In addition, registries and stem cell banks were screened for the appearance of novel hESC lines:
- • Human pluripotent stem cell registry of the European Union (hPSCreg): https://hpscreg.eu/
- • hESC registry of the NIH: http://stemcells.nih.gov/research/registry/
- • International Stem Cell Registry of the University of Massachusetts (UMass) Medical School: https://www.umassmed.edu/iscr/
- • List of acceptably derived embryonic stem cell lines, California Institute for Regenerative Medicine: https://www.cirm.ca.gov/our-funding/acceptably-derived-embryonic-stem-cell-lines
- • Coriell's Stem Cell Biobank: https://www.coriell.org/1/Stem-Cells/Stem-Cell-Services
- • eagle-i iPS search tool: https://search.eagle-i.net/central/iPSCellSearch.html
- • Integrated Collection of Stem Cell Bank data by MIACARM (ISCBI): http://icscb.stemcellinformatics.org/
- • UK Stem Cell Bank: http://www.nibsc.org/ukstemcellbank
- • Canadian National Registry of Human Embryonic Stem Cell Lines: http://www.cihr-irsc.gc.ca/e/39580.html
- • Korean Stem Cell Bank: http://www.cdc.go.kr/CDC/eng/contents/CdcEngContentView.jsp?cid=60433&menuIds=HOME002-MNU1628-MNU1629-MNU1631
- • Stem Cell Bank of Barcelona: https://www.cmrb.eu/banco-lineas-celulares/en_index.html
- • List of hESC lines derived in France, Agence de la Biomédecine: https://www.agence-biomedecine.fr/liste-et-caracteristiques?lang=fr
- • Andalusian Stem Cell Bank, Biobanco del Sistema Sanitario Público de Andalucía: http://www.juntadeandalucia.es/salud/biobanco/servicios/provision?hijo=356
- • Stem Cell Repository of the New York Stem Cell Foundation: https://nyscf.org/research-institute/repository-stem-cell-search/
- • University of Connecticut-Wesleyan University Stem Cell Core: https://health.uconn.edu/stem-cell-core/
- • WiCell Stem Cell Bank: https://www.wicell.org/home/stem-cell-lines/stem-cell-lines.cmsx
- • Genea Biocells Stem Cell Bank: http://geneabiocells.com/technology-platform/stem-cell-bank/
Furthermore, additional public information on novel hESCs released in the press was obtained by continuously examining Google News Alerts containing the terms “stem cell” or “stem cells.”
To determine hESC usage in individual studies, full text and supplementary information of hESC research papers were manually inspected. Sublines (e.g., clonal derivatives or genetically modified sublines) were allocated to the respective parental hESC line.
Determination of Clinical Trials Involving hPSCs
Clinical trials were identified by screening the ClinicalTrials.gov, by the US National Library of Medicine (https://clinicaltrials.gov/) and the International Clinical Trials Registry Platform of the World Health Organization (http://apps.who.int/trialsearch/). In addition, the sponsor's Web pages were evaluated for additional information on the respective clinical trials, and public information in the press was obtained by continuously examining Google News Alerts containing the terms “stem cell” or “stem cells” and “clinical trials.”
Author Contributions
A.G., S.K., S.S., and P.L. performed the collection and assembly of data. A.E.M.S.W., A.K., and P.L. interpreted the data. A.G., A.E.M.S.W., A.K., S.K., S.S., and P.L., performed the data analysis. A.E.M.S.W., A.K., and P.L. conceived and designed the study and wrote the manuscript.
Acknowledgments
This work was supported by EU-grant 726320 (hPSCreg).
Notes
Published: July 19, 2018
Footnotes
Supplemental Information includes three tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.06.012.
Supplemental Information
References
- Blair N.F., Barker R.A. Making it personal: the prospects for autologous pluripotent stem cell-derived therapies. Regen. Med. 2016;11:423–425. [Abstract] [Google Scholar]
- De Sousa P.A., Steeg R., Wachter E., Bruce K., King J., Hoeve M., Khadun S., McConnachie G., Holder J., Kurtz A. Rapid establishment of the European Bank for Induced Pluripotent Stem Cells (EBiSC) - the hot start experience. Stem Cell Res. 2017;20:105–114. [Abstract] [Google Scholar]
- Fortunato A., Grainger D.W., Abou-El-Enein M. Enhancing patient-level clinical data access to promote evidence-based practice and incentivize therapeutic innovation. Adv. Drug Deliv. Rev. 2018 [Abstract] [Google Scholar]
- Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotechnol. 2015;33:890–891. [Abstract] [Google Scholar]
- Guhr A., Kurtz A., Friedgen K., Löser P. Current state of human embryonic stem cell research: an overview of cell lines and their use in experimental work. Stem Cells. 2006;24:2187–2191. [Abstract] [Google Scholar]
- Holm S. Time to reconsider stem cell ethics–the importance of induced pluripotent cells. J. Med. Ethics. 2008;34:63–64. [Abstract] [Google Scholar]
- Hyun I., Hochedlinger K., Jaenisch R., Yamanaka S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1:367–368. [Abstract] [Google Scholar]
- Kobold S., Guhr A., Kurtz A., Löser P. Human embryonic and induced pluripotent stem cell research trends: complementation and diversification of the field. Stem Cell Rep. 2015;4:914–925. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurtz A., Seltmann S., Bairoch A., Bittner M.S., Bruce K., Capes-Davis A., Clarke L., Crook J.M., Daheron L., Dewender J. A standard nomenclature for referencing and authentication of pluripotent stem cells. Stem Cell Rep. 2018;10:1–6. [Europe PMC free article] [Abstract] [Google Scholar]
- Löser P., Schirm J., Guhr A., Wobus A.M., Kurtz A. Human embryonic stem cell lines and their use in international research. Stem Cells. 2010;28:240–246. [Europe PMC free article] [Abstract] [Google Scholar]
- McCormick J.B., Owen-Smith J., Scott C.T. Distribution of human embryonic stem cell lines: who, when, and where. Cell Stem Cell. 2009;4:107–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Muller F.J., Goldmann J., Löser P., Loring J.F. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6:412–414. [Abstract] [Google Scholar]
- Negoro T., Okura H., Matsuyama A. Induced pluripotent stem cells: global research trends. Biores. Open Access. 2017;6:63–73. [Europe PMC free article] [Abstract] [Google Scholar]
- Owen-Smith J., McCormick J. An international gap in human ES cell research. Nat. Biotechnol. 2006;24:391–392. [Abstract] [Google Scholar]
- Power C., Rasko J.E. Will cell reprogramming resolve the embryonic stem cell controversy? A narrative review. Ann. Intern. Med. 2011;155:114–121. [Abstract] [Google Scholar]
- Schuldt B.M., Guhr A., Lenz M., Kobold S., MacArthur B.D., Schuppert A., Löser P., Muller F.J. Power-laws and the use of pluripotent stem cell lines. PLoS One. 2013;8:e52068. [Europe PMC free article] [Abstract] [Google Scholar]
- Scott C.T., McCormick J.B., Owen-Smith J. And then there were two: use of hESC lines. Nat. Biotechnol. 2009;27:696–697. [Europe PMC free article] [Abstract] [Google Scholar]
- Seltmann S., Lekschas F., Muller R., Stachelscheid H., Bittner M.S., Zhang W., Kidane L., Seriola A., Veiga A., Stacey G. hPSCreg–the human pluripotent stem cell registry. Nucleic Acids Res. 2016;44:D757–D763. [Europe PMC free article] [Abstract] [Google Scholar]
- Sipp D. Gold standards in the diamond age: the commodification of pluripotency. Cell Stem Cell. 2009;5:360–363. [Abstract] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. [Abstract] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. [Abstract] [Google Scholar]
- Trounson A., DeWitt N.D. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 2016;17:194–200. [Abstract] [Google Scholar]
- Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. [Abstract] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. [Abstract] [Google Scholar]
Articles from Stem Cell Reports are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.stemcr.2018.06.012
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S2213671118302753/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.stemcr.2018.06.012
Article citations
An African perspective on genetically diverse human induced pluripotent stem cell lines.
Nat Commun, 15(1):8581, 03 Oct 2024
Cited by: 0 articles | PMID: 39362853 | PMCID: PMC11450012
Guidelines for managing and using the digital phenotypes of pluripotent stem cell lines.
Stem Cell Reports, 19(10):1369-1378, 26 Sep 2024
Cited by: 0 articles | PMID: 39332404 | PMCID: PMC11561460
Review Free full text in Europe PMC
Manufacturing Parameters for the Creation of Clinical-Grade Human-Induced Pluripotent Stem Cell Lines From Umbilical Cord Mesenchymal Stromal Cells.
Stem Cells Transl Med, 13(5):454-461, 01 May 2024
Cited by: 0 articles | PMID: 38402590 | PMCID: PMC11092272
Ultrasound-triggered three dimensional hyaluronic acid hydrogel promotes in vitro and in vivo reprogramming into induced pluripotent stem cells.
Bioact Mater, 38:331-345, 09 May 2024
Cited by: 0 articles | PMID: 38764447 | PMCID: PMC11101682
Spinal Cord Organoids to Study Motor Neuron Development and Disease.
Life (Basel), 13(6):1254, 25 May 2023
Cited by: 3 articles | PMID: 37374039 | PMCID: PMC10303776
Review Free full text in Europe PMC
Go to all (40) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 27 of 27)
- (1 citation) ClinicalTrials.gov - NCT02749734
- (1 citation) ClinicalTrials.gov - NCT03163511
- (1 citation) ClinicalTrials.gov - NCT02286089
- (1 citation) ClinicalTrials.gov - NCT03102138
- (1 citation) ClinicalTrials.gov - NCT02057900
- (1 citation) ClinicalTrials.gov - NCT02903576
- (1 citation) ClinicalTrials.gov - NCT03178149
- (1 citation) ClinicalTrials.gov - NCT01691261
- (1 citation) ClinicalTrials.gov - NCT03046407
- (1 citation) ClinicalTrials.gov - NCT02941991
- (1 citation) ClinicalTrials.gov - NCT01344993
- (1 citation) ClinicalTrials.gov - NCT01345006
- (1 citation) ClinicalTrials.gov - NCT01217008
- (1 citation) ClinicalTrials.gov - NCT02939118
- (1 citation) ClinicalTrials.gov - NCT03167203
- (1 citation) ClinicalTrials.gov - NCT01625559
- (1 citation) ClinicalTrials.gov - NCT01674829
- (1 citation) ClinicalTrials.gov - NCT02755428
- (1 citation) ClinicalTrials.gov - NCT03162926
- (1 citation) ClinicalTrials.gov - NCT02923375
- (1 citation) ClinicalTrials.gov - NCT02302157
- (1 citation) ClinicalTrials.gov - NCT02590692
- (1 citation) ClinicalTrials.gov - NCT01469832
- (1 citation) ClinicalTrials.gov - NCT02463344
- (1 citation) ClinicalTrials.gov - NCT02239354
- (1 citation) ClinicalTrials.gov - NCT02445612
- (1 citation) ClinicalTrials.gov - NCT03119636
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human embryonic and induced pluripotent stem cell research trends: complementation and diversification of the field.
Stem Cell Reports, 4(5):914-925, 09 Apr 2015
Cited by: 15 articles | PMID: 25866160 | PMCID: PMC4437486
HLA and Histo-Blood Group Antigen Expression in Human Pluripotent Stem Cells and their Derivatives.
Sci Rep, 7(1):13072, 12 Oct 2017
Cited by: 5 articles | PMID: 29026098 | PMCID: PMC5638960
Haematopoietic developmental potential of human pluripotent stem cell lines.
Folia Biol (Praha), 60 Suppl 1:90-94, 01 Jan 2014
Cited by: 2 articles | PMID: 25369348
The safety of human pluripotent stem cells in clinical treatment.
Ann Med, 47(5):370-380, 06 Jul 2015
Cited by: 39 articles | PMID: 26140342
Review





