Abstract
Free full text

89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges
Abstract
Molecular imaging—and especially Positron Emission Tomography (PET)—is of increasing importance for the diagnosis of various diseases and thus is experiencing increasing dissemination. Consequently, there is a growing demand for appropriate PET tracers which allow for a specific accumulation in the target structure as well as its visualization and exhibit decay characteristics matching their in vivo pharmacokinetics. To meet this demand, the development of new targeting vectors as well as the use of uncommon radionuclides becomes increasingly important. Uncommon nuclides in this regard enable the utilization of various selectively accumulating bioactive molecules such as peptides, antibodies, their fragments, other proteins and artificial structures for PET imaging in personalized medicine. Among these radionuclides, 89Zr (t1/2 = 3.27 days and mean Eβ+ = 0.389 MeV) has attracted increasing attention within the last years due to its favorably long half-life, which enables imaging at late time-points, being especially favorable in case of slowly-accumulating targeting vectors. This review outlines the recent developments in the field of 89Zr-labeled bioactive molecules, their potential and application in PET imaging and beyond, as well as remaining challenges.
1. Introduction
The development of new radiotracers for application in personalized medicine with PET has experienced enormous progress over the last decade, which is reflected in the large and still growing number of valuable compounds used for imaging of different diseases. A tendency that can be observed in this PET tracer development is the utilization of labeled targeting vectors that accumulate with high specificity at the target site, allowing for a highly sensitive target visualization. Many of these vectors are relatively large structures exhibiting high molecular weights as well as slow pharmacokinetics, necessitating the use of radionuclides with long half-lives in order to fully exploit the potential of the used targeting vectors for diagnostic imaging.
Interest in 89Zr has increased over the last several years as it exhibits a long half-life, making it ideally suited for imaging studies with slowly-accumulating bioactive molecules, allowing for imaging of biological processes at late time-points after tracer application. 89Zr—about which excellent other reviews are available [1,2,3,4]—produces positrons with a probability of 22.3% [5,6] and a mean energy of 0.389 MeV, which is between the positron energies of 18F (mean β+ energy of 0.250 MeV) and 68Ga (mean β+ energy of 0.836 MeV) [7], and thus allows for high resolution PET images. 89Zr is produced via cyclotron using 89Y (which has a natural abundance of 100%) as target material in the 89Y(p,n)89Zr nuclear reaction [8,9,10,11,12,13] and can be obtained in high isolated yields of 94–99.5% and very high radionuclidic purity of 99.99% [5,8].
Although 18F exhibits very favorable physical decay characteristics of 96.7% positron emission probability and a low positron energy, it cannot be used for in vivo imaging of biomolecules with slow pharmacokinetics due to its rather short half-life of 109 min. Thus, the use of 18F, as well as that of 68Ga (t1/2 = 68 min) and 11C (t1/2 = 20 min) which are the common nuclides in routine PET imaging applications, is restricted to imaging of the biodistribution of smaller radiolabeled bioactive compounds such as peptides and small molecules. As a result, 64Cu has attracted attention as it exhibits a longer half-life of 12.7 h together with a main positron energy of 0.653 MeV and low spatial resolution loss of 0.7 mm, allowing for an extended imaging of biological processes. However, 89Zr is even more interesting for the radiolabeling of slowly-accumulating radiopharmaceuticals as it exhibits a longer half-life of 3.27 days, being particularly suited for in vivo imaging of antibodies, nanoparticles and other large bioactive molecules [14,15]. However, this results in higher absorbed organ doses than in case of 18F-labeled tracers such as [18F]FDG [16,17]. 124I could also serve as a possible alternative for long-term imaging, exhibiting a half-life of 4.18 days, but in comparison to 89Zr it exhibits higher main positron energies of 1.54 and 2.14 MeV resulting in a relatively high intrinsic spatial resolution loss of 2.3 mm, whereas 89Zr with an intrinsic spatial resolution loss of 1.0 mm gives much better imaging results [18]. Furthermore, 124I is a so-called non-pure positron-emitter, producing in significant amount high-energy photons of different energy (603 keV (63.0%), 1691 keV (10.9%) and 723 keV (10.4%) [7]) which can result in random and scatter non-true coincidences and thus background noise, whereas 89Zr produces mainly one additional γ-line at 909 keV (909 keV (99.9%), 1657 (0.1%), 1713 keV (0.77%) and 1.744 keV (0.13%) [19]). Thus, the application of 124I necessitates the use of appropriate image reconstruction techniques in order to achieve a reasonable image quality [7,20].
Although 89Zr is mainly used for in vivo PET imaging, its use is not restricted to PET alone, as it can also be used for in vivo Cerenkov luminescence imaging (CLI) [21,22]. This imaging modality is based on luminescence that can be observed when a particle travels faster than light in the examined medium, is fully quantifiable and can be correlated to the respective PET signal [22]. Thus, Cerenkov luminescence imaging was shown to be applicable in image-guided surgery using 89Zr-labeled antibodies [23]. However, the main application of 89Zr is–and probably will remain–in vivo PET imaging of biological processes. Among 89Zr-labeled bioactive molecules used for PET imaging, mainly antibodies were labeled with 89Zr so far [24,25,26,27,28,29] although its use is not limited to these biomolecules. Beyond antibodies, 89Zr-labeling was also shown to be applicable in long-term PET studies of peptides and peptide multimers [30], nanoparticles [31], microspheres [32], targeted nanotubes [33], liposomes [34] and proteins [35,36].
In the following paragraphs, the radiolabeling with 89Zr, the synthesis strategies to introduce desferrioxamine B (DFO) into bioactive molecules, the stability of the 89Zr-DFO complex and its chemical conjugation as well as an overview of 89Zr-labeled biomolecules and their applications will be presented and critically discussed.
2. Radiolabeling of Bioactive Molecules with 89Zr
Although direct labeling approaches without a chelator have been described for 89Zr-introduction into liposomes [34] and serum proteins [37], reasonable labeling approaches with 89Zr have to be carried out using an appropriate chelator system in order to prevent the liberation of the radionuclide from the targeting vector. Liberated 89Zr4+ can bind to plasma proteins [38] and accumulates in mineral bone over time depositing significant doses there [39,40] which has to be avoided.
Diethylenetriaminepentaacetic acid (DTPA) was shown to not stably bind 89Zr [41], even though it exhibits a higher complex stability than the corresponding ethylenediaminetetraacetic acid (EDTA) complex [42]. Desferrioxamine B (a chelating agent binding various metal-ions [43,44]) was introduced for 89Zr complexation in 1992 and found to exhibit superior properties compared to DTPA [41]. DFO is still the commonly used chelator for 89Zr-complexation and is conjugated to the biomolecule before the radiolabeling reaction takes place.
Desferrioxamine B is commercially available in different chemical forms as DFO salt (e.g., from Sigma Aldrich, Schnelldorf, Germany) or in reactive form as the isothiocyanate derivative (Macrocyclics, Dallas, TX, USA), the maleimide derivative (Macrocyclics, Dallas, TX, USA) and the tetrafluorophenolic active ester chelated with Fe3+ (ABX, Radeberg, Germany). 89Zr is also commercially available on a regular basis from IBA Molecular (Richmond, VA, USA) and BV Cyclotron VU (Amsterdam, The Netherlands).
In the desferrioxamine B complex, the oxophile Zr4+ ion is supposed to be stabilized by the three hydroxamates and two additional anions or water molecules resulting in an expansion of the first coordination sphere, geometry relaxation and an octadentate complex structure [45] (Scheme 1). However, instead of a crystal structure, only DFT calculations of the 89Zr-DFO-complex are available so far [46].
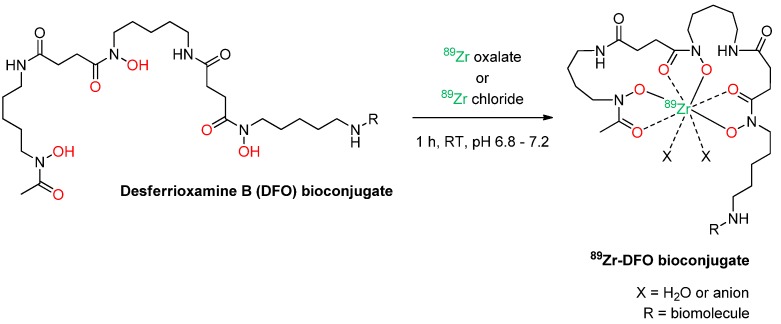
Schematic depiction of the radiolabeling reaction of a DFO-derivatized biomolecule with 89Zr (in red: hydroxamate coordination sphere of DFO).
A typical procedure for 89Zr-labeling consists of the following steps: (i) dilution of the 89Zr oxalic acid solution with saline or oxalic acid solution and adjustment of the pH of the solution to the optimal pH-range of 6.8 to 7.2 [47] by addition of Na2CO3 and/or HEPES buffer; (ii) addition of the DFO-derivatized bioactive compound and reaction for 30-60 min at ambient temperature; and (iii) purification of the radiolabeled product via HPLC, size exclusion chromatography or ultrafiltration. The radiolabeled products can be obtained in variable yields between 35% [48] and 98% [24], but in the vast majority of cases, radiochemical yields exceeding 75% are observed [8,26,47,49]. When presented, the radiochemical purities (RCPs) of the products were consistently reported to be over 95% after purification. However, upon storage, the reported RCPs strongly vary from no decrease in RCP after several days of storage in saline or buffered solution [8,26,46,50,51,52] to a significant 5% to 20% decrease in RCP after 48h or 168h [8,24,53]. DTPA was shown to significantly challenge the 89Zr-DFO-complex resulting in a 25–45% decrease in product RCPs after 7 days [5,50]. Other effects can also decrease the RCP of a 89Zr-preparation upon storage. Factors such as storage medium, pH as well as the applied temperature were found to significantly influence the RCP of the 89Zr-radiotracer over time and can result in a reduction of RCP of up to 48% after 144 h [47].
3. Introduction of Desferrioxamine B into Bioactive Molecules
Although the chelator chemistry available for 89Zr complexation is limited to desferrioxamine B alone, the introduction of this chelator into bioactive molecules can be carried out using different approaches. The most commonly used ones are based on acid-amide coupling and thiourea-formation, although also other methodologies such as click chemistry reactions can be used (see below). The different DFO conjugation approaches do not only result in different conjugation and radiolabeling efficiencies, but also in partially differing biodistribution properties of the radiolabeled compounds.
3.1. Introduction of DFO via Thiol Conjugation
One of the first described possibilities to introduce DFO into bioactive molecules is via the addition of thiols to maleimides. For this purpose, DFO can be reacted with N-succinimidyl-S-acetylthioacetate (SATA), resulting in an S-acetyl-protected thiol derivatization of the chelator (Scheme 2). In parallel, the biomolecule is reacted with 4-(N-maleimidomethyl) cyclohexane carboxylic acid N-hydroxysuccinimide ester (SMCC) to introduce maleimide moieties. Subsequently, both derivatized compounds can, in the presence of hydroxylamine, be reacted at physiologic pH to yield the DFO-biomolecule-conjugate (Scheme 2). Different antibody-chelator-conjugates were prepared using this method, labeled with 89Zr and evaluated in tumor-bearing mice, all showing a bone accumulation of approximately 2–5% at 72 h p.i. which can be attributed to the liberation of 89Zr [54]. However, the 89Zr-immunoconjugates also accumulated significantly in the target tumor tissues and even showed a higher tumor accumulation than the reference antibodies labeled with 123I and 99mTc after 72 h. Unfortunately, the 89Zr-labeled immunoglobulins also showed a higher background accumulation in other tissues, impairing the promising results.
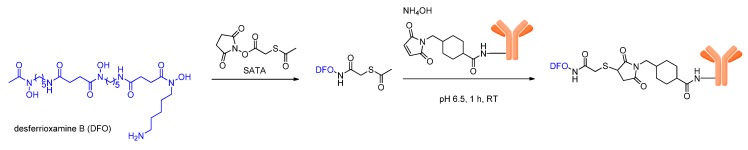
Schematic depiction of the conjugation reaction of thiol-derivatized DFO to maleimide-modified biomolecules.
Another approach utilizes antibodies bearing free thiols which can be reacted with thiol-reactive DFO derivatives. Different thiol-reactive DFO derivatives such as bromoacetyl-DFO, iodoacetyl-DFO and maleimidocyclohexyl-DFO were synthesized and could be conjugated to thiol-bearing trastuzumab which was recombinantly modified with unpaired cysteines that allowed for a site-specific conjugation of the thiol-reactive chelators (Scheme 3) [55].
The obtained 89Zr-labeled conjugates were evaluated in vitro and in vivo in a HER2-positive breast cancer mouse model and compared to non-site-specifically derivatized and 89Zr-labeled trastuzumab with regard to biodistribution and tumor visualization. Interestingly, the site-specific conjugation of the DFO chelator did not result in improved biodistribution properties compared to a non-site-specific modification using either the active ester or isothiocyanate approach (see section 3.3) for antibody derivatization as comparable in vivo properties were observed for all 89Zr-labeled immunoconjugates at different time points p.i.. This is astonishing as highly preserved antigen binding affinities of 0.87 ± 0.15 to 1.22 ± 0.22 nM could be found for the site-specifically modified antibodies (0.91 ± 0.15 nM KD for unmodified trastuzumab) which cannot be assumed in case of the non-site-specifically derivatized mAbs, although the respective data were not presented.
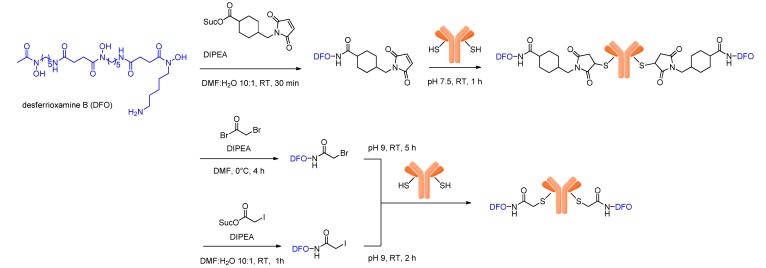
Schematic depiction of a site-specific DFO-conjugation by reaction of maleimide-, bromoacetyl- and iodoacetyl-derivatized DFO with biomolecules exhibiting unpaired cysteines.
Comparing the different thiol-reactive DFO derivatives in terms of reactivity, the conjugation efficiency varied. The best results were obtained for the maleimide-modified DFO whereas bromoacetyl- and iodoacetyl-DFO showed lower conjugation efficiencies and lower radiochemical purities after radiolabeling. In terms of in vivo stability and 89Zr-release, the different 89Zr-labeled antibodies gave similar results as reflected in similar bone accumulations that increased over time to 7–10% after 4 days p.i. [55].
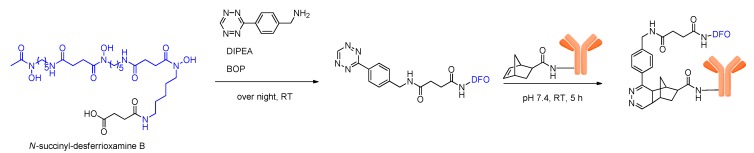
3.2. Introduction of DFO via Click Chemistry
Another method to introduce DFO into antibodies was described recently, using a Diels-Alder click chemistry reaction between norbornenes and tetrazines on the example of trastuzumab [56]. Using this approach, the antibody and DFO were first reacted with a norbornene and a tetrazine moiety, respectively, before the conjugation of DFO to the antibody was carried out. This Diels-Alder conjugation reaction was performed under mild conditions at ambient temperature, but required relatively long reaction times of 5 h (Scheme 4).
The DFO-modified antibody was subsequently labeled with 89Zr and evaluated in vitro and in vivo. Besides the expected very favorable accumulation in the HER2-positive tumor that could be observed, which is mediated by the highly specific antibody, also a high in vivo bone accumulation of 13.2% ± 2.4% could be detected as early as 24 h p.i.. This is especially astonishing as the in vitro stability tests of the same labeled antibody in serum revealed a liberation of only ~2% of 89Zr from the complex after 7 days of incubation. As other 89Zr-labeled antibodies do not show such an extensive liberation of 89Zr from the complex within such a short period, this seems to be a result of a susceptibility of the conjugating linker which might also destabilize the 89Zr-DFO-complex making it sensitive to detachment and metabolization.
3.3. Introduction of DFO via Acid-Amide or Thiourea Formation
The direct conjugation of functionalized DFO to side chain amino groups of lysine is still the main approach for DFO introduction. Here, two different routes can be pursued: conjugation via an isothiocyanate or acid-amide coupling using an active ester of the chelator.
The active ester strategy, first described by Verel et al. [8], is rather complex and consists of six steps: (i) succinylation of DFO; (ii) complexation of Fe3+ to prevent side-reactions involving the hydroxamate groups; (iii) formation of the tetrafluorophenolic active ester; (iv) conjugation of the DFO-active ester to the antibody; (v) removal of Fe3+ from the DFO complex by transchelation with EDTA (ethylenediamine tetraacetic acid) and vi) radiolabeling with 89Zr (Scheme 5).

Schematic depiction of biomolecule-DFO-conjugation via reaction of amino functionalities of the biomolecule with a DFO-active ester.
Despite the rather complex synthesis strategy, this approach has the advantage of resulting in 89Zr-labeled antibodies exhibiting favorable in vitro and in vivo properties as was shown by a study directly comparing antibodies using the active ester and the thiol-maleimide approach described before (Scheme 2) [8]. The in vitro experiments for example revealed strongly differing stabilities in human serum for both radiopharmaceuticals. The antibody conjugate prepared by the acid-amide coupling showed no degradation within 24 h in human serum, whereas in the case of the thiol-maleimide conjugate, the 89Zr-DFO-complex was partially transferred from the antibody to serum albumin although not resulting in the liberation of the radiometal ion. It was suggested that this observed conjugation instability in case of the thiol-maleimide conjugation product could be due to a Zr-catalyzed opening of the succinimide ring.
This observed complex-transfer from the antibody to albumin of course also resulted in differing in vivo imaging results for both antibodies. The antibody modified with DFO by the active ester strategy exhibited a longer blood pool circulation and also much higher tumor-to-background ratios than the thiol-maleimide-conjugate that showed a faster excretion and thus could not achieve the high tumor accumulation that was observed in case of the more stable acid-amide antibody-DFO conjugate. This study impressively shows that for obtaining a stable 89Zr-labeling resulting in radiopharmaceuticals with reasonable in vivo properties, the conjugation chemistry is a crucial factor. In this study–comparable to the results found by others–a significant bone accumulation resulting from 89Zr liberation could be observed that was similar for both antibodies [8].
Thus, the active ester strategy to introduce DFO into biomolecules is rather complex, but produces 89Zr-labeled compounds with favorable in vitro and in vivo properties and therefore is still one of the most often used methodologies for protein 89Zr-labeling [24,26,57,58,59]. Moreover, as the active ester complexed with Fe3+ (which can be directly applied for biomolecule modification) became commercially available recently, this approach for DFO-conjugation significantly gained simplicity, making it even more attractive for DFO-conjugation.
A methodology to efficiently introduce DFO into biomolecules was described recently and is based on a p-isothiocyanatobenzyl-derivative of DFO that can be directly conjugated at mildly basic pH within a relatively short reaction time of only ½ to 1 h (Scheme 6) and subsequently reacted with 89Zr to give the radiolabeled biomolecule [47].
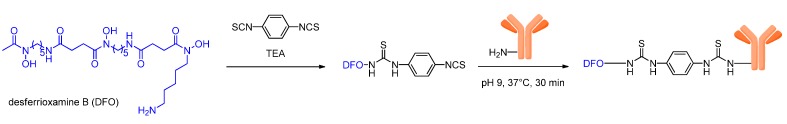
Schematic depiction of biomolecule-DFO-conjugation via reaction of lysine amino functionalities of the biomolecule with a DFO-isothiocyanate.
The antibodies cetuximab and cU36 were both derivatized and radiolabeled according to this procedure and compared to the same antibodies derivatized and labeled using the six reaction steps comprising active ester strategy described before. In vitro, the antibodies revealed significant differences concerning stability, depending on the derivatization method and the buffer used for storage [47]. It could be shown that the active ester-derivatized 89Zr-conjugate had a much higher stability upon storage in buffers (0.25 M sodium acetate + 5 mg gentisic acid/ml buffer at pH 5.5 and 0.9% NaCl + 5 mg gentisic acid/ml at pH 5.0) with a ≤ 5% release of 89Zr from the complex within 6 days. In contrast to these findings, the isothiocyanate-modified antibodies 89Zr-DFO-SCN-mAb showed comparably low stabilities under the same conditions, liberating 10.5 ± 2.1% 89Zr upon storage in 0.25 M sodium acetate + 5 mg gentisic acid/ml buffer at pH 5.5 and even 48.4 ± 5.7% upon storage in 0.9% NaCl + 5 mg gentisic acid/ml at pH 5.0 after 6 days. It was suggested by the authors that this limited stability of the isothiocyanate-derivatized compounds could be a result of a radiation-induced formation of reactive hypochlorite ions affecting the integrity of the isothiocyanate [47]. Interestingly, the storage of the isothiocyanate-modified and 89Zr-labeled antibody in serum resulted in a release of only ~5% of 89Zr from the complex after 7 days. Confirming these in vitro results obtained in serum, the subsequently performed in vivo experiments in tumor-bearing mice revealed similar in vivo biodistribution properties together with a ~5% sternum accumulation at 72 h p.i. for both antibodies modified by isothiocyanate- and acid-amide-conjugation. As the isothiocyanate methodology does not seem to negatively influence the obtained in vivo results of 89Zr-labeled compounds although showing a significant instability upon storage in some buffers, several studies were performed recently using this simpler conjugation and radiolabeling approach [27,30,60,61,62,63].
4. Stability of the 89Zr-Desferrioxamine B Complex
In contrast to the 89Zr-desferrioxamine B complex, which is quickly cleared via the renal system when cleaved from the targeting biomolecule in vivo [37,39,46], 89Zr4+ liberated from the complex is first incorporated into plasma proteins [38] and later embedded into mineral bone [37,39,40] comparable to other large radiometal ions such as 90Y or 177Lu [40,64]. The accumulation of radiometal ions can result in bone marrow ablation [65,66] and thus should be reduced to an absolute minimum. DFO has not only been used for 89Zr-complexation in the past, but also for that of other radionuclides such as gallium and indium isotopes [67,68,69,70], but has been replaced in these cases by other chelators due to the observed instability of the formed complexes. In case of 89Zr, the stability of the complex is also limited as could be shown in vitro and in vivo in several studies, which is reflected in a bone accumulation ranging from 3 to 15% at 72–168 h p.i. [27,29,46,56,59,71].
This limited stability of the 89Zr-DFO complex can be explained by the structure of desferrioxamine B, being a linear and not a macrocyclic ligand. This is corroborated by a study comparing antibodies that were modified with either linear or macrocyclic chelators and radiolabeled with 88Y, 177Lu and 89Zr. As anticipated, the complexes with linear chelators (88Y-DTPA, 177Lu-DTPA and 89Zr-DFO) showed a comparably low stability of the complexes, whereas the macrocyclic 88Y-DOTA and 177Lu-DOTA complexes showed a high stability even after an incubation period of 16 days in human serum [72]. In other studies comparing 88Y- and 177Lu-DOTA to 89Zr-DFO-labeled antibodies, the lower stability of the 89Zr-DFO complex compared to the 88Y- and 177Lu-DOTA complexes was obvious as well, which was reflected in a significantly higher bone accumulation of 89Zr compared to 88Y and 177Lu [19,53,58]. Other experiments, in which the specific binding of the applied 89Zr-radiopharmaceuticals was prevented by blocking, showed an even more pronounced effect of the liberation of 89Zr from the complex and its accumulation in mineral bone [23,46,59,61].
Another factor influencing the complex stability is the correlation between the size of the radiometal ion and the cavity size that is formed by the chelator. If cavity size and metal ion radius are similar, stable complexes are formed that are normally characterized by a complete envelopment of the radiometal by the chelator, thus exhibiting no weak point in complex geometry that would allow for a complex challenge. Vice versa, an incomplete envelopment was shown to result in less stable complexes [73,74,75]. DFO however is reported to not completely envelope the Zr4+ ion in the complex by occupying only six of the eight coordinate positions [46] in the assumedly preferred octadentate complex geometry [42]. The resulting complex stability is therefore expected to be limited, which can be confirmed by the experimental results obtained in vivo. Thus, it was proposed recently that a more stable chelating agent exhibiting four bidentate hydroxamates could be better-suited for a stable 89Zr complexation in vivo than the three hydroxamates-containing DFO [76].
In addition to the observed instability of the 89Zr-DFO complex itself, the linker chemistry that is used for DFO-conjugation has been shown to have a subordinate but yet measurable effect on the stability of the 89Zr-DFO complex. It has been shown for example in an in vivo study comparing 89Zr-DFO-trastuzumab conjugates obtained using different DFO conjugation strategies, that the radioactivity accumulation in mineral bone depended on the linker chemistry used [55] and decreased in the following order: 89Zr-DFO-Chx-Mal mAb (conjugation via thiol-maleimide coupling, Scheme 3) > 89Zr-DFO-Ac-mAb (conjugation via bromoacetyl-thiol- and iodoacetyl-thiol-coupling, Scheme 3) > 89Zr-DFO-Bz-SCN-mAb (conjugation via isothiocyanate coupling, Scheme 6) > 89Zr-N-SucDFO-mAb (conjugation via acid-amide coupling, Scheme 5). This observed effect might be a result of the functional groups–such as thiol ethers or thio-ureas–compromising the integrity of the 89Zr-DFO complex by acting as additional ligands disturbing the 89Zr-DFO coordination sphere and resulting in an enhanced probability of complex-challenge by other potential ligands.
5. 89Zr-labeled Bioactive Compounds
As the favorable properties of 89Zr can only be fully exploited in imaging applications of compounds exhibiting slow in vivo pharmacokinetics, mostly 89Zr-labeled antibodies have been described so far. In case of smaller bioactive compounds with fast pharmacokinetics such as small molecules and peptides, other radionuclides such as 18F or 68Ga are commonly applied due to their more favorable physical decay characteristics such as a higher positron emission probability, γ-radiation purity and lower radiation doses due to the shorter half-lives of these nuclides. Nevertheless, not only antibodies labeled with 89Zr have been described and the following sections give an overview of the 89Zr-labeled bioactive compounds studied so far and their application in preclinical and/or clinical settings. Unless otherwise stated, the mentioned studies are preclinical.
5.1. Peptides, Antibody Fragments and Serum Proteins
So far, only few examples of 89Zr-labeled peptides can be found. This is due to the generally fast pharmacokinetics of this substance class that enables a successful PET imaging using other more common nuclides such as 18F or 68Ga for radiolabeling. However, when evaluating peptide multimers, the use of 89Zr becomes more reasonable as the pharmacokinetics of these relatively large bioactive compounds can be rather slow [77,78]. Thus, one study evaluating DFO-Bz-SCN-derivatized and 89Zr-labeled c(RGDfK) monomers and dimers in vitro and in vivo was published recently. In this study, the anticipated results of higher in vitro binding avidity and in vivo tumor uptake together with higher tumor-to-background-ratios were found for the dimers compared to the respective monomers. As also observed in other studies using 89Zr-labeled biomolecules, a moderate bone accumulation was found in the in vivo evaluation that increased over the study period of 24h which can be attributed to liberated 89Zr [30]. Although the peptide dimers evaluated in these experiments could also have been labeled with other radiometal nuclides with shorter half-lives such as 68Ga or 64Cu, 89Zr could be a very favorable nuclide for evaluating larger peptidic multimers.
Besides peptide multimers, the in vivo evaluation of antibody fragments could also profit from the long half-life of 89Zr enabling PET imaging at late time-points after tracer application. Thus, different nanobodies and an antibody fragment were labeled with 89Zr and successfully evaluated regarding their in vivo biodistribution and pharmacokinetics at late time-points [63,79]. Furthermore, 89Zr-labeling and subsequent PET imaging was also successfully used for an estimation of therapy efficiency and therapy planning in case of antibody fragments [80].
Apart from antibody fragments, also other proteins such as albumin and transferrin can be radiolabeled with 89Zr as was shown in a study using 89Zr-DFO-albumin for the successful nontargeted visualization of tumors through their increased vascularization and enhanced permeability and retention (EPR) effect [81]. In another study, the iron-transport protein transferrin was labeled with 89Zr to be applied in brain tumor imaging as well as in imaging of transferrin receptor expression [35]. While the 89Zr-transferrin showed favorable results in brain tumor imaging, exhibiting a higher tumor-to-brain ratio than 18F-fluorodeoxyglucose ([18F]FDG), it also showed high uptake in kidneys, liver and bone as well as inflammatory abscesses, hindering an efficient transferrin receptor detection in solid tumors [36].
5.2. Nanoparticles and Microspheres
Besides peptides and medium scale proteins, 89Zr has also been used in radiolabeling and in vivo imaging of large synthetic structures such as microspheres, nanoparticles, nanotubes and liposomes.
Examples include 89Zr-labeled microspheres (microspheres with a diameter of approximately 20–40 µm can be used in 90Y-labeled form in selective internal radiation therapy (SIRT) of tumors) that were evaluated regarding their in vivo biodistribution in terms of therapy planning and dosimetry purposes with PET before therapy with the 90Y-labeled particles takes place [32]. It was found in this study that of all the different diagnostic radiolabeled spheres (64Cu-, 86Y- and 89Zr-labeled microspheres were prepared and studied), the 89Zr-labeled derivatives were the most valuable PET imaging surrogates for the 90Y-labeled spheres and could be useful for therapy planning and dosimetry calculations due to their valuable in vivo PET imaging properties and the comparable half-lives of 89Zr and 90Y of 78.4 and 64.1 h, respectively. The 86Y-labeled spheres (t1/2(86Y) = 14.7 h) were also shown to be useful for this application whereas the 64Cu-labelled derivatives showed an insufficient in vitro stability.
Nanocolloids of 89Zr-DFO-albumin were shown to be applicable for the detection of sentinel lymph nodes and were demonstrated to accumulate within several minutes in a rabbit lymphogenic metastasis model [82]. These nanocolloidal 89Zr-DFO-albumin particles where subsequently used in a continuing clinical study enabling a successful identification of tumorous sentinel lymph nodes even in direct vicinity to the primary tumor and yielding even more reliable results than the commonly applied 99mTc-albumin nanocolloids [83]. Another example for 89Zr-labeled nanoparticles, short chain dextran nanoparticles (DNPs) that were intended for imaging of macrophages and inflammation, was recently described. Here, it could be shown that 89Zr-DFO-cross-linked dextran nanoparticles accumulated specifically in tissue resident macrophages within 24h of administration, which could also be useful for the detection of tumor associated macrophages (TAMs) in tumor diagnosis and therapy [31].
Further examples of 89Zr-labeled synthetic structures include single wall carbon nanotubes (SWCNT) which were shown to be useful as scaffold structures for surface-modification with targeting vectors and radionuclides due to their large surface and low immunogenicity. For example, carbon nanotubes derivatized with the tumor neovasculature-targeting antibody E4G10 and DFO were described. These nanotubes were subsequently labeled with 89Zr and evaluated using in vivo PET tumor imaging studies in tumor-bearing mice. The 89Zr-labeled constructs showed a rapid blood clearance and specific tumor accumulation but also a very high liver and kidney accumulation limiting the application of these constructs for imaging or therapy of tumors [33].
In addition to nanotubes, 89Zr-labeled liposomes were described recently. These liposomes, comprising the somatostatin receptor-affine peptide octreotide for tumor targeting, were labeled with 89Zr (for visualization with PET) as well as gadolinium ions (for visualization with MRI) to offer a platform for combined PET/MRI [34]. Although representing an interesting new approach for the design of multimodal and target-specific imaging agents, the in vitro stability of the liposomes was shown to be suboptimal: the incubation in mouse serum or in a solution of bovine serum albumin at 37 °C for 24 h to 96 h resulted in a release of approximately 50% of the radioactivity being a consequence of the direct labeling with 89Zr without using a chelator in the lipid monolayer of the liposome.
5.3. Antibodies
The main application of 89Zr is still the labeling of antibodies for preclinical as well as clinical PET imaging studies [18,84,85]. This can be attributed to the slow pharmacokinetics and target accumulation of antibodies which necessitate a radionuclide exhibiting a long half-life such as 89Zr.
An important point in antibody derivatization and radiolabeling is the immunoreactivity of the obtained conjugates as only a preserved immunoreactivity can result in a high bioactivity of the radioimmunoconjugates allowing for a successful in vivo target visualization. However, the immunoreactivity was determined in only about half of all studies. When determined, the immunoreactivity in the vast majority of cases was in a reasonable range between 70 and 96% [26,27,28,46,47,58,62,79]. Furthermore, the antibody derivatization was shown to not result in an aggregation of the biomolecules over time [52,54].
A summary of 89Zr-labeled antibodies and their application in preclinical or clinical imaging studies is given in Table 1. 89Zr-labeling and subsequent in vivo imaging was shown to provide valuable information about antibody biodistribution properties via PET, allowing the visualization of target tissues (usually a tumor entity) as well as receptor-expression also enabling to assess the efficiency of tumor therapeutics and pre-therapeutic dosimetry [24,25,29,46,50,51,57,60,61,62,86,87,88,89,90,91,92].
Table 1
89Zr-labeled antibodies used for diagnostic imaging or therapy planning in preclinical and clinical studies.
| Antibody used | Epitope | Target tissue | Preclinical | Clinical |
|---|---|---|---|---|
| 323/A3 | 17.1 A | squamous-cell carcinoma and others | [54] | |
| 5A10 | “free” prostate-specific antigen (fPSA) | osseous prostate cancer lesions | [87] | |
| 7E11 | prostate-specific membrane antigen (PSMA) | prostate cancer | [51] | |
| bevacizumab | vascular endothelial growth factor (VEGF) | tumor angiogenic vessels | [24,93,86,92,97] | |
| cetuximab | epidermal growth factor receptor | various cancers | [29,47,72,97] | |
| CD45R | B220 | B cells | [48] | |
| cG250 | G250 | renal cell carcinoma (RCC) | [94] | |
| cU36 | CD44v6 | head and neck squamous cell carcinoma (HNSCC) human studies | [8,19,47,58] | [16,28] |
| DN30 | c-Met receptor | gastric cancer, Met/head and neck cancer | [57] | |
| E48 | 22 kDa antigen | squamous-cell carcinoma and others | [54] | |
| E4G10 | vascular endothelial cadherin (VE-cad) | tumor angiogenic vessels | [33] | |
| fresolimumab | Transforming growth factor-β (TGF-β) | highly invasive or metastatic tumors as glioblastomas and human breast cancer | [52] | |
| hRS7 | epithelial glycoprotein-1 (EGP-1) | epithelial carcinomas | [59] | |
| ibritumomab tiuxetan (Zevalin) | CD20 | NHL (non-Hodgkin’s lymphoma) human studies | [53] | [53,95] |
| J591 | prostate-specific membrane antigen (PSMA) | prostate cancer | [22,46] | |
| Onartuzumab | hepatocyte growth factor receptor (Met) | gastric carcinoma and glioblastoma | [89] | |
| panitumumab | (EGFR/HER1) | colorectal cancer, head and neck tumors | [61,90,98] | |
| PGN635 | phosphatidylserine | apoptosis | [91] | |
| R1507 | insulin-like growth factor receptor 1 | breast cancer | [71] | |
| ranibizumab | VEGF | tumor induced angiogenesis | [88] | |
| rituximab | CD20 | malign lymphoma cells | [47,62] | |
| trastuzumab | human epidermal growth factor receptor 2 (HER2) | breast cancer, ovarian, colorectal carcinoma human studies | [23,25,26,27,50,55,56,96] | [93,99,100] |
| TRC105 | CD105 | angiogenic endothelial cells | [60,101] |
The efficient in vivo tumor visualization using 89Zr-labeled antibodies in clinical studies could be shown to depend on the specific activity of the radiolabeled antibody. It was demonstrated in a human imaging study with patients suffering from metastatic breast cancer that the ideal time-point for imaging as well as the optimal specific activity of 89Zr-trastuzumab depend on the pretreatment the patient had received. In patients who had already received trastuzumab for treatment purposes, the best imaging results were obtained using a mixture of 89Zr-trastuzumab and 10 mg of the unlabeled antibody (otherwise, target saturation is likely), whereas a mixture of 89Zr-trastuzumab and 50 mg of the unlabeled antibody had to be applied in trastuzumab-naive patients (otherwise, the antibody was cleared too fast, hampering an adequate visualization of the lesions) and the best tumor-to-background-contrasts could be achieved 4–5 days after 89Zr-trastuzumab application [93].
Furthermore, some studies are available that directly compare 89Zr- and 111In-labeled antibodies in order to evaluate the potential of the 89Zr-labeled compounds as surrogates for the 111In-labeled ones in order to make use of the advantages of PET imaging versus SPECT. Although the 89Zr-labeled antibodies showed a significant liberation of the radionuclide from the complex reflected in higher bone and liver radioactivity accumulation, they were found to give almost identical in vivo biodistribution results regarding tumor accumulation and uptake into other tissues compared to the respective 111In-labeled analogs in preclinical studies [26,52,59,94].
The use of 89Zr-labeled substances is not limited to diagnostic imaging alone. 89Zr-labeled radiopharmaceuticals were also shown to be useful as surrogates for the respective therapeutic 90Y- or 177Lu-labeled antibodies in order to perform dosimetry calculations and therapy planning in preclinical as well as clinical studies [19,53,72,95].
Another possible application of 89Zr-labeled drugs is in combined multimodal imaging using PET/OI (OI = optical imaging). This has been shown to be a successful approach by the example of a 89Zr- and 800CW-NIR dye-labeled TRC105 antibody that was used for a pre-surgical tumor detection and intraoperative optical visualization of tumor material [101].
6. Conclusions and Outlook
89Zr shows very promising results in the in vivo PET imaging of slowly accumulating radiopharmaceuticals. However, some challenges remain such as the high energy photons emitted by this nuclide which can result in background noise of the images as well as the limited stability of the current chelator complexes. Without any doubt, work will be carried out to develop chelators and conjugation strategies that allow for a stable 89Zr-introduction into biomolecules, yielding compounds with optimized properties for the imaging of biological processes in clinical imaging applications. In addition, improvements in image reconstruction will overcome the obstacles of image background noise. These developments will thus enable to exploit the full potential of 89Zr.
The numerous successful examples of imaging studies with 89Zr-labeled bioactive molecules show the general applicability of this radiometal nuclide for long-term in vivo PET imaging. Furthermore, GMP-compliant 89Zr is already commercially available which allows using this most favorable nuclide in clinical routine. With regards to individual patient-tailored diagnostics and therapies, the development of new target-specific compounds—and among these presumably many antibodies or other large biomolecules—is still under way. Thus, 89Zr possesses a high potential for PET imaging applications in the future.
Acknowledgments
G.F. and C.W. would like to thank the Fonds der Chemischen Industrie for financial support.
References
Articles from Molecules are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/molecules18066469
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/1420-3049/18/6/6469/pdf?version=1403114782
Citations & impact
Impact metrics
Article citations
Development of 52Mn Labeled Trastuzumab for Extended Time Point PET Imaging of HER2.
Mol Imaging Biol, 26(5):858-868, 27 Aug 2024
Cited by: 0 articles | PMID: 39192059 | PMCID: PMC11436409
Intranasal delivery of imaging agents to the brain.
Theranostics, 14(13):5022-5101, 19 Aug 2024
Cited by: 0 articles | PMID: 39267777 | PMCID: PMC11388076
Review Free full text in Europe PMC
Development of a Novel, Easy-to-Prepare, and Potentially Valuable Peptide Coupling Technology Utilizing Amide Acid as a Linker.
Pharmaceuticals (Basel), 17(8):981, 24 Jul 2024
Cited by: 0 articles | PMID: 39204086 | PMCID: PMC11356999
Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate.
Pharmaceuticals (Basel), 17(4):480, 09 Apr 2024
Cited by: 0 articles | PMID: 38675440 | PMCID: PMC11053460
Beyond Small Molecules: Antibodies and Peptides for Fibroblast Activation Protein Targeting Radiopharmaceuticals.
Pharmaceutics, 16(3):345, 29 Feb 2024
Cited by: 0 articles | PMID: 38543239 | PMCID: PMC10974899
Review Free full text in Europe PMC
Go to all (60) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies.
J Vis Exp, (96), 12 Feb 2015
Cited by: 51 articles | PMID: 25741890 | PMCID: PMC4354640
Radiolabeling and Imaging of Adoptively Transferred Immune Cells by Positron Emission Tomography.
Methods Mol Biol, 2097:267-272, 01 Jan 2020
Cited by: 2 articles | PMID: 31776932
Recent Advances in Zirconium-89 Chelator Development.
Molecules, 23(3):E638, 12 Mar 2018
Cited by: 31 articles | PMID: 29534538 | PMCID: PMC6017441
Review Free full text in Europe PMC
Development of 68Ga- and 89Zr-Labeled Exendin-4 as Potential Radiotracers for the Imaging of Insulinomas by PET.
J Nucl Med, 56(10):1569-1574, 06 Aug 2015
Cited by: 14 articles | PMID: 26251418