Abstract
Objective
Acute pneumonitis with acute lung injury is a cause of significant mortality related to the 2009 pH1N1 influenza A virus. Widespread lung inflammation and increased pulmonary vascular permeability has been noted on autopsy. Also, many of these patients present with significant hemodynamic compromise suggesting systemic cytokine release. Therefore, attenuating circulating cytokines, and other mediators, by blood purification techniques is a theoretically attractive strategy. We report the use therapeutic plasma exchange in three children with 2009 H1N1 related acute lung injury with severe hemodynamic compromise that had failed conventional therapeutic interventions.Design
Case series.Setting
Pediatric intensive care unit in a university children's hospital.Patients
Three children, aged 8, 11, and 17 yrs, with acute respiratory distress syndrome and hemodynamic compromise related to the 2009 pH1N1 influenza A virus documented by polymerase chair reaction. All patients were on mechanical ventilation and inhaled nitric oxide, and one patient was on extracorporeal membrane oxygenation. Therapeutic plasma exchange was used as a rescue strategy.Interventions
Each patient received three exchanges of 35-40 mL/kg on consecutive days.Measurements
All three patients had dramatic reduction in pediatric logistic organ dysfunction scores, oxygen requirements, and vasopressor requirements after two exchanges. All survived with good functional recovery.Main results
In this small series of patients with H1N1/acute respiratory distress syndrome and hemodynamic compromise, therapeutic plasma exchange appeared to benefit as a method of mitigating the associated cytokine storm. The procedure was well tolerated with no reported side effects. All three patients survived, defying the predicted mortality. Because these procedures used the filtration exchange method, it was performed in a timely fashion by intensive care unit personnel and on equipment already available in the intensive care unit for renal support.Conclusions
This very limited case series suggest there may be a role for therapeutic plasma exchange as a rescue therapy in severe shock and acute lung injury related to pH1N1 that has not responded to traditional therapy.Free full text

Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—An associated respiratory failure and hemodynamic shock
Abstract
Objective:
Acute pneumonitis with acute lung injury is a cause of significant mortality related to the 2009 pH1N1 influenza A virus. Widespread lung inflammation and increased pulmonary vascular permeability has been noted on autopsy. Also, many of these patients present with significant hemodynamic compromise suggesting systemic cytokine release. Therefore, attenuating circulating cytokines, and other mediators, by blood purification techniques is a theoretically attractive strategy. We report the use therapeutic plasma exchange in three children with 2009 H1N1 related acute lung injury with severe hemodynamic compromise that had failed conventional therapeutic interventions.
Design:
Case series.
Setting:
Pediatric intensive care unit in a university children’s hospital.
Patients:
Three children, aged 8, 11, and 17 yrs, with acute respiratory distress syndrome and hemodynamic compromise related to the 2009 pH1N1 influenza A virus documented by polymerase chair reaction. All patients were on mechanical ventilation and inhaled nitric oxide, and one patient was on extra-corporeal membrane oxygenation. Therapeutic plasma exchange was used as a rescue strategy.
Interventions:
Each patient received three exchanges of 35–40 mL/kg on consecutive days.
Measurements:
All three patients had dramatic reduction in pediatric logistic organ dysfunction scores, oxygen requirements, and vasopressor requirements after two exchanges. All survived with good functional recovery.
Main Results:
In this small series of patients with H1N1/acute respiratory distress syndrome and hemodynamic compromise, therapeutic plasma exchange appeared to benefit as a method of mitigating the associated cytokine storm. The procedure was well tolerated with no reported side effects. All three patients survived, defying the predicted mortality. Because these procedures used the filtration exchange method, it was performed in a timely fashion by intensive care unit personnel and on equipment already available in the intensive care unit for renal support.
Conclusions:
This very limited case series suggest there may be a role for therapeutic plasma exchange as a rescue therapy in severe shock and acute lung injury related to pH1N1 that has not responded to traditional therapy. (Pediatr Crit Care Med 2011; 12:e87–e89)
Acute pneumonitis with severe acute lung injury (ALI) is a cause of significant mortality related to infection with the 2009 pH1N1 influenza A virus. At autopsy, histopathologic examination of patients who sucumbed to respiratory failure revealed severe lung inflammation and edema along with microvascular thrombosis (1).
Acute lung injury (ALI) is a syndrome of widespread lung inflammation and increased pulmonary vascular permeability. Cytokines activate alveolar macrophages and recruit neutrophils, which in turn release leukotrienes, oxidants, platelet-activating factor, and proteases. These substances damage capillary endothelium and alveolar epithelium, disrupting the barriers between capillaries and airspaces (2). Attenuating circulating cytokines, and other mediators, by blood purification techniques is a theoretically attractive strategy in treating this disease process because it has been shown to attenuate cytokine burdon in other inflammatory conditions (3–5).
Therapeutic plasma exchange (TPE) is an extracorporeal modality in which a volume of plasma is exchanged that is equal to the estimated plasma volume. The goal of TPE is to remove pathogenic substances in plasma, such as auto reactive antibodies, immune complexes, paraproteins, lipoproteins, and inflammatory mediators, such as cytokines (6). Acute influenza is one of many diseases that can produce significant cytokine “surges” in the acute phase (7). We report a case series of three patients infected with the 2009 pH1N1 influenza virus with shock and/or ALI who were treated with TPE as a strategy for inflammatory mediator attenuation.
This report was submitted to the Institutional Review Board and received exempt status.
Cases
We report the use of TPE in treating three children, aged 8, 11, and 17 yrs, with ALI and hemodynamic compromise related to the 2009 pH1N1 influenza A virus. In each case, the disease was confirmed by polymerase chain reaction.
Two of the three patients required significant vasopressor support to maintain mean arterial pressures of >60 mm Hg. All received mechanical ventilatory support. One patient had sickle cell disease without recent occlusive crisis, whereas other two had no medical history. All three patients received oseltamivir, mechanical ventilation, and inhaled nitric oxide with one patient requiring veno-venous extra-corporeal membrane oxygenation.
In spite of initial resuscitation and other therapuetic interventions, all had clinical deterioration defined by increasing oxygen and/or vasopressor requirements. TPE was initiated as a blood purification therapy. All therapies were performed on the Prisma continuous renal replacement therapy machine (Gambro, Lakewood, CO), using the Prisma TPE filtration system. This is a polypropylene membrane with a pore size of 0.05 μm and molecular clearance up to 3 million daltons. Each patient received daily exchanges of 38–40 mL/kg for 3 consecutive days. There was no ultrafiltration. One patient had a coagulopathy defined by an elevated international normalized ratio and decreased platelet count (<100,000 mm3) and received fresh-frozen plasma as the sole replacement solution, as the other two, including the patient on extracorporeal membrane oxygenation (ECMO), received a 50/50 mix of fresh-frozen plasma and 5% albumin. Two of the three patients did not receive continuous renal replacement therapy. The one patient who was also receiving ECMO support did receive continuous veno-venous hemodiafiltration after developing oilguria 6 days after presentation and 3 days after the last TPE was administered.
All three patients survived, two without sequela and one (the patient who received ECMO) with myopathy of critical illness but fully recovered at 4 months. The admission Pediatric Risk of Mortality score and the Pediatric Logistic Organ Dysfunction score, pre and post TPE, are presented in Table 1, along with the predicted mortality based on the admission Pediatric Risk of Mortality score. The two patients requiring vasopressor suport had immediate reduction in vasopressor requirements after the first TPE and both were off vasopressor support after completing the second treatment. All three patients had reduction in oxygen requirements and the two who were not on ECMO had a reduction in positive end-expiratory pressure requirements with no progression of their ALI after the first TPE. As shown in Table 1, the reduction in PaO2/FIO2 ratio was significantly improved from an average of 77 to 202 (p = .0213; 95% confidence interval [CI], 210.84 – 46.49). Each patient had a >50% reduction in Pediatric Logistic Organ Dysfunction scoring after the second treatment, and all had a reduction in Pediatric Logistic Organ Dysfunction score of <3 (p = .001, based on paired Student’s t test) by the third exchange as demonstrated in Figure 1.
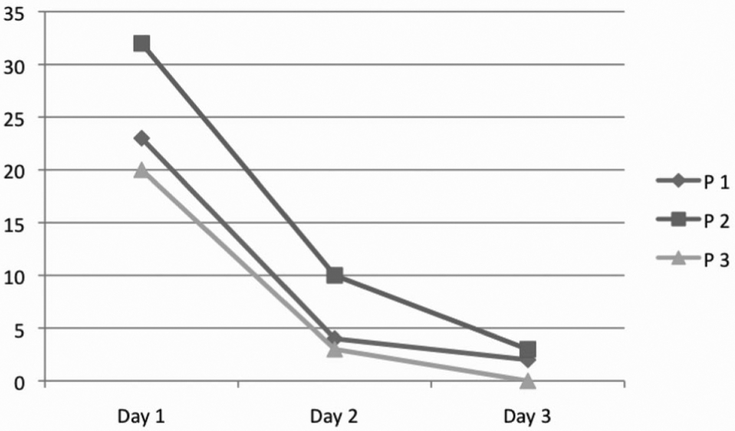
Pediatric Logistic Organ Dysfunction score for each patient. All patients received therapeutic plasma exchange on days 1–3. There is a significant reduction in Pediatric Logistic Organ Dysfunction scoring (p = .001 based on paired t test).
Table 1.
The admission Pediatric Risk of Mortality and Pediatric Logistic Organ Dysfunction score of the three patients in this series
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| PRISM | 20 | 31 | 14 |
| PELOD Pre | 23 | 32 | 20 |
| PELOD Posta | 2 | 3 | 0 |
| Pred. mortality | 32% | 85% | 16% |
| Prior to TPE | |||
 Lactate Lactate | 6.3 | 2.2 | 1.4 |
 PaO2/FIO2 PaO2/FIO2 | 70 | 54 | 97 |
 Norepi dose Norepi dose | 0.01–0.2 | 0.05–0.2 | – |
 Dobutamine dose Dobutamine dose | 2.5–5 | 2.5–5 | – |
| Post TPE | |||
 Lactate Lactate | 1.9 | 0.6 | 1.1 |
 PaO2/FIO2b PaO2/FIO2b | 212 | 207 | 188 |
 Norepi dosec Norepi dosec | Off | Off | – |
 Dobutamine dosec Dobutamine dosec | Off | Off | – |
PRISM, Pediatric Risk of Mortality; PELOD, Pediatric Logistic Organ Dysfunction; –, ————-; TPE, therapeutic plasma exchange; Norepi, norepinephrine.
The predicated mortality was derived from the admission PRISM scores. The PELOD score was significantly reduced after the third TPE (ap = .01). Also, the PaO2/FIO2 ratio was significantly reduced (bp = .021) in all three patients after three daily exchanges. The average lactate was reduced but was not significant. Patient 3 did not require vasopressor support but had a sudden increase in oxygen requirements and additional resuscitation fluid that prompted the initiation of TPE.
DISCUSSION
There have been several reports (8) that described the salutary effects of TPE in sepsis. In a case series review, survival rates with TPE were >70% compared with the expected rate of <50%. An additional series in patients with multiple organ failure showed a survival rate of 82% (9). In the only randomized trial published to date, Busund and colleagues (10) randomized 106 patients with severe sepsis and septic shock to receive either plasma exchange in addition to standard care or standard therapy alone. Their results showed a tendency toward improved outcome with the 28-day all-cause mortality rate of 33.3% in the plasma exchange group and 53.8% in controls. Finally, in a preliminary report, Nguyen and colleagues (11) reported that children with thrombocytopenia-associated (platelet count of <100 000/mm3) multi-organ failure had reduced or absent von Willebrand factor cleaving protease activity, along with markedly increased plasminogen activator inhibitor-1 activity, both of which were reversed by plasma exchange therapy. A median of 11 days of plasma exchange was administered. These findings suggest that prolonged plasma exchange may be required to reverse the thrombotic microangiopathy of thrombocytopenia-associated multiorgan failure. Outcomes connected to plasma exchange therapy may be related to the number of treatments and the degree of underlying thrombotic microangiopathy along with the cytokine burden.
Based on the limited data available, it is difficult to know whether plasma exchange is useful in the management of sepsis either alone or in combination with other forms of blood purification (12). However, the degree of blood purification obtained with TPE, and impressive results with other disease processes that have circulating inflammatory mediators, makes TPE an attractive strategy as a rescue modality in patients who are deteriorating or not responding to standard therapuetic interventions.
TPE as a primary therapy for ALI/acute respiratory distress syndrome has not been investigated. A review (13) of seven patients with suspected TTP with ALI/acute respiratory distress syndrome as its manifestation reported a reduction in oxygen requirements, ventilator days, and outcome after TPE. A study (14) in children with acute respiratory distress syndrome after bone marrow transplantation showed that these patients did seem to respond to hemofiltration as a blood purification strategy. This study was not designed to shed light on a mechanism, so the improvement seen with hemofiltration may have been related to fluid balance and not necessarily blood purification. The patients in this series did not receive hemofiltration or extracorporeal fluid removal during the days reported. It is, however, our standard-of-care to strive for euvolemia.
CONCLUSION
In this limited case series, there was a substantial reduction in vasopressor requirements and PaO2/FIO2 ratio after three daily TPEs. This series suggest there may be a role for TPE as a strategy for attenuation of cytokines and other inflammatory mediators in severe shock and/or ALI related to pH1N1 that has failed to responded to standard therapy.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1097/pcc.0b013e3181e2a569
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6328374?pdf=render
Citations & impact
Impact metrics
Article citations
Indications and Outcomes of Patients Receiving Therapeutic Plasma Exchange under Critical Care Conditions: A Retrospective Eleven-Year Single-Center Study at a Tertiary Care Center.
J Clin Med, 12(8):2876, 14 Apr 2023
Cited by: 1 article | PMID: 37109212 | PMCID: PMC10141205
Use of therapeutic plasma exchange in COVID-19: A systematic review of current evidence.
Ther Apher Dial, 26 Suppl 1:3-11, 05 Dec 2022
Cited by: 1 article | PMID: 36468346 | PMCID: PMC9877855
Review Free full text in Europe PMC
Apheresis and COVID-19 in intensive care unit (ICU).
Transfus Apher Sci, 61(6):103593, 01 Nov 2022
Cited by: 0 articles | PMID: 36335074 | PMCID: PMC9624107
Plasma exchange and COVID 19.
Transfus Apher Sci, 61(6):103598, 12 Nov 2022
Cited by: 1 article | PMID: 36379843 | PMCID: PMC9652706
Acute Kidney Injury and Blood Purification Techniques in Severe COVID-19 Patients.
J Clin Med, 11(21):6286, 25 Oct 2022
Cited by: 1 article | PMID: 36362514 | PMCID: PMC9658337
Review Free full text in Europe PMC
Go to all (49) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Fulminant pH1N1-09 influenza-associated myocarditis in pediatric patients.
Pediatr Crit Care Med, 12(2):e99-e101, 01 Mar 2011
Cited by: 10 articles | PMID: 20601924 | PMCID: PMC4425292
Rescue therapy in adult and pediatric patients with pH1N1 influenza infection: a tertiary center intensive care unit experience from April to October 2009.
Crit Care Med, 38(11):2103-2107, 01 Nov 2010
Cited by: 21 articles | PMID: 20711068 | PMCID: PMC3739437
Influenza A (pH1N1) infection in children admitted to a pediatric intensive care unit: differences with other respiratory viruses.
Pediatr Crit Care Med, 12(3):e136-40, 01 May 2011
Cited by: 7 articles | PMID: 20431501
Extracorporeal membrane oxygenation for 2009 influenza A (H1N1)-associated acute respiratory distress syndrome.
Semin Respir Crit Care Med, 32(2):188-194, 19 Apr 2011
Cited by: 19 articles | PMID: 21506055
Review





