Abstract
Free full text

The relationship of maternal glycemia to childhood obesity and metabolic dysfunction‡
Abstract
Objective:
To determine the association of maternal glycemia with childhood obesity and metabolic dysfunction.
Study design:
Secondary analysis of follow-up data 5–10 years after a mild gestational diabetes mellitus (GDM) treatment trial. The relationship between maternal oral glucose tolerance testing (OGTT) at 24–31-week gestation and body mass index (BMI), fasting glucose, insulin, and anthropometric measurements (sum of skinfolds, subscapular/triceps ratio, and waist circumference) in the offspring of untreated mild GDM and non-GDM (abnormal 50-g screen/normal OGTT) women was assessed. Multivariable regression modeling controlling for maternal and neonatal characteristics was employed.
Results:
A cohort of 236 untreated mild GDM and 480 non-GDM offspring were analyzed. In the combined cohort, significant correlations existed between fasting, 1, 2, and 3 h maternal glucose and subscapular/triceps ratio (all p < .04) and in all OGTT values other than the 2-hour value for homeostatic model assessment-estimated insulin resistance (HOMA-IR) (all p < .04) and sum of skinfold measurements (all p < .03). No correlation was found between OGTT values and childhood BMI Z-score. Multivariable regression modeling showed that OGTT values were associated with only sum of skinfolds and subscapular/triceps ratio and not with childhood BMI Z-score. Hispanic ethnicity and prepregnancy maternal BMI were most consistently related to childhood BMI Z-score and HOMA-IR, and Hispanic ethnicity with fasting glucose.
Conclusions:
Among women with untreated mild GDM and those without GDM, maternal glycemia is associated with childhood anthropometric measures of obesity but not childhood BMI, fasting glucose, or insulin resistance. Hispanic ethnicity, maternal BMI, and gestational weight gain were consistently related to childhood BMI.
Introduction
Childhood obesity, which is associated with a subsequent risk for both metabolic and cardiovascular abnormalities into adulthood, is a major public health problem [1]. The incidence of obesity in the USA has doubled in children and quadrupled in adolescents over the past 30 years with more than one-third of children and adolescents being classified as either overweight or obese [2]. The intergenerational cycle of obesity and diabetes may be related to the altered hormonal milieu of pregnancy in which maternal hyperglycemia by way of fetal programing could have long range effects on the developing fetus resulting in behavioral, anthropometric, and metabolic functions [3]. The landmark Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and others have shown that with increasing maternal glucose levels, the frequency of large for gestational age infants increases proportionately and the risk remains after adjustment for maternal BMI [4,5]. Longitudinal studies in selected populations have demonstrated a link between in-utero exposure to maternal hyperglycemia and the development of obesity in both childhood and adolescence [6,7]. In utero hyperglycemia as reflected by amniotic fluid insulin levels is also a predictor of insulin resistance during childhood and these effects apparently may extend into adult life [7,8]. While it is well accepted that fetal growth and neonatal adiposity are related to a continuum of maternal glucose levels, the effect of maternal glycemia on subsequent childhood growth and metabolism has been less well studied [9]. We, therefore, sought to determine the relationship between maternal glycemia and childhood obesity and metabolic dysfunction.
Materials and methods
This study represents a secondary analysis of the mild gestational diabetes mellitus (GDM) treatment trial follow-up (GDMFU) study conducted between February 2012 and September 2013 [10]. The GDMFU study was an unplanned follow-up study of women, and their offspring who participated in the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network randomized clinical trial (RCT) for the treatment of mild GDM [11]. In the parent study, mild GDM was defined by a fasting glucose less than 95 mg/dL with two of the three post glucose load measurements meeting Carpenter-Coustan criteria for the diagnosis of GDM [12]. The population reported in this study consisted of offspring of untreated mild GDM as well as off-spring of non-GDM women defined as women with a 50 g 1-hour screening test between 135 to 200 mg/dL who underwent a 100 g 3 h oral glucose tolerance test (OGTT) with normal results between 24 and 31 weeks gestation. Follow-up results comparing offspring of women treated for mild GDM versus controls has been previously reported [11]. Enrollment for the follow-up study included enrollment in the RCT at a center still participating in the MFMU Network at the time of the follow-up study (12 of 16 centers; 94% of the original participants eligible). Following informed consent of the mothers and child assent when appropriate, children underwent a study visit between the ages of 5–10 years of the index pregnancy. At the study visit, trained nursing personnel collected height and weight measurements using a calibrated scale and stationary stadiometer. Determination of overweight and obesity status was based on a BMI ≥ 85th percentile and ≥ 95th percentile, respectively, for child age and sex per 2000 Centers for Disease Control and Prevention growth charts [13]. In addition, waist circumference and anthropometric measurements including subscapular and triceps skinfolds were obtained in a standard manner [14,15]. The study visit took place after an overnight fast, and blood samples were sent to a central laboratory for glucose and insulin measurements. HOMA-estimated insulin resistance (HOMA-IR) was calculated as (fasting glucose (mmol/L) × fasting insulin micro units/mL/22.5) [16]. The study was approved by the institutional review board of all participating centers.
For the present analysis, the association of maternal OGTT levels with the following child outcomes was examined: BMI percentile for child age and sex converted to a Z−score, fasting glucose, HOMA-IR, sum of skinfold thicknesses (thigh, tricep, subscapular, and suprailiac areas), subscapular to triceps skinfold ratio, and waist circumference. The chi-square test and the Wilcoxon rank sum test we used to examine differences between males and females. The Spearman correlation coefficients were calculated to assess the association between maternal OGTT values and the childhood outcomes of interest. Chi-square tests for trend were used to examine the relationship between ordered categories of the OGTT and childhood BMI greater than both the 85th and 95th percentiles. Multiple variable linear regression analysis was also used to examine the relationship between maternal OGTT levels and childhood outcomes, adjusting for maternal factors including age, race/ethnicity, prepregnancy maternal BMI and maternal weight gain based on prepregnancy weight, neonatal factors, such as, large for gestational age (LGA), small for gestational age (SGA) [17], fat mass, and elevated cord c-peptide level, and sex and age of the child in the follow-up examination. Additional models excluding the neonatal factors were also generated. For regression analysis, a composite OGTT variable was created by calculating the area under the curve for an individual’s fasting, 1, 2, and 3 h OGTT values using a trapezoidal algorithm [18]. Additional models that examined the fasting, 1, 2, and 3 h OGTT values separately were also generated. Because their distributions were positively skewed, sum of skin-folds, subscapular/triceps skinfold ratio, and HOMA-IR were logarithmically transformed for the regression analysis. A nominal p-value of less than .05 was considered to indicate statistical significance and no adjustments were made for multiple comparisons. Statistical analysis was conducted with SAS software (SAS Institute Inc, Cary, NC).
Results
Of 1404 potential enrollees, a total of 716 (51%) children participated in this unplanned follow-up study. The mean follow-up age was 7 years. The study population included 236 offspring of untreated mild GDM and 480 offspring women with a normal OGTT. The baseline maternal characteristics of age (mean 27.8 ± 5.5) years, race/ethnicity (56% Hispanic, 29% Caucasian, 11% African American) and, BMI (mean 26.7 ± 5.6) and BMI category (underweight 2.6%, normal 39.4%, overweight 34.6%, obese 23.5%) were not different among those in the follow-up study compared to those not followed in the original study population. The mean OGTT values of those in the follow-up study were as follows: fasting 85.6 ± 5.7, 1 h 166.3 ± 29.8, 2 h 143.8 ± 29.1, 3 h 117.6 ± 28.1 mg/dL, respectively. Baseline neonatal characteristics revealed a mean gestational age of 39.1 ± 1.7 weeks, 50% males, mean birthweight of 3394 ± 534 g, 12% large-for-gestational age, and 6% small for gestational age.
Child characteristics at follow-up examination are presented in Table 1. There were no differences in age, weight or BMI according to sex. Anthropometric measurements showed a greater sum of skinfolds for females (p < .001). Females also had a greater degree of insulin resistance as determined by HOMA-IR (p = .01) whereas males demonstrated a slightly increased fasting glucose level (p =.03). The prevalence of both childhood overweight(≥85th percentile) and obesity (≥95th percentile) according to various glucose categories for each data point of the 3 h OGTT are displayed in Figure 1. For all 3 h OGTT values, we found no significant trend with subsequent childhood BMI.
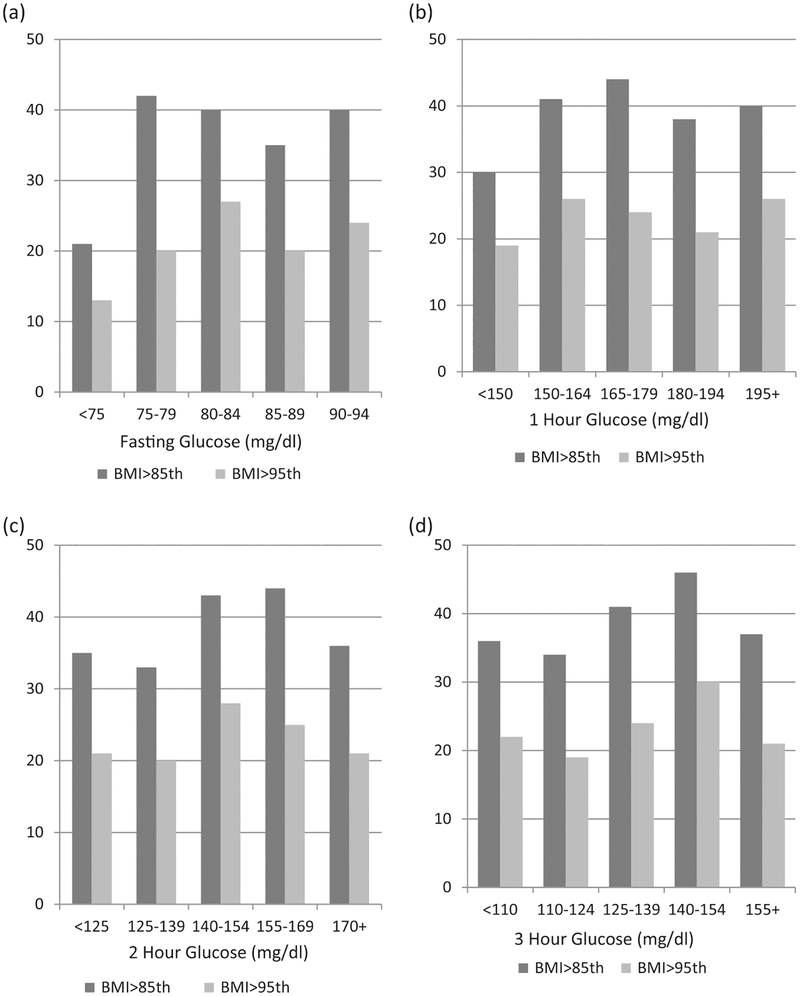
Percent of children >85th and >95th BMI percentile versus maternal baseline OGTT: (a) Fasting, (b) 1 hour, (c) 2 hours, (d) 3 hours glucose levels.
Table 1.
Child characteristics at the follow-up exam.
| Characteristic | Male | Female | p-Value |
|---|---|---|---|
| Age at Exam (y) | 7 [6, 8] | 7 [6, 8] | .83 |
| Weight (kg) | 25.0 [21.3, 30.1] | 24.2 [20.5,30.4] | .49 |
| BMI (kg/m2) | 16.6 [15.3, 18.8] | 16.5 [15.2, 18.8] | .69 |
| BMI percentile | 76 [43, 94] | 72 [43, 93] | .61 |
| Overweight (BMI ≥85th percentile) | 138 (38.6%) | 133 (37.2%) | .70 |
| Obese (BMI ≥95th percentile) | 85 (23.7%) | 77 (21.5%) | .48 |
| Sum of skinfolds (mm) | 39.5 [29.7, 58.0]N = 353 | 46.5 [35.1, 63.0]N = 349 | <.001 |
| Subscapular to tricep skinfold ratio | 0.65 [0.55, 0.77]N = 356 | 0.64 [0.54, 0.78]N = 357 | .37 |
| Waist circumference (cm) | 56.5 [52.5, 61.7] | 56.9 [52.2, 64.5] | .73 |
| HOMA – IR | 1.08 [0.71, 1.72]N = 276 | 1.32 [0.79, 1.96]N = 279 | .01 |
| Fasting glucose (mg/dL) | 90 [86, 95]N = 276 | 89 [84, 93]N = 280 | .03 |
Data are median [interquartile range] or n (%), N = 358 for males and N = 358 for females except where otherwise indicated.
The correlation between maternal OGTT values and childhood outcomes is presented in Table 2. Significant correlations existed between fasting, 1, 2, and 3 h maternal glucose and subscapular/triceps skin-fold ratio and in all but the 2-hour values for the sum of skinfolds and HOMA-IR in the offspring. However, no significant positive correlation was observed between any OGTT value and childhood BMI percentile, and between 1, 2, and 3 h values and child fasting glucose and child waist circumference. Fat mass, birth-weight, and cord c-peptide level were correlated with childhood BMI among other outcomes (Table 3).
Table 2.
Correlation of maternal oral glucose tolerance testing (OGTT) with child outcomes.
| Child Outcome | Fasting glucose | 1 Hour glucose | 2 Hour glucose | 3 Hour glucose |
|---|---|---|---|---|
| BMI percentile | 0.02 | 0.05 | 0.00 | 0.04 |
| N = 715 | N = 715 | N = 715 | N = 715 | |
| Sum of skinfolds (mm) | 0.08a | 0.09a | 0.07 | 0.12a |
| N = 702 | N = 702 | N = 702 | N = 702 | |
| Subscapular to tricep skinfold ratio | 0.10a | 0.08a | 0.11a | 0.12a |
| N = 713 | N = 713 | N = 713 | N = 713 | |
| HOMA-IR | 0.09a | 0.10a | 0.06 | 0.12a |
| N = 555 | N = 555 | N = 555 | N = 555 | |
| Fasting glucose (mg/dL) | 0.09a | 0.06 | 0.02 | 0.06 |
| N = 556 | N = 556 | N = 556 | N = 556 | |
| Waist circumference (cm) | 0.08a | 0.06 | 0.05 | 0.05 |
| N = 716 | N = 716 | N = 716 | N = 716 |
Data are Spearman correlation coefficients.
Table 3.
Correlation of neonatal factors with child outcomes.
| Child outcome | Fat mass | Birth weight | C-peptide |
|---|---|---|---|
| BMI percentile | 0.16a | 0.16a | 0.09a |
| N = 638 | N = 714 | N = 626 | |
| Sum of skinfolds (mm) | 0.14a | 0.11a | 0.09a |
| N = 626 | N = 701 | N = 614 | |
| Subscapular to tricep skinfold ratio | 0.03 | 0.00 | 0.05 |
| N = 636 | N = 712 | N = 624 | |
| HOMA-IR | 0.12a | 0.08 | 0.10a |
| N = 505 | N = 555 | N = 495 | |
| Fasting glucose (mg/dL) | 0.11a | 0.08 | −0.02 |
| N = 506 | N = 556 | N = 496 | |
| Waist circumference (cm) | 0.15a | 0.13a | 0.09a |
| N = 639 | N = 715 | N = 627 |
Data are Spearman’s correlation coefficients.
In multivariable regression analyses in which we adjusted for maternal age, race/ethnicity, prepregnancy BMI, maternal weight gain, and neonatal factors that included sex, fat mass, LGA, SGA, elevated c-peptide, and age at follow-up examination, we found no association between maternal OGTT values and childhood BMI, HOMA-IR, fasting glucose levels, or waist circumference. However, we did find that 1, 2, and 3 h OGTT values were associated with both the sum of skinfolds and the subscapular to triceps skinfold ratio (Table 4). Similar associations between maternal OGTT values and the childhood outcomes of interest were found in additional regression model analysis that excluded the neonatal factors of fat mass, LGA, SGA, and elevated c-peptide. Among baseline factors in the regression analysis, Hispanic ethnicity, maternal BMI, and maternal weight gain were all significantly associated with childhood BMI. Hispanic ethnicity and maternal BMI were also associated with childhood HOMA-IR, the sum of skinfolds, the subscapular to triceps skin-fold ratio and waist circumference, and Hispanic ethnicity alone was associated with childhood fasting glucose levels (Table 5).
Table 4.
Linear regression analyses for the association of maternal OGTT with child outcomes.
| Child BMI Z-score | HOMA-IR | Fasting glucose (mg/dL) | Sum of Skin Folds (mm) | Subscapular to Triceps Skinfold Ratio | Waist Circumference (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 553 | N = 439 | N = 439 | N = 541 | N = 551 | N = 554 | |||||||
| Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | |
| Composite OGTT* | 0.050 ± 0.091 | .58 | 0.085 ± 0.058 | .14 | 0.208 ± 0.621 | .74 | 0.080 ± 0.029 | .005 | 0.074 ± 0.022 | .001 | 0.963 ± 0.579 | .10 |
| Fasting | −0.004 ± 0.010 | .65 | 0.003 ± 0.006 | .56 | 0.099 ± 0.063 | .12 | 0.002 ± 0.003 | .50 | 0.004 ± 0.002 | .07 | −0.009 ± 0.061 | .88 |
| 1 hour | 0.0007 ± 0.002 | .73 | 0.001 ± 0.001 | .26 | −0.005 ± 0.013 | .69 | 0.001 ± 0.0006 | .029 | 0.001 ± 0.0005 | .035 | 0.016 ± 0.012 | .18 |
| 2 hour | −0.0001 ± 0.002 | .96 | 0.001 ± 0.001 | .36 | 0.007 ± 0.013 | .59 | 0.001 ± 0.0006 | .031 | 0.001 ± 0.0005 | .002 | 0.015 ± 0.012 | .22 |
| 3 hour | 0.004 ± 0.002 | .07 | 0.002 ± 0.001 | .07 | 0.011 ± 0.013 | .41 | 0.002 ± 0.0006 | .004 | 0.001 ± 0.0005 | .004 | 0.023 ± 0.012 | .07 |
Variables included in models were maternal glucose, maternal age, race/ethnicity, prepregnancy maternal BMI, maternal weight gain, neonatal factors of large for gestational age (LGA), small for gestational age (SGA), fat mass, and elevated cord c-peptide level, gender, and age of the child at exam. HOMA-IR, sum of skin folds, and subscapular to triceps skinfold ratios were log-transformed.
Table 5.
Linear regression analysis for the association of maternal characteristics with child outcomes.
| Child BMI Z-score | HOMA-IR | Fasting glucose(mg/dL) | Sum of Skin Folds (mm) | Subscapular to Triceps Skinfold Ratio | Waist Circumference (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 553 | N = 439 | N = 439 | N = 541 | N = 551 | N = 554 | |||||||
| Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | Beta ± SE | p-Val | |
| Hispanic Ethnicity | 0.685 ± 0.109 | <.001 | 0.332 ± 0.066 | <.001 | 2.93 ± 0.711 | <.001 | 0.212 ± 0.034 | <.001 | 0.116± 0.027 | <.001 | 2.522 ± 0.694 | <.001 |
| Pre-pregnancy BMI (kg) | 0.077 ± 0.010 | <.001 | 0.018 ± 0.007 | .007 | 0.107 ± 0.070 | .13 | 0.0169 ± 0.003 | <.001 | 0.006 ± 0.0025 | .015 | 0.409 ± 0.065 | <.001 |
| Maternal Weight Gain (kg) | 0.024 ± 0.009 | .007 | 0.001 ± 0.005 | .82 | −0.053 ± 0.058 | .36 | 0.005 ± 0.003 | .071 | −0.002 ± 0.0021 | .44 | 0.086 ± 0.056 | .13 |
Variables included in models were composite maternal OGTT*, age, race/ethnicity, prepregnancy maternal BMI, maternal weight gain, neonatal factors of large for gestational age (LGA), small for gestational age (SGA), fat mass, and elevated cord c-peptide level, gender, and age of the child at exam. HOMA-IR, sum of skin folds, and subscapular to triceps skinfold ratios were log-transformed.
Discussion
In this childhood follow-up study, we found that maternal glycemia was associated with anthropometric measures of obesity but not with childhood BMI, fasting glucose, or insulin resistance. In contrast, maternal BMI, gestational weight gain, and Hispanic ethnicity were all consistently associated with childhood BMI. This finding is of interest in as much as a monotonic relationship appears to exist between maternal glucose levels during pregnancy and neonatal outcomes such as birthweight and adiposity even at levels far below the criteria for the diagnosis of GDM [4]. The causal relationship between both preexisting and gestational diabetes and fetal/neonatal hyperinsulinism and overgrowth is well established. Moreover, large scale treatment trials of mild gestational diabetes conducted over the last decade have provided evidence that treatment leads to a reduction in birthweight and the frequency of large-for gestational age infants [10,19]. However, follow-up studies of the offspring from these studies have failed to demonstrate a lasting treatment effect as reflected by assessment of childhood BMI [11,20]. These results have challenged the developmental over nutrition hypothesis which suggests that offspring of diabetic mothers have larger infants who in turn are at risk for both childhood obesity and metabolic abnormalities that may persist into adulthood. A better understanding of the relationship between maternal glycemia and offspring obesity remains critical to our understanding of the pathogenesis of childhood obesity and for planning potential interventions for this significant problem.
Two recent systematic reviews and meta-analysis have examined the relationship of gestational diabetes to childhood overweight and obesity [9,21]. In both analyses, maternal diabetes was strongly associated with increasing offspring BMI Z−score, although this association was no longer apparent after adjustment for maternal BMI [9,21]. Similarly, in the present study, we found maternal BMI but not glucose to be consistently associated with childhood obesity and insulin resistance. Our results are also in agreement with the report of Catalano and colleagues who found in 8 to 9-year old children that the only variable of significance related to dual energy X-ray absorptiometry (DXA)-estimated obesity in their population was maternal pregravid BMI [22]. In the only prospective long-term study of offspring of women with diabetes, Pettitt and colleagues [6] found that maternal diabetes in a Pima Indian population, independent of both birthweight and maternal BMI, was associated with obesity in 5 to 19-year-old offspring. These findings as well as other studies showing an association between GDM and childhood obesity may be explained by ethnic differences among various study populations as well as methodology. In most pregnancy follow-up studies of offspring, the population is dichotomized into GDM (exposed) and non-GDM controls. In our analysis, we considered glucose as a continuous variable and could not demonstrate a relationship between maternal glucose levels and childhood BMI or metabolic measures. In contrast, a recent 7-year follow-up study of Danish offspring of women with treated GDM found that maternal fasting plasma glucose (diagnostic 75 g OGTT) was significantly associated with subsequent overweight/obesity in childhood. In this study, however, post glucose load measurements were not analyzed and one-third of women had a fasting glucose greater than or equal to 99 mg/dL [23]. Similarly, in Pettit’s study, women with prediabetes or GDM included those with pronounced hyperglycemia (2-hour post load values exceeding 200 mg/dL). In contrast, our cohort included only mild untreated GDM [6]. It thus remains possible that a risk for childhood obesity exists for the offspring of women with pronounced hyperglycemia during pregnancy. Increased background rates of childhood obesity may also obscure the relationship between maternal glycemia and childhood outcomes. A large scale German retrospective cohort of 7355 mother–child dyads which revealed an association between GDM and childhood obesity interestingly reported an overall obesity rate of 2.5%, which is markedly lower than our rate of 22.6% [24]. Thus, it is possible that any effect of maternal glycemia on subsequent offspring obesity could be attenuated by a higher prevalence of childhood obesity, which exists in our study population.
Classic studies conducted in the Pima Indian population of siblings exposed to maternal diabetes who have significantly greater rates of childhood and adult obesity and diabetes compared to those unexposed clearly support the concept of intrauterine programing [25]. While our study might suggest a minimal role for maternal glycemia in this process in pregnancies with mild GDM or those women with lesser degrees of hyperglycemia, the concept of nutrient excess during intrauterine life having profound effects on subsequent growth and development in the setting of maternal obesity is well supported. Maternal obesity which likely bears a relationship to postnatal nutrient exposure can also provide a milieu of nutrient excess for the developing fetus, which includes substrates other than glucose such as lipids. A high-fat diet during pregnancy has been demonstrated to result in epigenetic modifications of the fetal hypothalamic appetite center leading to altered production of specific peptides which can in turn lead to overeating and adiposity [26]. Impaired insulin action in the proinflammatory state present in obese women can also affect placental programing of genes related to adipokine expression and lipid metabolism which may promote fat accretion in the fetus, which in turn affects the long-term development [27,28].
While our study provided detailed assessment of the children obtained at the follow-up visit, there are inherent limitations to this analysis. Our unplanned follow-up study included only 51% of participants and might suggest potential for selection bias. It is possible that those who chose to participate in follow up might be different from those who we could not locate or refused participation. We, however, did not observe any differences in demographic measures between these groups. The inclusion of women who failed their 50 g challenge in the overall population may compromise the analysis as these women may be considered relatively hyperglycemic despite their normal OGTT results. Moreover, the OGTT represents only a specific point in time and may not reflect maternal glycemia over the course of pregnancy. Other confounders which must be considered in determining the relationship between maternal glycemia and childhood obesity include both genetic and postnatal factors. Thus, a potential weakness of this analysis is that we did not include childhood dietary or physical activity data in the regression model. We did, however, in additional analyses, adjust for breastfeeding which may be associated with a reduction in childhood obesity in offspring of diabetic women [29]. The age of follow up of the offspring must also be considered. Similar to our results, the Belfast group of the Hyperglycemia and Adverse Pregnancy Outcomes study examined offspring 2 years of age and found some associations with various anthropometrics but no significant relationship between maternal glucose levels and subsequent childhood obesity [30]. We followed children ages 5–10 years, yet the emergence of both obesity and metabolic dysfunction in offspring of diabetic women may not occur until adolescence or adulthood [31]. As most of the children in our study were prepubertal (average age 7 years), we acknowledge that the development of obesity related to glycemia during pregnancy may not be apparent in this cohort. Importantly, however, is the recognition that the period of “adiposity rebound” which occurs by an average age of 6 years is believed to be the critical period for risk for adult obesity [32]. The use of BMI as a primary measure of childhood obesity has also been challenged. However, BMI remains a widely utilized metric in the clinical assessment of childhood and adult obesity. Childhood anthropometric measurements and BMI both correlate with densitometry measurement of fat mass [33]. We did find a relationship between maternal glucose and various childhood anthropometric measures. This included subscapular/triceps ratio indicating increased central obesity. To the extent that these measurements reflect childhood adiposity, it is thus possible that mild maternal hyperglycemia may be an important contributor to offspring obesity.
In addition to maternal BMI, we found that weight gain during pregnancy was strongly related to childhood BMI. (In multivariate regression analysis, for each kilogram increase in maternal weight gain, there was a corresponding increase in childhood BMI Z−score of 0.024 (p = .007).) Weight gain during pregnancy compared with maternal BMI and diabetes has been demonstrated to be the strongest contributor to the delivery of large-for-gestational age infants [34]. Excessive gestational weight gain is also associated with childhood adiposity among women with normal weight status [35]. It has been suggested that preventing excess weight gain during pregnancy may be more feasible than preventing obesity and GDM [36]. A randomized controlled study of a low glycemic index diet during pregnancy resulted in a decrease in gestational weight gain in the intervention group, but no difference in birthweight or macrosomia [37]. Metaanalyses of lifestyle interventions during pregnancy have demonstrated only moderate success in curbing weight gain with little effect on fetal growth or the frequency of GDM [38]. Whether any long-term childhood benefit exists to these interventions awaits further study.
In summary, our study confirms that an important relationship exists between maternal obesity and childhood BMI and metabolic dysfunction. Despite a minimal association of glucose related to long-term outcomes, our results should not diminish the efforts to establish optimal glycemia in pregnancies complicated by diabetes mellitus, as our study population consisted primarily of non-GDM women. Breaking the cycle of intergenerational obesity remains a public health priority. Interventions during pregnancy focused on limiting weight gain have met with modest success and, it is now apparent that we should focus on reducing preconceptional obesity. Preconception care including specific services to reduce weight in obese women has been advocated and remains a goal for those treating women of childbearing age [39]. Such intervention strategies to reduce childhood obesity hold promise as preliminary data indicates that inter-pregnancy weight loss lowers the risk for excessive birthweight and neonatal fat mass [40]. These efforts may be considered the first step to stemming the rising tide of childhood obesity.
Acknowledgments
The authors thank Celeste Durnwald, M.D (University of Pennsylvania, School of Medicine, Philadelphia, PA, United States of America) for contributions to the design of this study; Patrick Catalano, MD (Case Western Reserve University–MetroHealth Medical Center, Cleveland, OH) for contributions to the design of this study and review of the manuscript; and subcommittee members Francee Johnson, RN, BSN (the Ohio State University College of Medicine, Columbus, OH, USA) and Lisa Moseley, R.N. (University of Texas Southwestern Medical Center, Dallas, TX, USA) for protocol development and coordination between clinical research centers, Lindsey Doherty, M.S. (George Washington University Biostatistics Center, Washington, DC, USA) for protocol/data management, and Madeline M. Rice, Ph.D. (George Washington University Biostatistics Center, Washington, DC, USA), Elizabeth Thom, Ph.D. (George Washington University Biostatistics Center, Washington, DC, USA) and Catherine Y. Spong, M.D. (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA) for protocol development and oversight.
Funding
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27915, HD36801, HD34208, HD34116, HD40485, HD40500, HD27869, HD40560, HD40544, HD53097, HD40512, HD40545] and the National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS) [UL1TR001070, UL1TR000439].
Appendix
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
The Ohio State University, Columbus, OH – F. Johnson, S. Wylie, D. Habash, S. Heintzman, E. Nini, J. Iams, C. Durnwald
University of Utah Health Sciences Center, Salt Lake City, UT – K. Hill, M. Thompson, A. Sowles, G. Anderson (Intermountain Healthcare)
University of Texas Southwestern Medical Center, Dallas, TX – L. Moseley, J. Price, A. Sias, K. Gonzales, Y. Delira
Columbia University, New York, NY – S. Bousleiman, M. Talucci, V. Carmona, I. Quezada, A. Ranzini (St. Peter’s University Hospital), M. Lake (St. Peter’s University Hospital), S. Davis (St. Peter’s University Hospital), M. Hoffman (Christiana Care), S. Lynch (Christiana Care), J. Benson (Christiana Care), C. Kitto (Christiana Care), L. Plante (Drexel U.), C. Tocci (Drexel U.), Y. Williams (Drexel U.)
Brown University, Providence, RI – D. Allard, B. Anderson, K. Pereda, E. Hipolito, J. McNamara
University of Alabama at Birmingham, Birmingham, AL – S. Harris, J. Biggio, A. McClain, J. Sheppard
University of North Carolina at Chapel Hill, Chapel Hill, NC – K. Clark, B. Eucker, S. Timlin, K. Pena, T. Varney
MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH – W. Dalton, C. Milluzzi, P. Catalano,B. Mercer
University of Texas Medical Branch, Galveston, TX – A.Salazar, A. Acosta, S. Bouse, G. Hankins, S. Jain
Northwestern University, Chicago, IL – G. Mallet, M. Ramos-Brinson, C. Collins, L. Stein, A. Peaceman, M. Dinsmoor (NorthShore Health Systems-Evanston Hospital)
University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX – F. Ortiz, B. Sibai, B. Rech, L. Garcia
The George Washington University Biostatistics Center, Washington, DC –E. Thom, M. Rice, L. Doherty, T. Spangler
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
Footnotes
‡Presented in part at the 2015 Annual Scientific Meeting of the Society for Maternal-Fetal Medicine, 2–7 February 2015, San Diego, CA, USA.
Disclosure statement
Comments and views of the authors do not necessarily represent views of the NICHD or the NIH. No potential conflict of interest was reported by the authors.
References
Full text links
Read article at publisher's site: https://doi.org/10.1080/14767058.2018.1484094
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6338534?pdf=render
Citations & impact
Impact metrics
Article citations
Continuous glucose monitoring metrics in pregnancy with type 1 diabetes mellitus.
World J Methodol, 14(1):90316, 20 Mar 2024
Cited by: 0 articles | PMID: 38577196 | PMCID: PMC10989406
Review Free full text in Europe PMC
Metabolic Profile of Offspring of Mothers with Gestational Diabetes Mellitus.
Indian J Endocrinol Metab, 28(2):192-196, 01 Mar 2024
Cited by: 1 article | PMID: 38911115 | PMCID: PMC11189277
Associations between maternal and offspring glucose metabolism: a 9-year follow-up of a randomised controlled trial.
Front Endocrinol (Lausanne), 14:1324925, 10 Jan 2024
Cited by: 1 article | PMID: 38269252 | PMCID: PMC10806570
An unwelcome inheritance: childhood obesity after diabetes in pregnancy.
Diabetologia, 66(11):1961-1970, 13 Jul 2023
Cited by: 4 articles | PMID: 37442824 | PMCID: PMC10541526
Review Free full text in Europe PMC
Gestational Diabetes-Placental Expression of Human Equilibrative Nucleoside Transporter 1 (hENT1): Is Delayed Villous Maturation an Adaptive Pattern?
Diagnostics (Basel), 13(12):2034, 12 Jun 2023
Cited by: 1 article | PMID: 37370929 | PMCID: PMC10297314
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: An interim analysis from a longitudinal mother-child cohort study.
PLoS Med, 15(10):e1002681, 29 Oct 2018
Cited by: 33 articles | PMID: 30372451 | PMCID: PMC6205663
Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism.
Diabetes Care, 42(3):372-380, 17 Jan 2019
Cited by: 214 articles | PMID: 30655380 | PMCID: PMC6385693
Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study.
Diabetologia, 62(4):598-610, 15 Jan 2019
Cited by: 115 articles | PMID: 30648193 | PMCID: PMC6421132
Offspring body size and metabolic profile - effects of lifestyle intervention in obese pregnant women.
Dan Med J, 61(7):B4893, 01 Jul 2014
Cited by: 29 articles | PMID: 25123127
Review
Funding
Funders who supported this work.
Eunice Kennedy Shriver National Institute of Child Health and Human Development
NCATS (2)
Grant ID: UL1TR000439
Grant ID: UL1TR001070
NCATS NIH HHS (4)
Grant ID: UL1 TR001070
Grant ID: UL1 TR001863
Grant ID: UL1 TR002548
Grant ID: UL1 TR000439
NICHD (12)
Grant ID: HD40545
Grant ID: HD53097
Grant ID: HD27869
Grant ID: HD40485
Grant ID: HD34116
Grant ID: HD27915
Grant ID: HD34208
Grant ID: HD40544
Grant ID: HD40560
Grant ID: HD40512
Grant ID: HD36801
Grant ID: HD40500
NICHD NIH HHS (26)
Grant ID: U10 HD036801
Grant ID: U10 HD040500
Grant ID: U24 HD036801
Grant ID: UG1 HD034116
Grant ID: UG1 HD040544
Grant ID: U10 HD034116
Grant ID: UG1 HD040500
Grant ID: U10 HD053097
Grant ID: UG1 HD027915
Grant ID: UG1 HD040560
Grant ID: U10 HD040512
Grant ID: U10 HD040560
Grant ID: UG1 HD034208
Grant ID: U10 HD040545
Grant ID: UG1 HD040485
Grant ID: P2C HD050924
Grant ID: U10 HD040485
Grant ID: UG1 HD027869
Grant ID: UG1 HD040545
Grant ID: UG1 HD040512
Grant ID: U01 HD036801
Grant ID: U10 HD027869
Grant ID: U10 HD027915
Grant ID: U10 HD034208
Grant ID: U10 HD040544
Grant ID: UG1 HD053097





