Abstract
Free full text

Volatile Compounds in Citrus Essential Oils: A Comprehensive Review
Abstract
The essential oil fraction obtained from the rind of Citrus spp. is rich in chemical compounds of interest for the food and perfume industries, and therefore has been extensively studied during the last decades. In this manuscript, we provide a comprehensive review of the volatile composition of this oil fraction and rind extracts for the 10 most studied Citrus species: C. sinensis (sweet orange), C. reticulata (mandarin), C. paradisi (grapefruit), C. grandis (pummelo), C. limon (lemon), C. medica (citron), C. aurantifolia (lime), C. aurantium (bitter orange), C. bergamia (bergamot orange), and C. junos (yuzu). Forty-nine volatile organic compounds have been reported in all 10 species, most of them terpenoid (90%), although about half of the volatile compounds identified in Citrus peel are non-terpenoid. Over 400 volatiles of different chemical nature have been exclusively described in only one of these species and some of them could be useful as species biomarkers. A hierarchical cluster analysis based on volatile composition arranges these Citrus species in three clusters which essentially mirrors those obtained with genetic information. The first cluster is comprised by C. reticulata, C. grandis, C. sinensis, C. paradisi and C. aurantium, and is mainly characterized by the presence of a larger abundance of non-terpenoid ester and aldehyde compounds than in the other species reviewed. The second cluster is comprised by C. junos, C. medica, C. aurantifolia, and C. bergamia, and is characterized by the prevalence of mono- and sesquiterpene hydrocarbons. Finally, C. limon shows a particular volatile profile with some sulfur monoterpenoids and non-terpenoid esters and aldehydes as part of its main differential peculiarities. A systematic description of the rind volatile composition in each of the species is provided together with a general comparison with those in leaves and blossoms. Additionally, the most widely used techniques for the extraction and analysis of volatile Citrus compounds are also described.
Introduction
Citrus essential oils, widely used to the procurance of natural fruity perfumes and as flavoring ingredients in food, pharmaceutical and cosmetic products (Tranchida et al., 2012; Palazzolo et al., 2013; Othman et al., 2017), are obtained mainly from the fruit rind (flavedo), although flowers or leaves have also been used. Volatile and semi-volatile compounds represent 85–99% of the entire oil fraction (Dugo and Mondello, 2011; Tranchida et al., 2012; Sarrou et al., 2013), which is represented typically by over 200 compounds. Hydrocarbon and derivate mono-and sesquiterpenes are the compounds most frequently reported, followed by aliphatic and olefinic C6–C12 non-terpene aldehydes, alcohols, ketones, esters and acids, along with several aromatic compounds. The non-volatile residue is mainly composed of flavonoids, coumarins, diterpenoids, sterols, and fatty acids.
Most of the studies regarding the composition of the volatile and semi-volatile fraction in Citrus ssp. show data from rind EOs (Dugo and Mondello, 2011). Besides, there are several papers reporting the volatile composition either from neroli (Citrus flowers EO) (Boussaada and Chemli, 2006; Boussaada et al., 2007; Sarrou et al., 2013; Družić, 2016), and petitgrain (Citrus leaves and little branches EO) (Lota et al., 1999, 2001a,b, 2002; Alonzo et al., 2000; Huang et al., 2000; Brophy et al., 2001; Vekiari et al., 2002, 2004; Fanciullino et al., 2005, 2006; Smadja et al., 2005; Boussaada and Chemli, 2006; Boussaada et al., 2007; Tomi et al., 2008; Singh et al., 2010; Guerrini et al., 2014; Družić, 2016; Paoli et al., 2016) or from extracts of Citrus flowers (Choi, 2003a; Flamini et al., 2007; Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Cheong et al., 2011b) and leaves (Gancel et al., 2002, 2003, 2005).
The main objective of this review is to gather in a single manuscript the information on volatile and semi-volatile compounds that has been described up to date in rinds, flowers and leaves of the most studied Citrus species over the last two decades, when most of the work on Citrus essential oils has been performed. These species include C. sinensis (L.) Osb. (sweet orange), C. reticulata Bl. (mandarin), C. paradisi Macfad. (grapefruit), C. grandis (L.) Osb. (also C. maxima Burm.; pummelo), C. limon (L.) Burm. f. (lemon), C. medica L. (citron), C. aurantifolia (Christm.) Swingle (lime), C. aurantium L. (bitter orange), C. bergamia Risso et Poit. (bergamot orange) and C. junos Sieb. ex. Tanaka (yuzu), according to the classification of Tanaka (1954). Moreover, we used all the data of volatile compounds reviewed to perform a hierarchical clustering of these Citrus species based on the similarity of their rind volatile profiles. Additionally, the most widely used techniques for the extraction and analysis of volatile Citrus compounds are also described.
Techniques to Extract Citrus Volatile Compounds
The EOs extraction from Citrus vegetal material (peel, flowers, and leaves) is based on steam distillation (Blanco Tirado et al., 1995), mainly in Clevenger-type hydrodistillation (Alonzo et al., 2000; Lota et al., 2001a,b; Vekiari et al., 2004; Boussaada and Chemli, 2006; Boussaada et al., 2007; Karioti et al., 2007; Darjazi, 2011a; Spadaro et al., 2012; Sarrou et al., 2013; Asikin et al., 2015; Fancello et al., 2016; Ben Hsouna et al., 2017). Recently, an improved Clevenger-type apparatus with a second condenser preventing thermal reactions and reducing the oxidation of some monoterpene compounds has been described (Chen et al., 2014). Moreover, EOs obtained from Citrus peel can also be performed by cold press extraction, whereas this technique should not be applied for Citrus flowers and leaves (Blanco Tirado et al., 1995; Merle et al., 2004; Choi, 2005, 2006; Dugo et al., 2005; Njoroge et al., 2005a,b; Sawamura et al., 2005; Asikin et al., 2012a,b; Tranchida et al., 2012; Sun et al., 2014b). The oil from C. aurantium (Dugo et al., 2011), C. aurantifolia (Craske et al., 2005) and specially C. bergamia (Poiana et al., 2003; Costa et al., 2010) peel obtained by cold pressing or solvent extraction, contains phototoxic furocoumarins of the bergapten type and it cannot be used neither in pharmaceutical nor in cosmetic industries. Then, Belsito et al. (2007) described a vacuum distillation which resulted in a high-quality C. bergamia essential oil, and therefore appropriate for commercial uses.
Other studies of Citrus peel composition use organic solvents such as pentane (Feger et al., 2001a; Miyazawa et al., 2010), pentane: ether (1:1) (Chisholm et al., 2003a,b), hexane (Inafuku-Teramoto et al., 2011; Miyazato et al., 2012, 2013), dichloromethane (Buettner et al., 2003; Craske et al., 2005; Cheong et al., 2011a; Omori et al., 2011; Cannon et al., 2015) or ethyl acetate (Jiang et al., 2011) to obtain different extracts. Also the organic system solvent n-pentane: diethyl ether (1:2) has been used to obtain the volatile organic compounds (VOCs) from Citrus flower (Alissandrakis et al., 2003). However, these extracts are not actually EOs, according to European Pharmacopeia (Spadaro et al., 2012). The EO should only be obtained either by distillation (water steam distillation or Clevenger hydrodistillation) or cold pressing. To prevent confusion, an organic extract from Citrus peel should not be named EO, (Alissandrakis et al., 2003; Chisholm et al., 2003b) although it could simply be named oil (Feger et al., 2001b; Buettner et al., 2003; Craske et al., 2005; Fisher et al., 2008). In fact, medium polarity solvents (diethyl ether, dichloromethane or ethyl acetate) extract more polar and higher MW compounds such as hexadecanal (Naef and Velluz, 2001; Gancel et al., 2002; Chisholm et al., 2003a,b; Cannon et al., 2015), squalene (Cheong et al., 2011b; Jiang et al., 2011), linoleic acid (Cheong et al., 2011b, 2012; Jiang et al., 2011), heptadecanoic acid (Delort and Jaquier, 2009; Jiang et al., 2011) or neophytadiene (Delort and Jaquier, 2009; Delort et al., 2015) than distillation or cold press extraction, and it fails to extract many monoterpene and sesquiterpene compounds which are characteristic of Citrus EOs (Jiang et al., 2011). Moreover, there are other minority extraction methods that use also organic solvents such as simultaneous distillation and extraction (SDE) (Akakabe et al., 2008; Kerdchoechuen et al., 2010; Sun et al., 2014a), microwave-assisted extraction (MAE) (Sun et al., 2014a; Liu et al., 2015), and ultrasonic-assisted extraction (UAE) (Alissandrakis et al., 2003; Darjazi, 2011b; Liu et al., 2012; Sun et al., 2014a; Zhang et al., 2017).
Additionally, several other extraction methods not involving the use of water or organic solvents have been employed. This is the case of water-free microwave extraction (Ferhat et al., 2007; Uysal et al., 2011); or supercritical CO2 fluid extraction (Akakabe et al., 2010; Dong et al., 2014; Sun et al., 2014a), a technique which extracts more efficiently higher MW compounds, such as squalene (Benelli et al., 2010) or linoleic acid (Dong et al., 2014). Headspace techniques have also been often used to extract volatile compounds, such as headspace extraction using a gas-washing bottle and a Porapak-Q sorbent tube (Elmaci and Onogur, 2012), and most frequently HS-SPME. HS-SPME has been used to extract volatiles and semi-volatile compounds of flowers and leaves, predominantly (Flamini et al., 2007; Jabalpurwala et al., 2009; Lin et al., 2010; Cheong et al., 2011b). When using this technique, the plant material is introduced in a septum-capped vial and after an equilibration period, a pre-conditioned fiber is exposed to the headspace of the vial for several minutes either at room temperature or at higher temperatures. There are currently several types of fibers suitable for adsorbing Citrus VOCs. A 100 μm PDMS-coated fiber was used to analyze the aroma of C. unshiu Marcov. fresh and dry blossoms (Choi, 2003a), C. limon flower organs and pollen (Flamini et al., 2007; Flamini and Cioni, 2010) and Taiwan Citrus leaves (Lin et al., 2010). In other studies, a 75 μm/85 μm CAR/PDMS-coated fiber was chosen to extract the VOCs from Citrus flowers (Jabalpurwala et al., 2009; Cheong et al., 2011b; Chung, 2012). Also, an additional two fibers have been used for Citrus peel oil. One fiber was a 65 μm DVB/PDMS coated fiber, which was used to analyze C. clementine EOs (González-Mas et al., 2015). For this analysis the oil was diluted 1:100 with dichloromethane and 10 μL were introduced in the septum-cap vial for HS-SPME with 990 μL of milli-Q water. Choi and Min (2004) also used this last fiber to analyze the VOCs in fresh flavedo of Hallabong Citrus. The other fiber used for Citrus oil and fresh flavedo was a 50 μm/30 μm DVB/CAR/PDMS fiber (Qiao et al., 2008; Mitropoulou et al., 2017), which is more polar than the other named fibers, and often used to extract volatile and semi-volatile compounds from the Citrus juices (González-Mas et al., 2011; Benjamin et al., 2013; Rambla et al., 2014; Qiu and Wang, 2015). Although recently it has also been used to analyze VOCs in Citrus flowers (Huang et al., 2017). According to the results reported in our literature review, the SPME-fibers used most often to analyze Citrus flower and leaf volatiles seems be the minor polar 100 μm PDMS or 75 μm/85 μm CAR/PDMS fibers, while the 65 μm DVB/PDMS fiber is more used for Citrus essential oil.
Techniques Used to Analyze Citrus Volatile Compounds
The technique most widely used for the analysis of volatile and semi-volatile compounds in Citrus species currently is gas chromatography coupled to mass spectrometry (GC-MS). The principal option used to introduce the oil in the chromatograph is by direct injection of a dilution in organic solvent: most often pentane (Verzera et al., 2003; Boussaada and Chemli, 2006; Jazet Dongmo et al., 2009; Hosni et al., 2010) or hexane (Alonzo et al., 2000; Ahmed et al., 2001; Mondello et al., 2003; Dugo et al., 2005; Hamdan et al., 2010; Wang and Liu, 2014), but also dichloromethane (Blanco Tirado et al., 1995; Frizzo et al., 2004), diethyl ether (Merle et al., 2004) or even acetone (Viuda-Martos et al., 2009). Sun et al. (2014b) diluted the C. maxima EO in two steps: by 5,000-fold dilution in ethanol for analysis of the most abundant volatiles and 100-fold dilution for the minor volatiles. However, in other studies, the oil is not diluted (Mitiku et al., 2000; Song et al., 2000b; Choi et al., 2001; Minh Tu et al., 2002b, 2003b; Choi, 2005; Njoroge et al., 2005a,b, 2008; Sawamura et al., 2005; Akakabe et al., 2010; Cheong et al., 2011b; Omori et al., 2011). When volatile and semi-volatile compounds are extracted with an organic solvent, the samples are concentrated before direct injection using a rotary evaporator and further reduced under a stream of N2 (Hamdan et al., 2010; Miyazato et al., 2012). However, during these concentration steps, highly volatile compounds tend to be lost (Kerry et al., 2002). When VOCs are extracted with a fiber (HS-SPME), they are transferred directly to the injection port of the GC-MS system (Flamini et al., 2007; Jabalpurwala et al., 2009).
Different GC capillary columns have been used to analyze the volatile and semi-volatile Citrus compounds. Supplementary Table S1 in the Supporting Information shows the most often GC columns reported since 2010. Additionally, multidimensional gas chromatography consisting on the use of two columns in tandem with different separation characteristics (typically one polar and the other non-polar) has also been often used in the last decade, in order to achieve a higher chromatographic resolution of the diverse compounds comprising complex matrices such as EOs (Tranchida et al., 2012; Cannon et al., 2015).
There also exist more complex techniques based on carbon isotope ratios which allow the authentication of Citrus VOCs for the control of adulteration of Citrus essential oils. Thus, the employment of GC hyphenated to carbon isotope ratio mass spectrometry (GC-C-IRMS) can detect compounds of different botanical origin such as, for example, the presence of citral extracted from lemon grass (Cymbopogon citratus) (Schipilliti et al., 2018) in lemon EO. Moreover, this technique provides the authenticity assessment to differentiate natural and synthetic compounds in different Citrus essential oils, to discriminate Citrus peel oils of different geographical origin. Also, GC-C-IRMS is useful to eliminate the environmental influences and to differentiate genuine Citrus essential oils belonging to different species. However, this technique presents limitations related to chromatographic resolution due to instrumentation engineering. It will be necessary the optimization of the multidimensional GC separations to more effectively establish the ranges of authenticity of Citrus essential oils.
Despite the utility of GC-MS to analyze the volatile fractions of different biological samples, neither the profile of compounds identified by this technique nor the sensitivity in their detection correspond exactly with those the human nose perceives. To know the characteristic aroma of a Citrus oil or extract or fresh vegetal material, and to make an organoleptic evaluation of them, GC coupled to olfactometry (GC-O) should be used (Song et al., 2000a,b). This technique determinates the odor-threshold values of the volatile components eluted from the GC column and requires trained sniffers (panelists). It is well known that even though food products usually contain several hundreds of volatiles, most of them do not possess aroma activity in the existing concentration in the product. GC-O includes aroma extracts dilution analysis (AEDA) to identify the most potent odorants. For that, aroma extracts are successively diluted, and the compounds are separated by GC and sniffed. Results are then expressed as a FD factor. The FD factor of a particular odorant is the highest dilution at which it is detected. An odorant with a high FD factor can be judged as an important contributor to the characteristic flavor of that particular product. Comparatively, there are fewer studies about the Citrus volatile and semi-volatile fraction using GC-O as compared to GC-MS. Up to date, GC-O has been used for the analysis of peel oil of the following species: C. sinensis (Högnadóttir and Rouseff, 2003; Qiao et al., 2008), C. reticulata (Buettner et al., 2003; Chisholm et al., 2003a; Miyazaki et al., 2012), C. grandis (Cheong et al., 2011a; Chung et al., 2012; Li et al., 2016), C. aurantium (Song et al., 2000a), C. paradisi (Lin and Rouseff, 2001), C. aurantifolia (Chisholm et al., 2003b), C. limon (Cannon et al., 2015), C. bergamia (Sawamura et al., 2006), C. junos (Song et al., 2000b; Lan Phi et al., 2009; Miyazawa et al., 2009; Miyazato et al., 2012, 2013; Tomiyama et al., 2012), and the minor species C. tamurana Hort. ex Tanaka (Choi et al., 2001; Tao et al., 2014), C. flaviculpus Hort. ex Tanaka (Choi et al., 2002), C. inflata Hort. ex Tanaka (Minh Tu et al., 2003b), C. natsudaidai Hayata (Lan Phi et al., 2006), C. kinokuni Hort. ex Tanaka (kinokuni mandarin) (Miyazawa et al., 2010), C. unshiu Marcov (satsuma mandarin) (Miyazawa et al., 2010), C. nobilis Lauriro (wild Citrus Mangshanyegan) (Liu et al., 2012), C. sudachi Hort. ex Shirai (sudachi) (Tomiyama et al., 2012), C. sphaerocarpa Hort. ex Tanaka (kabosu) (Minh Tu et al., 2002b; Tomiyama et al., 2012), C. jabara Hort. ex Tanaka (Omori et al., 2011), C. hystrix D.C. (kaffir lime) (Jirapakkul et al., 2013), Citrus sp. (Kiyookadaidai) (Minh Tu et al., 2003a), Citrus hallabong (C. unshiu Marcov ×C. sinensis Osbeck) (Choi, 2003b), C. nobilis Lour. var. microcarpa Hassk. (Pontianak oranges) (Fisher et al., 2008; Dharmawan et al., 2009), Fortunella japonica Swingle (kumquat) (Choi, 2005) and the hybrid C. aurantifolia ×Fortunella japonica Swingle (Eustis limequat) (Casilli et al., 2014).
Volatile Compounds Identified in Essential Oils From Peel, Leaves, and Flowers of Citrus Species
We have listed in this review over one thousand volatile and semi-volatile compounds that have been reported during the last two decades in rinds, leaves or flowers (EOs and solvent extracts) of Citrus species cultivated worldwide, although some early studies have also been included (Supplementary Table S2). In this review we have focused on the composition of C. reticulata, C. grandis (also named C. maxima), C. sinensis, C. paradisi, C. aurantium, C. junos, C. medica, C. aurantifolia, C. bergamia, and C. limon. This study also allowed us to establish which species are more similar or distant to each other according to the rind composition of their volatile and semi-volatile fractions as described in the literature. Finally, the volatile pattern of Citrus rinds has been compared to those of flowers and leaves.
Our literature review on VOCs in Citrus species corroborates some commonalities for the 10 most widely used and analyzed Citrus species. Thus, the EOs obtained from Citrus rinds always show limonene, a hydrocarbon monoterpene, as the most abundant compound, its concentration generally representing about 60–95% of the oil (Jing et al., 2014). However, limonene can show lower levels, as in C. bergamia, in which it can decrease down to 30% (Jing et al., 2014), or in C. limon, where it can decrease down to 48% (Lota et al., 2002). The following compounds in abundance are also monoterpenes, usually representing less than 15% (Supplementary Table S2), although γ-terpinene (Fanciullino et al., 2006) and linalyl acetate (Verzera et al., 2003) can reach an abundance of 23% and 36%, respectively. Non-terpenoid compounds very rarely represent more than 1%, although this does not necessarily mean that these compounds do not have an impact on the EO aroma. On the other hand, Citrus flowers and leaves show a different volatile profile. In these organs, limonene is not always the major volatile compound. Depending on the species, other monoterpenes such as linalool, β-myrcene or β-citronellol, in the case of flowers (Boussaada and Chemli, 2006; Jabalpurwala et al., 2009; Sarrou et al., 2013), or sabinene, in the case of leaves (Tomi et al., 2008), may be the most prominent compounds.
Attending to the number of compounds identified, sesquiterpene hydrocarbons are the most diverse group in most of the species. Monoterpene hydrocarbons and oxygenated monoterpene alcohols also tend to be among the most copious groups (Figure 1). Nevertheless, in some species such as C. reticulata or C. limon, the most numerous group corresponds to non-terpenoid aldehydes (Figure 2).
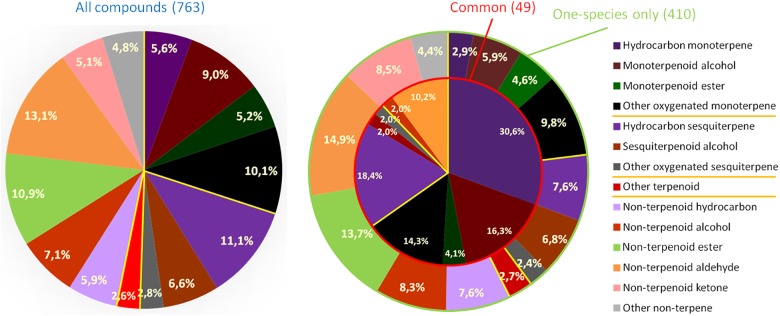
Relative frequency of each group of compounds present in the 10 most common Citrus species. Left, all the compounds described; right outer circle, compounds up to date detected in only one species (one-species only); right inner circle, compounds detected in all 10 species (common). Orange bars indicate separation between mono-, sesqui-, and other terpenoids, and non-terpenoid compounds. The qualitative data used is available in Supplementary Table S4.
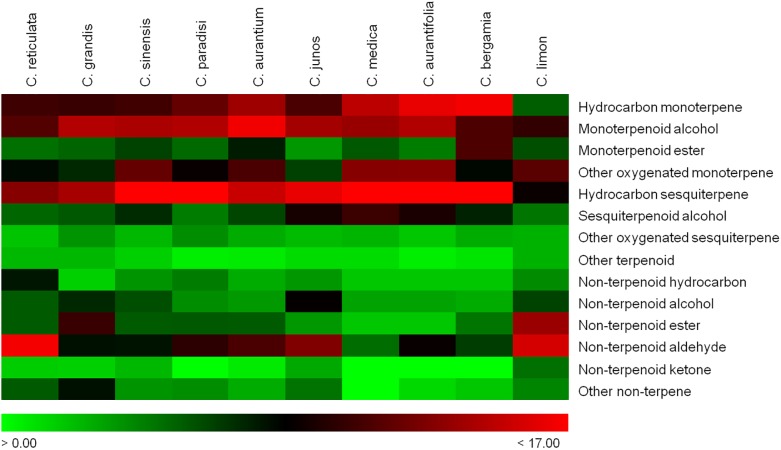
Heatmap showing the relative frequency of each group of compounds is presented for each of the most common Citrus species. Data represent the number of compounds reported in each category divided by the total number of compounds reported in each particular species, according to the scale below: red, high frequency; black, intermediate frequency; green, low frequency. The qualitative data used is available in Supplementary Table S5.
It became obvious when performing this review that the nomenclature of Citrus VOCs compounds, especially for mono- and sesquiterpene derivates needs standardization, as in its present form it can lead to misinterpretation of the results. Compounds are named according to IUPAC in some papers, but this nomenclature is very complex and results in very long names (Yu et al., 2009). Most of the papers use shorter non-systematic names, but this results in many synonyms for the same compound. An example of this is p-cymenene (Lota et al., 2002; Fanciullino et al., 2006; Pino et al., 2006), also named α-p-dimethylstyrene (Huang et al., 2000; Jabalpurwala et al., 2009; Cannon et al., 2015) and dehydro-4-cymene (Tomiyama et al., 2012). Similarly, (Z)-limonene oxide (Minh Tu et al., 2003b; Sun et al., 2014b) is also named (Z)-epoxylimonene (Blanco Tirado et al., 1995), (Z)-limonene,1,2-epoxide (Lota et al., 1999; Omori et al., 2011) or 1,2-epoxy-limonene (Casilli et al., 2014). Monoterpene compounds are named in many occasions according to the structure of p-menthane (Supplementary Figure S1A). This is the case of pseudolimonene (Lan Phi et al., 2009; Sun et al., 2014b), α-terpineol (Lota et al., 2002; Cheong et al., 2011b; Liu et al., 2012; Sun et al., 2014a), α-terpinyl acetate (Lota et al., 2002) (also α-terpenyl acetate) (Verzera et al., 2004), 4-terpinenol (Cheong et al., 2011b; Liu et al., 2012) and dihydrocarveol (Njoroge et al., 2005a) (also dehydrocarveol) (Shen et al., 2002), which have also been named as p-mentha-1(7),8-diene (Tomiyama et al., 2012), p-menth-1-en-8-ol (Chen et al., 2014), p-menth-1-en-8-yl acetate (Kirbaslar et al., 2001), 1-p-menthen-4-ol (Delort and Jaquier, 2009), and p-menth-8-en-2-ol (Delort et al., 2015), respectively. It seems necessary to reach a scientific agreement to name these compounds with a single criterion or, at the least, to indicate the several common synonyms for each compound in related articles. Along this review we have used one of the most common names for each compound, but all the different terms used in the scientific literature have been kept in Supplementary Table S2.
Volatile Composition of Peel Extracts and Peel Essential Oil
In this study, we have particularly focussed on the presence of volatile compounds in the peel of the 10 Citrus species most commonly analyzed, for which a significant amount of information is available in the scientific literature. Depending on each particular study, compounds have been identified with a different degree of confidence. We have systematically classified the reliability of identification for each compound in each of the revised articles in a scale from A+++ (highest confidence) to H (lowest confidence), as detailed in Supplementary Table S2. We considered it a key feature in this review, as our own experience in Citrus juice volatile profiling is that misidentification of volatile compounds is far to be rare in the scientific literature, particularly for those tentatively identified based on retention indices (González-Mas et al., 2011). Therefore, we have used a conservative approach all along this review for the description of the volatile profiles, as well as for the generation of figures and statistical analyses. We have selected only the 763 volatile and semi-volatile compounds which have been identified by, at least, both retention index and mass spectrum (reliability classified from A+++ to B in Supplementary Table S2), while discarding those identified with a lower degree of confidence. We used this approach to perform a qualitative description (described/never described) of the volatile profiles of these species, summarized in Figure 1, ,2,2, and to elaborate the HCA shown in Figure 3. Applying this criterion, we observed that slightly more than half of these 763 reliably identified compounds are terpenoids, whilst the other half corresponds to a wide range of non-terpenoid compounds. Oxygenated monoterpenes is the most numerous group, comprising almost a quarter of all the compounds identified. Forty-nine of these volatile compounds have been described in all 10 species (Table 1 and Supplementary Figures S1B–H), almost 90% of which are terpenoid. Therefore, in some way they could be considered as those defining the characteristic Citrus volatile profile. Interestingly, most of these shared compounds are monoterpenoids (15 monoterpene hydrocarbons; 17 oxygenated monoterpenes) or sesquiterpenoids (9 sesquiterpene hydrocarbons; 2 oxygenated sesquiterpenes). The only non-terpenoid common volatiles are 1-octanol and the five C8 to C12 linear aliphatic aldehydes from octanal to dodecanal. On the other hand, 410 compounds of ample chemical diversity have been described up to date in only one of these species, most often in only one study (Figure 1 and Supplementary Table S2). It cannot be discarded that in some cases this might be due to misidentification, particularly when compound identification was not confirmed with a pure standard. Nevertheless, when unambiguously identified, they could be considered as biomarkers for each particular species.
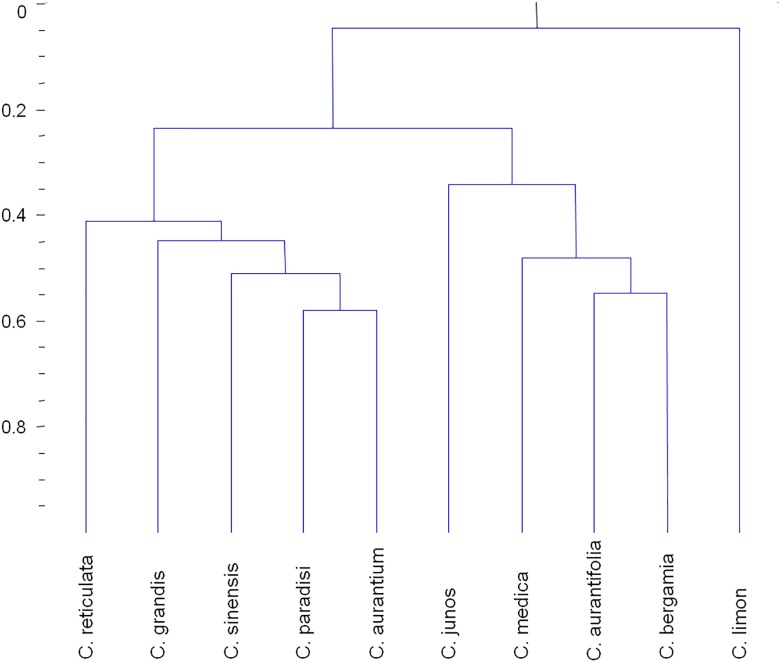
Hierarchical clustering of the 10 most common Citrus species according to their volatile profile as described in the scientific literature. For each species, data were normalized by dividing the number of times a particular compound was reported by the total number of reports of all the compounds in that species. The statistical parameters used were as described in Rambla et al. (2015). The qualitative data used to create HCA is available in Supplementary Table S4.
Table 1
Common volatile compounds identified in C. sinensis, C. reticulata, C. paradisi, C. grandis, C. aurantium, C. limon, C. medica, C. aurantifolia, C. bergamia, and C. junos.
| Groups of common compounds | Common compounds |
|---|---|
| Monoterpene hydrocarbons | β-Myrcene, (Z)-β-ocimene, (E)-β-ocimene, α-phellandrene, β-phellandrene, α-terpinene, γ-terpinene, limonene, terpinolene, sabinene, α-thujene, α-pinene, β-pinene, camphene, p-cymene |
| Monoterpenoid alcohols | linalool, (Z)-linalool oxide, β-citronellol, nerol, geraniol, terpinen-4-ol, α-terpineol, (Z)-sabinene hydrate |
| Monoterpenoid aldehydes | β-citronellal, neral, perillaldehyde |
| Other oxygenated monoterpenes | Citronellyl acetate, geranyl acetate, camphor, (Z)-limonene oxide, (E)-limonene oxide, carvone |
| Sesquiterpene hydrocarbons | (E,E)-α-farnesene, (E)-β-farnesene, β-elemene, δ-elemene, germacrene D, bicyclogermacrene, α-humulene, (E)-β-caryophyllene, δ-cadinene |
| Sesquiterpenoid alcohols | (E)-nerolidol |
| Other oxygenated sesquiterpenes | (E)-caryophyllene oxide |
| Non-terpenoid alcohols | 1-octanol |
| Non-terpenoid aldehydes | Octanal, nonanal, decanal, undecanal, dodecanal |
As previously described, several different -and somewhat complementary- analytical techniques have been used for the identification of the VOCs in the EOs along the scientific literature. In the case of these 10 species we considered that the number of studies already published is sufficient to rely on the total list of compounds identified as quite representative of their actual volatile profile. Therefore, we addressed the classification of these species by HCA based on their respective volatile profiles as described in the literature. This approach allowed us to classify these species into three clusters based on their volatile profile as described in the literature (Figure 3) (Rambla et al., 2015). The first cluster is comprised by C. reticulata, C. grandis, C. sinensis, C. paradisi, and C. aurantium, and is mainly characterized by the presence of a larger abundance of aliphatic and olefinic non-terpene compounds, principally esters and aldehydes (Figure 2). The second cluster is comprised by C. junos, C. medica, C. aurantifolia, and C. bergamia, and is characterized by the prevalence of mono- and sesquiterpene hydrocarbons. Finally, C. limon shows a particular volatile profile with some sulfur monoterpenoids and non-terpenoid esters and aldehydes as part of its main differential peculiarities (Figure 2, ,33 and Supplementary Table S2).
This clustering shares some similarities with a recent phylogenetic analysis based on the chloroplast genomes of several Citrus species (Carbonell-Caballero et al., 2015). Most notably, C. grandis, C. paradisi, C. sinensis, and C. aurantium form a clade independent of another clade including C. medica and C. aurantifolia in both cluster analyses. Nevertheless, a remarkable difference between them is that C. reticulata is phylogenetically very different from the C. sinensis clade, whilst their volatile profiles are quite similar. Additionally, C. limon clustered with C. grandis, C. paradisi, C. sinensis, and C. aurantium in the chloroplast genome phylogenetic analysis, whilst it clusters separately from all these species when the volatile profile is considered.
However, a recent study about the origin and evolution of Citrus based on the total genome shows higher similarities with the volatile profiles here summarized. Our HCA clustered C. sinensis, C. paradisi and C. aurantium with C. reticulata and C. grandis (C. maxima). In fact, Wu et al. (2018) showed that the first three species are actually genetical hybrids and admixtures of the latter two. This genomic study also revealed that C. aurantifolia is a hybrid of C. medica. In accordance with this, our HCA based on the volatile profile of the fruit peel also clustered them together.
In the following sections, the volatile and semi-volatile profiles of the peel are presented independently, describing the peculiarities of each species. Species are presented following the results of the HCA, which provides information about the similarities and differences between them. The compounds up to date described in only one species have been highlighted (Supplementary Table S3), as well as those that allowed to establish correlations between species. In addition, the compounds quantitatively most abundant in each species have been indicated. Finally, the compounds frequently isolated in most Citrus but never reported in a particular species have also been mentioned here.
C. reticulata
Most of the VOCs identified in any of these Citrus species have also been isolated in C. reticulata. The EO of this species seems to differ principally from the others due to the presence of some exclusive non-terpenoid aldehyde compounds, such as (E,E)-2,4-heptadienal (Naef and Velluz, 2001) or (Z)-2-dodecenal (Naef and Velluz, 2001; Chisholm et al., 2003a) (Figure 2 and Supplementary Tables S2, S3). Moreover, several other non-terpenoid aldehyde compounds have been mainly isolated in the peel of C. reticulata and only sporadically in the peel of other Citrus species. This is the case of (Z)-4-decenal [C. reticulata (Naef and Velluz, 2001; Buettner et al., 2003; Chisholm et al., 2003a), C. junos (Miyazato et al., 2012) and C. limon (Cannon et al., 2015)], heptanal [C. reticulata (Dugo et al., 2005; Pino and Quijano-Celís, 2007; Zhang et al., 2017), C. grandis (Zhang et al., 2017), C. sinensis (Dugo and Mondello, 2011), and C. limon (Cannon et al., 2015)] or (E)-2-nonenal [C. reticulata (Naef and Velluz, 2001; Buettner et al., 2003; Pino and Quijano-Celís, 2007), C. junos (Miyazawa et al., 2009) and C. limon (Cannon et al., 2015)]. Although C. limon is the species with the volatile profile furthest from that of C. reticulata, also some non-terpenoid aldehyde compounds have only been reported so far in both C. limon (Cannon et al., 2015) and C. reticulata such as (E)-2-octenal (Naef and Velluz, 2001; Chisholm et al., 2003a), (E,Z)-2,4-nonadienal (Buettner et al., 2003), (2E,4Z,7Z)-decatrienal (Naef and Velluz, 2001), (Z)-5-dodecenal (Chisholm et al., 2003a) and (Z)-6-dodecenal (Ruberto and Rapisarda, 2002; Chisholm et al., 2003a). Among other compounds described in rinds of C. reticulata, we highlight the sesquiterpene hydrocarbon β-copaene, which has only been reported in the five species in this cluster according to their volatile profile [C. reticulata (Merle et al., 2004; Hosni et al., 2010; Luciardi et al., 2016), C. sinensis (Mondello et al., 2003; Hosni et al., 2010; Petretto et al., 2016), C. paradisi (Petretto et al., 2016), C. aurantium (Hosni et al., 2010), and C. grandis (Sawamura et al., 1991; Hosni et al., 2010)].
From a quantitative perspective, although non-terpenoid aldehyde compounds could help to differentiate this species from others, the most abundant compounds in C. reticulata EO are monoterpene hydrocarbons. Among them, the most relevant is limonene, usually representing about 95% of the total EO, but occasionally down to 60% in some studies (Fanciullino et al., 2006; Tao et al., 2014). The next compounds in abundance are γ-terpinene, sometimes reaching values above 15% (Mondello et al., 2003; Petretto et al., 2016), β-myrcene (7.43–0.1%) (Fanciullino et al., 2006; Tao et al., 2014), α-pinene (3.93–0.1%) (Fanciullino et al., 2006; Tao et al., 2014) or β-pinene (4%-traces) (Fanciullino et al., 2006). Monoterpenoids linalool and β-citronellal can reach up to 2.9% and 0.6%, respectively (Tao et al., 2014). Among other compounds with abundances approximately between 0.7 and 0.1% we found the sesquiterpene α-sinensal, the non-terpene aliphatic compounds octanal and decanal, and the aromatic compound methyl N-methylanthranilate (Supplementary Table S2). The rest of the compounds, including the non-terpenoid aldehydes, do not usually reach percentages higher than 0.1%, although this does not necessarily imply that they do not influence the final aroma of the EO.
C. grandis
The C. grandis peel is interesting as it presents several aromatic compounds that are almost exclusive for this species, such as indole [only described in C. grandis (Cheong et al., 2011a,b; Liu et al., 2012, 2015; Sun et al., 2014a,b; Li et al., 2016), C. bergamia (Verzera et al., 2003), and C. limon (Cannon et al., 2015)], or ethyl anthranilate (Cheong et al., 2011b), benzothiazole (Cheong et al., 2011a) (nitrogenous and sulfurous compound), 2-phenylethanol (Cheong et al., 2011b) [also in blossom and leaves of other Citrus species (Alissandrakis et al., 2003; Družić, 2016)], 3-ethylphenol (Cheong et al., 2011b), and methyl benzoate (Cheong et al., 2011a,b). Also, the non-terpenoid alcohols 1-hexanol and (E)-2-hexen-1-ol have been reported in the peel of C. grandis and other few Citrus species [C. grandis (Cheong et al., 2011a,b; Liu et al., 2012, 2015), C. sinensis (Högnadóttir and Rouseff, 2003; Liu et al., 2012), C. reticulata (Liu et al., 2012), C. limon (Cannon et al., 2015), and C. junos (Tomiyama et al., 2012)]. However, these compounds have been identified in a very low percentage, in many cases lower than 0.1%. The most abundant compounds are the same described in C. reticulata, also in very similar proportions. Only linalool has been usually identified with percentages lower than C. reticulata, close to 0.1% (Minh Tu et al., 2002a; Njoroge et al., 2005b; Cheong et al., 2011b), and γ-terpinene appears in this species between 0.05%-traces (Njoroge et al., 2005b; Cheong et al., 2011b). In any case, it is difficult to compare the results of different studies, because concentration of each compound is not always expressed in percentages; sometimes the authors express it in % w/w (Njoroge et al., 2008) or μg/g oil (Zhang et al., 2017). In addition, important variations in the VOCs concentration can occur within the same species, depending on cultivation conditions or varieties (Lota et al., 2002; Verzera et al., 2003) or even according to the type of oil extraction (Sun et al., 2014a).
The volatile profile most apart from C. grandis is that of C. limon (Figure 3). However, provided that C. limon has been widely studied by Cannon et al. (2015), several compounds have been exclusively isolated in C. limon and C. grandis peels, specially some non-terpenoid aliphatic ester compounds such as (Z)-3-hexenyl hexanoate (Sun et al., 2014a; Cannon et al., 2015) and methyl octanoate, methyl nonanoate and methyl decanoate (Cheong et al., 2011b; Cannon et al., 2015). Moreover, for this same reason, there are compounds only isolated in C. grandis, and C. limon and also C. reticulata or C. sinensis or C. paradisi such as (E)-piperitol [C. grandis (Sun et al., 2014a; Liu et al., 2015), C. reticulata (Liu et al., 2012), and C. limon (Cannon et al., 2015)].
One of the most relevant features of C. grandis volatile profile is the absence of some compounds that are typical of other common species of Citrus, mainly some sesquiterpene compounds. This is the case of the sesquiterpene (E)-α-bergamotene, which has never been reported in C. grandis peel but frequently identified in all the other common Citrus species studied (Mondello et al., 2003; Lan Phi et al., 2009; Aliberti et al., 2016; Petretto et al., 2016; Zhang et al., 2017). As far as we know, neither the compound thymol has been reported in C. grandis nor C. paradisi nor C. bergamia peel and neither (E)-2-decenal had been reported in C. grandis nor C. aurantifolia, but in the rest of the species reviewed.
Finally, it is important to indicate that when Citrus essential oils are exposed to sunlight, UV radiation or the air, the volatile profile can be modified due to chemical reactions. It has been reported in C. grandis oil that the concentration of several compounds, especially aldehydes such as decanal, dodecanal, neral or geranial, drops dramatically when exposed to these conditions. Instead, other compounds increase their concentration or appear novelly such as β-citronellal, α-humulene, linalool oxides, limonene oxides, (E)-carveol, (Z)-carveol, perilla alcohol, carvone, α-pinene oxide, γ-elemene or caryophyllene oxide (Supplementary Table S2) (Sun et al., 2014b; Li et al., 2016). This aldehyde transformation in C. grandis promoted an increase of strong oily notes and a decrease of fresh notes. Recently, sensory evaluation, GC-MS and GC-O demonstrated the pummelo rind oil co-treated by both oxygen and heating (70°C O2) showed significant differences in both volatile composition and aromatic profile from those of the corresponding fresh, nitrogen-protected (25°C N2), heated (70°C N2) and oxygen-exposed (25°C O2) oils (Sun et al., 2018). This study indicates that oxygen and heating co-treatment increases the content of limonene oxides and carvone in pummelo EO. The first compounds enhanced the sweet and floral notes, while the latter promoted the intensity of the minty note. These results indicate the importance of a correct production and storage of Citrus essential oil to preserve its original freshness quality.
C. sinensis
The volatile composition of C. sinensis peel EO is the most studied of all Citrus species along with those of C. reticulata and C. limon (Supplementary Table S2). This species seems to be richer in the diversity of sesquiterpene hydrocarbons than other species as C. reticulata or C. limon. An example of these compounds is aromadendrene (Njoroge et al., 2005a; Hosni et al., 2010) or sesquiphellandrene (Ruberto and Rapisarda, 2002; Sawamura et al., 2005), although it should not be forgotten that many of these sesquiterpenes are identified ambiguously, because there are no commercial standards or are unattainable for their price for many scientific studies. Also, in this species have been reported a relative large group of monoterpenoid compounds, being some of them almost exclusive compounds as limonene diepoxide (Högnadóttir and Rouseff, 2003; Njoroge et al., 2005a; Sawamura et al., 2005; Esquivel-Ferriño et al., 2014).
However, these distinctive VOCs of C. sinensis are minor compounds with a concentration usually lower than 0.1%. The major C. sinensis VOCs are very similar to that of C. reticulata, with comparable proportions. Limonene is usually reported between 90 and 97% in C. sinensis, although this percentage decreased down to 64% in some studies (Chen et al., 2014). Quantitatively, the most important difference with C. reticulata is for α-sinensal, since in C. reticulata this compound can reach a percentage above 0.7% (Lota et al., 2001b), while in C. sinensis its percentage does not rise of 0.05% (Njoroge et al., 2005a; Sawamura et al., 2005).
Recently the non-terpene aldehydes 2-butyl-2-octenal, 2-hexyl-2-decenal, and 2-octyl-2-dodecenal have each been detected in folded cold press orange oil at trace concentrations, confirmed with synthesized standards (Abreu et al., 2017). These compounds are hexanal, octanal, and decanal self-aldol condensation products, respectively. These self-aldol condensation products have attractive organoleptic qualities according to aroma and taste evaluations.
C. paradisi
The volatile profile from this species is very close to those of C. aurantium and C. sinensis, and a little less so to C. grandis and C. reticulata (Figure 3), and further apart from the other species studied. As in C. sinensis, numerous sesquiterpene hydrocarbons in have been reported in C. paradisi (Figure 2 and Supplementary Table S2), such as the sesquiterpene α-cedrene, isolated in C. paradisi (Njoroge et al., 2005b), C. grandis (Njoroge et al., 2005b), C. sinensis (Njoroge et al., 2005a; Sawamura et al., 2005), C. aurantifolia (Minh Tu et al., 2002a), and C. medica (Wu et al., 2013).
The major compounds in C. paradisi are very similar to those in C. grandis, often with lower levels of γ-terpinene and linalool to those with C. sinensis and C. reticulata (Njoroge et al., 2005b; Petretto et al., 2016), and with higher levels of nootkatone, which can reach 0.2% in C. paradisi (Njoroge et al., 2005b) and 177.02–5.05 μg/g in C. grandis (Zhang et al., 2017), while in the other two varieties they usually appear in lower percentages (Supplementary Table S2) (Ruberto et al., 1997; Zhang et al., 2017).
Again, although C. limon is the furthest variety of C. paradisi, there are compounds reported in C. paradisi and also in C. limon such as the compound undecanoic acid [C. paradisi (Lin and Rouseff, 2001; Njoroge et al., 2005b), C. sinensis (Njoroge et al., 2005a; Sawamura et al., 2005), and C. limon (Cannon et al., 2015)].
C. aurantium
The volatile profile of Citrus aurantium (sour orange) is dominated by monoterpenoid compounds, such as isomenthol, geranyl propionate or eucalyptol (Figure 2) (Song et al., 2000a,b; Jabri Karoui and Marzouk, 2013). In fact, monoterpenoids comprise almost half of all the volatiles described in this species. Even so, the major compounds in C. aurantium are very similar to those of C. sinensis and C. reticulata (limonene, β-myrcene, β-pinene, linalool, (E)-β-ocimene, octanal) although in C. aurantium the percentages of linalyl acetate and geranyl acetate may reach 5% and 0.9%, respectively (Lota et al., 2001a; Petretto et al., 2016), while in C. sinensis and C. reticulata the percentages of these compounds do not usually exceed 0.1% (Mondello et al., 2003; Njoroge et al., 2005a, 2008). In addition, according to Jabri Karoui and Marzouk (2013), C. aurantium peel presents eucalyptol levels around 0.87%, significantly higher than those reported in other species. C. aurantium also seems to have higher levels of sesquiterpene germacrene D than in any other species (Lota et al., 2001a).
Some VOCS have been exclusively described in Citrus aurantium, principally monoterpene compounds, hydrocarbons such as α-ocimene (Baik et al., 2008) and oxygenated compounds such as α-terpinen-4-ol acetate (Pino and Rosado, 2000). The set of species most different to C. aurantium include C. junos, C. bergamia, C. medica, C. aurantifolia and especially C. limon, although some of them have several compounds in common with C. aurantium and some species in other clusters. This is the case of thymol, reported in C. aurantium, C. reticulata, C. sinensis, C. aurantifolia, C. medica, C. junos and C. limon (Supplementary Table S2) (Miyazawa et al., 2009; Dugo and Mondello, 2011; Aliberti et al., 2016; Zhang et al., 2017).
C. junos
According to the volatiles described in the peel, C. junos (yuzu) clusters with C. medica and C. aurantifolia and C. bergamia, although the similarity between their respective profiles is lower than those in the previous cluster (Figure 3). Its peel shows some exclusive VOCs, which could influence the aroma of its oil and peel extracts. In fact, in some cases, it has been proved by olfactometry that some of these compounds are strongly involved in the characteristic flavor of yuzu (Song et al., 2000b). These C. junos specific compounds belong to the group of sulfur compounds, aromatic compounds, terpenoid compounds, and non-terpenoid compounds of 7 to 12 carbons, most of which are also oxygenated, principally alcohols and aldehydes. These exclusive compounds are: methyltrisulfide (Song et al., 2000b) (odor characteristic component), 4-methyl-4-mercapto-pentan-2-one (Oh et al., 2007; Miyazawa et al., 2009), isoeugenol (Song et al., 2000b), the terpenoid compounds p-mentha-1,4,8-triene (Song et al., 2000b; Tomiyama et al., 2012), p-mentha-1(7),2-dien-8-ol (Tomiyama et al., 2012), geranyl butanoate (Song et al., 2000b), cedryl acetate (Song et al., 2000b), and δ-muurolene (Song et al., 2000b), (odor characteristic component), and the non-terpenoid hydrocarbon compounds (3E,5Z)-undeca-1,3,5-triene (Naef and Velluz, 2001; Miyazawa et al., 2009), (3E,5Z,8Z)-undeca-1,3,5,8-tetraene (Miyazawa et al., 2009; Miyazato et al., 2012), 6-methyl-5-hepten-2-ol (Song et al., 2000b) (odor characteristic component), (6Z,8E)-undeca-6,8,10-trien-3-ol (yuzuol) (Miyazawa et al., 2009; Tomiyama et al., 2012), (E)-6-nonenal (Miyazawa et al., 2009), (E)-4-decenal (Miyazawa et al., 2009), (Z)-4,5-epoxy-(E)-2-decenal (Miyazawa et al., 2009; Miyazato et al., 2012; Tomiyama et al., 2012), (E)-4,5-epoxy-(E,Z)-2,7-decadienal (Miyazato et al., 2012) (odor-active component), (E)-4-methyl-3-hexenoic acid (Miyazato et al., 2013) (odor-active component) and (6Z,8E)-undeca-6,8,10-trien-3-one (yuzunone) (Miyazawa et al., 2009; Miyazato et al., 2012; Tomiyama et al., 2012). It can be highlighted that some VOCs have only been reported so far in yuzu oil and in some of the other nine reviewed Citrus species (Supplementary Table S2). This is the case of the compound (Z)-9-dodecen-12-olide, also named yuzu lactone or oxacyclotridec-10-en-2-one, only isolated in C. junos (Miyazawa et al., 2009; Tomiyama et al., 2012) and C. limon (Cannon et al., 2015), or the compound decanoic acid, identified in C. junos (Song et al., 2000b; Tomiyama et al., 2012), C. grandis (Cheong et al., 2011b), C. limon (Cannon et al., 2015), C. reticulata (Pino and Quijano-Celís, 2007) and C. aurantium (Dugo and Mondello, 2011).
Most of the C. junos characteristic compounds previously mentioned are compounds that have been reported at trace levels, with percentages lower than 0.02%. In this species the major compounds are also very similar to those described in C. sinensis or C. reticulata. Thus, the most abundant compound remains limonene, although in concentrations between 63 and 77%, lower than usually those of the species closest to C. reticulata (Song et al., 2000b; Lan Phi et al., 2009). This decrease is associated to the increase of some monoterpene and sesquiterpene hydrocarbons, such as α-phellandrene, β-phellandrene, terpinolene or bicyclogermacrene (Song et al., 2000b; Lan Phi et al., 2009; Tomiyama et al., 2012).
It is important to emphasize that some VOCs frequently isolated in the other common Citrus species were not found in C. junos peel, such as geranial, isolated in the peel of all the other nine Citrus species, terpenyl acetate, neryl acetate, eucalyptol (also missing in C. medica peel), tetradecanal, β-bisabolene or decyl acetate (the latter two compounds found in the juice but not in the peel of C. junos) (Tomiyama et al., 2012).
C. medica
According to our review, the volatile profile most similar to that of C. medica are those of C. aurantifolia and C. bergamia, whilst C. junos also clusters together in terms of volatile profile similarity (Figure 3). As in C. bergamia, C. aurantifolia and C. junos peel, and also in C. sinensis and C. paradisi, many sesquiterpene hydrocarbons have been described in C. medica (Figure 2). The recent study realized by Aliberti et al. (2016) has reported some compounds in C. medica not described, or very rarely identified, in other Citrus species so far as like the monoterpenes cuminyl alcohol, dehydrosabina ketone or cis-4-caranone, and specially sesquiterpene like longifolene, 9-epi-caryophyllene, α-cuprenene, γ-cuprenene, italicene, nootkatol, or β-oplopenone, among many others. However, it is important to note that most of these compounds were tentatively identified, because a standard was not used for their identification, with some exception like the monoterpene trans-4-caranone and the sesquiterpenes longifolene or 9-epi-caryophyllene.
As in all the revised species, the most abundant compound in C. medica is limonene, but its percentage can decrease down to 51% (Verzera et al., 2005), while other monoterpene compounds such as γ-terpinene, β-pinene or camphene are present at higher concentration, in comparison with species of C. reticulata cluster where these compounds are usually described in percentages below 1%. Thus, in C. medica oil, γ-terpinene, β-pinene or camphene can reach percentage of 31%, 9.7%, and 10%, respectively (Lota et al., 1999; Aliberti et al., 2016; Petretto et al., 2016). Also C. medica presents higher concentrations of some sesquiterpenes, as is the case of (E)-α-bergamotene (Aliberti et al., 2016) or germacrene D (Petretto et al., 2016), although their abundance is usually lower than 0.5%.
Finally, C. medica peel lacks some compounds especially non-terpenoid aldehyde that many times are also absent in C. aurantifolia and/or C. bergamia peel, but which have been detected in the rind of all the other species analyzed. Thus, (E,E)-2,4-decadienal has never been reported in C. medica and C. bergamia, and (Z)-3-hexenal, (E)-2-dodecenal and the sesquiterpenoid α-sinensal have not been reported in C. medica, C. aurantifolia and C. bergamia. As far as we know, other compounds which have not been reported in C. medica peel are: (E)-2-hexenal, neither reported in C. aurantium nor in C. bergamia peel, or hexanal, neither present in C. bergamia, C. aurantium nor in C. junos peels. Also, the aromatic monoterpenoid carvacrol has not been described in C. medica, C. bergamia, C. aurantifolia or C. paradisi.
C. aurantifolia
Many mono- and sesquiterpene hydrocarbons and oxygenated monoterpenes have been reported in C. aurantifolia peel (Figure 2). In fact, C. aurantifolia has some exclusive terpenes such as the sesquiterpene santal-10-en-2-ol (Lota et al., 2002) (Supplementary Tables S2, S3). According to our cluster, the species most similar to C. aurantifolia is C. bergamia and further are C. medica and C. junos (Figure 3).
Despite C. sinensis, C. reticulata, C. paradisi, C. grandis and C. aurantium and specially C. limon clustering further away from C. aurantifolia, some terpenes are present in C. aurantifolia peel and in some of these other species, such as the oxygenated monoterpene fenchol, only isolated in C. aurantifolia (Selvaraj et al., 2002; Chisholm et al., 2003b; Fouad and da Camara, 2017), C. grandis (Cheong et al., 2011a) and C. limon (Ferhat et al., 2007) peels (Figure 3 and Supplementary Table S2). Despite the distance with C. limon, there are some terpene compounds which have been exclusively reported in C. aurantifolia and C. limon, such as the monoterpene geranic oxide [C. aurantifolia (Chisholm et al., 2003b) and C. limon (Cannon et al., 2015)]. Interestingly, the monoterpenoid isogeranial and the sesquiterpene α-santalene have been described only in C. aurantifolia (Feger et al., 2000; Costa et al., 2014), C. medica (Venturini et al., 2014; Mitropoulou et al., 2017) and C. limon (Vekiari et al., 2002; Cannon et al., 2015).
As in C. medica, the percentage of limonene may drop to 39.9% in the oil of C. aurantifolia (Lota et al., 2002), and the abundance of other terpene compounds is increased, such as β-pinene, neryl acetate, geranyl acetate, β-bisabolene, (E)-α-bergamotene, germacrene D and β-caryophyllene (Lota et al., 2002; Minh Tu et al., 2002a), similarly to what occurs in C. medica.
Despite C. aurantifolia peel being rich in total terpenes, it is striking that we do not find some terpene compounds found in other Citrus species, as indicated in the previous section about C. medica. Among the terpenes absent in C. aurantifolia, that were not mentioned above, we highlight the sesquiterpenoid nootkatone, also not reported in C. junos.
C. bergamia
As in C. aurantifolia and in C. medica peel, copious mono- and sesquiterpene hydrocarbons have been reported in C. bergamia (Figure 2 and Supplementary Table S2). Thus, several sesquiterpenoid compounds are principally isolated in C. bergamia, and in its more related species and in C. limon. A notable example is the sesquiterpene α-bisabolol, frequently isolated in C. bergamia (Sawamura et al., 2006; Belsito et al., 2007; Costa et al., 2010; Furneri et al., 2012), C. limon (Blázquez and Carbó, 2015; Cannon et al., 2015; Loizzo et al., 2016; Zhang et al., 2017), C. aurantifolia (Gancel et al., 2002; Minh Tu et al., 2002a; Chisholm et al., 2003b; Zhang et al., 2017), and C. medica (Lota et al., 1999; Verzera et al., 2005; Aliberti et al., 2016). Even there are terpene compounds that have been reported in C. bergamia and just once in C. limon as the monoterpene linalyl propionate [C. bergamia (Poiana et al., 2003; Verzera et al., 2003; Costa et al., 2010) and C. limon (Cannon et al., 2015)]. Also, several hydrocarbon and also oxygenated monoterpenes such as δ-terpinene (Poiana et al., 2003), (Z)-sabinene hydrate acetate (Poiana et al., 2003), and (E)-sabinene hydrate acetate (Verzera et al., 2003), seems exclusive of C. bergamia (Supplementary Table S2). In contrast, the sesquiterpenes α-copaene and valencene have not been found in C. bergamia, although it has been identified in all the other species studied (Song et al., 2000b; Zhang et al., 2017), excepting valencene, neither reported in C. junos.
The major compound in C. bergamia is limonene, but its percentage can decrease down to 33% (Poiana et al., 2003). Instead, C. bergamia shows higher percentage of other monoterpene compounds, most notably linalyl acetate, which is sometimes almost as abundant as limonene, which does not happen for any other species of the reviewed, as far as we know (Poiana et al., 2003; Verzera et al., 2003). Linalool is also more abundant in C. bergamia EO than in any other of these species; it can reach up to 13.9% in SC-CO2 extracts (Poiana et al., 2003). Moreover, other terpenes which are abundant in C. medica and C. aurantifolia are also increased in C. bergamia, when compared to the species of the C. reticulata cluster, such as α-pinene, β-pinene, neryl acetate, geranyl acetate, β-bisabolene, (E)-α-bergamotene, β-caryophyllene (Mondello et al., 2003; Poiana et al., 2003; Verzera et al., 2003; Sawamura et al., 2006; Belsito et al., 2007). Finally, compounds such as octyl acetate are more abundant in C. bergamia than in other species, while compounds such as octanal seems to decrease, with values lower than 0.1% (Poiana et al., 2003; Verzera et al., 2003).
On the other hand, several coumarin and psoralen (furocoumarin) compounds have been isolated in C. bergamia EOs. These compounds are more polar and less volatile than most of the compounds in Citrus EOs. Not surprisingly, they has been mainly reported in oils obtained by cold press (Costa et al., 2010; Russo et al., 2015), organic solvent (polar extracts) (Chisholm et al., 2003b; Craske et al., 2005), or more recently by ultrasonic (Zhang et al., 2017) extractions, and are usually identified by means of HPLC techniques (Costa et al., 2010; Russo et al., 2015). In some studies, such compounds are considered as part of the non-volatile peel fraction (Costa et al., 2010). Following this criterion, we have not included them as part of the volatile profile, although some of them have been listed in Supplementary Table S2. Although this type of compounds are usually not included in Citrus peel oil studies, when reported they have been principally described in C. bergamia, C. aurantifolia and C. aurantium EOs, and also in other oils such as those from C. limon (Bonaccorsi et al., 1999; Cannon et al., 2015; Russo et al., 2015). These compounds have not been found in C. sinensis or C. reticulata peel, which instead are the richest in polymethoxyflavones such as tangeretin (Bonaccorsi et al., 1999; Russo et al., 2015). Among them, the furocoumarins bergaptene (Poiana et al., 2003; Belsito et al., 2007; Costa et al., 2010; Russo et al., 2015) and bergamottin (Costa et al., 2010; Russo et al., 2015) are characteristic of the rind of C. bergamia, but they are not exclusive for this species as they have also been described in other species as C. aurantifolia (Craske et al., 2005; Russo et al., 2015), C. limon (only bergamottin) (Bonaccorsi et al., 1999; Russo et al., 2015), C. paradisi (Russo et al., 2015), and C. aurantium (Dugo et al., 1996, 2011; Pino and Rosado, 2000; Russo et al., 2015). Other examples are citropten [C. aurantifolia (Craske et al., 2005; Russo et al., 2015; Zhang et al., 2017), C. bergamia, and C. limon] or 5-geranyloxy-7-methoxy-coumarin [C. aurantifolia (Russo et al., 2015), C. bergamia and C. limon].
C. limon
Lemon EO is the most different of the commonly studied Citrus species in terms of volatiles. The volatile profile of C. limon has been analyzed in numerous studies (Dugo and Mondello, 2011; Liu et al., 2012; Spadaro et al., 2012; Cannon et al., 2015; Zhang et al., 2017). As far as we could find in our review mining, the majority of the volatile and semi-volatile compounds found in any other of these 10 Citrus species have also been described in C. limon. Moreover, Cannon et al. (2015) identified over 150 compounds by dichloromethane extraction in C. limon peel, which we could not find in any other study on VOCs in Citrus. Among them, we highlight those identified without ambiguity such as sulfur monoterpenoid compounds 2-(5-isopropyl-2-methyltetrahydrothiophen-2-yl)ethanol, 3-mercapto-3,7-dimethyl-6-octenyl acetate (odor-active component), 2-(5-isopropyl-2-methyltetrahydrothiophen-2-yl) ethyl acetate, 2-(5-isopropylidene-2-methyltetrahydrothiophen-2-yl) ethyl acetate and 2-[5-(1-hydroxy-1-methylethyl)2-methyltetrahydrothiophen-2-yl] ethyl acetate; other two sulfur non-terpenoid compounds, 2-propanoylthiophene and 2-(2-methyltetrahydrothiophen-2-yl) ethyl acetate; and the branched-chain aliphatic aldehydes 6-methylnonanal, 4-methyldecanal, 6-methyldecanal, 3-methylundecanal, 4-methylundecanal, 3-methyldodecanal, 4-methyldodecanal, and 4-methyltridecanal. All these exclusive compounds were identified as traces.
Quantitatively, the major compound of C. limon EO is limonene, at levels usually ranging between 70 and 48%, similar to those in C. medica and C. aurantifolia, and lower than those in the species comprising the first cluster. The following more abundant compounds are terpenoids similar to those higher in C. medica and C. aurantifolia. Among these compounds which have not been named so far, we can highlight geranial and neral, that in C. limon (Lota et al., 2002; Loizzo et al., 2016) can reach abundances of 2.9% and 1.5%, respectively, compared to percentages between 0.3%-traces in the species in the C. reticulata cluster (Minh Tu et al., 2002a; Ruberto and Rapisarda, 2002; Mondello et al., 2003; Tao et al., 2014).
It is also important to note that there is a set of sesquiterpene compounds that have been very frequently identified in C. limon peel, and with relative high frequency also in C. aurantifolia and C. bergamia. These compounds seem to form a small subgroup that characterizes these three species, although some of them may sporadically be found in other Citrus common species, such as C. medica. This is the case of the previously named α-bisabolol, and also of (E)-α-bisabolene, which has been very frequently isolated in C. limon (Verzera et al., 2001; Lota et al., 2002; Liu et al., 2012; Spadaro et al., 2012; Blázquez and Carbó, 2015; Cannon et al., 2015; Petretto et al., 2016), in C. aurantifolia (Feger et al., 2000; Craske et al., 2005; Costa et al., 2014) and occasionally in C. paradisi (Flamini and Cioni, 2010), C. medica (Lota et al., 1999) and C. bergamia (Costa et al., 2010); (Z)-α-bisabolene, very frequently isolated in C. limon (Verzera et al., 2001; Ferhat et al., 2007; Spadaro et al., 2012; Blázquez and Carbó, 2015; Cannon et al., 2015; Zhang et al., 2017) and C. bergamia (Verzera et al., 2003; Cosimi et al., 2009; Costa et al., 2010), and occasionally in C. aurantifolia (Feger et al., 2000; Zhang et al., 2017), C. medica (Verzera et al., 2005), and C. aurantium (Lota et al., 2001a); γ-curcumene, frequently reported in C. limon (Ferhat et al., 2007; Blázquez and Carbó, 2015; Cannon et al., 2015; Petretto et al., 2016), C. bergamia (Costa et al., 2010) and C. aurantifolia (Feger et al., 2000; Costa et al., 2014); β-santalene, isolated mainly in C. limon (Spadaro et al., 2012; Blázquez and Carbó, 2015; Cannon et al., 2015; Petretto et al., 2016), C. bergamia (Verzera et al., 2003; Cosimi et al., 2009; Costa et al., 2010; Furneri et al., 2012), and C. aurantifolia (Feger et al., 2000; Minh Tu et al., 2002a; Costa et al., 2014; Zhang et al., 2017); and campherenol, reported mainly in C. limon (Verzera et al., 2004; Spadaro et al., 2012; Cannon et al., 2015) and C. bergamia (Mondello et al., 2003; Verzera et al., 2003; Costa et al., 2010; Furneri et al., 2012), and sporadically in C. aurantifolia (Dugo and Mondello, 2011) and C. medica (Lamine et al., 2018). Unfortunately, the identification of most of these sesquiterpenes is not totally reliable, since it seems that standards of these compounds are not commercially available. Other compound with a similar pattern is the norcarotenoid 6-methyl-5-hepten-2-one, frequently reported in C. limon (Lota et al., 2002; Verzera et al., 2004; Spadaro et al., 2012; Blázquez and Carbó, 2015; Cannon et al., 2015) and C. bergamia (Mondello et al., 2003; Verzera et al., 2003; Sawamura et al., 2006; Cosimi et al., 2009; Costa et al., 2010), and only occasionally identified in C. grandis (Cheong et al., 2011a,b), C. reticulata (Pino and Quijano-Celís, 2007), C. sinensis (Dugo and Mondello, 2011), C. aurantifolia (Dugo and Mondello, 2011) and C. medica (Lota et al., 1999).
Volatile Composition of Citrus Blossoms
There are fewer studies about the VOCs in the flowers of Citrus most common species in comparation with Citrus rind. These analyses have been mostly performed on C. grandis, C. aurantium, C. limon, C. reticulata, C. sinensis, C. aurantifolia, and C. paradisi blossoms. According to our review, many of the volatile compounds present in the Citrus peel have also been reported in Citrus blossom and neroli (Alissandrakis et al., 2003; Boussaada and Chemli, 2006; Jabalpurwala et al., 2009; Cheong et al., 2011b; Dugo et al., 2011; Sarrou et al., 2013; Družić, 2016). This is the case of many monoterpene hydrocarbons such as limonene, some monoterpene alcohol such as linalool and geraniol, some sesquiterpene hydrocarbons such as (E)-β-farnesene and β-caryophyllene, some alcohol sesquiterpene such as (E)-nerolidol and (Z,E)-farnesol, the norcarotenoid 6-methyl-5-hepten-2-one, and a few non-terpenoid aliphatic compounds such as hexanal. The common VOCs identified in the peel of all the 10 species described (Table 1) are not always reported in their corresponding blossoms. This is the case of the terpene compounds perillaldehyde, carvone, valencene or nootkatone (Supplementary Table S2). Interestingly, the non-terpenoid C8–C12 aliphatic aldehyde compounds, all of them common in the peel of all the revised species, have been sporadically described in flowers of some species as C. paradisi (Flamini and Cioni, 2010) or C. grandis (Huang et al., 2017).
Unlike in the peel EO, the most abundant compound in neroli is not always the monoterpene limonene, as it is sometimes replaced by linalool (Boussaada and Chemli, 2006; Sarrou et al., 2013). Moreover, according to Flamini and Cioni (2010) and Huang et al. (2017), C. limon and C. grandis neroli composition are different to those of whole flowers, because these neroli are richer in monoterpenes such as limonene, while their corresponding whole flowers are richer in alcohol monoterpenoids such as linalool. Moreover, some studies show that VOCs are produced in distinctive amounts by the different flower organs (Flamini et al., 2007; Flamini and Cioni, 2010; Huang et al., 2017).
In addition, there are some VOCs almost exclusively isolated in Citrus blossoms, which have been reported occasionally in Citrus peel and leaves, although they do not appear to be characteristic of any Citrus species in particular. Among them, we highlight nitrogenous compounds such as benzeneacetonitrile (Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Družić, 2016), isolated in flowers of C. reticulata, C. sinensis, C. aurantifolia, C. aurantium, C. paradisi and C. limon; N-phenylformamide (Jabalpurwala et al., 2009), isolated in flowers of the same species except C. limon, or methyl anthranilate (Flamini et al., 2007; Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Cheong et al., 2011b; Dugo et al., 2011; Sarrou et al., 2013; Ben Hsouna et al., 2017; Huang et al., 2017) and indole (Alissandrakis et al., 2003; Boussaada and Chemli, 2006; Boussaada et al., 2007; Flamini et al., 2007; Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Zakaria et al., 2010; Družić, 2016; Ben Hsouna et al., 2017; Huang et al., 2017), identified in flowers of C. grandis, C. limon, C. reticulata, C. sinensis, C. aurantium, C. aurantifolia, and C. paradisi. Other compounds mainly detected in Citrus flowers are the aromatic compounds 2-phenylethanol [C. grandis (Cheong et al., 2011b), C. reticulata (Alissandrakis et al., 2003; Jabalpurwala et al., 2009), C. paradisi (Jabalpurwala et al., 2009; Flamini and Cioni, 2010), C. aurantifolia (Jabalpurwala et al., 2009), and C. aurantium (Jabalpurwala et al., 2009; Dugo et al., 2011; Družić, 2016)], benzaldehyde [C. grandis (Zakaria et al., 2010; Cheong et al., 2011b), C. reticulata (Jabalpurwala et al., 2009), C. paradisi (Jabalpurwala et al., 2009), C. aurantifolia (Jabalpurwala et al., 2009), C. sinensis (Jabalpurwala et al., 2009), C. aurantium (Družić, 2016), and C. limon (Jabalpurwala et al., 2009)], and phenylacetaldehyde [C. grandis (Cheong et al., 2011b), C. reticulata (Alissandrakis et al., 2003; Jabalpurwala et al., 2009), C. sinensis (Jabalpurwala et al., 2009), C. aurantium (Družić, 2016), and C. limon (Jabalpurwala et al., 2009)]. There is also a group of non-terpenoid aliphatic and olefinic compounds frequently reported in several Citrus flowers, but not in rinds or leaves, including acetone (Jabalpurwala et al., 2009; Cheong et al., 2011b), isopropanol (Jabalpurwala et al., 2009), (Z)-jasmone (Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Dugo et al., 2011; Družić, 2016), or pentadecane (Jabalpurwala et al., 2009; Flamini and Cioni, 2010; Zakaria et al., 2010).
Volatile Composition of Citrus Leaves
All the common VOCs reported in the peel of the 10 Citrus species studies (Table 1) have also been identified in the leaves of most of these species, except perillaldehyde, only reported in C. limon (Guerrini et al., 2014) (Supplementary Table S2). Almost all the terpenoid compounds described in Citrus peels have also been reported in leaves, except a few of them such as carvyl acetate, perillyl acetate, bornyl acetate or nootkatone. In contrast, only a few aromatic and nitrogen compounds have been described in Citrus leaves, with the exception of some compounds, such as methyl N-methylanthranilate in C. reticulata (Fanciullino et al., 2006; Tomi et al., 2008), C. aurantium (Kirbaslar and Kirbaslar, 2004; Dugo et al., 2011; Guerrini et al., 2014), C. junos (Huang et al., 2000) and C. limon (Kirbaslar and Kirbaslar, 2004). Citrus peel and leaves share many non-terpenoid aliphatic compounds, although some of them have not been reported in Citrus leaves, such as nonyl acetate, decyl acetate, dodecyl acetate or (E,E)-2,4-decadienal. At the same time, some non-terpenoid olefinic compounds are reported more frequently in Citrus leaves than in the peel, such as (E)-2-hexenal (Huang et al., 2000; Ahmed et al., 2001; Gancel et al., 2003, 2005) or (Z)-3-hexen-1-ol (Huang et al., 2000; Ahmed et al., 2001; Gancel et al., 2003, 2005), both with a distinctive grassy-green odor (Elmaci and Onogur, 2012). Even some compounds have only been found in leaves, such as (Z)-2-penten-1-ol (Gancel et al., 2003, 2005) or (Z)-3-hexenyl butanoate (Flamini and Cioni, 2010).
Recently a volatilome of Citrus leaves about differentially accumulated volatiles has been developed (Lamine et al., 2018). This study was performed in the leaves of four Citrus species (C. limon, C. reticulata, C. sinensis and C. aurantium) and these four species were clearly discriminated, especially C. limon. The number of taxonomic classifiers was narrow to eight (linalyl acetate, geranyl acetate, neryl acetate, β-cis-ocimene, δ-3-carene, β-myrcene, sabinene and α-terpineol). However, according to our review, it is very risky to establish taxonomic volatile metabolites with a single study, given the great variability of the volatile profile for the same species.
Conclusion
This review about the volatiles in the Citrus essential oil summarized critically the volatile compounds identified up to date in the 10 most frequently studied Citrus species. It allowed their clustering based on the qualitative profile of the volatiles more reliably identified, which was in concordance with their genomic similitude. It also provides the possibility of developing species biomarkers based on this type of compounds, and points toward those which could be useful for the industry to monitor quality and consumer safety (Salgueiro et al., 2010). In addition, traceability, quality control, and adulterations of foods and drugs could be better addressed and optimized by using the Citrus volatile chemical database presented here. This review also provides important information to help in the selection of the most adequate species and the best source for specialized volatile chemicals for pharmaceutical, food, beverages, fragrances, and cosmetic industries.
Author Contributions
MG-M reviewed the literature and elaborated the tables. JR performed statistical analysis and figures. ML-G elaborated Supplementary Figure S1. MG-M and JR drafted the manuscript. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| CAR | carboxen |
| DVB | divinylbenzene |
| EO | essential oil |
| FD | flavor dilution |
| GC-MS | gas chromatography-mass spectrometry |
| GC-O | gas chromatography-olfactometry |
| HCA | hierarchical cluster analysis |
| HS-SPME | headspace-solid phase microextraction |
| PDMS | polydimethylsiloxane |
| VOCs | volatile organic compounds |
Footnotes
Funding. This work was supported in part by the European Commission Horizon 2020 program TRADITOM grant 634561 and TomGEM grant 679796 to JR and AG.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00012/full#supplementary-material
References
- Abreu I., da Costa N. C., van Es A., Kim J.-A., Parasar U., Poulsen M. L. (2017). Natural occurrence of aldol condensation products in Valencia orange oil. J. Food Sci. 82 2805–2815. 10.1111/1750-3841.13948 [Abstract] [CrossRef] [Google Scholar]
- Ahmed M., Arpaia M. L., Scora R. W. (2001). Seasonal variation in lemon (Citrus limon L. Burm. F) leaf and rind oil composition. J. Essent. Oil Res. 13 149–153. 10.1080/10412905.2001.9699646 [CrossRef] [Google Scholar]
- Akakabe Y., Kusunoki A., Tanaka R., Kanetsune Y. (2010). A comparison of volatile components of Setomi with its parent cultivars. Biosci. Biotechnol. Biochem. 74 659–662. 10.1271/bbb.90722 [Abstract] [CrossRef] [Google Scholar]
- Akakabe Y., Sakamoto M., Ikeda Y., Tanaka M. (2008). Identification and characterization of volatile components of the Japanese Sour Citrus fruit Citrus nagato-yuzukichi Tanaka. Biosci. Biotechnol. Biochem. 72 1965–1968. 10.1271/bbb.80144 [Abstract] [CrossRef] [Google Scholar]
- Aliberti L., Caputo L., de Feo V., de Martino L., Nazzaro F., Souza L. F. (2016). Chemical composition and in vitro antimicrobial, cytotoxic, and central nervous system activities of the essential oils of Citrus medica L. cv. ‘Liscia’ and C. medica cv. ‘Rugosa’ cultivated in Southern Italy. Molecules 21:E1244. 10.3390/molecules21091244 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alissandrakis E., Daferera D., Tarantilis P. A., Polissiou M., Harizanis P. C. (2003). Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 82 575–582. 10.1016/S0308-8146(03)00013-X [CrossRef] [Google Scholar]
- Alonzo G., Fatta del Bosco S., Palazzolo E., Saiano F., Tusa N. (2000). Citrus cybrid leaf essential oil. Flavour Frag. J. 15 91–95. 10.1002/(SICI)1099-1026(200003/04)15:2<91::AID-FFJ872>3.0.CO;2-X [CrossRef] [Google Scholar]
- Asikin Y., Maeda G., Tamaki H., Mizu M., Oku H., Wada K. (2015). Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant anlyses. Food Res. Int. 67 102–110. 10.1016/j.foodres.2014.11.015 [CrossRef] [Google Scholar]
- Asikin Y., Taira I., Inafuku-Teramoto S., Sumi H., Ohta H., Takara K., et al. (2012a). The composition of volatile aroma components, flavanones, and polymethoxylated flavones in shiikuwasha (Citrus depressa Hayata) peels of different cultivation lines. J. Agric. Food Chem. 60 7973–7980. 10.1021/jf301848s [Abstract] [CrossRef] [Google Scholar]
- Asikin Y., Taira I., Inafuku S., Sumi H., Sawamura M., Takara K., et al. (2012b). Volatile aroma components and antioxidant activities of the flavedo peel extract of unripe shiikuwasha (Citrus depressa Hayata). J. Food Sci. 77 469–475. 10.1111/j.1750-3841.2011.02604.x [Abstract] [CrossRef] [Google Scholar]
- Baik J. S., Kim S.-S., Lee J.-A., Oh T.-H., Kim J.-Y., Lee N. H., et al. (2008). Chemical composition and biological activities of essential oils extracted from Korean endemic Citrus species. J. Microbiol. Biotechnol. 18 74–79. [Abstract] [Google Scholar]
- Belsito E. L., Carbone C., Di Gioa M. L., Leggio A., Liguori A., Perri F., et al. (2007). Comparison of the volatile constituents in cold-pressed bergamot oil and a volatile oil isolated by vacuum distillation. J. Agric. Food Chem. 55 7847–7851. 10.1021/jf070997q [Abstract] [CrossRef] [Google Scholar]
- Ben Hsouna A., Ben Halima N., Smaoui S., Hamdi N. (2017). Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 16:146. 10.1186/s12944-017-0487-5 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Benelli P., Riehl C. A. S., Smânia J. A., Smânia E. F. A., Ferreira S. R. S. (2010). Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques: mathematical modeling and extract composition. J. Supercrit. Fluids 55 132–141. 10.1016/j.supflu.2010.08.015 [CrossRef] [Google Scholar]
- Benjamin G., Tietel Z., Porat R. (2013). Effects of rootstock/scion combinations on the flavor of Citrus fruit. J. Agric. Food Chem. 61 11286–11294. 10.1021/jf402892p [Abstract] [CrossRef] [Google Scholar]
- Blanco Tirado C., Stashenko E. E., Combariza M. Y., Martinez J. R. (1995). Comparative study of Colombian citrus oils by high-resolution gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. A 697 501–513. 10.1016/0021-9673(94)00955-9 [CrossRef] [Google Scholar]
- Blázquez M. A., Carbó E. (2015). Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crops Prod. 76 515–521. 10.1016/j.indcrop.2015.07.019 [CrossRef] [Google Scholar]
- Bonaccorsi I. L., McNair H. M., Brunner L. A., Dugo P., Dugo G. (1999). Fast HPLC for the analysis of oxygen heterocyclic compounds of Citrus essential oils. J. Agric. Food Chem. 47 4237–4239. 10.1021/jf990417s [Abstract] [CrossRef] [Google Scholar]
- Boussaada O., Chemli R. (2006). Chemical composition of essential oils from flowers, leaves and peel of Citrus aurantium L. var. amara from Tunisia. J. Essent. Oil Bear. Pl. 9 133–139. 10.1080/0972060X.2006.10643484 [CrossRef] [Google Scholar]
- Boussaada O., Skoula M., Kokkalou E., Chemli R. (2007). Chemical variability of flowers, leaves, and peels oils of four sour orange provenances. J. Essent. Oil Bear Plants 10 453–464. 10.1080/0972060X.2007.10643579 [CrossRef] [Google Scholar]
- Brophy J. J., Goldsack R. J., Forster P. I. (2001). The leaf oils of the Australian species of Citrus (Rutaceae). J. Essent. Oil Bear Plant 13 264–268. 10.1080/10412905.2001.9699690 [CrossRef] [Google Scholar]
- Buettner A., Mestres M., Fischer A., Guasch J., Schieberle P. (2003). Evaluation of the most odour-active compounds in the peel oil of clementines (Citrus reticulata blanco cv. clementine). Eur. Food Res. Technol. 216 11–14. 10.1007/s00217-002-0586-y [CrossRef] [Google Scholar]
- Cannon R. J., Kazimierski A., Curto N. L., Li J., Trinnaman L., Janczuk A. J., et al. (2015). Identification, synthesis, and characterization of novel sulfur-containing volatile compounds from the in-depth analysis of Lisbon lemon peels (Citrus limon L. Burm. f. cv. Lisbon). J. Agric. Food Chem. 63 1915–1931. 10.1021/jf505177r [Abstract] [CrossRef] [Google Scholar]
- Carbonell-Caballero J., Alonso R., Ibañez V., Terol J., Talon M., Dopazo J. (2015). A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol. Biol. Evol. 32 2015–2035. 10.1093/molbev/msv082 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Casilli A., Decorzant E., Jaquier A., Delort E. (2014). Multidimensional gas chromatography hyphenated to mass spectrometry and olfactometry for the volatile analysis of Citrus hybrid peel extract. J. Chromatogr. A 1373 169–178. 10.1016/j.chroma.2014.11.023 [Abstract] [CrossRef] [Google Scholar]
- Chen Y., Wu J., Xu Y., Fu M., Xiao G. (2014). Effect of second cooling on the chemical components of essential oils from orange peel (Citrus sinensis). J. Agric. Food Chem. 62 8786–8790. 10.1021/jf501079r [Abstract] [CrossRef] [Google Scholar]
- Cheong M. W., Chong Z. S., Liu S.-Q., Zhou W., Curran P., Yu B. (2012). Characterization of calamansi (Citrus microcarpa). Part I: volatiles, aromatic profiles and phenolic acids in the peel. Food Chem. 134 686–695. 10.1016/j.foodchem.2012.02.162 [Abstract] [CrossRef] [Google Scholar]
- Cheong M. W., Liu S.-Q., Yeo J., Chionh H.-K., Pramudya K., Curran P., et al. (2011a). Identification of aroma-active compounds in Malaysian pomelo (Citrus grandis (L.) Osbeck) peel by gas chromatography-olfactometry. J. Essent. Oil Res. 23 34–42. 10.1080/10412905.2011.9712279 [CrossRef] [Google Scholar]
- Cheong M. W., Loke X.-Q., Liu S.-Q., Pramudya K., Curran P., Yu B. (2011b). Characterization of volatile compounds and aroma profiles of Malaysian pomelo (Citrus grandis (L.) Osbeck) blossom and peel. J. Essent. Oil Res. 23 33–44. 10.1080/10412905.2011.9700445 [CrossRef] [Google Scholar]
- Chisholm M. G., Jell J. A., Cass D. M. J. (2003a). Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography-olfactometry. Flavour Frag. J. 18 275–281. [Google Scholar]
- Chisholm M. G., Wilson M. A., Gaskey G. M. (2003b). Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle). Flavour Frag. J. 18 106–115. [Google Scholar]
- Choi H.-S. (2003a). Characterization of Citrus unshiu (C. unshiu Marcov. forma Miyagawa-wase) blossom aroma by solid-phase microextraction in conjunction with an electronic nose. J. Agric. Food Chem. 51 418–423. 10.1021/jf0114280 [Abstract] [CrossRef] [Google Scholar]
- Choi H.-S. (2003b). Character impact odorants of Citrus hallabong [(C.unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 51 2687–2692. 10.1021/jf021069o [Abstract] [CrossRef] [Google Scholar]
- Choi H.-S. (2005). Characteristic odor components of kumquat (Fortunella japonica Swingle) peel oil. J. Agric. Food Chem. 53 1642–1647. 10.1021/jf040324x [Abstract] [CrossRef] [Google Scholar]
- Choi H.-S. (2006). Lipolytic effects of citrus peel oils and their components. J. Agric. Food Chem. 54 3254–3258. 10.1021/jf052409j [Abstract] [CrossRef] [Google Scholar]
- Choi H.-S., Kondo Y., Sawamura M. (2001). Characterization of the odor-active volatiles in Citrus Hyuganatsu (Citrus tamurana Hort. ex Tanaka). J. Agric. Food Chem. 49 2404–2408. 10.1021/jf001467w [Abstract] [CrossRef] [Google Scholar]
- Choi H.-S., Min K.-C. (2004). Headspace-SPME Analysis of Citrus Hybrid, Hallabong. Food Sci. Biotechnol. 13 126–129. [Google Scholar]
- Choi H. S., Sawamura M., Kondo Y. (2002). Characterization of the key aroma compounds of Citrus flaviculpus Hort. ex Tanaka by aroma extraction dilution analysis. Food Chem. Toxicol. 67 1713–1718. 10.1111/j.1365-2621.2002.tb08711.x [CrossRef] [Google Scholar]
- Chung H., Chung W.-Y., Yoo E.-S., Cho S. K., Oh S.-K., Kim Y.-S. (2012). Characterization of volatile aroma-active compounds in Dangyooja (Citrus grandis Osbeck). J. Korean Soc. Appl. Biol. Chem. 55 133–136. 10.1007/s13765-012-0023-2 [CrossRef] [Google Scholar]
- Chung M. S. (2012). Volatile compounds of the Hallabong (Citrus kiyomi x Citrus ponkan) blossom. Food Sci. Biotechnol. 21 285–290. 10.1007/s10068-012-0038-9 [CrossRef] [Google Scholar]
- Cosimi S., Rossi E., Cioni P. L., Canale A. (2009). Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pest: Evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J. Stored Prod. Res. 45 125–132. 10.1016/j.jspr.2008.10.002 [CrossRef] [Google Scholar]
- Costa R., Bisignano C., Filocamo A., Grasso E., Occhiuto F., Spadaro F. (2014). Antimicrobial activity and chemical composition of Citrus aurantifolia (Christm.) Swingle essential oil from Italian organic crops. J. Essent. Oil Res. 26 400–408. 10.1080/10412905.2014.964428 [CrossRef] [Google Scholar]
- Costa R., Dugo P., Navarra M., Raymo V., Dugo G., Mondello L. (2010). Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oil. Flavour Frag. J. 25 4–12. 10.1002/ffj.1949 [CrossRef] [Google Scholar]
- Craske J. D., Suryadi N., Wooton M. (2005). A comparison of the peel oil components of Australian native lime (Microcitrus australe) and Mexican lime (Citrus aurantifolia Swingle). J. Sci. Food Agric. 85 522–525. 10.1002/jsfa.2038 [CrossRef] [Google Scholar]
- Darjazi B. B. (2011a). A comparison of volatile components of flower of page mandarin obtained by ultrasound-assisted extraction and hydrodistillation. J. Med. Plants Res. 5 2839–2847. [Google Scholar]
- Darjazi B. B. (2011b). Comparison of volatile components of flower, leaf, peel and juice of ’Page’ mandarin [(Citrus reticulata var ’Dancy’ x Citrus paradisi var ’Duncan’) x Citrus clementina]. Afr. J. Biotechnol. 10 10437–10446. 10.5897/AJB11.1069 [CrossRef] [Google Scholar]
- Delort E., Jaquier A. (2009). Novel terpenyl esters from Australian finger lime (Citrus australasica) peel extract. Flavour Frag. J. 24 123–132. 10.1002/ffj.1922 [CrossRef] [Google Scholar]
- Delort E., Jaquier A., Decorzant E., Chapuis C., Casilli A., Frérot E. (2015). Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique Citrus chemotypes and new volatile molecules. Phytochemistry 109 111–124. 10.1016/j.phytochem.2014.10.023 [Abstract] [CrossRef] [Google Scholar]
- Dharmawan J., Kasapis S., Sriramula P., Lear M. J., Curran P. (2009). Evaluation of aroma-active compounds in Pontianak orange peel oil (Citrus nobilis Lour. Var. microcarpa Hassk.) by gas chromatography-olfactometry, aroma reconstitution, and omission test. J. Agric. Food Chem. 57 239–244. 10.1021/jf801070r [Abstract] [CrossRef] [Google Scholar]
- Dong Z. B., Shao W. Y., Liang Y. R. (2014). Isolation and characterization of essential oil extracted from tangerine peel. Asian J. Chem. 26 4975–4978. 10.14233/ajchem.2014.16277 [CrossRef] [Google Scholar]
- Družić J., Jerković I., Marijanović Z., Roje M. (2016). Chemical biodiversity of the leaf and flower essential oils of Citrus aurantium L. from Dubrovnik area (Croatia) in comparison with Citrus sinensis L. Osbeck cv. Washington navel, Citrus sinensis L. Osbeck cv. Tarocco and Citrus sinensis L. Osbeck cv. Doppio Sanguigno. J. Essent. Oil Res. 28 283–291. 10.1080/10412905.2016.1159258 [CrossRef] [Google Scholar]
- Dugo G., Bonaccorsi I., Sciarrone D., Costa R., Dugo P., Mondello L., et al. (2011). Characterization of oils from the fruits, leaves and flowers of the bitter orange tree. J. Essent. Oil Res. 23 45–59. 10.1080/10412905.2011.9700446 [Abstract] [CrossRef] [Google Scholar]
- Dugo P., Mondello L. (2011). Citrus Oils: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity, Vol. 49 Boca Raton, FL: CRC Press. [Google Scholar]
- Dugo P., Mondello L., Cogliandro E., Verzera A., Dugo G. (1996). On the genuineness of citrus essential oils. Oxygen heterocyclic compounds of bitter orange oil (Citrus aurantium L.). J. Agric. Food Chem. 44 544–549. 10.1021/jf950183m [CrossRef] [Google Scholar]
- Dugo P., Mondello L., Favoino O., Cicero L., Rodríguez-Zenteno N. A., Dugo G. (2005). Characterization of cold-pressed Mexican dancy tangerine oils. Flavour Frag. J. 20 60–66. 10.1002/ffj.1367 [CrossRef] [Google Scholar]
- Elmaci Y., Onogur T. A. (2012). Mandarin peel aroma: estimation by using headspace/GC/MS and descriptive analysis techniques. Acta Aliment. 41 131–139. 10.1556/AAlim.41.2012.1.15 [CrossRef] [Google Scholar]
- Esquivel-Ferriño P. C., Clemente-Soto A. F., Ramírez-Cabriales M. Y., Garza-González E., Camacho-Corona M. R. (2014). Volatile constituents identified in hexane extract of Citrus sinensis peel and anti-mycobacterial tuberculosis activity of some of its constituents. J. Mex. Chem. Soc. 58 431–434. [Google Scholar]
- Fancello F., Petretto G. L., Zara S., Sanna M. L., Addis R., Maldini M., et al. (2016). Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT Food Sci. Technol. 69 579–585. 10.1016/j.lwt.2016.02.018 [CrossRef] [Google Scholar]
- Fanciullino A.-L., Gancel A.-L., Froelicher Y., Luro F., Ollitrault P., Brillouet J.-M. (2005). Effects of nucleo-cytoplasmic interactions on leaf volatile compounds from Citrus somatic diploid hybrids. J. Agric. Food Chem. 53 4517–4523. 10.1021/jf0502855 [Abstract] [CrossRef] [Google Scholar]
- Fanciullino A.-L., Tomi F., Luro F., Desjobert J. M., Casanova J. (2006). Chemical variability of peel and leaf oils of mandarins. Flavour Frag. J. 21 359–367. 10.1002/ffj.1658 [Abstract] [CrossRef] [Google Scholar]
- Feger W., Brandauer H., Ziegler H. (2000). Sesquiterpene hydrocarbons of cold-pressed lime oils. Flavour Frag. J. 15 281–284. 10.1002/1099-1026(200007/08)15:4<281::AID-FFJ916>3.0.CO;2-W [CrossRef] [Google Scholar]
- Feger W., Brandauer H., Ziegler H. (2001a). Analytical investigation of sweetie peel oil. J. Essent. Oil Res. 13 309–313. 10.1080/10412905.2001.9712221 [CrossRef] [Google Scholar]
- Feger W., Brandauer H., Ziegler H. (2001b). Germacrenes in citrus peel oils. J. Essent. Oil Res. 13 274–277. 10.1080/10412905.2001.9699692 [CrossRef] [Google Scholar]
- Ferhat M. A., Meklati B. Y., Chemat F. (2007). Comparison of different isolation methods of essential oil from Citrus fruits: cold pressing, hydrodistillation and microwave ’dry’ distillation. Flavour Frag. J. 22 494–504. 10.1002/ffj.1829 [CrossRef] [Google Scholar]
- Fisher A., Grab W., Schieberle P. (2008). Characterisation of the most odour-active compounds in a peel oil extract from Pontianak oranges (Citrus nobilis var. Lour. microcarpa Hassk.). Eur. Food Res. Technol. 227 735–744. 10.1007/s00217-007-0781-y [CrossRef] [Google Scholar]
- Flamini G., Cioni P. L. (2010). Odour gradients and patterns in volatile emission of different plant parts and developing fruits of grapefruit (Citrus paradisi L.). Food Chem. 120 984–992. 10.1016/j.foodchem.2009.11.037 [CrossRef] [Google Scholar]
- Flamini G., Tebano M., Cioni P. L. (2007). Volatiles emission patterns of different plant organs and pollen of Citrus limon. Anal. Chim. Acta 589 120–124. 10.1016/j.aca.2007.02.053 [Abstract] [CrossRef] [Google Scholar]
- Fouad H., da Camara C. A. G. (2017). Chemical composition and bioactivity of peel oils from Citrus aurantifolia and Citrus reticulata and enantiomers of their major constituents against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 73 30–36. 10.1016/j.jspr.2017.06.001 [CrossRef] [Google Scholar]
- Frizzo C. D., Lorenzo D., Dellacassa E. (2004). Composition and seasonal variation of the essential oils from two mandarin cultivars of Southern Brazil. J. Agric. Food Chem. 52 3036–3041. 10.1021/jf030685x [Abstract] [CrossRef] [Google Scholar]
- Furneri P. M., Mondello L., Mandalari G., Paolino D., Dugo P., Garozzo A., et al. (2012). In vitro antimycoplasmal activity of Citrus bergamia essential oil and its major components. Eur. J. Med. Chem. 52 66–69. 10.1016/j.ejmech.2012.03.005 [Abstract] [CrossRef] [Google Scholar]
- Gancel A.-L., Ollé D., Ollitrault P., Luro F., Brillouet J.-M. (2002). Leaf and peel volatile compounds of an interspecific citrus somatic hybrid [Citrus aurantifolia (Christm.) Swing. + Citrus paradisi Macfayden]. Flavour Frag. J. 17 416–424. 10.1002/ffj.1119 [CrossRef] [Google Scholar]
- Gancel A.-L., Ollitrault P., Froelicher Y., Tomi F., Jacquemond C., Luro F., et al. (2003). Leaf volatile compounds of seven citrus somatic tetraploid hybrids sharing willow leaf mandarin (Citrus deliciosa Ten.) as their common parent. J. Agric. Food Chem. 51 6006–6013. 10.1021/jf0345090 [Abstract] [CrossRef] [Google Scholar]
- Gancel A.-L., Ollitrault P., Froelicher Y., Tomi F., Jacquemond C., Luro F., et al. (2005). Leaf volatile compounds of six citrus somatic allotetraploid hybrids originating from various combinations of lime, lemon, citron, sweet orange, and grapefruit. J. Agric. Food Chem. 53 2224–2230. 10.1021/jf048315b [Abstract] [CrossRef] [Google Scholar]
- González-Mas M. C., Rambla J. L., Alamar M. C., Gutiérrez A., Granell A. (2011). Comparative analysis of the volatile fraction of fruit juice from different Citrus species. PLoS One 6:e22016. 10.1371/journal.pone.0022016 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- González-Mas M. C., Rambla J. L., Gómez-Torrente M. L., Buj A., López-Gresa M. P., Granell A. (2015). Análisis no dirigido del aceite esencial de las variedades de mandarino Clemenules y Clemenpons. Levante Agrícola 429 232–241. [Google Scholar]
- Guerrini A., Rossi D., Grandini A., Scalvenzi L., Noriega Rivera P. F., Andreotti E., et al. (2014). Biological and chemo-diverse characterization of Amazonian (Ecuador) Citrus petitgrains. J. Appl. Bot. Food Qual. 87 108–116. [Google Scholar]
- Hamdan D., El-Readi M. Z., Nibret E., Sporer F., Farrag N., El-Shazly A., et al. (2010). Chemical composition of the essential oils of two Citrus species and their biological activities. Pharmazie 65 141–147. [Abstract] [Google Scholar]
- Högnadóttir Á., Rouseff R. L. (2003). Identification of arome active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A 998 201–211. 10.1016/S0021-9673(03)00524-7 [Abstract] [CrossRef] [Google Scholar]
- Hosni K., Zahed N., Chrif R., Abid I., Medfei W., Kallel M., et al. (2010). Composition of peel essential oils from four selected Tunisian Citrus species: evidence for the genotypic influence. Food Chem. 123 1098–1104. 10.1016/j.foodchem.2010.05.068 [CrossRef] [Google Scholar]
- Huang H.-H., Lin L.-Y., Chiang H.-M., Lay S.-J., Wu C.-S., Chen H.-C. (2017). Analysis of volatile compounds from different parts of Citrus grandis (L.) Osbeck flowers by Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. J. Essent. Oil Bear Plant 20 1057–1065. 10.1080/0972060X.2017.1377112 [CrossRef] [Google Scholar]
- Huang Y., Pu Z., Chen Q. (2000). The chemical composition of the leaf essential oils from 110 Citrus species, cultivars, hybrids and varieties of Chinese origin. Perfum. Flavor. 25 53–66. [Google Scholar]
- Inafuku-Teramoto S., Suwa R., Fukuzawa Y., Kawamitsu Y. (2011). Polymethoxyflavones, synephrine and volatile constitution of peels of citrus fruit grown in Okinawa. J. Jpn. Soc. Hortic. Sci. 80 214–224. 10.2503/jjshs1.80.214 [CrossRef] [Google Scholar]
- Jabalpurwala F. A., Smoot J. M., Rouseff R. L. (2009). A comparison of citrus blossom volatiles. Phytochemistry 70 1428–1434. 10.1016/j.phytochem.2009.07.031 [Abstract] [CrossRef] [Google Scholar]
- Jabri Karoui I., Marzouk B. (2013). Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. Biomed. Res. Int. 2013: 345415. 10.1155/2013/345415 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jazet Dongmo P. M., Tatsadjieu L. N., Tchinda Sonwa E., Kuate J., Amvam Zollo P. H., Menut C. (2009). Essential oils of Citrus aurantifolia from Cameroon and their antifungal activity against Phaeoramularia angolensis. Afr. J. Agric. Res. 4 354–358. [Google Scholar]
- Jiang M.-H., Yang L., Zhu L., Piao J.-H., Jiang J.-G. (2011). Comparative GC/MS analysis of essential oils extracted by 3 methods from the bud of Citrus aurantium L. var. amara Engl. J. Food Sci. 76 1219–1225. 10.1111/j.1750-3841.2011.02421.x [Abstract] [CrossRef] [Google Scholar]
- Jing L., Lei Z., Li L., Xie R., Xi R., Xi W., et al. (2014). Antifungal activity of citrus essential oils. J. Agric. Food Chem. 62 3011–3033. 10.1021/jf5006148 [Abstract] [CrossRef] [Google Scholar]
- Jirapakkul W., Tinchan P., Chaiseri S. (2013). Effect of drying temperature on key odourants in kaffir lime (Citrus hystrix D.C., Rutaceae) leaves. Int. J. Food Sci. Tech. 48 143–149. 10.1111/j.1365-2621.2012.03170.x [CrossRef] [Google Scholar]
- Karioti A., Skaltsa H., Gbolade A. A. (2007). Constituents of the distilled essential oils of Citrus reticulata and C. paradisi from Nigeria. J. Essent. Oil Res. 19 520–522. 10.1080/10412905.2007.9699320 [CrossRef] [Google Scholar]
- Kerdchoechuen O., Laohakunjit N., Singkornard S., Matta F. B. (2010). Essential oils from six herbal plants for biocontrol of the maixe weevil. Hortscience 45 592–598. [Google Scholar]
- Kerry J. P., Kerry J. F., Ledward D. (2002). Meat Processing, Improvent Quality, 1st Edn Cambridge: Woodhead Publishing, 480. [Google Scholar]
- Kirbaslar F. G., Kirbaslar S. I., Dramur U. (2001). The compositions of Turkish bergamot oils produced by cold-pressing and steam distillation. J. Essent. Oil Res. 13 411–415. 10.1080/10412905.2001.9699710 [CrossRef] [Google Scholar]
- Kirbaslar G., Kirbaslar S. I. (2004). Composition of Turkish bitter orange and lemon leaf oils. J. Essent. Oil Res. 16 105–108. 10.1080/10412905.2004.9698663 [Abstract] [CrossRef] [Google Scholar]
- Lamine M., Rahali F. Z., Hammami M., Mliki A. (2018). From differentially accumulated volatiles to the search of robust metabolic classifiers: exploring the volatome of Citrus leaves. Microchem. J. 138 321–327. 10.1016/j.microc.2018.01.030 [CrossRef] [Google Scholar]
- Lan Phi N. T., Nishiyama C., Choi H.-S., Sawamura M. (2006). Evaluation of characteristic aroma compounds of Citrus natsudaidai Hayata (Natsudaidai) cold-pressed peel oil. Biosci. Biotechnol. Biochem. 70 1832–1838. 10.1271/bbb.50705 [Abstract] [CrossRef] [Google Scholar]
- Lan Phi N. T., Shimamura T., Ukeda H., Sawamura M. (2009). Chemical and aroma profiles of yuzu (Citrus junos) peel oils of different cultivars. Food Chem. 115 1042–1047. 10.1016/j.foodchem.2008.12.024 [CrossRef] [Google Scholar]
- Li L. J., Hong P., Chen F., Sun H., Yang Y. F., Yu X., et al. (2016). Characterization of the aldehydes and their transformations induced by uv irradiation and air exposure of white guanxi honey pummelo (Citrus grandis (L.) Osbeck) essential oil. J. Agric. Food Chem. 64 5000–5010. 10.1021/acs.jafc.6b01369 [Abstract] [CrossRef] [Google Scholar]
- Lin J., Rouseff R. L. (2001). Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time-intensity GC-olfactometry and GC-MS. Flavour Frag. J. 16 457–463. 10.1002/ffj.1041 [CrossRef] [Google Scholar]
- Lin S. Y., Roan S. F., Lee C. L., Chen I. Z. (2010). Volatile organic components of fresh leaves as indicators of indigenous and cultivated citrus species in Taiwan. Biosci. Biotechnol. Biochem. 74 806–811. 10.1271/bbb.90891 [Abstract] [CrossRef] [Google Scholar]
- Liu C., Cheng Y., Zhang H., Deng X., Chen F., Xu J. (2012). Volatile constituents of wild Citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil. J. Agric. Food Chem. 60 2617–2628. 10.1021/jf2039197 [Abstract] [CrossRef] [Google Scholar]
- Liu C., Yan F., Gao H., He M., Wang Z., Cheng Y., et al. (2015). Features of citrus terpenoid production as revealed by carotenoid, limonoid and aroma profiles of two pummelos (Citrus maxima) with different flesh color. J. Sci. Food Agric. 95 111–119. 10.1002/jsfa.6689 [Abstract] [CrossRef] [Google Scholar]
- Loizzo M. R., Tundis R., Bonesi M., Di Sanzo G., Verardi A., Lopresto C. G., et al. (2016). Chemical profile and antioxidant properties of extracts and essential oils from Citrus x limon (L.) BURM. cv. Femminello comune. Chem. Biodiversity 13 571–581. 10.1002/cbdv.201500186 [Abstract] [CrossRef] [Google Scholar]
- Lota M.-L., de Rocca Serra D., Jacquemond C., Tomi F., Casanova J. (2001a). Chemical variability of peel and leaf essential oils of sour orange. Flavour Frag. J. 16 89–96. [Abstract] [Google Scholar]
- Lota M.-L., de Rocca Serra D., Tomi F., Casanova J. (2001b). Chemical variabiliy of peel and leaf essential oils of 15 species of mandarins. Biochem. Syst. Ecol. 29 77–104. 10.1016/S0305-1978(00)00029-6 [Abstract] [CrossRef] [Google Scholar]
- Lota M.-L., de Rocca Serra D., Tomi F., Bessiere J.-M., Casanova J. (1999). Chemical composition of peel and leaf essential oils of Citrus medica L. and C. limonimedica Lush. Flavour Frag. J. 14 161–166. 10.1002/(SICI)1099-1026(199905/06)14:3<161::AID-FFJ801>3.0.CO;2-8 [CrossRef] [Google Scholar]
- Lota M.-L., de Rocca Serra D., Tomi F., Jacquemond C., Casanova J. (2002). Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 50 796–805. 10.1021/jf010924l [Abstract] [CrossRef] [Google Scholar]
- Luciardi M. C., Blázquez M. A., Cartagena E., Bardón A., Arena M. E. (2016). Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci. Technol. 68 373–380. 10.1016/j.lwt.2015.12.056 [Abstract] [CrossRef] [Google Scholar]
- Merle H., Morón M., Blázquez M. A., Boira H. (2004). Taxonomical contribution of essential oils in mandarins cultivars. Biochem. Syst. Ecol. 32 491–497. 10.1016/j.bse.2003.09.010 [CrossRef] [Google Scholar]
- Minh Tu N. T., Onishi Y., Choi H. S., Kondo Y., Ukeda H., Sawamura M. (2003a). Characteristic odour components of Citrus sp. (Kiyookadaidai) cold-pressed peel oil. Flavour Frag. J. 18 515–520. [Google Scholar]
- Minh Tu N. T., Onishi Y., Son U. S., Ogawa E., Ukeda H., Sawamura M. (2003b). Characteristic odour components of Citrus inflata Hort. ex Tanaka (Mochiyu) cold-pressed peel oil. Flavour Frag. J. 18 454–459. [Google Scholar]
- Minh Tu N. T., Onishi Y., Choi H. S., Kondo Y., Bassore S. M., Ukeda H., et al. (2002a). Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J. Agric. Food Chem. 50 2908–2913. 10.1021/jf011578a [Abstract] [CrossRef] [Google Scholar]
- Minh Tu N. T., Thanh L. X., Une A., Ukeda H., Sawamura M. (2002b). Volatile constituents of Vietnamese pummelo, orange, tangerine and lime peel oils. Flavour Frag. J. 17 169–174. [Google Scholar]
- Mitiku S. B., Sawamura M., Itoh T., Ukeda H. (2000). Volatile components of peel cold-pressed oils of two cultivars of sweet orange (Citrus sinensis (L.) Osbeck) from Ethiopia. Flavour Frag. J. 15 240–244. 10.1002/1099-1026(200007/08)15:4<240::AID-FFJ902>3.0.CO;2-F [CrossRef] [Google Scholar]
- Mitropoulou G., Fitsiou E., Spyridopoulou K., Tiptiri-Kourpeti A., Bardouki H., Vamvakias M., et al. (2017). Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT Food Sci. Technol. 84 344–352. 10.1016/j.lwt.2017.05.036 [CrossRef] [Google Scholar]
- Miyazaki T., Plotto A., Baldwin E. A., Reyes-de-Corcuera J. I., Gmitter F. G. J. (2012). Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J. Sci. Food Agric. 92 727–735. 10.1002/jsfa.4663 [Abstract] [CrossRef] [Google Scholar]
- Miyazato H., Hashimoto S., Hayashi S. (2012). Identification of the odour-active aldehyde trans-4,5-epoxy-(E,Z)-2,7-decadienal in yuzu (Citrus junos Sieb. ex Tanaka). Eur. Food Res. Technol. 235 881–891. 10.1007/s00217-012-1820-x [CrossRef] [Google Scholar]
- Miyazato H., Hashimoto S., Hayashi S. (2013). First identification of the odour-active unsaturated aliphatic acid (E)-4-methyl-3-hexenoic acid in yuzu (Citrus junos Sieb. ex Tanaka). Flavour Frag. J. 28 62–69. 10.1002/ffj.3128 [CrossRef] [Google Scholar]
- Miyazawa N., Fujita A., Kubota K. (2010). Aroma character impact compounds in Kinokuni mandarin orange (Citrus kinokuni) compared with satsuma mandarin orange (Citrus unshiu). Biosci. Biotechnol. Biochem. 74 835–842. 10.1271/bbb.90937 [Abstract] [CrossRef] [Google Scholar]
- Miyazawa N., Tomita N., Kurobayashi Y., Nakanishi A., Ohkubo Y., Maeda T., et al. (2009). Novel character impact compounds in yuzu (Citrus junos Sieb. ex Tanaka) peel oil. J. Agric. Food Chem. 57 1990–1996. 10.1021/jf803257x [Abstract] [CrossRef] [Google Scholar]
- Mondello L., Casilli A., Tranchida P. Q., Cicero L., Dugo P., Dugo G. (2003). Comparison of fast and conventional GC analysis for Citrus essential oils. J. Agric. Food Chem. 51 5602–5606. 10.1021/jf0341971 [Abstract] [CrossRef] [Google Scholar]
- Naef R., Velluz A. (2001). Volatile constituents in extracts of mandarin and tangerine peel. J. Essent. Oil Res. 13 154–157. 10.1080/10412905.2001.9699647 [Abstract] [CrossRef] [Google Scholar]
- Njoroge S. M., Karanja P. N., Sawamura M. (2008). Essential oil components of common mandarins (Citrus reticulata Blanco) from Uganda. J. Essent. Oil Bear. Plant 11 609–614. 10.1080/0972060X.2008.10643675 [CrossRef] [Google Scholar]
- Njoroge S. M., Koaze H., Karanja P. N., Sawamura M. (2005a). Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis). Flavour Frag. J. 20 80–85. [Google Scholar]
- Njoroge S. M., Koaze H., Karanja P. N., Sawamura M. (2005b). Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 53 9790–9794. 10.1021/jf051373s [Abstract] [CrossRef] [Google Scholar]
- Oh H. J., Ahn H. M., Kim S. S., Yung P. Y., Jeon G. L., Ko Y. H., et al. (2007). Composition and antimicrobial activities of essential oils in the peel of Citrus fruits. J. Appl. Biol. Chem. 50 148–154. [Google Scholar]
- Omori H., Nakahara K., Umano K. (2011). Characterization of aroma compounds in the peel extract of Jabara (Citrus jabara Hort. ex Tanaka). Flavour Frag. J. 26 396–402. 10.1002/ffj.2066 [CrossRef] [Google Scholar]
- Othman S. N. A. M., Hassan M. A., Nahar L., Basar N., Jamil S., Sarker S. D. (2017). Essential oils from the Malaysian Citrus (Rutaceae) medicinal plants. Medicines 3:13. 10.3390/medicines3020013 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Palazzolo E., Laudicina V. A., Germanà M. A. (2013). Current and potential use of Citrus essential oils. Curr. Org. Chem. 17 3042–3049. 10.2174/13852728113179990122 [CrossRef] [Google Scholar]
- Paoli M., de Rocca Serra D., Tomi F., Luro F., Bighelli A. (2016). Chemical composition of the leaf essential oil of grapefruits (Citrus paradisi Macf.) in relation with the genetic origin. J. Essent. Oil Res. 28 265–271. 10.1080/10412905.2016.1140090 [CrossRef] [Google Scholar]
- Petretto G. L., Sarais G., Maldini M. T., Foddai M., Tirillini B., Rourke J. P., et al. (2016). Citrus monstruosa discrimination among several Citrus species by multivariate analysis of volatiles: a metabolomic approach. J. Food Process. Preserv. 40 950–957. 10.1111/jfpp.12674 [CrossRef] [Google Scholar]
- Pino J. A., Muñoz Y., Quijano-Celís C. E. (2006). Analysis of cold-pressed mandarin peel oil from Cuba. J. Essent. Oil Bear. Plant 9 271–276. 10.1080/0972060X.2006.10643503 [CrossRef] [Google Scholar]
- Pino J. A., Quijano-Celís C. E. (2007). Chromatographic deterpenation of mandarin cold-pressed oil. J. Essent. Oil Bear. Plant 10 504–509. 10.1080/0972060X.2007.10643587 [Abstract] [CrossRef] [Google Scholar]
- Pino J. A., Rosado A. (2000). Composition of cold-pressed bitter orange oil from Cuba. J. Essent. Oil Res. 12 675–676. 10.1080/10412905.2000.9712187 [CrossRef] [Google Scholar]
- Poiana M., Mincione A., Gionfriddo F., Castaldo D. (2003). Supercritical carbon dioxide separation of bergamot essential oil by a countercurrent process. Flavour Frag. J. 18 429–435. 10.1002/ffj.1245 [Abstract] [CrossRef] [Google Scholar]
- Qiao Y., Xie B. J., Zhang Y., Zhang Y., Fan G., Yao X. L., et al. (2008). Characterization of aroma active compounds in fruit juice and peel oil of jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 13 1333–1344. 10.3390/molecules13061333 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Qiu S., Wang J. (2015). Application of sensory evaluation, HS-SPME GC-MS, E-nose, and E-tongue for quality detection in Citrus fruits. J. Food Sci. 80 S2296–S2304. 10.1111/1750-3841.13012 [Abstract] [CrossRef] [Google Scholar]
- Rambla J. L., Alfaro C., Medina A., Zarzo M., Primo J., Granell A. (2015). Tomato fruit volatile profiles are highly dependent on sample processing and capturing methods. Metabolomics 11 1708–1720. 10.1007/s11306-015-0824-5 [CrossRef] [Google Scholar]
- Rambla J. L., González-Mas M. C., Pons C., Bernet G. P., Asins M. J., Granell A. (2014). Fruit volatile profiles of two Citrus hybrids are dramatically different from those of their parents. J. Agric. Food Chem. 62 11312–11322. 10.1021/jf5043079 [Abstract] [CrossRef] [Google Scholar]
- Ruberto G., Rapisarda P. (2002). Essential oils of new pigmented citrus hybrids: Citrus sinensis L. Osbeck x C. clementina Hort. ex Tanaka. J. Food Sci. 67 2778–2780. 10.1111/j.1365-2621.2002.tb08815.x [CrossRef] [Google Scholar]
- Ruberto G., Renda A., Piattelli M., Rapisarda P., Starrantino A. (1997). Essential oil of two new pigmented Citrus hybrids, Citrus clementina x Citrus sinensis. J. Agric. Food Chem. 45 467–471. 10.1021/jf960109j [CrossRef] [Google Scholar]
- Russo M., Bonaccorsi I., Costa R., Trozzi A., Dugo P., Mondello L. (2015). Reduced time HPLC analyses for fast quality control of citrus essential oils. J. Essent. Oil Res. 27 307–315. 10.1080/10412905.2015.1027419 [CrossRef] [Google Scholar]
- Salgueiro L., Martins A. P., Correia H. (2010). Raw materials: the importance of quality and safety. A review. Flavour Frag. J. 25 253–271. 10.1002/ffj.1973 [Abstract] [CrossRef] [Google Scholar]
- Sarrou E., Chatzopoulou P., Dimassi-Theriou K., Therios I. (2013). Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 18 10639–10647. 10.3390/molecules180910639 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sawamura M., Minh Tu N. T., Yu X., Xu B. (2005). Volatile constituents of the peel oils of several sweet oranges in China. J. Essent. Oil Res. 17 2–6. 10.1080/10412905.2005.9698813 [CrossRef] [Google Scholar]
- Sawamura M., Onishi Y., Ikemoto J., Minh Tu N. T., Lan Phi N. T. (2006). Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Frag. J. 21 609–615. 10.1002/ffj.1604 [CrossRef] [Google Scholar]
- Sawamura M., Shichiri K., Ootani Y., Zheng X. H. (1991). Volatile constituents of several varieties of pummelos and characteristics among citrus species. Agric. Biol. Chem. 55 2571–2578. [Google Scholar]
- Schipilliti L., Bonaccorsi I. L., Occhiuto C., Dugo P., Mondello P. (2018). Authentication of citrus volatiles based on carbon isotope ratios. J Essent. Oil Res. 30 1–15. 10.1080/10412905.2017.1377123 [CrossRef] [Google Scholar]
- Selvaraj Y., Prasad M. B. N. V., Venkateshwarlu G. (2002). Profiles of essential oils of peel and leaf of a new Citrus hybrid, Citrus latifolia Tanaka x Citrus aurantifolia Swingle. J. Essent. Oil Res. 14 369–371. 10.1080/10412905.2002.9699887 [CrossRef] [Google Scholar]
- Shen Z., Mishra V., Imison B., Palmer M., Fairclough R. (2002). Use of adsorbent and supercritical carbon dioxide to concentrate flavor compounds from orange oil. J. Agric. Food Chem. 50 154–160. 10.1021/jf010582j [Abstract] [CrossRef] [Google Scholar]
- Singh P., Shukla R., Prakash B., Kumar A., Singh S., Kumar Mishra P., et al. (2010). Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm., and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterperne, DL-limonene. Food Chem. Toxicol. 48 1734–1740. 10.1016/j.fct.2010.04.001 [Abstract] [CrossRef] [Google Scholar]
- Smadja J., Rondeau P., Sing A. S. C. (2005). Volatile constituents of five Citrus petitgrain essential oils from Reunion. Flavour Frag. J. 20 399–402. 10.1002/ffj.1438 [CrossRef] [Google Scholar]
- Song H. S., Sawamura M., Ito T., Ido A., Ukeda H. (2000a). Quantitative determination and characteristic flavour of daidai (Citrus aurantium L. var. cyathifera Y. Tanaka) peel oil. Flavour Frag. J. 15 323–328. [Google Scholar]
- Song H. S., Sawamura M., Ito T., Kawashimo K., Ukeda H. (2000b). Quantitative determination and characteristic flavour of Citrus junos (yuzu) peel oil. Flavour Frag. J. 15 245–250. [Google Scholar]
- Spadaro F., Circosta C., Costa R., Pizzimenti F., Palumbo D. R., Occhiuto F. (2012). Volatile fraction composition and biological activity of lemon oil (Citrus limon L. Burm.): comparative study of oils extracted from conventionally grown and biological fruits. J. Essent. Oil Res. 24 187–193. 10.1080/10412905.2012.659518 [CrossRef] [Google Scholar]
- Sun H., Ni H., Chen F., Jiang Z., Huang G., Yang Y. (2018). Effect of oxygen and heating on aromas of pummelo (Citrus maxima) essential oil. J. Essent. Oil Res. 30 92–104. 10.1080/10412905.2017.1420553 [CrossRef] [Google Scholar]
- Sun H., Ni H., Yang Y., Chen F., Cai H., Xiao A. (2014a). Sensory evaluation and gas chromatography-mass spectrometry (GC-MS) analysis of the volatile extracts of pummelo (Citrus maxima) peel. Flavour Frag. J. 29305–312. [Google Scholar]
- Sun H., Ni H., Yang Y., Wu L., Cai H.-N., Xiao A.-F., et al. (2014b). Investigation of sunlight-induced deterioration of aroma of pummelo (Citrus maxima) essential oil. J. Agric. Food Chem. 62 11818–11830. 10.1021/jf504294g [Abstract] [CrossRef] [Google Scholar]
- Tanaka C. (1954). Species Problem in Citrus, a Critical Study of Wild and Cultivated Units of Citrus, Based Upon Field Studies in Their Native Homes. Tokyo: Japanese Society for the Promotion of Science. [Google Scholar]
- Tao N., Jia L., Zhou H. (2014). Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 153 265–271. 10.1016/j.foodchem.2013.12.070 [Abstract] [CrossRef] [Google Scholar]
- Tomi F., Barzalona M., Casanova J., Luro F. (2008). Chemical variability of the leaf oil of 113 hybrids from Citrus clementina (Commun) x Citrus deliciosa (Willow Leaf). Flavour Frag. J. 23 152–163. 10.1002/ffj.1867 [CrossRef] [Google Scholar]
- Tomiyama K., Aoki H., Oikawa T., Sakurai K., Kashara Y., Kawakami Y. (2012). Characteristic volatile components of Japanese sour citrus fruits: Yuzu, Sudachi and Kabosu. Flavour Frag. J. 27 341–355. 10.1002/ffj.3104 [CrossRef] [Google Scholar]
- Tranchida P. Q., Bonaccorsi I., Dugo P., Mondello L., Dugo G. (2012). Analysis of Citrus essential oils: state of the art and future perspectives. A review. Flavour Frag. J. 27 98–123. 10.1002/ffj.2089 [CrossRef] [Google Scholar]
- Uysal B., Sozmen F., Aktas O., Oksal B. S., Kose E. O. (2011). Essential oil composition and antibacterial activity of the grapefruit (Citrus paradisi L.) peel essential oils obtained by solvent-free microwave extraction: comparison with hydrodistillation. Int. J. Food Sci. Tech. 46 1455–1461. 10.1111/j.1365-2621.2011.02640.x [CrossRef] [Google Scholar]
- Vekiari S. A., Protopapadakis E. E., Gianovits-Argyriadou N. (2004). Composition of the leaf and peel oils of Citrus medica L ’Diamante’ from Crete. J. Essent. Oil Res. 16 528–530. 10.1080/10412905.2004.9698789 [CrossRef] [Google Scholar]
- Vekiari S. A., Protopapadakis E. E., Papadopoulou P., Papanicolaou D., Panou C., Vamvakias M. (2002). Composition and seasonal variation of the essential oil from leaves and peel of a Cretan lemon variety. J. Agric. Food Chem. 50 147–153. 10.1021/jf001369a [Abstract] [CrossRef] [Google Scholar]
- Venturini N., Barboni T., Curk F., Costa J., Paolini J. (2014). Volatile and flavonoid composition of the peel of Citrus medica L. var. Corsican fruit for quality assessment of its liqueur. Food Technol. Biotechnol. 52 403–410. 10.17113/ftb.52.04.14.3717 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Verzera A., Russo C., la Rosa G., Bonaccorsi I., Cotroneo A. (2001). Influence of cultivar on lemon oil composition. J. Essent. Oil Res. 13 343–347. 10.1080/10412905.2001.9712228 [CrossRef] [Google Scholar]
- Verzera A., Trozzi A., Dugo G., Di Bella G., Cotroneo A. (2004). Biological lemon and sweet orange essential oil composition. Flavour Frag. J. 19 544–548. 10.1002/ffj.1348 [CrossRef] [Google Scholar]
- Verzera A., Trozzi A., Gazea F., Cicciarello G., Cotroneo A. (2003). Effects of rootstock on the composition of bergamot (Citrus bergamia Risso et Poiteau) essential oil. J. Agric. Food Chem. 51 206–210. 10.1021/jf0206872 [Abstract] [CrossRef] [Google Scholar]
- Verzera A., Trozzi A., Zappalá M., Condurso C., Cotroneo A. (2005). Essential oil composition of Citrus meyerii Y. Tan. and Citrus medica L. cv. Diamante and their lemon hybrids. J. Agric. Food Chem. 53 4890–4894. 10.1021/jf047879c [Abstract] [CrossRef] [Google Scholar]
- Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J. (2009). Chemical composition of mandarin (C. reticulata L.), grapefruit (C. paradisi L.), lemon (C. limon L.), and orange (C. sinensis L.) essential oils. J. Essent. Oil Bear. Plant 12 236–243. 10.1080/0972060X.2009.10643716 [CrossRef] [Google Scholar]
- Wang J., Liu Y. (2014). Comparative study on the volatiles in Citrus reticulata ’Dahongpao’ peel from the same plant and their antioxidant activities. J. Essent. Oil Bear. Plant 17 303–308. 10.1080/0972060X.2014.895162 [CrossRef] [Google Scholar]
- Wu G. A., Terol J., Ibanez V., López-García A., Pérez-Román E., Borredá C., et al. (2018). Genomics of the origin and evolution of Citrus. Nature 554:311. 10.1038/nature25447 [Abstract] [CrossRef] [Google Scholar]
- Wu Z., Li H., Yang Y., Zhan Y., Tu D. (2013). Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stage of maturity. Ind. Crops Prod. 46 311–316. 10.1016/j.indcrop.2013.02.015 [CrossRef] [Google Scholar]
- Yu L., Li X., Liu S., Xu G., Liang Y. (2009). Comparative analysis of volatile constituents in Citrus reticulata Blanco using GC-MS and alternative moving window factor analysis. J. Sep. Sci. 32 3457–3465. 10.1002/jssc.200900267 [Abstract] [CrossRef] [Google Scholar]
- Zakaria Z., Zakaria S., Ishak M. Z. M. (2010). Analysis of major fragrant compounds from Citrus grandis flowers extracts. Sains Malays. 39 565–569. [Google Scholar]
- Zhang H., Xie Y., Liu C., Chen S., Hu S., Xie Z., et al. (2017). Comprehensive comparative analysis of volatile compounds in Citrus fruits of different species. Food Chem. 230 316–326. 10.1016/j.foodchem.2017.03.040 [Abstract] [CrossRef] [Google Scholar]
Articles from Frontiers in Plant Science are provided here courtesy of Frontiers Media SA
Full text links
Read article at publisher's site: https://doi.org/10.3389/fpls.2019.00012
Read article for free, from open access legal sources, via Unpaywall:
https://www.frontiersin.org/articles/10.3389/fpls.2019.00012/pdf
Citations & impact
Impact metrics
Article citations
In Situ Antimicrobial Properties of Sabinene Hydrate, a Secondary Plant Metabolite.
Molecules, 29(17):4252, 07 Sep 2024
Cited by: 0 articles | PMID: 39275100 | PMCID: PMC11396819
A cleaner and eco-friendly approach to simultaneous extraction and characterization of essential oil and pectin from Assam lemon peel and its application for energy generation through TENG devices.
Heliyon, 10(15):e32999, 18 Jul 2024
Cited by: 0 articles | PMID: 39165985 | PMCID: PMC11334901
Preparation of Perilla frutescens L. essential oil hydrogel beads and preservation application research in strawberry.
Heliyon, 10(13):e33689, 26 Jun 2024
Cited by: 0 articles | PMID: 39027485 | PMCID: PMC11255502
Analysis of the Volatile Components in Different Parts of Three Species of the Genus Amomum via Combined HS-SPME-GC-TOF-MS and Multivariate Statistical Analysis.
Foods, 13(12):1925, 18 Jun 2024
Cited by: 0 articles | PMID: 38928865
A Female-Biased Chemosensory Protein PxutCSP19 in the Antennae of Papilio xuthus Tuned to Host Volatiles and Insecticides.
Insects, 15(7):501, 05 Jul 2024
Cited by: 0 articles | PMID: 39057234 | PMCID: PMC11276849
Go to all (82) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Biological Activities and Safety of Citrus spp. Essential Oils.
Int J Mol Sci, 19(7):E1966, 05 Jul 2018
Cited by: 83 articles | PMID: 29976894 | PMCID: PMC6073409
Review Free full text in Europe PMC
Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya.
J Agric Food Chem, 53(25):9790-9794, 01 Dec 2005
Cited by: 31 articles | PMID: 16332132
Determination of Volatile Flavour Profiles of Citrus spp. Fruits by SDE-GC-MS and Enantiomeric Composition of Chiral Compounds by MDGC-MS.
Phytochem Anal, 28(5):392-403, 25 Apr 2017
Cited by: 6 articles | PMID: 28444796
Comparison of Resistance to Asiatic Citrus Canker Among Different Genotypes of Citrus in a Long-Term Canker-Resistance Field Screening Experiment in Brazil.
Plant Dis, 99(2):207-218, 01 Feb 2015
Cited by: 8 articles | PMID: 30699570
Funding
Funders who supported this work.





