Abstract
Objective
To examine the associations of physical activity, Alzheimer disease (AD), and other brain pathologies and cognition in older adults.Methods
We studied 454 brain autopsies from decedents in a clinical-pathologic cohort study. Nineteen cognitive tests were summarized in a global cognitive score. Total daily physical activity summarized continuous multiday recordings of activity during everyday living in the community setting. A global motor ability score summarized 10 supervised motor performance tests. A series of regression analyses were used to examine associations of physical activity, AD, and other brain pathologies with global cognition proximate to death controlling for age, sex, education, and motor abilities.Results
Higher levels of total daily activity (estimate 0.148, 95% confidence interval 0.053-0.244, SE 0.049, p = 0.003) and better motor abilities (estimate 0.283, 95% confidence interval, 0.175-0.390, SE 0.055, p < 0.001) were independently associated with better cognition. These independent associations remained significant when terms for AD and other pathologies were added as well as in sensitivity analyses excluding cases with poor cognition or dementia. Adding interaction terms, the associations of total daily activity and motor abilities with cognition did not vary in individuals with and without dementia. The associations of AD and other pathologies with cognition did not vary with the levels of total daily activity or motor abilities.Conclusions
Physical activity in older adults may provide cognitive reserve to maintain function independent of the accumulation of diverse brain pathologies. Further studies are needed to identify the molecular mechanisms underlying this potential reserve and to ensure the causal effects of physical activity.Free full text

Physical activity, common brain pathologies, and cognition in community-dwelling older adults
Associated Data
Abstract
Objective
To examine the associations of physical activity, Alzheimer disease (AD), and other brain pathologies and cognition in older adults.
Methods
We studied 454 brain autopsies from decedents in a clinical-pathologic cohort study. Nineteen cognitive tests were summarized in a global cognitive score. Total daily physical activity summarized continuous multiday recordings of activity during everyday living in the community setting. A global motor ability score summarized 10 supervised motor performance tests. A series of regression analyses were used to examine associations of physical activity, AD, and other brain pathologies with global cognition proximate to death controlling for age, sex, education, and motor abilities.
Results
Higher levels of total daily activity (estimate 0.148, 95% confidence interval 0.053–0.244, SE 0.049, p = 0.003) and better motor abilities (estimate 0.283, 95% confidence interval, 0.175–0.390, SE 0.055, p < 0.001) were independently associated with better cognition. These independent associations remained significant when terms for AD and other pathologies were added as well as in sensitivity analyses excluding cases with poor cognition or dementia. Adding interaction terms, the associations of total daily activity and motor abilities with cognition did not vary in individuals with and without dementia. The associations of AD and other pathologies with cognition did not vary with the levels of total daily activity or motor abilities.
Conclusions
Physical activity in older adults may provide cognitive reserve to maintain function independent of the accumulation of diverse brain pathologies. Further studies are needed to identify the molecular mechanisms underlying this potential reserve and to ensure the causal effects of physical activity.
Lifestyle factors such as physical activity are being intensely studied as a means to maintain brain health and reduce Alzheimer disease (AD) dementia in our aging population.1 We previously reported that a higher level of total daily physical activity was related to lower risk of AD dementia and a slower rate of cognitive decline in older adults.2 Yet, the mechanisms underlying these associations are poorly understood.
The concept of cognitive reserve or resilience refers to an individual's ability to maintain function despite damage from injury or the accumulation of brain pathologies. Several mechanisms may underlie behaviors that provide reserve.3 Some factors such as BDNF gene expression may provide reserve by modifying the association of AD pathology with cognitive function.4,5 We have also found that AD and other brain pathologies are associated with motor phenotypes including physical activity.6,–8 Furthermore, we found that physical activity modified the relation of white matter changes to motor function in older persons.9 This raises the possibility that a more active lifestyle may modify the association of brain pathologies with cognition. By contrast, late-life cognitive activities are independently associated with better cognition and seem to bypass indices of AD and other brain pathologies.10
To examine the associations of physical activity, AD, and other brain pathologies and cognition proximate to death in older adults, we used data from participants in the Rush Memory and Aging Project (MAP), a community-based cohort study. In this study, older adults agree to annual detailed clinical examination and brain donation at the time of death.11
Methods
Participants
Participants were individuals from the MAP, an ongoing cohort study of older adults agreeing to annual clinical assessments and brain donation at time of death.11 MAP began in 1997 and actigraphy data collection was begun in 2005. There were 454 decedents with valid actigraphic recordings and cognitive testing proximate to death with completed autopsy at the time of these analyses.
Assessment of cognition and diagnoses
Each participant underwent annual medical history and examination with cognitive testing. Trained technicians administered 21 cognitive tests. Composite global cognition was derived from z scores, using the baseline mean and SD as previously published.11 Mini-Mental State Examination was used to describe the cohort but was not included in the global cognition score. The diagnosis of dementia and its causes was based on guidelines of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association.11
Assessment of the quantity of total daily physical activity
Total daily physical activity including all exercise and nonexercise activities was measured continuously 24 h/d for up to 10 days with an omnidirectional accelerometer worn on the nondominant wrist (Actical; Mini Mitter, Bend, OR). Total daily physical activity was the average sum of all daily activity counts for each 15-second epoch for full days of data.2 The first 7 days of complete data were analyzed in this study.
Assessment of motor abilities and other covariates
A global motor score summarized 10 motor abilites.12 Self-report date of birth, sex, and years of education were recorded at the study entry. Age was computed from self-report date of birth and date of actigraphic testing.
Assessment of postmortem histopathology postmortem indices
Brain removal, tissue sectioning, and preservation, and a uniform gross and microscopic examination with quantification of postmortem indices followed a standard protocol, which was collected by staff blinded to any clinical data.13
Alzheimer pathology
Bielschowsky silver stain was used to visualize neuritic plaques, diffuse plaques, and neurofibrillary tangles in the frontal, temporal, parietal, and entorhinal cortex, and the hippocampus, as previously described.14 A global measure of AD pathology for each participant used in these analyses was constructed from summary scores of the 3 AD markers from all 5 regions.14 N-terminus–directed monoclonal antibody (1:1,000, 10D5; Elan Pharmaceuticals, Dublin, Ireland) was used to determine β-amyloid burden in each of the 5 regions. The area occupied by β-amyloid–immunoreactive pixels in each region was measured and a summary measure for β-amyloid burden across all regions was calculated as previously described.15 Computer-assisted sampling was used to quantify the density of tau immunoreactive tangles stained with an anti-paired helical filament tau antibody clone AT8 (1:2,000; Thermo Fisher Scientific, Rockford, IL). Tangle density/mm2 in each region was standardized and the mean tangle density for all 5 regions was used in these analyses.15
Nigral neuronal loss
Dissection of diagnostic blocks included a hemisection of midbrain that included substantia nigra. We assessed nigral neuronal loss in the substantia nigra in the midbrain near or at the exit of the third nerve using a semiquantitative scale (0–3) as previously described.16
Lewy body disease pathology
As previously described, Lewy bodies were assessed in 6 regions (substantia nigra, anterior cingulate cortex, entorhinal cortex, midfrontal cortex, superior or middle temporal cortex, inferior parietal cortex) using a monoclonal phosphorylated antibody to α-synuclein (1:20,000; Wako Chemical, Richmond, VA) with alkaline phosphatase as the chromogen. Lewy bodies were treated as present or absent.16
TAR DNA-binding protein 43
Immunohistochemistry was performed on sections of the amygdala, hippocampus (CA1 and dentate), and midfrontal, midtemporal, and entorhinal cortices using a rat phosphorylated monoclonal TAR5P-1D3 (pS409/410, 1:100; Ascenion, Munich, Germany) TAR DNA-binding protein 43 (TDP-43) antibody. Three stages of TDP-43 distribution were recognized as described previously.17
Hippocampal sclerosis
Hippocampal sclerosis was evaluated unilaterally in a coronal section of the mid hippocampus, and graded as absent or present based on severe neuronal loss and gliosis in CA1 and/or subiculum as previously described.18
Macroscopic cerebral infarcts
We reviewed 1-cm slabs and recorded the age, volume (in cubic millimeters), side, and location of all cerebral infarcts visible to the naked eye as previously reported.19 All suspected macroscopic infarcts were confirmed microscopically. Only chronic macroinfarcts were included in these analyses and coded as present or absent.
Cerebral atherosclerosis
Atherosclerosis was assessed via gross inspection of the anterior, middle, and posterior cerebral arteries and their proximal branches at the circle of Willis using a semiquantitative scale of none (0) to severe (5) indicating near total or total involvement of visualized arteries as previously described.11
Microscopic cerebral infarcts
Microscopic infarcts were defined as any infarct seen only by microscopic examination of hematoxylin & eosin–stained 6-μm sections. The following regions were examined in all cases: middle frontal cortex, middle temporal cortex, anterior cingulate cortex, inferior parietal cortex, entorhinal cortex, hippocampus, anterior basal ganglia, anterior thalamus, and hemisection of midbrain including substantia nigra. Microinfarcts were treated as present or absent as previously described.20
Cerebral arteriolosclerosis
Arteriolosclerosis describes the histologic changes frequently found in the deep penetrating small vessels of the brain in aging. The vessels of the anterior basal ganglia were assessed using a semiquantitative grading system from 0 (none) to 4 (severe) indicating more than twice the normal wall thickness, as previously described.13
Cerebral amyloid angiopathy
Cerebral amyloid angiopathy (CAA) was assessed for meningeal and parenchymal vessels in 5 regions (midfrontal, middle temporal, angular, and calcarine cortices). A semiquantitative scale (0–4) was used to quantify vascular deposition of β-amyloid in each region. The maximum score between the meningeal and parenchymal amyloid angiopathy scores was used as the CAA pathology score for that region. Scores were then averaged across the 5 regions. We used this overall average score to develop a 4-level rating scale, based on the data distribution and pathology severity at the different levels as determined by the neuropathologist.20
Analyses
Bivariate correlations were used to examine the relationship of total daily physical activity with other covariates; a t test was used to compare men and women. We used a series of linear regression models to examine the association of total daily physical activity with level of cognition before and after adjusting for indices of AD and related pathologies. Since higher levels of physical activity may be related to better motor abilities, all models included a previously validated summary measure of 10 motor performances assessed during supervised annual motor testing.12 All models also controlled for age, sex, and education. Then we added interaction terms to determine whether the associations of postmortem indices and cognition varied with the level of total daily physical activity or motor abilities. Finally, we repeated regression analyses including demographics, postmortem indices, total daily physical activity, and motor abilities replacing cognition with dementia status proximate to death.
Standard protocol approvals, registrations, and patient consents
The study was approved by the institutional review board of Rush University Medical Center. Written informed consent was obtained from all study participants as was an Anatomical Gift Act for organ donation.
Data availability
All data included in these analyses are available via the Rush Alzheimer's Disease Center Research Resource Sharing Hub, which can be found at radc.rush.edu. It has descriptions of the studies and variables and a dynamic query function to aid searches for data and biospecimens for selected data. There is a login, after which any qualified investigator can submit requests for deidentified data.
Results
Characteristics of study participants
Clinical characteristics
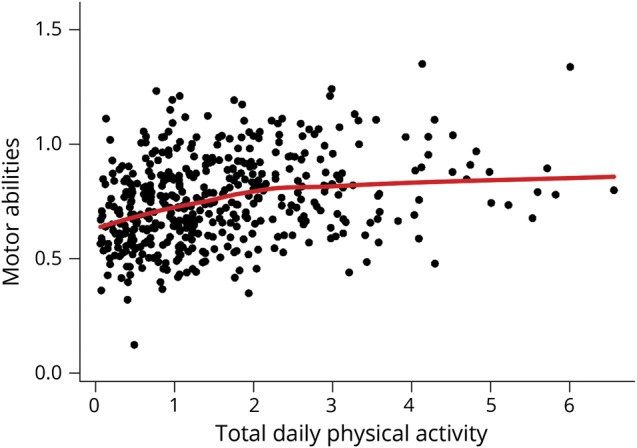
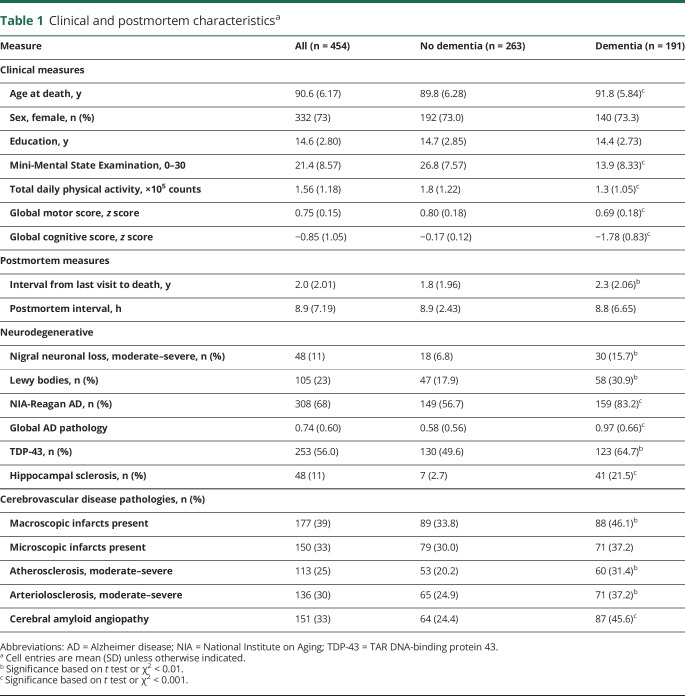
There were 454 participants. Their clinical and postmortem characteristics are included in table 1. At their last visit, about 2 years before death when quantitative physical activity metrics were obtained, total daily physical activity levels ranged from 0.06 × 105 counts/d to 6.56 × 105 counts/d (mean = 1.55 × 105 counts/d, SD = 1.16 × 105 counts/d) and global cognition score ranged from −3.82 to 1.15 (mean = −0.85, SD = 1.05). Total daily physical activity was related to age (r = −0.18, p < 0.001) and education (r = −0.11, p = 0.016). Total daily physical activity was similar in women and men (women 1.60 × 105 counts/d [SD = 1.22 × 105 counts/d] vs men 1.46 × 105 counts/d [SD = 1.05 × 105 counts/d] [t452 = 1.10, p = 0.271]). There was a modest association of total daily physical activity with motor abilities (r = 0.29, p < 0.001); a scatterplot of their interrelationship illustrates the modest correlation and that high daily activity was observed in individuals with a range of motor abilities (figure 1).
Table 1
Clinical and postmortem characteristicsa
Postmortem indices
On average, participants had 3 different brain pathologies. One or more pathologies were observed in nearly all cases (95.6%): 0: n = 16 (3.5%); 1: n = 72 (12.6%); 2: n = 81 (17.8%); 3: n = 104 (22.9%); 4: n = 93 (20.5%); 5: n = 55 (12.1%); and 6–9: n = 48 (13.7%).
Physical activity, AD pathology, and cognition
Higher levels of physical activity and motor abilities were associated with better cognition (table 2, models A and B) and both were independently associated with cognition when included together in a single model (table 2, model C).
Table 2
Association of total daily physical activity, motor abilities, and AD pathology with global cognition proximate to deatha
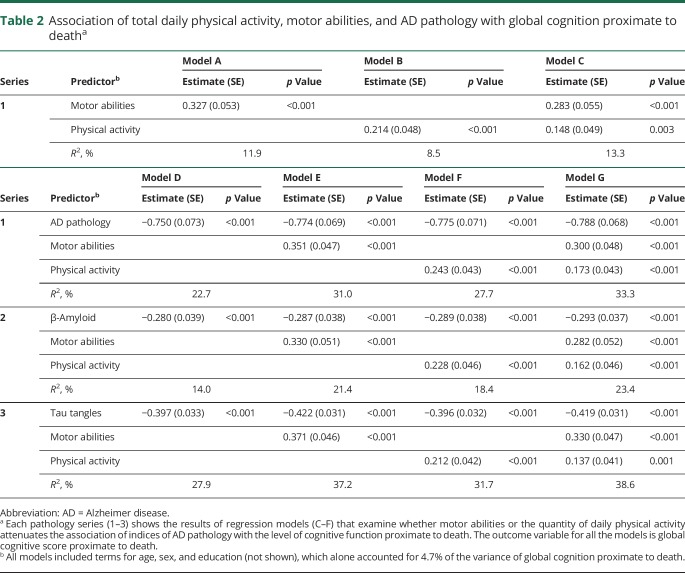
A higher level of AD pathology alone was associated with poorer cognition proximate to death indicated by the negative β estimate (table 2, model D). When a term for AD pathology was added to the model with terms for both physical activity and motor abilities, the associations of higher levels of both physical activity and motor abilities with higher levels of cognition remained significant (table 2, models E–G). In these same joint models, the association of AD pathology and cognition was unaffected by the inclusion of terms for physical activity and motor abilities in the same model (table 2, models E–G).
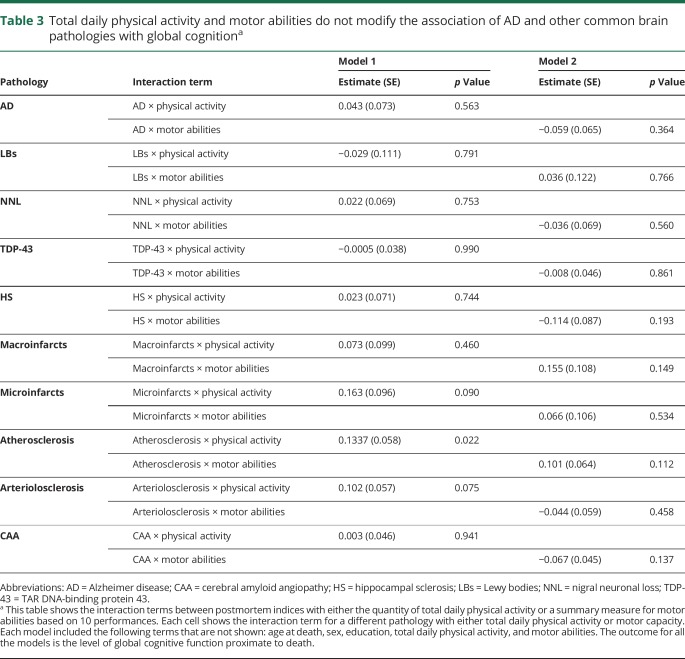
Similar findings were noted when we examined the burden of β-amyloid and the density of tau tangles in place of the summary measure for AD pathology (table 2, series 2 and 3). In further analyses after adding interaction terms, the association of the summary measure of AD pathology and cognitive function did not vary with the level of physical activity or motor abilities (table 3).
Table 3
Total daily physical activity and motor abilities do not modify the association of AD and other common brain pathologies with global cognitiona
We repeated these analyses for each of the 5 cognitive abilities, which were used to construct global cognitive function. Both total daily physical activity and motor abilities were independently associated with each of the 5 cognitive abilities with a term for AD pathology included in the model: semantic memory (motor abilities: estimate 0.295, SE 0.058, p < 0.001; total daily activity: estimate 0.151, SE 0.051, p = 0.003); episodic memory (motor abilities: estimate 0.247, SE 0.058, p < 0.001; total daily activity: estimate 0.204, SE 0.052, p < 0.001); working memory (motor abilities: estimate 0.240, SE 0.053, p < 0.001; total daily activity: estimate 0.163, SE 0.047, p < 0.001); perceptual speed (motor abilities: estimate 0.385, SE 0.045, p < 0.001; total daily activity: estimate 0.100, SE 0.040, p = 0.013); visuospatial abilities (motor abilities: estimate 0.295, SE 0.051, p < 0.001; total daily activity: estimate 0.141, SE 0.046, p = 0.002).
Physical activity, other common brain pathologies, and cognition
Other common brain pathologies are related to poorer cognitive function in older adults. The associations of higher levels of both physical activity and motor abilities with higher levels of cognition were unchanged in models that included indices of other common brain pathologies (table 4, models 1–9).
Table 4
Total daily physical activity and motor abilities are independently associated with global cognition when controlling for other common brain pathologiesa

In further analyses, we added interaction terms between physical activity and motor abilities and indices of brain pathologies. Overall, with the exception of atherosclerosis, the association of the other 8 indices of pathology with cognition did not vary with the levels of physical activity or with level of motor abilities (table 3).
As noted above, about 85% of individuals in this cohort showed evidence of 2 or more brain pathologies. Therefore, we examined a regression model that included all the postmortem indices together with and without terms for physical activity and motor abilities proximate to death with global cognition as the outcome. In a single model that included terms for all postmortem indices together, total daily physical activity and motor abilities remained independently associated with global cognition and accounted for an additional 8% of the variance of cognition proximate to death (table 5, column B). In this joint model, AD pathology, nigral neuronal loss, hippocampal sclerosis, and CAA were also independently associated with global cognition. The point estimates for the associations of macroinfarcts, microinfarcts, and arteriolosclerosis with cognition were attenuated by 58% to 68% with terms for motor function in the model as compared to the model with only indices of pathology alone (table 5, column A vs B). Of note, the presence of macroinfarcts was no longer associated with cognitive function.
Table 5
Associations of 10 common age-related brain pathologies and global cognition with and without total daily physical activity and motor abilities in a single modela
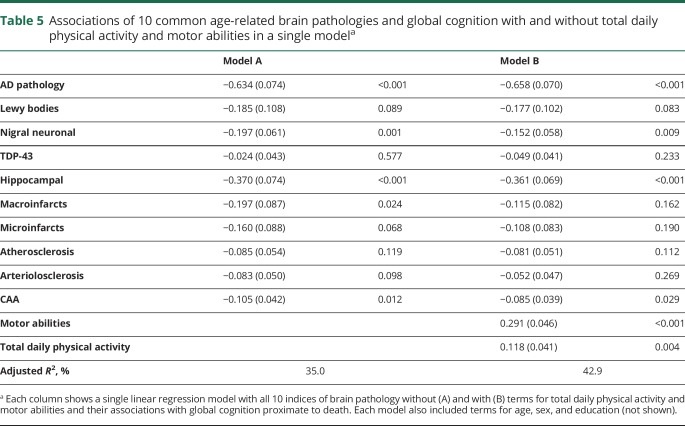
Figure 2B illustrates the strong association of total daily activity with residual cognition proximate to death when controlling for demographics (red line) is not significantly attenuated when adding terms controlling for 10 postmortem indices (blue line). Figure 2A illustrates that similar to total daily activity, the association of motor abilities with residual cognition proximate to death (red line) is not attenuated when adding terms for postmortem indices (blue).
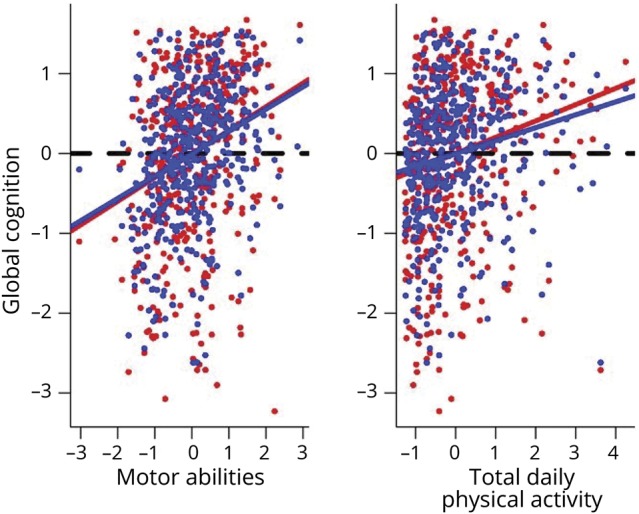
(A) Illustration of the association of residual cognition with motor abilities controlling for age and sex (red line average regression line, and individual data points red circles) and after controlling for demographics and brain pathologies (blue line average regression line, and individual data points blue circles). Dashed line shows a line with a slope = 0 if there was no association between 2 predictors. (B) Model generated figure showing residual cognition and total daily physical activity after controlling for age and sex (red line average regression line, and individual values red circles) and after adding indices for 10 brain pathologies (blue line average regression line, and individual values blue circles). To highlight the association between motor function and cognition in both figures, a dashed line shows a line with a slope = 0 as would be seen if there was no association between 2 predictors. Blue lines in both figures show a significant association between higher total daily physical activity and higher motor abilities even after controlling for demographics and indices of 10 brain pathologies.
The association between total daily activity and cognition may have been observed because higher total daily activity may lead to better cognition or because poorer cognition, i.e., dementia, might lead to reduced total daily activity. We examined a series of sensitivity analyses to inform on these possibilities.
First, we repeated model B (table 5) sequentially excluding cases with the lowest 5%, 10%, and 15% of global cognition. In all 3 models, total daily activity and motor abilities remained independently associated with cognition, suggesting that poorer cognition did not account for the association of total daily activity and motor abilities with cognition proximate to death: lowest 5% of global cognition: motor abilities (estimate 0.168, SE 0.035, p < 0.001) and total daily activity (estimate 0.165, SE 0.031, p < 0.001); lowest 10% of global cognition: motor abilities (estimate 0.143, SE 0.034, p < 0.001) and total daily activity (estimate 0.145, SE 0.030, p < 0.001); lowest 15% of global cognition: motor abilities (estimate 0.103, SE 0.033, p = 0.002) and total daily activity (estimate 0.131, SE 0.028, p < 0.001).
To further examine whether poorer cognition accounts for the association of total daily physical activity with cognition, we repeated model B (table 5) examining individuals with and without dementia in separate models. One would expect the regression coefficient to be much larger in the model for individuals with dementia as compared to individuals without dementia, if poor cognition drives the association of total daily physical activity with cognition. In the model with only individuals without dementia (n = 274), higher levels of total daily physical activity (estimate 0.069, SE 0.032, p = 0.030) and motor abilities (estimate 0.118, SE 0.037, p = 0.002) remained independently associated with better cognition. In contrast, in the model with only individuals with dementia (n = 172), total daily physical activity was no longer associated with cognition (estimate 0.033, SE 0.069, p = 0.630), but motor abilities remained independently associated with better cognition (estimate 0.200, SE 0.072, p = 0.006). These results suggest that poorer cognition did not account for the association of total daily activity and motor abilities with cognition proximate to death.
As both estimates in stratified models were reduced compared to the primary model (table 5, model B), we were concerned that the differences observed regarding individuals with and without dementia was attributable to reduced sample sizes in the stratified models. If our findings are attributable to dementia leading to reduced total daily physical activity, there might be an interaction between dementia status and the association between total daily activity and cognitive function. Interaction terms added to model B (table 5) were not significant (dementia × total daily activity: estimate −0.039, SE 0.064, p = 0.543; dementia × motor abilities: estimate 0.079, SE 0.069, p = 0.249). Thus, although clinical characteristics including cognitive and motor function differ in individuals with and without dementia (table 1), the associations between total daily activity and motor abilities with cognition did not vary with cognitive status.
To contextualize our findings with clinical categories, we repeated the model replacing global cognition with the diagnosis of dementia proximate to death. A 1-SD increase of total daily activity or motor capacity was associated with a reduction in the risk of dementia proximate to death (total daily activity 31%, motor abilities 55%). These associations were additive as the association of total daily physical activity with cognition did not vary with motor abilities (total daily physical activity × motor abilities: estimate −0.008, SE 0.040, p = 0.837).
Discussion
In a group of more than 450 cognitively well-characterized community-dwelling older adults, a more active lifestyle and better motor abilities proximate to death were independently associated with better cognitive function and reduced odds of dementia when controlling for AD and 9 other common age-related brain pathologies. Moreover, there was also no evidence that a more active lifestyle or better motor abilities modified the associations of these brain pathologies with cognitive function proximate to death. Together, these data suggest that the cognitive reserve associated with physical activities and motor abilities is unrelated to the presence of common brain pathologies and that the molecular mechanisms that underlie this reserve remain to be identified.
Many previous studies, including a prior study in this cohort, suggest that higher levels of physical activity may slow the rate of cognitive decline and reduce the risk of AD dementia, but the mechanisms underlying this potential benefit are unknown.21,–23 Some preclinical animal studies suggest that higher levels of physical activity may prevent the accumulation and progression of AD pathology.24,–27 Moreover, antemortem brain imaging studies in older adults suggest that higher levels of physical activity are associated with lower levels of infarcts and better white matter brain integrity.28 In addition, several clinical studies using PET of cerebral β-amyloid deposition suggest that higher levels of β-amyloid burden are associated with poorer motor function.29,30 However, in the handful of studies that have focused specifically on physical activity, there has not been a consistent relationship with level of physical activity and brain imaging levels of β-amyloid or CSF biomarkers of AD.31,–33 The paucity of human data makes it difficult to explicate the pathologic mechanisms underlying the association of a more active lifestyle with better cognition in older adults.
The current results provide evidence that both physical activity and motor abilities are associated with better cognition and reduced odds of dementia. These associations were independent and additive. Physical activity is a volitional behavior controlled by dissociable neural systems that begin in the brain and extend throughout the entire CNS and via the peripheral nervous system to activate muscle contraction in the periphery. Damage to any portion of the distributed motor system has potential to degrade motor abilities underlying movement while leaving motor decision-making regions necessary for the initiation of movement intact. Thus, an individual with poor motor abilities may nonetheless have higher levels of total daily physical activity compared to an individual with good motor abilities, who might elect to sit in a chair for the entire day. This may explain why the quantity of daily physical activity is only modestly related to motor abilities and not everyone with a high level of physical activity necessarily has good motor abilities.
Physical movement is a volitional activity underscoring the importance of trying to disentangle the cause and effect when examining its interrelationship with global cognition. The cognitive benefits of a more active lifestyle may be small, derive from diverse late-life activities, and accrue over many years. Thus, assessing the benefits of a more active lifestyle may be beyond the resolution of a randomized clinical trial. Thus, inferences from well-designed epidemiologic studies may be crucial in assessing the benefits of a more active lifestyle. Thus, no single study is likely to be able to provide unambiguous support for the efficacy of a more active lifestyle in brain health. Nonetheless, while lower cognition may lead to reduced total daily activity, a more active lifestyle and better motor abilities remained independently associated with cognition after sensitivity analyses in which we excluded cases with poor cognition and examined individuals with and without dementia separately. In addition, the association of total daily activity with cognition did not vary in individuals with and without dementia. Thus, while our cross-sectional results must be interpreted with caution, these data provide support for the idea that strategies or behaviors that lead to a more active lifestyle and better motor abilities may provide cognitive reserve, which may maintain cognitive function in older adults despite the accumulation of AD and other common brain pathologies. Further work is needed to clarify to what extent the risk factors and the types and duration of interventions to increase total daily physical activity and motor abilities are distinct and can be disentangled.
There are several mechanisms that can underlie cognitive resilience or reserve and nosology of these and related terms is evolving.34 For example, in prior work, we have shown that some instances of cognitive resilience may be attributable to factors that modify AD and related pathologies through an interaction with these pathologies either directly or indirectly.4,5,35,–37 However, other factors may provide reserve through unidentified molecular mechanisms that do not have a known pathologic footprint or that have a more direct association with biological indices such as neuronal or structural elements that promote cognitive resilience.10,38,–41 Recent work using systems biology has identified molecular networks whose genes are associated with cognitive decline but are not explained by known pathologies and which may provide resilience.42
The current analyses found that the association of physical activity motor abilities with cognition is not explained by AD and other common brain pathologies, suggesting these factors may provide cognitive reserve in older adults. The association of physical activity and motor abilities with cognition was not accounted for by any of the brain pathologies we examined. Moreover, we did not find evidence that higher levels of physical activity or better motor abilities modify the association of indices of brain pathologies with cognition. Nonetheless, these findings suggest that a more active lifestyle may provide cognitive reserve or resilience for older adults. These findings may have important public health implications because they suggest that resilience factors such as more cognitive activities or physical activity might mitigate late-life cognitive impairment even in the absence of effective therapies to reduce AD and other common brain pathologies. However, much remains unknown about the optimal behaviors, molecular mechanisms, and sites within and outside the brain through which a more active lifestyle might provide cognitive reserve in old age. The current postmortem study focused on brain substrate; antemortem studies using brain imaging and other clinical proxies will be needed to determine the extent to which resilient mechanisms affect brain substrate and cognitive processing and efficiency.34 Work filling these knowledge gaps is needed to facilitate interventions that modify lifestyles as a means to maintain cognition and brain health in older adults even in the absence of effective therapies to reduce AD and other common brain pathologies.
Cerebrovascular and neurodegenerative pathologies did not affect the association of physical activity and motor abilities with cognitive function. However, an unexpected finding was that the well-known association of higher levels of several cerebrovascular disease pathologies with poorer cognition, most notably macroinfarcts, were attenuated and no longer significant when terms for motor function were included in the model.43 Physical activity has been reported to facilitate vascular plasticity in preclinical studies, but evidence showing similar effects on cognitive function in humans has been elusive.44 Further work is needed to determine the mechanisms that might account for these findings, which may offer novel interventions to mitigate the harmful effects of cerebrovascular diseases and pathologies on cognition in older adults.
There are several limitations to this study. These data were cross-sectional and concerns about reverse causality underscore that causal inferences must be drawn with caution. The physical activity data analyzed are from an observational study and were not from a physical-activity intervention study. The data extracted were derived from multiday recordings during daily living, providing a quantitative metric of how active an individual's lifestyle was during these recordings. These results support the need for further studies to determine the degree to which physical interventions in late life can provide the cognitive reserve suggested by this study. Moreover, the study does not have prior data on how active these individuals were over the course of their lifespan. Thus, it is unknown to what extent the metrics of physical activity in late life reflect the accumulation of higher physical activity over many years or might provide reserve during which they were recorded. Thus, these results underscore the gaps in our knowledge about the utility of chronic physical activity vs late-life interventions that increase physical activity. Other brain pathologies such as white matter integrity were not examined in these analyses. Moreover, since the motor system extends beyond the brain, further work will be needed to show that the cognitive reserve does not derive from the effects of physical activity in other key motor regions outside the brain.45,46 Finally, the activity monitors used in this study do not differentiate between different physical activities, so further studies are necessary to determine whether the cognitive reserve provided by physical activity derives from any increases in movement or whether particular types of activity drive this reserve, i.e., steps vs arm movements, and removal of the device cannot always be distinguished from periods of no activity.
The main strength of this study is that we obtained objective measures of both physical activity and motor abilities from a relatively large number of cognitively well-characterized older persons who may be more representative of the cognitive and physical function spectrum observed in the community setting. In addition, all cases were evaluated with a uniform clinical evaluation and structured postmortem brain assessment, providing a unique combination of novel data.
Acknowledgment
The authors thank all the participants in the Rush Memory and Aging Project. They also thank the staff of the Rush Alzheimer's Disease Center. More information regarding obtaining (MAP) data for research use can be found at the RADC Research Resource Sharing Hub (radc.rush.edu).
Glossary
| AD | Alzheimer disease |
| CAA | cerebral amyloid angiopathy |
| MAP | Memory and Aging Project |
| TDP-43 | TAR DNA-binding protein 43 |
Footnotes
Editorial, page 362
Podcast: NPub.org/9rs1v3
Author contributions
Drafting/revising manuscript for content: A.S.B., L.Y., R.S.W., A.L., R.J.D., C.G., S.E.L., J.A.S., D.A.B. Study concept or design: A.S.B., L.Y., R.S.W., D.A.B. Analyses or interpretation of the data: A.S.B., L.Y., R.S.W., A.L., R.J.D., C.G., S.E.L., J.A.S., D.A.B. Acquisition of data: A.S.B., D.A.B. Statistical analysis: A.S.B., S.E.L., L.Y. Study supervision or coordination: A.S.B., J.A.S., D.A.B. Obtaining funding: A.S.B., D.A.B.
Study funding
This work was supported by NIH (R01AG17917, R01NS78009, R01AG56352 R01AG052488), the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
Disclosure
A. Buchman, L. Yu, R. Wilson, A. Lim, R. Dawe, C. Gaiteri, and S. Leurgans report no disclosures relevant to the manuscript. J. Schneider reports disclosures for this manuscript including serving on the scientific advisory board for Grifols, Lilly, and Genentech, and as a consultant for the Michael J. Fox Foundation and the National Hockey League. D. Bennett reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0000000000006954
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6396972?pdf=render
Citations & impact
Impact metrics
Article citations
Dissecting the causal relationship between moderate to vigorous physical activity levels and cognitive performance: a bidirectional two-sample Mendelian randomization study.
Front Psychol, 15:1368241, 06 Sep 2024
Cited by: 0 articles | PMID: 39309156 | PMCID: PMC11412864
The efficacy of a mobile-based multidomain program on cognitive functioning of residents in assisted living facilities.
Public Health Pract (Oxf), 8:100528, 04 Jul 2024
Cited by: 0 articles | PMID: 39081699 | PMCID: PMC11286991
Accelerometer-based and self-reported physical activity and sedentary time and their relationships with the P300 in a Go/No-Go task in older adults.
Brain Cogn, 178:106168, 15 May 2024
Cited by: 0 articles | PMID: 38754283
Cognitive and Cerebrospinal Fluid Alzheimer's Disease-related Biomarker Trajectories in Older Surgical Patients and Matched Nonsurgical Controls.
Anesthesiology, 140(5):963-978, 01 May 2024
Cited by: 0 articles | PMID: 38324729
2024 Alzheimer's disease facts and figures.
Alzheimers Dement, 20(5):3708-3821, 30 Apr 2024
Cited by: 59 articles | PMID: 38689398 | PMCID: PMC11095490
Go to all (42) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
MIND Diet, Common Brain Pathologies, and Cognition in Community-Dwelling Older Adults.
J Alzheimers Dis, 83(2):683-692, 01 Jan 2021
Cited by: 25 articles | PMID: 34334393 | PMCID: PMC8480203
Healthy Lifestyle and Cognition in Older Adults With Common Neuropathologies of Dementia.
JAMA Neurol, 81(3):233-239, 01 Mar 2024
Cited by: 9 articles | PMID: 38315471
Total daily physical activity, brain pathologies, and parkinsonism in older adults.
PLoS One, 15(4):e0232404, 29 Apr 2020
Cited by: 8 articles | PMID: 32348372 | PMCID: PMC7190120
The relationship of Alzheimer-type pathology to dementia in Parkinson's disease.
J Neural Transm Suppl, 49:23-31, 01 Jan 1997
Cited by: 3 articles | PMID: 9266411
Review
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: R01 AG052488
Grant ID: R01 AG017917
NINDS NIH HHS (1)
Grant ID: R01 NS078009