Abstract
Purpose
To assess the safety of the superoxide dismutase mimetic GC4419 in combination with radiation and concurrent cisplatin for patients with oral cavity or oropharyngeal cancer (OCC) and to assess the potential of GC4419 to reduce severe oral mucositis (OM).Patients and methods
Patients with locally advanced OCC treated with definitive or postoperative intensity modulated radiation therapy (IMRT) plus cisplatin received GC4419 by 60-minute intravenous infusion, ending <60 minutes before IMRT, Monday through Friday for 3 to 7 weeks, in a dose and duration escalation study. Oral mucositis was assessed twice weekly during and weekly after IMRT.Results
A total of 46 patients received GC4419 in 11 separate dosing and duration cohorts: dose escalation occurred in 5 cohorts receiving 15 to 112 mg/d over 3 weeks (n=20), duration escalation in 3 cohorts receiving 112 mg/d over 4 to 6 weeks (n=12), and then 3 additional cohorts receiving 30 or 90 mg/d over 6 to 7 weeks (n=14). A maximum tolerated dose was not reached. One dose-limiting toxicity (grade 3 gastroenteritis and vomiting with hyponatremia) occurred in each of 2 separate cohorts at 112 mg. Nausea/vomiting and facial paresthesia during infusion seemed to be GC4419 dose-related. Severe OM occurred through 60 Gy in 4 of 14 patients (29%) dosed for 6 to 7 weeks, with median duration of only 2.5 days.Conclusions
The safety of GC4419 concurrently with chemoradiation for OCC was acceptable. Toxicities included nausea/vomiting and paresthesia. Doses of 30 and 90 mg/d administered for 7 weeks were selected for further study. In an exploratory analysis, severe OM seemed less frequent and briefer than expected.Free full text

Phase 1b/2a Trial of The Superoxide Dismutase Mimetic GC4419 to Reduce Chemoradiotherapy-induced Oral Mucositis in Patients with Oral Cavity or Oropharyngeal Carcinoma
Associated Data
Abstract
Purpose:
Oral mucositis (OM) remains a critical problem; 70% of patients receiving chemoradiation (CRT) for oral cavity or oropharynx cancers (OCC) develop severe OM. Superoxide (O2•-) generated by CRT plays a significant role in initiating OM. Pre-clinical studies demonstrated that conversion of (O2•-) to O2 and H2O2 by the superoxide dismutase mimetic GC4419 interdicts this essential step. We hypothesized that GC4419 could safely be administered with CRT and reduce severe OM.
Patients and Methods:
Patients with locally-advanced OCC treated with definitive or post-operative intensity-modulated (IM)RT plus cisplatin received GC4419 by 60-minute IV infusion, ending <60 minutes before IMRT, M-F for 3–7 weeks, in a dose and duration escalation study. OM was assessed twice weekly during and weekly after IMRT.
Results:
46 patients received GC4419 in 11 separate dosing and duration cohorts: dose escalation occurred in 5 cohorts receiving 15–112 mg/day over 3 weeks (N=20); duration escalation in 3 cohorts receiving 112 mg/day over 4–6 weeks (N=12), then 3 additional cohorts receiving 30 or 90 mg/day over 6–7 weeks (N=14). A maximum tolerated dose was not reached. One dose-limiting toxicity (Grade 3 gastroenteritis and vomiting with hyponatremia) occurred in each of two separate cohorts @112 mg. Nausea/vomiting and facial paresthesia during infusion appeared to be GC4419 dose-related. Severe OM occurred through 60 Gy in 4 of 14 patients (29%) dosed for 6–7 weeks, with median duration of only 2.5 days.
Conclusion:
Safety of GC4419 concurrently with CRT for OCC was acceptable. Toxicities included nausea/vomiting and paresthesia. Doses of 30 and 90 mg/d administered for 7 weeks were selected for further study. In an exploratory analysis, severe OM appeared less frequent and briefer than expected.
Introduction
Oral mucositis (OM) is a common, disruptive, and painful complication of radiation and chemoradiation (CRT) for head and neck squamous cell carcinoma (HNSCC).1 About 70% of patients receiving CRT for oral cavity or oropharyngeal cancer (OCC) develop severe OM, defined as Grade 3–4 by the World Health Organization (WHO) scale.2,3 OM causes marked pain requiring narcotic analgesics, and adversely affects nutrition, hydration, speech, swallowing, quality of life, bacteremia risk, and feeding tube placement and use rates.4,5 Severe OM is also associated with radiation treatment breaks, which harms successful tumor management.6–8 The financial cost of managing patients with severe OM is substantial and is attributable to increased hospitalization and emergency room use.9.10
Recommended approaches to managing OM are limited to palliation and pain control with topical agents and systemic analgesics.11–19 The only approved drug or biological to reduce OM, palifermin, is limited to patients at risk for OM associated with conditioning regimens prior to stem cell transplant for the treatment of hematologic malignancies.20,21
The pathogenesis of mucositis is a complex sequence of biologic events in which oxidative stress plays a pivotal initiating role.22 Therapeutic radiation causes radiolytic hydrolysis and the formation of reactive oxygen species (ROS), including superoxide anion (O2•-). Superoxide is extremely reactive, triggering a cascade of signaling pathways in the cells and tissues of the submucosa resulting in apoptosis of epithelial stem cells, consequent loss of epithelial renewal, atrophy, and mucosal ulceration.23
A complement of naturally-occurring superoxide dismutase (SOD) enzymes exists to dispose of superoxide.24 However, large, rapidly produced amounts of superoxide due to therapeutic radiation can overwhelm these native SOD enzymes. GC4419 is a highly stable manganese-containing macrocyclic complex (MW=483), whose activity mimics the native enzymes, selectively removing superoxide anions without reacting with other reactive oxygen species, including nitric oxide, hydrogen peroxide, and peroxynitrite. An active enantiomer of GC4419 protected mice from lethal total body irradiation25 and reduced radiation-induced OM in a hamster cheek pouch model in a dose-related fashion.26 GC4419 had equivalent effects in the same OM model, but did not spare tumor from the effects of CRT in multiple preclinical models (Galera Therapeutics, unpublished data). Further, GC4419 protected mice from radiation-induced pulmonary fibrosis.27
The present study was done to assess the safety of GC4419 in combination with radiation and concurrent cisplatin for patients with OCC, and to assess the potential of GC4419 to reduce severe OM.
Patients and Methods
Patients
Eligible patients had oral cavity (OC) or oropharyngeal (OP), Stage III-IVb HNSCC, ECOG performance status ≤2, and a treatment plan that called for standard fractionation intensity modulated radiation therapy (IMRT) with concurrent cisplatin (80–100mg/m2 q3wk or 30–40 mg/m2 qwk). The IMRT plan had to include at least 2 oral mucosal sites (right or left buccal mucosa, right or left ventral/lateral oral tongue, floor of mouth, or soft palate) within the cumulative 50 Gy isodose line. IMRT plans were centrally reviewed by an independent radiation oncologist to confirm adherence to protocol requirements. Adequate marrow, renal and hepatic functions were required. Prophylactic PEG placement was allowed at enrollment. Patients were excluded if they had prior induction chemotherapy, significant dietary compromise due to reduced oral/pharyngeal function, or concurrent treatment with nitrates.
The protocol was approved by each institution’s IRB and was registered at clinicaltrials.gov. Investigators obtained written informed consent from each participant. Data were anonymized to protect the study subjects’ identities.
Treatment and Study Design
IMRT was administered once daily, Monday-Friday, at 2.0–2.2 Gy/day, to a cumulative tumor dose between 60–72 Gy. The assigned dose of GC4419 was delivered IV in normal saline over 60 minutes, ending within 60 minutes prior to each radiation fraction. Oral rinses limited to sodium bicarbonate, lidocaine, and antifungal agents were permitted. Other concurrent available or experimental systemic or topical pharmaceuticals or devices, or low-level laser therapy, were excluded. Supportive care per ASCO guidelines, including antiemetic drugs for cisplatin, was encouraged.
The study followed a serial cohort dose-escalation design with 3–6 patients enrolled per cohort. The first 5 cohorts received GC4419 before each of the first 14 IMRT fractions (over approximately 3 weeks), reflecting the duration of IND-supporting animal toxicology studies of GC4419. These 5 serial cohorts received 15, 30, 50, 75, or 112 mg of GC4419 per dose. These doses were based on previous results with a single 15-minute infusion administered to healthy human volunteers; the 60-minute infusion duration was chosen because dogs tolerated higher doses administered more slowly (i.e., 12.5 mg/kg over 60 minutes vs 5 mg/kg over 15 minutes—( Galera Therapeutics, unpublished observation). Based on observed safety results in the first 5 cohorts, the protocol was then amended to allow “duration extension” of GC4419 administered progressively longer during IMRT. Three serial cohorts received 112 mg of GC4419 before IMRT for 4, 5, or 6 weeks. Subsequent review of safety and OM results through these cohorts led to a decision by the sponsor add three cohorts to extend dosing further at reduced doses: 90 mg for 6 or 7 weeks or 30 mg for 7 weeks.
Dose-limiting toxicity (DLT) was defined as: Grade 3 or 4 nausea or vomiting despite maximal antiemetic therapy; Grade 4 anemia; Grade 4 thrombocytopenia or Grade 3 associated with hemorrhage; Grade 4 neutropenia lasting > 7 days; Grade 3 or 4 febrile neutropenia; or other Grade 3+ events (except oral mucositis), judged by the investigator not attributable to IMRT, cisplatin, or complications of HNSCC. For adverse events attributable to IMRT or cisplatin but judged to be exacerbated by the presence of GC4419, dose modification of GC4419 was at the investigator’s discretion. For any given patient, DLT required the GC4419 dose to be reduced one dose level (i.e. from 112mg to 75mg). Up to two such dose reductions per patient were permitted. Six patients/cohort were enrolled if DLT was observed in 1 of the first 3 patients. The maximum tolerated dose (MTD) of GC4419 was defined as the highest dose and longest schedule at which DLT was observed in >1 patient in a single dose and schedule cohort.
Study Assessments and Analysis
Adverse events were assessed using the NCI-CTCAE, version 4.0.
OM was assessed by trained investigator-evaluators using World Health Organization (WHO) criteria, in which: Grade 0 = No mucositis; Grade 1 = Pain and erythema; Grade 2 = Ulceration, able to eat solid food; Grade 3 = Ulceration, able to eat only liquids; Grade 4 = Ulceration, inability to eat requiring tube or parenteral feeding. OM was assessed twice weekly with at least a 48-hour interval between assessments during IMRT and weekly thereafter for up to 8 weeks or until the WHO score was < 2. OM assessment training and quality control were performed by Clinical Assistance Programs (Framingham, MA) to assure (1) all oral assessments were performed in a consistent manner using standardized questions, oral cavity examination technique and order, and data collection and (2) WHO grade scoring was correctly assigned per assessment findings for all OM assessments. To reduce the variability in assessing a patient’s diet, investigator-evaluators were trained to carefully elucidate whether dietary compromise was due to oral pain. If not, and the diet was compromised by confounding factors (e.g., dysgeusia, edentulous, nausea, mucous, throat pain, functional dysphagia), the WHO score was determined based on what the patient said they could eat absent these confounding factors.
Plasma concentrations of GC4419 and major metabolites were measured, using a validated liquid chromatography/mass spectrometry (LC/MS) method, pre-infusion, at the end of infusion, and 1, 2, 4, and 6–8 hours after infusion with two dosing cycles: Week 1/Day 2, and Day 3 during the last week of the infusion schedule.
Tumor status was assessed by clinical examination at the end of IMRT and 3, 6, 9, and 12 months thereafter. Standard-of-care imaging (CT, PET or MRI) was done before, and 3 and 12 months after IMRT. After completion of the 12-month assessment, study follow-up ended.
Statistical analysis of clinical endpoints was descriptive. Severe OM incidence, time to onset, and duration were tabulated. Patients who never developed OM > Grade 2 had values of 0 days and > 50 days imputed for duration and onset, respectively, of severe OM. Pharmacokinetics were analyzed by noncompartmental analysis (NCA) using the software program Phoenix (WinNonlin Professional version 6.4, PharSight Corp., Mountain View, CA).
Results
Nine US centers enrolled 46 patients between August 2013 and June 2015. Patient characteristics are summarized in Table 1. Forty-three patients completed OM assessments and were considered evaluable for OM; 44 completed tumor follow up through one year after IMRT and were evaluable for tumor endpoints (Table 2).
Table 1.
Patient characteristics
| Total patients enrolled | 46 |
| Men/Women | 38/8 |
| Median Age (Range), yrs | 58.5 (37–81) |
| Primary tumor | |
 Oral cavity Oral cavity | 7 |
 Oropharyngeal Oropharyngeal | 38 |
 Unknown Unknown | 1 |
| Post-operative/Definitive treatment | 18/28 |
| Overall Stage | |
 III III | 2 |
 IV A IV A | 39 |
 IV B IV B | 4 |
 Not available Not available | 1 |
| Tumor HPV status | |
 Positive Positive | 25 |
 Negative Negative | 2 |
 Not available Not available | 19 |
| Cisplatin schedule | |
 Q 3 week Q 3 week | 39 |
 Weekly Weekly | 7 |
| Evaluable for OM assessment | 43 |
| Evaluable for tumor follow up | 44 |
Table 2:
Distribution of patients by dose cohort
| Dose Cohort | # enrolled | # evaluable for safety | # evaluable for OM | # evaluable for recurrence |
|---|---|---|---|---|
| 15 mg/d x 14 doses | 4 | 4 | 4 | 4 |
| 30 mg/d x 14 doses | 3 | 3 | 3 | 3 |
| 50 mg/d x 14 doses | 4 | 4 | 4 | 4 |
| 75 mg/d x 14 doses | 3 | 3 | 3 | 3 |
| 112 mg/d x 14 doses | 8 | 8 | 6* | 6* |
| 112 mg/d x 20 doses | 3 | 3 | 3 | 3 |
| 112 mg/d x 25 doses | 3 | 3 | 3 | 3 |
| 112 mg/d x 30 doses | 5 | 5 | 3† | 5 |
| 90 mg/d x 30 doses | 3 | 3 | 4‡ | 3 |
| 90 mg/d x 35 doses | 6 | 6 | 6 | 6 |
| 30 mg/d x 35 doses | 4 | 4 | 4 | 4 |
| Totals | 46 | 46 | 43 | 44 |
Safety
Initial cohorts received GC4419 for the first 14 days of IMRT at 15 mg (N=4), 30 mg (N=3), 50 mg (N=4), 75 mg (N=3) or 112 mg (N=6). In these five cohorts, one toxicity event—Grade 3 nausea occurring at 112 mg—was considered potentially drug-related and therefore dose limiting. Ten of the 20 patients in these five cohorts completed treatment with no severe OM (Figure 1). Based on the favorable tolerability profile of GC4419 in these cohorts, GC4419 dosing duration was extended by protocol amendment, initially in three serial cohorts at 112 mg—20 doses/4 weeks, 25 doses/5 weeks, or 30 doses/6 weeks (N=3 each). In these three cohorts, one patient in the 112 mg/6 weeks cohort had Grade 3 nausea, which was considered dose limiting. Because no single cohort had >1 patient with DLT, the MTD was not considered exceeded. However, to reduce the potential risk of nausea, the GC4419 dose was lowered in the next two additional cohorts, to 90 mg for 30 doses/6 weeks (N=4), and then 35 doses/7 weeks (N=6). Concurrently, another cohort received 30 mg for 35 doses/7 weeks (N=4).
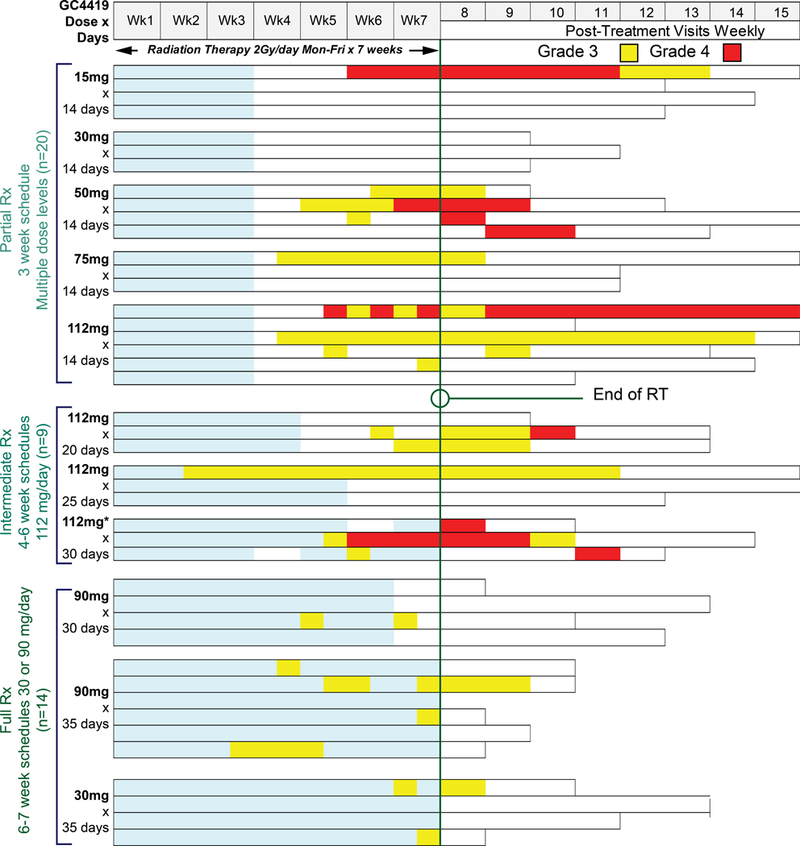
Swimmers’ plot of OM scores for each evaluable patient (n=43) over time stratified by the three dosing duration groups. Each horizontal bar (lane) provides WHO scores for an individual patient during the period of CRT (weeks 1–7) and post-CRT (weeks 8–15, top of figure). Severe OM Grade 3 is denoted by yellow shading and Grade 4 by red. Light blue within a lane denotes administration of GC4419.
41/46 patients (89%) received all planned GC4419 infusions. Five patients, all at 112 mg, stopped GC4419 early for adverse events (2) or patient request (3), receiving 2/14, 3/14, 9/14, 10/30, or 26/30 infusions. One additional patient (112 mg x 30 doses) had a permanent reduction to 75 mg for the last 6 doses because of Grade 3 nausea; the other 40 received their full doses.
The median total RT dose was 70 Gy (<10 Gy for two early-withdrawing patients, 81.3 Gy for one patient, 66–70 Gy for all others). RT breaks of 5 or more consecutive fractions occurred in 3/46 (6.5%) of patients (attributed to Grade 3 nausea; respiratory failure unrelated to GC4419; patient noncompliance). A total of 9 patients (one at 15 mg of GC4419, 2 at 30 mg, 3 at 90 mg, 3 at 112 mg), all receiving q 3 week platinum, had their platinum doses reduced by 20–60% for the second and/or third scheduled dose, at the discretion of the treating investigator, because of adverse events attributed to cisplatin. Four of the 9 patients received the full schedule of GC4419 (one 30mg/d x 35 doses, two 90mg/d x 35 doses and one 90mg/d x 30 doses) and another 3 of the 9 patients received 112mg/d (one x14 doses, one x20 doses and one x 30 doses).
Overall, adverse events, the most common of which were cytopenias, nausea, fatigue, constipation, dysgeusia, and dry mouth, were considered attributable to IMRT/cisplatin, complications of HNSCC, or other concomitant conditions. Although dose escalation of GC4419 was curtailed before reaching a formal MTD, Grade 3 nausea or vomiting were more frequent at higher GC4419 doses (Table 3), as was transient, infusion-related Grade 1 facial paresthesia that spontaneously resolved shortly following the infusion and did not limit dosing.
Table 3:
Adverse events of note. Tabulated events are listed without regard to attribution to GC4419.
| Daily dose | 15 mg | 30 mg | 50 mg | 75 mg | 90 mg | 112 mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total doses/cohort | 14 | 14 | 35 | 14 or 35 | 14 | 14 | 30 | 35 | 30 or 35 | 14* | 20 | 25 | 30* | 14, 20, 25, or 30 |
| N | 4 | 3 | 4 | 7 | 4 | 3 | 4 | 6 | 10 | 8 | 3 | 3 | 4 | 18 |
| Paresthesia (Grade 1) | 0 | 0 | 1 (25%) | 1 (14%) | 1 (25%) | 0 | 2 (50%) | 3 (50%) | 5 (50%) | 5 (63%) | 1 (33%) | 1 (33%) | 4 (100%) | 11 (61%) |
| Nausea (Grade 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (33%) | 2 (20%) | 2 (25%) | 0 | 1 (33%) | 1 (25%) | 4 (22%) |
| Vomiting (Grade 3) | 0 | 0 | 1 (25%) | 1 (14%) | 0 | 0 | 0 | 1 (16%) | 1 (10%) | 2 (25%) | 0 | 1 (33%) | 1 (25%) | 4 (22%) |
| Nausea (any Grade) | 4 (100%) | 2 (67%) | 4 (100%) | 6 (86%) | 4 (100%) | 2 (67%) | 3 (75%) | 6 (100%) | 9 (90%) | 6 (75%) | 2 (67%) | 3 (100% | 4 (100%) | 15 (83%) |
OM Efficacy
For patients who received 30 or 90 mg over the full 6–7 weeks of CRT, the cumulative incidence of severe OM was 29% (4/14) through 6 weeks of RT (60 Gy), and 50% (7/14) at any time, with a median time to onset >50 days, and a median duration of 2.5 days (Table 4, Figure 1). In contrast, in the initial 5 cohorts, GC4419 for 3 weeks was not as effective in reducing cumulative severe OM through 60Gy (incidence 40% and duration 4.5 days). For patients in the intermediate treatment group, cumulative incidence at 60Gy was 44%, at any time 67%, median onset 43 days and duration 18 days. Duration of all grades of OM appeared shorter as GC4419 administration was extended further in the IMRT treatment period (Figure 2).
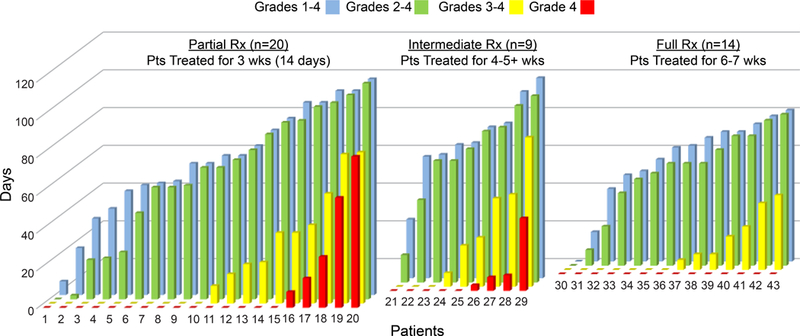
Duration of all grades of OM decreased with longer GC4419 dosing. Each vertical set of bars represents OM duration by grade for an individual subject. Data are presented by the three dosing duration groups (14 doses/3 weeks; 4–5+ weeks; or 6–7 weeks), and arranged to increase from left to right for each subgroup for viewing clarity.
Table 4:
Key OM efficacy parameters compared with historical controls. Results for the 14 patients receiving 30 or 90 mg for 6–7 weeks are compared with those in the initial dose escalation phase (N=20), the intermediate schedule (N=9) and representative historical controls. Patients who never developed OM > Grade 2 had values of 0 days and > 50 days imputed for duration and onset, respectively, of severe (Grade 3–4) OM.
| SEVERE ORAL MUCOSITIS (Grade 3 & 4) Comparison Of Key Efficacy Parameters | Comparative Historical Control | GC4419 Phase Ib/2a | |||||
|---|---|---|---|---|---|---|---|
| Unpublished Data | Placebo Data from Phase III Trials | Partial Treatment (3 wks) | Intermediate Treatment (4–5+ wks) | Full Treatment (6–7 wks) | |||
| S. Sonis | Le | Henke | |||||
| #Patients | 380 | 94 | 94 | 20 | 9 | 14 | |
| INCIDENCE | Through 60 Gy | 60% | 57% | 62% | 40% | 44% | 29% |
| At any time | 70% | 69% | 67% | 50% | 67% | 50% | |
| ONSET | Median #Days [range] | ≈28 | 35 | 32 | >50 [25–57] | 43 [15->50] | >50 [19->50] |
| DURATION | Median #Days [range] | 26–30 | 26 | 22 | 4.5 [0–77] | 18 [0–68] | 2.5 [0–34] |
Pharmacokinetics
Peak concentration (Cmax) and area under the concentration time profile (AUC) were approximately dose-proportional for GC4419 in plasma (data not shown). The terminal elimination half-life was approximately 2 hours, with minimal accumulation upon repeated dosing. There were two primary metabolites: the major metabolite GC4520/parent GC4419 ratio was approximately 10% at all dose levels, and the minor metabolite GC4570/GC4419 ratio was less than 0.2% (data not shown).
Tumor Outcomes
44/46 patients were evaluable for the 1-year tumor outcome analysis (2 patients withdrew consent for follow-up, Table 5). Per study design, post-RT follow-up was completed through 12 months for all 44 patients. Three patients died during the follow-up phase: 2 patients with oropharyngeal cancer died of noncancer causes without evidence of progression and 1 patient with oropharyngeal cancer (HPV negative) died from locoregional and distant recurrence at 6 months. Two additional patients with oropharyngeal cancer had locoregional progression at Month 12 (one HPV positive and one HPV unknown) and 3 patients had distant metastases at that Month 12. One of the 5 patients who experienced recurrence was in the full dose cohort (90mg x 35 doses). None of the 5 patients who experienced recurrence required cisplatin dose reduction. Two of the 5 patients received two full doses of cisplatin 100mg/m2 but missed the last q 3 week dose of cisplatin, one due to patient non-compliance and one due to “administrative reasons”.
Table 5:
Summary of tumor outcome parameters for evaluable patients (N=44). LRC was defined as alive without receiving alternate therapy and without locoregional progression. Two patients who died of non-cancer causes were censored from the LRC and DM-free metrics, making the N=42 for those endpoints.
| 1-Yr LRC* | 1-Yr DM-free† | 1-Yr PFS‡ | 1-Yr OS§ | |
|---|---|---|---|---|
| Oral Cavity | 7/7 (100%) | 6/7 (86%) | 6/7 (86%) | 7/7 (100%) |
| Oropharyngeal | 32/35 (91%) | 32/35 (91%) | 31/37 (84%) | 34/37 (92%) |
| Overall | 39/42 (93%) | 38/42 (91%) | 37/44 (84%) | 41/44 (93%) |
Discussion
In this study, we found it feasible to add the superoxide dismutase mimetic GC4419 to IMRT and cisplatin. Observed safety was acceptable at all dose and duration schedules studied. Delivery of planned chemoradiotherapy was not compromised in the presence of GC4419. Grade 3 nausea and vomiting were more frequent at the highest dose (112 mg). Because of this, and a low threshold for additional toxicity in this patient population, GC4419 dose escalation was curtailed before reaching the study-defined MTD. The most common GC4419-associated adverse event--mild paresthesia--is similar to reports with sublingual nitroglycerine,28 and is likely due to potentiation of nitric oxide (NO) by GC4419 as has previously been reported to occur with this class of compounds.29 Superoxide reacts rapidly with NO to remove it, and abruptly reducing the amount of superoxide present would be expected to potentiate NO’s effects.
The anticipated incidence, duration, and time to onset of severe OM in this study were favorable compared to historical controls (Table 4). These controls included the placebo arms of two published studies of palifermin,2,3 and unpublished results known to one of the authors from control arms of multiple prospective OM studies. The unpublished historical controls are not case-matched with the present study and Stage and HPV status are not available. The unpublished experience was limited to patients with OC or OP primaries, post-op and definitive, who received IMRT plus concurrent cisplatin (q 3 week or weekly). However, for all of these published and unpublished historical data, mucositis was assessed at sites receiving > 50 Gy by the same OM assessment criteria (trained assessors, assessment method, assessment interval, WHO scale) as in the present trial. Although conclusions from historical, cross-study comparisons must be limited, the current results are sufficiently encouraging to warrant more rigorous assessment in a prospective, controlled trial.
OM results were best for the cohorts that received 30 or 90 mg of GC4419 for 6–7 weeks, consistent with expectations that GC4419 should be administered throughout the entire IMRT course to remove superoxide produced with each IMRT fraction. Patients receiving as little as 30 mg of GC4419 had little severe OM (Figure 1). Further, OM results through 6 weeks/60 Gy are of interest as this is a common landmark expected to be reached in IMRT of all patients with locally advanced HNSCC.
While most of our patients developed OM ≥ WHO Grade 2, consistent with prior reports,4 severe (WHO Grade 3–4) OM is arguably more relevant to clinical benefit. Reducing severe OM should decrease the substantial day-to-day burden of OM overall5. As a result, increased resource usage estimated to cost approximately $18,000 per patient 10, could be reduced. A further question is whether patients’ subjective reports of mouth and throat soreness correspond to the WHO score, a relationship that held for palifermin’s effects on OM in patients receiving total body irradiation/high dose chemotherapy and hematopoietic stem cell transplant30 but not in the trials of that drug in HNSCC patients.2,3
Of equal importance, IMRT treatment breaks are associated with compromised tumor outcomes in HNSCC.7,8 That only 3/46 (6.5%) of patients in this study had RT breaks of 5 or more consecutive fractions is promising. Treatment breaks of this duration were reported in 15% of both control and experimental patients in the two palifermin studies to reduce OM in HNSCC,2,3 which employed 3-field conformal radiation, and 15.1% of patients receiving IMRT/platinum and 26.9% receiving IMRT/cetuximab in the RTOG 0522 trial.31
For cancer supportive care agents, a major concern is that they not compromise or antagonize the efficacy of the underlying anti-tumor regimen. In the present study, tumor outcomes at one year did not appear unfavorable compared with contemporary expectations,31 but conclusions are limited due to the small sample size and lack of a placebo arm for control.
On a mechanistic basis, GC4419 is expected not to antagonize, but instead potentially enhance, tumor radiation response. Normal and cancer cells metabolize ROS differently. Specifically, normal cells tend to be more sensitive to elevations in superoxide anion, but more tolerant of increases in hydrogen peroxide flux. They utilize redox protective enzyme systems to convert superoxide into water and molecular oxygen, removing it rapidly to prevent normal tissue damage. While these same enzyme systems are typically active in cancer cells, moderate elevations in superoxide actually serve to promote tumor growth but significant increases in hydrogen peroxide flux are apparently less well tolerated than for normal cells.32 Thus, therapeutic radiation, by increasing superoxide, can overwhelm the SOD enzyme system and initiate normal tissue toxicity such as OM. GC4419 can convert this excess superoxide into hydrogen peroxide, which is less toxic to normal tissue, while simultaneously maintaining or even increasing anti-tumor efficacy. Consistent with this are non-clinical data demonstrating synergy, especially between GC4419 and higher dose-fraction RT regimens,27 as are becoming used in stereotactic body radiotherapy.
To our knowledge this is the first clinical trial of a selective SOD mimetic to reduce RT-induced severe OM. Results in animal models of radiation OM have been reported for a manganese SOD33, for non-selective oxygen radical scavengers34, and for manganese porphyrins that mimic both SOD and catalase35. None of these, however, have been tested in a clinical study of OM. A marketed bovine-sourced copper-zinc SOD was also tested in a small study of HNC patients for treatment of radiation toxicities in HNC patients36 but that product was subsequently withdrawn from all markets for safety reasons.
The promising OM results of this study, along with a comparative assessment of the contribution of GC4419 to the toxicity and tumor control outcomes of CRT, must be confirmed in a larger randomized, placebo-controlled setting. To that end, doses of 30 and 90 mg/day administered throughout CRT were selected for the randomized, double-blind, placebo-controlled GC4419 Phase 2b trial in progress. Tumor follow-up will extend through 24 months post-IMRT in that trial.
Acknowledgments
Research support: This study was funded by Galera Therapeutics, Inc.
Footnotes
The results of this study were presented in part at:
Multidisciplinary Head and Neck Cancer Symposium, Feb 18–20, 2016, oral presentation
ASCO Annual Meeting, June 3–7, 2016, poster discussion
58th Annual Meeting of the American Society of Therapeutic Radiation Oncology (ASTRO), September 24–28, 2016, oral presentation
Disclaimers: None
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ijrobp.2017.10.019
Read article for free, from open access legal sources, via Unpaywall:
http://www.redjournal.org/article/S0360301617340245/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ijrobp.2017.10.019
Article citations
Balanced Duality: H2O2-Based Therapy in Cancer and Its Protective Effects on Non-Malignant Tissues.
Int J Mol Sci, 25(16):8885, 15 Aug 2024
Cited by: 0 articles | PMID: 39201571 | PMCID: PMC11354297
Review Free full text in Europe PMC
The antioxidant and anti-inflammatory activities of avasopasem manganese in age-associated, cisplatin-induced renal injury.
Redox Biol, 70:103022, 01 Jan 2024
Cited by: 5 articles | PMID: 38215546 | PMCID: PMC10821164
Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation.
Antioxidants (Basel), 11(8):1613, 19 Aug 2022
Cited by: 14 articles | PMID: 36009331 | PMCID: PMC9405171
Review Free full text in Europe PMC
Inter-agency perspective: Translating advances in biomarker discovery and medical countermeasures development between terrestrial and space radiation environments.
Life Sci Space Res (Amst), 35:9-19, 14 Jun 2022
Cited by: 1 article | PMID: 36336375 | PMCID: PMC9832585
1-Isobutanoil-2-isopropylisothiourea Phosphate, T1082: A Safe and Effective Prevention of Radiotherapy Complications in Oncology.
Int J Mol Sci, 23(5):2697, 28 Feb 2022
Cited by: 1 article | PMID: 35269835 | PMCID: PMC8911053
Go to all (33) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer.
J Clin Oncol, 37(34):3256-3265, 16 Oct 2019
Cited by: 39 articles | PMID: 31618127 | PMCID: PMC6881100
Multivariable model for predicting acute oral mucositis during combined IMRT and chemotherapy for locally advanced nasopharyngeal cancer patients.
Oral Oncol, 86:266-272, 11 Oct 2018
Cited by: 18 articles | PMID: 30409311
Phase 2 Trial of De-intensified Chemoradiation Therapy for Favorable-Risk Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma.
Int J Radiat Oncol Biol Phys, 93(5):976-985, 22 Aug 2015
Cited by: 88 articles | PMID: 26581135
Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data.
Oncologist, 22(9):1056-1066, 22 May 2017
Cited by: 67 articles | PMID: 28533474 | PMCID: PMC5599190
Review Free full text in Europe PMC





