Abstract
Importance
Intraoperative electroencephalogram (EEG) waveform suppression, often suggesting excessive general anesthesia, has been associated with postoperative delirium.Objective
To assess whether EEG-guided anesthetic administration decreases the incidence of postoperative delirium.Design, setting, and participants
Randomized clinical trial of 1232 adults aged 60 years and older undergoing major surgery and receiving general anesthesia at Barnes-Jewish Hospital in St Louis. Recruitment was from January 2015 to May 2018, with follow-up until July 2018.Interventions
Patients were randomized 1:1 (stratified by cardiac vs noncardiac surgery and positive vs negative recent fall history) to receive EEG-guided anesthetic administration (n = 614) or usual anesthetic care (n = 618).Main outcomes and measures
The primary outcome was incident delirium during postoperative days 1 through 5. Intraoperative measures included anesthetic concentration, EEG suppression, and hypotension. Adverse events included undesirable intraoperative movement, intraoperative awareness with recall, postoperative nausea and vomiting, medical complications, and death.Results
Of the 1232 randomized patients (median age, 69 years [range, 60 to 95]; 563 women [45.7%]), 1213 (98.5%) were assessed for the primary outcome. Delirium during postoperative days 1 to 5 occurred in 157 of 604 patients (26.0%) in the guided group and 140 of 609 patients (23.0%) in the usual care group (difference, 3.0% [95% CI, -2.0% to 8.0%]; P = .22). Median end-tidal volatile anesthetic concentration was significantly lower in the guided group than the usual care group (0.69 vs 0.80 minimum alveolar concentration; difference, -0.11 [95% CI, -0.13 to -0.10), and median cumulative time with EEG suppression was significantly less (7 vs 13 minutes; difference, -6.0 [95% CI, -9.9 to -2.1]). There was no significant difference between groups in the median cumulative time with mean arterial pressure below 60 mm Hg (7 vs 7 minutes; difference, 0.0 [95% CI, -1.7 to 1.7]). Undesirable movement occurred in 137 patients (22.3%) in the guided and 95 (15.4%) in the usual care group. No patients reported intraoperative awareness. Postoperative nausea and vomiting was reported in 48 patients (7.8%) in the guided and 55 patients (8.9%) in the usual care group. Serious adverse events were reported in 124 patients (20.2%) in the guided and 130 (21.0%) in the usual care group. Within 30 days of surgery, 4 patients (0.65%) in the guided group and 19 (3.07%) in the usual care group died.Conclusions and relevance
Among older adults undergoing major surgery, EEG-guided anesthetic administration, compared with usual care, did not decrease the incidence of postoperative delirium. This finding does not support the use of EEG-guided anesthetic administration for this indication.Trial registration
ClinicalTrials.gov Identifier: NCT02241655.Free full text

Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery
Key Points
Question
Does electroencephalography-guided anesthetic administration decrease the incidence of postoperative delirium in older patients undergoing major surgery?
Findings
In this randomized clinical trial involving 1232 patients aged 60 years and older undergoing major surgery, postoperative delirium occurred in 26.0% of the electroencephalography-guided anesthetic group and 23.0% of the usual care group, a difference that was not statistically significant.
Meaning
These findings do not support the use of electroencephalography-guided anesthetic administration for the prevention of postoperative delirium among older adults undergoing major surgery.
Abstract
Importance
Intraoperative electroencephalogram (EEG) waveform suppression, often suggesting excessive general anesthesia, has been associated with postoperative delirium.
Objective
To assess whether EEG-guided anesthetic administration decreases the incidence of postoperative delirium.
Design, Setting, and Participants
Randomized clinical trial of 1232 adults aged 60 years and older undergoing major surgery and receiving general anesthesia at Barnes-Jewish Hospital in St Louis. Recruitment was from January 2015 to May 2018, with follow-up until July 2018.
Interventions
Patients were randomized 1:1 (stratified by cardiac vs noncardiac surgery and positive vs negative recent fall history) to receive EEG-guided anesthetic administration (n =
= 614) or usual anesthetic care (n
614) or usual anesthetic care (n =
= 618).
618).
Main Outcomes and Measures
The primary outcome was incident delirium during postoperative days 1 through 5. Intraoperative measures included anesthetic concentration, EEG suppression, and hypotension. Adverse events included undesirable intraoperative movement, intraoperative awareness with recall, postoperative nausea and vomiting, medical complications, and death.
Results
Of the 1232 randomized patients (median age, 69 years [range, 60 to 95]; 563 women [45.7%]), 1213 (98.5%) were assessed for the primary outcome. Delirium during postoperative days 1 to 5 occurred in 157 of 604 patients (26.0%) in the guided group and 140 of 609 patients (23.0%) in the usual care group (difference, 3.0% [95% CI, −2.0% to 8.0%]; P =
= .22). Median end-tidal volatile anesthetic concentration was significantly lower in the guided group than the usual care group (0.69 vs 0.80 minimum alveolar concentration; difference, −0.11 [95% CI, −0.13 to −0.10), and median cumulative time with EEG suppression was significantly less (7 vs 13 minutes; difference, −6.0 [95% CI, −9.9 to −2.1]). There was no significant difference between groups in the median cumulative time with mean arterial pressure below 60 mm Hg (7 vs 7 minutes; difference, 0.0 [95% CI, −1.7 to 1.7]). Undesirable movement occurred in 137 patients (22.3%) in the guided and 95 (15.4%) in the usual care group. No patients reported intraoperative awareness. Postoperative nausea and vomiting was reported in 48 patients (7.8%) in the guided and 55 patients (8.9%) in the usual care group. Serious adverse events were reported in 124 patients (20.2%) in the guided and 130 (21.0%) in the usual care group. Within 30 days of surgery, 4 patients (0.65%) in the guided group and 19 (3.07%) in the usual care group died.
.22). Median end-tidal volatile anesthetic concentration was significantly lower in the guided group than the usual care group (0.69 vs 0.80 minimum alveolar concentration; difference, −0.11 [95% CI, −0.13 to −0.10), and median cumulative time with EEG suppression was significantly less (7 vs 13 minutes; difference, −6.0 [95% CI, −9.9 to −2.1]). There was no significant difference between groups in the median cumulative time with mean arterial pressure below 60 mm Hg (7 vs 7 minutes; difference, 0.0 [95% CI, −1.7 to 1.7]). Undesirable movement occurred in 137 patients (22.3%) in the guided and 95 (15.4%) in the usual care group. No patients reported intraoperative awareness. Postoperative nausea and vomiting was reported in 48 patients (7.8%) in the guided and 55 patients (8.9%) in the usual care group. Serious adverse events were reported in 124 patients (20.2%) in the guided and 130 (21.0%) in the usual care group. Within 30 days of surgery, 4 patients (0.65%) in the guided group and 19 (3.07%) in the usual care group died.
Conclusions and Relevance
Among older adults undergoing major surgery, EEG-guided anesthetic administration, compared with usual care, did not decrease the incidence of postoperative delirium. This finding does not support the use of EEG-guided anesthetic administration for this indication.
Trial Registration
ClinicalTrials.gov Identifier: NCT02241655
Introduction
Older adults often become delirious after major surgery.1,2,3,4,5 Delirium is a reversible state of impaired cognition, inattention, and altered level of consciousness.1 It is associated with poorer functional recovery, longer intensive care stay, and increased use of health care resources; delirium is also distressing to patients, family members, and clinicians.1 The UK’s National Institute for Health and Care Excellence, the American Geriatric Society, the American College of Surgeons, and the American Society of Anesthesiologists have all identified the prevention of postoperative delirium as a public health priority.6,7,8,9
Meta-analyses of randomized trials have reported that electroencephalography-guided anesthetic administration reduced postoperative delirium incidence by one-third to one-half.10 A mechanism for delirium reduction might be the avoidance of burst suppression, an electroencephalographic pattern suggesting excessively deep anesthesia. Burst suppression is characterized by isoelectric periods (suppression) punctuated by large-amplitude waves (bursts).11 Burst suppression also occurs with coma and brain injury, but not during sleep.12 Electroencephalogram suppression during surgery has been associated with postoperative delirium.13 However, causality has not been established. Patients who are susceptible to delirium could coincidentally be prone to electroencephalogram suppression during general anesthesia.14
The pragmatic Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) trial was designed to investigate whether reducing anesthetic administration and minimizing electroencephalogram suppression during surgical anesthesia decreases the incidence of postoperative delirium.
Methods
Design, Setting, and Ethics
This was a single-center, pragmatic, patient- and evaluator-blinded, randomized clinical trial at Barnes-Jewish Hospital in St Louis, Missouri. The study was conducted at 3 Barnes-Jewish Hospital facilities (South, Southwest Tower, and Parkview Tower). There were 5 separate groups of operating rooms, and surgical procedures for patients enrolled in this trial were conducted in 48 different operating rooms across these locations. The ethics committee at the Washington University School of Medicine approved the study, and all patients provided written informed consent. The protocol and statistical plan for the trial (Supplement 1), which includes a description of its pragmatic elements, has been published.15 A manual of operations is also included (Supplement 2).
Study Population
Eligible patients were aged 60 years and older and undergoing major surgery with general anesthesia. Patients were excluded if they were unable to provide informed consent, delirious, blind, deaf, illiterate, not fluent in English, had a history of intraoperative awareness, or were scheduled for a second surgery within 5 days of the initial surgery. Sociodemographic information (eg, race, employment status, income) was collected to ensure that the study population was broadly reflective of the patient population at the hospital. Participants indicated their race using fixed categories from a list. Preoperative evaluations included a delirium assessment; the Eight-item Interview to Differentiate Aging and Dementia to screen for dementia (score range: 0 to 8; 0-1 indicates normal cognition and ≥2 indicates that cognitive impairment is likely present); the handgrip strength test (kg) and timed up-and-go (seconds) to measure frailty; a depression screen using the 8-question Personal Health Questionnaire Depression Scale (PHQ8) (score range: 0-24 score; ≥10 indicates major depression and ≥20 indicates severe major depression); the Short Blessed Test to measure cognition (score range: 0-28; 0-4 indicates normal cognition, 5-9 indicates questionable impairment, and ≥10 indicates impairment consistent with dementia); the Veterans Rand 12-Item Health Survey (physical and mental score ranges: 0-100; 50 represents the population average) to measure quality of life; and history of falls within 6 months. The Consolidated Standards of Reporting Trials guidelines for pragmatic trials and for nonpharmacological treatments were followed when reporting results.16,17
Randomization and Blinding
Patients were randomized 1:1 in blocks of 20 in 4 strata, using computer-generated assignment to usual anesthesia care (usual care group) or to electroencephalography-guided anesthesia (guided group).15 The strata were based on cardiac vs noncardiac surgery and a positive vs negative history of falling in the past 6 months.15 Preoperative falls and cardiac surgery have been associated with increased risk for both postoperative delirium and falls.4,18 Randomization was communicated to anesthesia clinicians after the patient was transported to the operating room. To minimize bias, all patients and the researchers assessing outcomes and adverse events were blinded to randomization assignments, except for the clinicians reporting the adverse event of undesirable intraoperative patient movement.16
Procedures
A Bispectral Index Quatro (Medtronic) frontal electroencephalogram sensor was applied to the forehead of each enrolled patient. This device employs a proprietary algorithm to display anesthetic depth on a scale of 0 to 100; values below 40 suggest excessive depth.19 In the usual care group, clinicians were blinded to all electroencephalogram waveforms and derived values, except the signal quality index. In the guided group, the electroencephalogram waveforms and derived parameters (suppression ratio, spectral edge frequency, electromyography, signal quality index, and bispectral index) were displayed. In the guided group, clinicians were encouraged to decrease volatile anesthetic administration based on electroencephalogram information and their clinical judgment.
Clinicians received education regarding typical electroencephalogram morphology during volatile anesthetic-based general anesthesia.15,20,21 They received reinforcement instruction regarding the conduct of the study (eFigure 1 in Supplement 3) and links to online educational modules.21 Clinicians were encouraged to minimize, primarily, epochs of electroencephalogram suppression and, secondarily, periods with bispectral index values below 40. During surgical procedures, researchers provided feedback to anesthesia clinicians on the cardinal features of the electroencephalogram waveforms (eFigure 2 in Supplement 3). In both groups, clinicians were discouraged from administering nitrous oxide and intravenous hypnotic agents (eg, midazolam, propofol, ketamine, dexmedetomidine) during the maintenance period of general anesthesia.
Patients in both groups also received a multicomponent safety intervention, including review of their baseline medications by a geriatric psychiatrist who provided recommendations to participants’ physicians regarding changes in medications with the potential to increase risk of falls or confusion. All patients were provided with information on improving safety in the hospital after surgical procedures and educational material about making the home environment safer to decrease the risk of falls and related injuries. Patients with a history of falls were eligible to receive a home occupational therapy visit, with the aim of increasing daily activity performance, improving safety, and preventing falls.
Outcomes
The primary outcome was delirium incidence on postoperative days 1 through 5. As prespecified exploratory objectives, we compared (1) additional delirium outcomes, including severe delirium incidence (defined as a score ≥10 on the Confusion Assessment Method [CAM]22 or ≥6 on the Confusion Assessment Method for the Intensive Care Unit [CAM-ICU]), duration of delirium, time to onset of delirium, delirium incidence on the day of surgery, and delirium incidence in the randomization strata (cardiac surgery with no history of falls, cardiac surgery with history of falls, noncardiac surgery with no history of falls, noncardiac surgery with history of falls); (2) intraoperative measures, including anesthetic concentrations, electroencephalogram suppression time, and hypotension duration; and (3) adverse events, including undesirable intraoperative movement, awareness with recall, nausea and vomiting, postoperative medical complications, and 30-day rate of death, between the groups. As prespecified exploratory objectives, we also compared 30-day outcomes between groups, including falls and measures of quality of life, functionality, and cognitive impairment. Prespecified secondary end points included health-related quality of life and falls at 1 year, which are not reported in this study.
Delirium Assessments
Diagnosis of incident delirium was based on established criteria according to the CAM22 or CAM-ICU23 assessments or chart review. All researchers were trained in CAM and CAM-ICU delirium assessment, and reliability among them was demonstrated.15,24,25 Patients were assessed daily, preferably with the CAM, after 1 pm on postoperative days 1 through 5, unless patients were discharged or sedated (Richmond Agitation and Sedation Scale score < −3). Delirium severity was based on the CAM-S or the CAM-ICU-7 instruments.26,27 When a researcher was uncertain regarding the scoring of a component of the CAM or CAM-ICU, the delirium assessment was discussed at a weekly research meeting, and, if uncertainty remained, the assessment was referred to an external expert panel for adjudication. A random selection of assessments was also adjudicated. Because delirium is a fluctuating condition that often manifests late at night, CAM assessments were supplemented with medical record review.15 A trained researcher, blinded to treatment allocation and CAM/CAM-ICU determinations, completed a structured chart review for evidence of delirium.28 All indeterminate chart reviews were referred to the expert panel.
Intraoperative and Postoperative Measures, Exploratory Outcomes, and Adverse Events
Anesthesia clinicians documented undesirable patient movement during surgical procedures (eFigure 3 in Supplement 3). Patients without delirium were assessed for intraoperative awareness with recall 24 to 48 hours after tracheal extubation, and patients with delirium were assessed following delirium resolution. Approximately 30 days after the surgical procedure, patients were contacted by phone, email, or letter to complete the Veterans RAND 12-Item Health Survey to asses quality of life, the Lawton Instrumental Activities of Daily Living Scale and Barthel Activities of Daily Living Scale to measure functionality, the Eight-item Informant Interview to Differentiate Aging and Dementia and the Short Blessed Test to assess cognitive impairment, and to report any falls after surgery. Intraoperative measures (ie, anesthetic concentration, electroencephalographic data, hemodynamic data), postoperative measures, and 30-day adverse event data were obtained from patients’ electronic medical records. Death was determined through chart review, phone calls to family, surveys returned by family members, and obituaries.
Sample Size Calculation and Statistical Analysis
On the basis of meta-analyses of randomized trials, we assumed an incidence of postoperative delirium in the usual care group of 25%3,10,13 and a relative reduction in delirium with electroencephalography-guided anesthesia of greater than one-third.3,10 Based on a 2-sided α <.05 and 1232 patients, we estimated greater than 90% power to detect an absolute decrease in delirium incidence of 8%.15 Continuous variables were presented as mean (SD) or median (interquartile range [IQR]), depending on their distributions. χ2 or Fisher exact tests were used for comparisons of discrete data. Unpaired t or Mann-Whitney U tests were used for comparisons of continuous data, depending on their distributions. CIs for median and between-proportions differences were calculated using Hodges-Lehmann estimates and Newcombe’s method with continuity correction, respectively.
For the primary outcome, we compared the proportion of patients with incident postoperative delirium between the groups. Patients were assessed based on their randomization groups (guided or usual care). Patients who could not be assessed for delirium were excluded from the primary outcome analysis. In 2 post hoc sensitivity analyses, the patients who could not be assessed for delirium (unless they died during the surgical procedure) were all either assumed to have had incident delirium or not to have had incident delirium. We conducted 3 post hoc sensitivity analyses to assess whether improved clinician fidelity to the guided protocol might have altered the primary outcome. We excluded 25% of cases in each of the 4 strata within the guided group with the most electroencephalogram suppression, the most time with bispectral index less than 40, and the highest median volatile anesthetic concentrations. We performed a post hoc sensitivity analysis using covariate adjustment, including likely risk factors for delirium, using 2 methods: logistic regression and standardized estimator combined with bootstrapped 95% CIs.29
To compare time to delirium onset between groups, we constructed Kaplan-Meier curves for each group, conducted a log-rank test, and tested for proportionality of hazards using the Cox model, which included patients’ age and group assignment as well as their interaction. We compared delirium incidence between groups in a post hoc analysis, segregated by Charlson Comorbidity Index categories, and tested for its interaction with the randomization groups. We conducted the following post hoc per protocol analysis: for cases in which clinicians in the usual care group insisted on viewing electroencephalogram data, these patients were included in the guided group and for cases in which there were technical difficulties in viewing electroencephalogram data in the guided group, these patients were included in the blinded group. Adverse events and 30-day outcomes were compared between groups. We compared preoperative characteristics between respondents and alive nonrespondents to 30-day postoperative surveys. No imputation was performed for missing baseline patient characteristics or any outcomes. Results were presented with 95% CIs. All significance testing was 2-sided, with P values <.05 considered as providing suggestive evidence and P values <.005 as providing a more stringent threshold for statistical significance.30 The statistical analyses were performed with SAS, version 9.4 (SAS Institute), and STATA, version 14.2 (StataCorp LP). A data and safety monitoring committee reviewed the study biannually, and an independent safety officer reviewed all serious adverse events.
Results
Of 39 144 patients screened for eligibility from January 16, 2015, to May 7, 2018, 1400 were enrolled. In total, 58 attending anesthesiologists, 12 anesthesiology fellows, 79 anesthesiology residents, 92 certified registered nurse anesthetists, and 14 student registered nurse anesthetists managed the anesthetic administration for patients in the trial. A total of 1232 patients undergoing diverse major surgeries (eg, cardiac, gastrointestinal, thoracic, gynecologic, hepatobiliary-pancreatic, urologic, vascular) were included in the trial (Table 1; eTables 1, 2, 4, and 5 in Supplement 3), of whom 1213 (98.5%) were assessed for the primary outcome (Figure 1). For 5 patients in the guided group, technical problems prevented clinicians from viewing the electroencephalogram. For 10 patients in the usual care group, clinicians considered it necessary to view the electroencephalogram for periods during the surgical procedure. The median duration of general anesthesia and doses of midazolam, propofol, opioids, and neuromuscular blocking agents were not significantly different between groups (Table 2; eTable 6 in Supplement 3). Median end-tidal volatile anesthetic concentration was lower in the guided group than the control group (0.69 vs 0.80 minimum alveolar concentration; difference, −0.11 [95% CI, −0.13 to −0.10], and median cumulative time with electroencephalogram suppression (7 vs 13-minute; difference, −6.0 [95% CI, −9.9 to −2.1]) and bispectral index less than 40 (32 vs 60-minute; difference, −28.0 [95% CI, −38.0 to −18.0]) were less in the guided group (Figure 2). Although the median duration of hypotension at various thresholds was not significantly different between the groups (eTable7 in Supplement 3), more phenylephrine was administered in the usual care group (Table 2). There were no significant differences in postoperative measures between groups (Table 2).
144 patients screened for eligibility from January 16, 2015, to May 7, 2018, 1400 were enrolled. In total, 58 attending anesthesiologists, 12 anesthesiology fellows, 79 anesthesiology residents, 92 certified registered nurse anesthetists, and 14 student registered nurse anesthetists managed the anesthetic administration for patients in the trial. A total of 1232 patients undergoing diverse major surgeries (eg, cardiac, gastrointestinal, thoracic, gynecologic, hepatobiliary-pancreatic, urologic, vascular) were included in the trial (Table 1; eTables 1, 2, 4, and 5 in Supplement 3), of whom 1213 (98.5%) were assessed for the primary outcome (Figure 1). For 5 patients in the guided group, technical problems prevented clinicians from viewing the electroencephalogram. For 10 patients in the usual care group, clinicians considered it necessary to view the electroencephalogram for periods during the surgical procedure. The median duration of general anesthesia and doses of midazolam, propofol, opioids, and neuromuscular blocking agents were not significantly different between groups (Table 2; eTable 6 in Supplement 3). Median end-tidal volatile anesthetic concentration was lower in the guided group than the control group (0.69 vs 0.80 minimum alveolar concentration; difference, −0.11 [95% CI, −0.13 to −0.10], and median cumulative time with electroencephalogram suppression (7 vs 13-minute; difference, −6.0 [95% CI, −9.9 to −2.1]) and bispectral index less than 40 (32 vs 60-minute; difference, −28.0 [95% CI, −38.0 to −18.0]) were less in the guided group (Figure 2). Although the median duration of hypotension at various thresholds was not significantly different between the groups (eTable7 in Supplement 3), more phenylephrine was administered in the usual care group (Table 2). There were no significant differences in postoperative measures between groups (Table 2).
Table 1.
| No. (%) | ||
|---|---|---|
Guided (n = = 614) 614) | Usual Care (n = = 618) 618) | |
| Age, median (IQR), y | 69.5 (65.0-74.7) | 69.4 (64.7-75.8) |
| Women | 282 (45.9) | 281 (45.5) |
| Men | 332 (54.1) | 337 (54.5) |
| BMI, median (IQR) | 29.0 (25-33) | 29.0 (25-33) |
| Race | ||
| White | 555 (90.4) | 558 (90.3) |
| Black | 54 (8.8) | 53 (8.6) |
| Other | 5 (0.8) | 7 (1.1) |
| Attended college | 198 (36.3) | 208 (37.3) |
| Lifetime tobacco usea | 376 (61.2) | 349 (56.8) |
| Current weekly alcohol useb | 289 (47.1) | 297 (48.1) |
| Current use of anticonvulsants | 94 (15.3) | 81 (13.1) |
| Regular use of opioids | 154 (25.1) | 149 (24.1) |
| Regular use of benzodiazepines | 86 (14.0) | 102 (16.5) |
| ASA physical classification >3c | 209 (34.0) | 221 (35.8) |
| Marginal exercise tolerance (<4 METs) | 297 (50.3) | 295 (50.4) |
| Pulmonary hypertension | 97 (15.8) | 95 (15.4) |
| Aortic stenosis | 90 (14.7) | 108 (17.5) |
| History of or high risk for obstructive sleep apnea | 230 (37.5) | 219 (35.4) |
| History of delirium | 78 (12.8) | 79 (12.9) |
| No. of comorbidities, median (IQR) | 5 (3-6) | 5 (3-6) |
| History of depression | 85 (13.8) | 83 (13.4) |
| PHQ8, median (IQR)d | 3 (1-6) | 3 (0-6) |
| Short Blessed Test for cognition score, median (IQR)e | 2 (0-4) | 2 (0-4) |
| 8-item Interview to Differentiate Aging and Dementia, median (IQR)f | 0 (0-1) | 0 (0-1) |
| Barthel Activities of Daily Living index, median (IQR)g | 15 (15-15) | 15 (15-15) |
| Handgrip strength score, mean (SD), kg | 26.4 (11.0) | 25.7 (10.7) |
| Timed up-and-go score, median (IQR), s | 10.5 (9.2-13.1) | 11.0 (9.4-13.4) |
| Lawton Instrumental Activities of Daily Living, median (IQR)h | 8 (7-8) | 8 (8-8) |
| VR-12 Component Score, mean (SD)i | ||
| Physical | 38.1 (11.9) | 38.2 (11.8) |
| Mental | 53.6 (10.6) | 53.6 (11.0) |
Abbreviations: ASA, American Society of Anesthesiologists; IQR, interquartile range; MET, metabolic equivalent of task; PHQ8, Eight-Item Personal Health Questionnaire; VR-12, Veterans RAND 12-Item Health Survey.
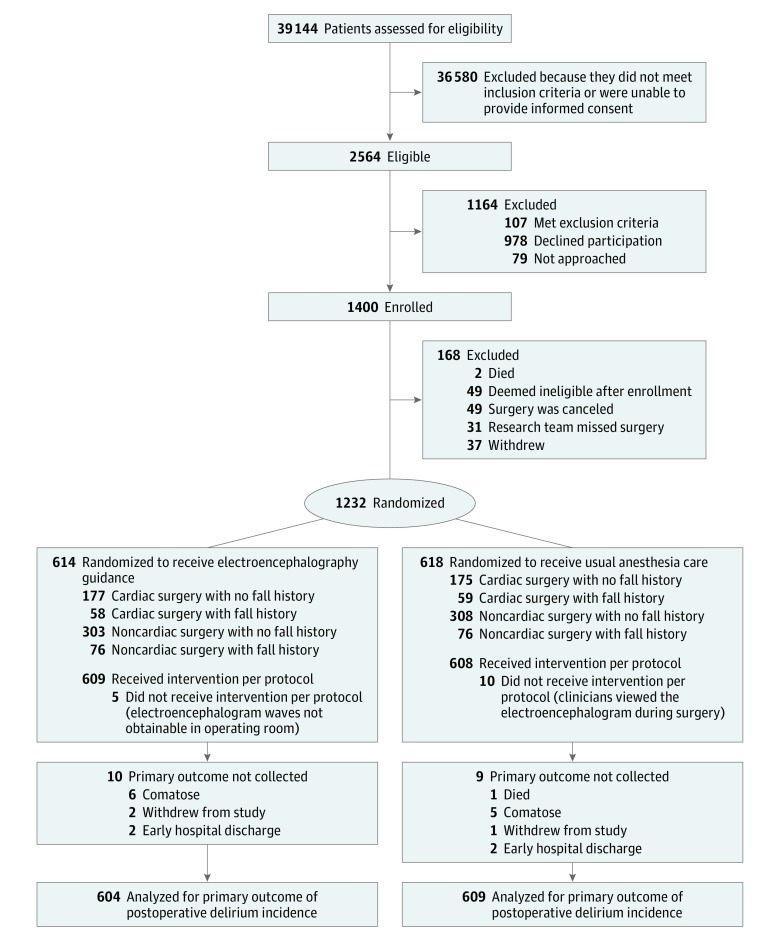
Table 2.
| Measure | Median (IQR) | Difference (95% CI)a | |
|---|---|---|---|
| Guided | Usual Care | ||
| Perioperative medications of interest | |||
| Received midazolam, No./total (%) | 306/614 (49.8) | 328/618 (53.1) | −3.2 (−8.9 to 2.5) |
| Received nondepolarizing neuromuscular blocker, No./total (%) | 570/614 (92.8) | 560/618 (90.6) | 2.2 (−1.0 to 5.5) |
| Intraoperative neuromuscular blocker dose, mg/kgb | 0.82 (0.55 to 1.22) | 0.78 (0.50 to 1.15) | 0.03 (−0.04 to 0.11) |
| Intraoperative opioid dose, mg/kgc | 0.65 (0.39 to 1.02) | 0.58 (0.34 to 1.02) | 0.06 (−0.02 to 0.13) |
| Fentanyl dose, µg | 400 (250 to 750) | 350 (250 to 750) | 50 (−4.45 to 104.45) |
| Hydromorphone dose, mg | 0.20 (0 to 1.50) | 0.23 (0 to 1.25) | 0 (−0.18 to 0.18) |
| Intraoperative phenylephrine dose, mg | 1.37 (0.20 to 5.14) | 2.02 (0.30 to 5.90) | −0.63 (−1.22 to −0.03) |
| Intraoperative measures | |||
| Duration of anesthesia, min | 264.5 (192 to 344) | 264.0 (186 to 349) | 0.5 (−16.7 to 16.7) |
| End-tidal volatile agent concentration, MACd | 0.69 (0.62 to 0.77) | 0.80 (0.71 to 0.86) | −0.11 (−0.13 to −0.10) |
| Duration of BIS <40, mine | 32 (9 to 81) | 60 (19 to 132) | −28 (−38.0 to −18.0) |
| Time with SR >1%, minf | 7 (1 to 23) | 13 (2 to 58) | −6 (−9.9 to −2.1) |
| MAP, mean (SD), mm Hg | 81.2 (8.26) | 79.6 (7.68) | 1.5 (0.63 to 2.42) |
| Duration of MAP <60 mm Hg, min | 7 (2 to 19) | 7 (1 to 19) | 0 (−1.7 to 1.7) |
| Postoperative measures | |||
| Admitted to PACU from OR, No./total (%) | 326/614 (53.1) | 339/618 (54.9) | −1.8 (−7.5 to 3.8) |
| Time spent in the PACU, min | 143 (103 to 183) | 147 (109 to 186) | −3 (−12.4 to 6.4) |
| Admitted to ICU, No./total (%) | 322/614 (52.4) | 297/618 (48.1) | 4.4 (−1.3 to 10.0) |
| Time spent in the ICU, d | 3 (2 to 5) | 3 (2 to 5) | 0 (−1 to 1) |
| Time spent intubated, min | 237.0 (175 to 317) | 231.5 (173 to 305) | 5.5 (−23.0 to 16.0) |
| Hospital length of stay, d | 7 (5 to 11) | 7 (5 to 11) | 0 (−1 to 1) |
Abbreviations: BIS, bispectral index; ICU, intensive care unit; IQR, interquartile range; MAC, minimum alveolar concentration; MAP, mean arterial pressure; OR, operating room; PACU, postanesthesia care unit; SR, electroencephalogram suppression ratio.
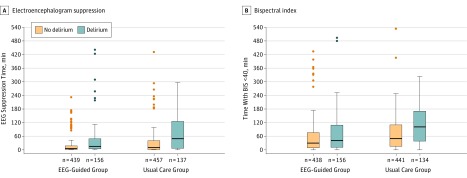
The box-and-whisker plots show the medians (thick horizontal lines) and interquartile ranges (IQRs; boundaries of the box) and ranges. Whisker boundaries are set at 1.5 ×
× IQR. Outliers <3 x IQR are not shown. Outliers shown are the most extreme values at >3 x IQR. The suppression time plots depict the cumulative times in each of the study groups during which the electroencephalogram suppression ratio was >1%. There was no imputation of missing suppression ratio or BIS data. Suppression ratio and BIS data were excluded when the EEG signal quality index was <50. In some cases, these data were missing because of technical difficulties with the BIS equipment. In a few cases, the EEG suppression ratio or BIS data were not stored in the electronic health record. Of the 1213 patients who were assessed for delirium, EEG suppression ratio data are shown for 595 of 604 patients (98.5%) in the guided group and 594 of 609 (97.5%) in the usual care group. BIS <40 data are shown for 594 of 604 patients (98.3%) in the guided group and 575 of 609 (94.4%) in the usual care group. The overall median differences between the randomization groups in times with EEG suppression (7 minutes in the guided group and 13 in the usual care group; difference, 6 minutes [95% CI, 2.2-9.9]) and BIS <40 (32 minutes in the guided group and 60 in the usual care group; difference, 28 minutes [95% CI, 18.0-38.0]) were significant (P
IQR. Outliers <3 x IQR are not shown. Outliers shown are the most extreme values at >3 x IQR. The suppression time plots depict the cumulative times in each of the study groups during which the electroencephalogram suppression ratio was >1%. There was no imputation of missing suppression ratio or BIS data. Suppression ratio and BIS data were excluded when the EEG signal quality index was <50. In some cases, these data were missing because of technical difficulties with the BIS equipment. In a few cases, the EEG suppression ratio or BIS data were not stored in the electronic health record. Of the 1213 patients who were assessed for delirium, EEG suppression ratio data are shown for 595 of 604 patients (98.5%) in the guided group and 594 of 609 (97.5%) in the usual care group. BIS <40 data are shown for 594 of 604 patients (98.3%) in the guided group and 575 of 609 (94.4%) in the usual care group. The overall median differences between the randomization groups in times with EEG suppression (7 minutes in the guided group and 13 in the usual care group; difference, 6 minutes [95% CI, 2.2-9.9]) and BIS <40 (32 minutes in the guided group and 60 in the usual care group; difference, 28 minutes [95% CI, 18.0-38.0]) were significant (P <
< .001). These comparisons between the groups were assessed with the Mann-Whitney U test.
.001). These comparisons between the groups were assessed with the Mann-Whitney U test.
Delirium: Primary Outcome
The delirium incidence during postoperative days 1 through 5 was 157 of 604 patients (26.0%) in the guided group and 140 of 609 (23.0%) in the usual care group (difference, 3.0% [95% CI, −2.0% to 8.0%]; P =
= .22). There were no missing delirium determinations for patients who were alive, in the hospital, and not comatose (eTables 8 and 9 in Supplement 3).
.22). There were no missing delirium determinations for patients who were alive, in the hospital, and not comatose (eTables 8 and 9 in Supplement 3).
Delirium: Exploratory and Post Hoc Outcomes
There were no significant differences between the groups with respect to the exploratory delirium outcomes. (Table 3; eTable 10 in Supplement 3). These outcomes included delirium incidence on the day of the surgical procedure, incidence of delirium in each randomization stratum, time to delirium onset, duration of delirium, and incidence of severe delirium. When the patients who could not be assessed for delirium because of death, coma, withdrawal from study, or early hospital discharge (Figure 1) were all assumed to have incident delirium, the difference between groups in delirium incidence was 3.2% ([95% CI, −2.0% to 8.2%]; P =
= .22). When these patients were all assumed not to have incident delirium, the difference was 2.9% ([95% CI, −2.0% to 7.8%]; P
.22). When these patients were all assumed not to have incident delirium, the difference was 2.9% ([95% CI, −2.0% to 7.8%]; P =
= .26) (eTable 11 in Supplement 3). Three post hoc sensitivity analyses, which modeled improved clinician fidelity to the guided protocol, demonstrated no significant differences in delirium incidence between groups (Table 3). Time to delirium onset was not significantly different between the groups (log-rank P
.26) (eTable 11 in Supplement 3). Three post hoc sensitivity analyses, which modeled improved clinician fidelity to the guided protocol, demonstrated no significant differences in delirium incidence between groups (Table 3). Time to delirium onset was not significantly different between the groups (log-rank P =
= .26) (eFigure 5 in Supplement 3). After covariate adjustment, there remained no significant effect of the intervention on incident delirium. The logistic regression odds ratio for delirium was 1.21 ([95% CI, 0.90-1.64]; P
.26) (eFigure 5 in Supplement 3). After covariate adjustment, there remained no significant effect of the intervention on incident delirium. The logistic regression odds ratio for delirium was 1.21 ([95% CI, 0.90-1.64]; P =
= .21) in the guided group with reference to the usual care group. Using the standardized estimator method with bootstrapping,29 the standardized marginal effect was 0.032 ([95% CI, −0.019 to 0.082]; P
.21) in the guided group with reference to the usual care group. Using the standardized estimator method with bootstrapping,29 the standardized marginal effect was 0.032 ([95% CI, −0.019 to 0.082]; P =
= .22) (eTable 10 in Supplement 3). The per-protocol analysis, based on actual treatment received, showed no significant difference in delirium incidence between the guided and usual care groups (difference, 4.0% [95% CI, −1.0% to 8.8%]; P
.22) (eTable 10 in Supplement 3). The per-protocol analysis, based on actual treatment received, showed no significant difference in delirium incidence between the guided and usual care groups (difference, 4.0% [95% CI, −1.0% to 8.8%]; P =
= .11) (eTable 12 in Supplement 3). The interaction between Charlson Comorbidity Index categories and the randomization groups was not significant (P
.11) (eTable 12 in Supplement 3). The interaction between Charlson Comorbidity Index categories and the randomization groups was not significant (P =
= .06) (eFigure 6 in Supplement 3).
.06) (eFigure 6 in Supplement 3).
Table 3.
| Outcome Category | No./Total No. (%) | Difference, % (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Guided | Usual Care | |||
| Primary outcome | ||||
| Overall delirium incidencec | 157/604 (26.0) | 140/609 (23.0) | 3.0 (−2.0 to 8.0) | .22 |
| Exploratory delirium outcomes | ||||
| Delirium incidence by randomization stratum | ||||
| Noncardiac | ||||
| No fall history | 63/299 (21.1) | 50/304 (16.4) | 4.6 (−1.9 to 11.1) | .15 |
| Fall history | 22/75 (29.3) | 19/76 (25.0) | 4.3 (−10.7 to 19.1) | .55 |
| Cardiac | ||||
| No fall history | 47/173 (27.2) | 47/170 (27.6) | −0.5 (−10.3 to 9.3) | .92 |
| Fall history | 25/57 (43.9) | 24/59 (40.7) | 3.2 (−15.5 to 21.6) | .73 |
| Delirium incidence sensitivity analysesd | ||||
| Excluding EEG suppression | 103/445 (23.2) | 140/609 (23.0) | 0.2 (−5.0 to 5.5) | .95 |
| Excluding BIS <40 | 105/445 (23.6) | 140/609 (23.0) | 0.6 (−4.6 to 6.0) | .82 |
| Excluding Highest MAC | 127/451 (28.2) | 140/608 (23.0) | 5.1 (−0.3 to 10.6) | .06 |
| Delirium duration, median (IQR),de | 1 (1 to 3) | 1 (1 to 3) | 0 (−1 to 1) | .17 |
| No. | 157 | 140 | ||
| Incidence of severe deliriumf | 59/585 (10.1) | 51/591 (8.6) | 1.5 (−2.0 to 4.9) | .39 |
| Delirium on day of surgical procedure | 106/552 (19.2) | 123/561 (21.9) | −2.7 (−7.6 to 2.2) | .26 |
| Adverse events | ||||
| Undesirable intraoperative movement | 137/614 (22.3) | 95/618 (15.4) | 6.9 (2.5 to 11.4) | .002 |
| Intraoperative awareness | 0/563 (0.0) | 0/568 (0.0) | 0 (−0.8 to 0.8) | NA |
| Postoperative nausea and vomiting | 48/614 (7.8) | 55/617(8.9) | −1.1 (−4.3 to 2.1) | .49 |
| Perioperative serious adverse eventsg | 124/614 (20.2) | 130/618 (21.0) | −0.8 (−5.5 to 3.8) | .72 |
| Mortality up to 30 days after surgical procedure | 4/614 (0.7) | 19/618 (3.1) | −2.42 (−4.3 to −0.8) | .004 |
| Exploratory 30-day outcomes | ||||
| 30-day fall incidenceh | 50/503 (9.9) | 38/503 (7.6) | 2.3 (−1.3 to 6.1) | .18 |
| Short Blessed Test score, median (IQR) | 0 (0 to 2) | 0 (0 to 2) | 0 (−0.7 to 0.7) | .48 |
| No. | 418 | 395 | ||
| 8-item Interview to Differentiate Aging and Dementia score, median (IQR) | 0 (0 to 1) | 0 (0 to 1) | 0 (−0.3 to 0.3) | .22 |
| No. | 474 | 451 | ||
| VR-12 Physical Component Score, mean (SD) | 35.5 (10.3) | 35.6 (10.0) | −0.11 (−1.2 to 1.4) | .87 |
| No. | 455 | 471 | ||
| VR-12 Mental Component Score, mean (SD) | 54.0 (10.7) | 53.4 (1.6) | 0.63 (−0.7 to 2.0) | .37 |
| No. | 455 | 471 | ||
Abbreviations: ADL, activities of daily living; BIS, bispectral index; EEG, electroencephalogram; IQR, interquartile range; MAC, minimum alveolar concentration; OSA, obstructive sleep apnea; PONV, postoperative nausea and vomiting; VR-12, Veterans RAND 12-Item Health Survey.
Adverse Events
Undesirable intraoperative movement was reported in 137 of 614 patients (22.3%) in the guided group and 95 of 618 (15.4%) in the usual care group (difference, 6.9% [95% CI, 2.5%-11.4%]). No patients reported intraoperative awareness. Postoperative nausea and vomiting was reported in 48 patients (7.8%) in the guided group and 55 (8.9%) in the usual care group (difference, −1.1% [95% CI, −4.3% to 2.1%]). The number of patients experiencing serious adverse events was not significantly different between the groups (124 [20.2%] in the guided group vs 130 [21.0%] in the usual care group; difference, −0.8% [95% CI, −5.5% to 3.8%]). Vital status was ascertained for all randomized patients 30 days after the surgical procedure. The 30-day mortality rate was 4 of 614 patients (0.7%) in the guided group and 19 of 618 (3.1%) in the usual care group (difference, −2.42% [ 95% CI, −4.3% to −0.8%]) (eFigure 4 in Supplement 3). Other adverse events occurred with similar frequencies in the groups (Table 3; eTable 13 in Supplement 3).
Thirty-Day Outcomes
There were 1209 patients (610 in the guided group and 599 in the usual care group) who were alive and, therefore, eligible to complete the 30-day survey. A total of 1036 patients (85.7%) completed at least part of this survey; 1006 completed the questionnaire on falls and 1010 completed the quality of life questionnaire. None of the 30-day outcomes were found to be statistically significantly different between the groups (Table 3). The 173 nonrespondents had similar preoperative characteristics to those who completed the survey (eTable 3 in Supplement 3). Mean physical component scores of the Veterans Rand 12-Item Health Survey were not significantly different between the groups (35.5 in the guided vs 35.6 in the usual care group; difference, −0.11 [95% CI, −1.2 to 1.4]). Similarly, the mean scores for the mental component of the Veterans Rand 12-Item Health Survey were not significantly different between the groups (54.0 in the guided vs 53.4 in the usual care group; difference, 0.63 [95% CI, −0.7 to 2.0]). Median Short Blessed Test scores (0 [range, 0 to 2]) and Eight-Item Interview to Differentiate Aging and Dementia scores (0 [range, 0 to 1]) were the same in both groups. Fifty patients in the guided group and 38 patients in the usual care group reported falls (difference, 2.3% [95% CI, −1.3 to 6.1]).
Discussion
The primary finding of this trial was that electroencephalography guidance of anesthesia in older adults undergoing major surgery did not decrease the incidence of postoperative delirium, despite successfully reducing anesthetic exposure and duration of electroencephalogram suppression. This finding contrasts with recent meta-analyses that reported greater than a one-third reduction in delirium incidence with electroencephalography guidance of anesthesia.3,10,31 However, the evidence from these meta-analyses has been appraised as moderate in quality.10 The following methodological differences between the current trial and trials comprising the meta-analyses might partially explain the discrepant findings: anesthetic techniques, compliance with trial protocols, population risk profiles, effect of electroencephalogram guidance on anesthetic management, rigor in delirium ascertainment, and reporting of missing data. In the study by Chan et al,2 921 of 1000 patients undergoing noncardiac surgery were randomized, and 902 were assessed for delirium. In contrast to the current trial, patients were healthier and on average underwent shorter surgical procedures. Bispectral index guidance of anesthesia was associated with a significant reduction in anesthetic administration, bispectral index values, and delirium incidence. However, the primary focus was postoperative cognitive dysfunction, and delirium was a secondary outcome only assessed daily with the CAM. Methodological details on the number of missing delirium assessments and training of raters were not reported. In the study by Radtke et al,5 1277 patients of 1600 patients undergoing noncardiac surgery were randomized, and 1155 patients were assessed for delirium. Patients were healthier than in the current trial. Bispectral index guidance of anesthesia was not associated with a significant decrease in average bispectral index values, but was associated with a significant decrease in delirium incidence. However, clinicians unblinded themselves for a quarter of the patients in the control group. With per-protocol analysis, by inclusion of these patients in the bispectral index guided group, the difference in delirium incidence between groups was not significant. Delirium was assessed twice daily in this study, but the number of missing assessments was not reported. In the study by Whitlock et al,3 310 patients undergoing mainly cardiac surgery were examined and delirium was a secondary outcome. In contrast to the study by Chan et al,2 higher volatile anesthetic concentration was associated with lower delirium incidence.3 Bispectral index guidance was not associated with meaningful differences in median anesthetic concentrations or bispectral index values between study groups. Trained intensive care unit nurses assessed patients for delirium twice daily with the CAM-ICU instrument; there were few missing assessments and there was not a statistically significant difference in delirium incidence between groups.
The current trial had methodological strengths. First, barriers to successful conduct of the trial were proactively identified and addressed in a pilot phase, where predetermined milestones were achieved.24 Second, the intervention was successful in modifying anesthetic exposure. This success is important because without demonstrable effect on anesthetic practice parameters, the biological plausibility of a positive finding (ie, a significant decrease in delirium incidence) would be questionable, and the relevance of a negative finding (ie, no significant decrease in delirium incidence) would be diminished. Third, delirium incidence was sufficiently high to allow detection of clinically meaningful intervention effects. Fourth, the CAM instrument has been validated against reference standard Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria in multiple studies, and has been appraised as 1 of the 2 most reliable instruments for detecting delirium in a research context.32 The CAM has been demonstrated to have excellent psychometric properties for both hypoactive and hyperactive delirium.33 Fifth, with programmatic training coupled with highly structured use of the CAM, excellent inter-rater reliability of the researchers was demonstrated.24,25 Sixth, by complementing the CAM with validated chart review,28 delirium detection was bolstered and missed primary outcome assessments were minimized.
The American Geriatric Society, the European Society of Anesthesiologists, and the UK’s National Institute for Health and Care Excellence all recommend that intraoperative electroencephalogram monitoring should be considered to prevent excessive anesthetic administration to patients at high risk of postoperative delirium.34,35,36 The majority of UK anesthesiologists have not adopted this recommendation.37 The results of the current trial challenge the evidence underpinning the recommendation. Moreover, attempting to minimize anesthetic exposure is labor intensive, and may distract from other priorities. Unintended negative consequences might occur, such as undesirable patient movement during surgery. On the other hand, the lower 30-day mortality in the guided group warrants further investigation.
Limitations
This trial had several limitations. First, particular practice patterns at the study’s single center might have negated the benefit of the intervention. Ongoing multi-center trials, like ENGAGES-Canada (NCT02692300) and the Balanced Anesthesia Study,38 might refine the interpretation of this trial. Second, delirium can be difficult to diagnose,39 with no corroborative biomarkers. Attempts to minimize this limitation included following established methods for delirium assessment15,24,25,39 and blinding assessors to treatment assignment. Third, delirium is a fluctuating disorder and could be missed with interval assessments. To address this issue, CAM assessments were complemented with independent structured medical record review for evidence of delirium. Fourth, enrollment in a clinical trial focused on the prevention of delirium could have decreased the likelihood of delirium occuring. However, the overall delirium incidence (24.5%) was in accordance with the a priori estimate for this patient population.15 Fifth, a multicomponent intervention, including postoperative medication simplification, educational materials, and postoperative fall safety planning was implemented15 for all patients in the trial. This implementation might have affected study outcomes, especially falls and quality of life, but the effect should have been the same in both groups. Sixth, the findings might not apply to general anesthesia based on intravenous anesthetic agents. Seventh, the bispectral index monitor’s suppression ratio parameter might underestimate electroencephalogram suppression.40 To mitigate this issue, clinicians were educated to recognize suppression from the electroencephalogram waveforms and not to rely on this derived parameter.
Conclusions
Among older adults undergoing major surgery, electroencephalography-guided anesthetic administration, compared with usual care, did not decrease the incidence of postoperative delirium. This finding does not support the use of electroencephalography-guided anesthetic administration for this indication.
Notes
Supplement 1.
Study protocol and statistical analysis plan
Supplement 3.
List of investigators and committees in the ENGAGES Trial
eTable 1. Patient Sociodemographic Characteristics
eTable 2. Pre-Operative Health Conditions: Comorbidities
eTable 3. Patient Sociodemographic Characteristics and Pre-Operative Health Conditions by 30-16 day follow-up Response
eTable 4. Laboratory Values
eTable 5. Surgery Information
eTable 6. Intraoperative Drug Administration
eTable 7. Intraoperative Mean Arterial Pressure (MAP) and Heart Rate
eTable 8. Number of Delirium Assessments Completed
eTable 9. Reasons CAM or CAM-ICU Assessments Were Not Conducted
eTable 10. Logistic Regression and Covariate Adjustment for Delirium
eTable 11. Sensitivity Analysis – Treating Patients with Missing Assessments as All Positive or 25 All Negative
eTable 12. Sensitivity Analysis – Switching Patients Between Groups per Actual Treatment Received (Per Protocol Analysis)
eTable 13. Frequency of All Serious Adverse Events by Body System and Preferred Term
eTable 14. Causes of Death up to 30 Days After Surgery
eFigure1. ENGAGES Study Electroencephalography (EEG) Guided Protocol Quick Reference 31 Guide
eFigure2. Real-Time Clinician Feedback.
eFigure3. Anesthesia Fidelity Checklist
eFigure4. Kaplan-Meier Curve: 30 Day Survival
eFigure5. Kaplan-Meier Curve: Cumulative Incidence of Delirium
eFigure6. Delirium Incidence by Comorbidity
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2018.22005
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jama/articlepdf/2724026/jama_wildes_2019_oi_180159.pdf
Citations & impact
Impact metrics
Article citations
Post-operative delirium in different age groups and subtypes: a systematic review of case reports.
Front Neurol, 15:1465681, 10 Oct 2024
Cited by: 0 articles | PMID: 39450048 | PMCID: PMC11499180
Review Free full text in Europe PMC
Index of Consciousness monitoring may effectively predict and prevent circulatory stress induced by endotracheal intubation under general anesthesia: a prospective randomized controlled trial.
BMC Anesthesiol, 24(1):316, 06 Sep 2024
Cited by: 0 articles | PMID: 39243003 | PMCID: PMC11378600
Emerging Trends and Hot Topics of Non-Invasive Electroencephalography Research in the Elderly: A Bibliometric Analysis from 2014 to 2023.
Med Devices (Auckl), 17:311-322, 27 Aug 2024
Cited by: 0 articles | PMID: 39219987 | PMCID: PMC11365532
Electroencephalography-Guided Anesthesia and Delirium in Older Adults After Cardiac Surgery: The ENGAGES-Canada Randomized Clinical Trial.
JAMA, 332(2):112-123, 01 Jul 2024
Cited by: 0 articles | PMID: 38857019
Effectiveness Assessment of Bispectral Index Monitoring Compared with Conventional Monitoring in General Anesthesia: A Systematic Review and Meta-Analysis.
Anesthesiol Res Pract, 2024:5555481, 07 Aug 2024
Cited by: 0 articles | PMID: 39149130 | PMCID: PMC11325011
Review Free full text in Europe PMC
Go to all (156) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT02241655
- (1 citation) ClinicalTrials.gov - NCT02692300
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Electroencephalography-Guided Anesthesia and Delirium in Older Adults After Cardiac Surgery: The ENGAGES-Canada Randomized Clinical Trial.
JAMA, 332(2):112-123, 01 Jul 2024
Cited by: 0 articles | PMID: 38857019
Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: The RAGA Randomized Trial.
JAMA, 327(1):50-58, 01 Jan 2022
Cited by: 100 articles | PMID: 34928310 | PMCID: PMC8689436
ADAPT-2: A Randomized Clinical Trial to Reduce Intraoperative EEG Suppression in Older Surgical Patients Undergoing Major Noncardiac Surgery.
Anesth Analg, 131(4):1228-1236, 01 Oct 2020
Cited by: 17 articles | PMID: 32925344 | PMCID: PMC7599075
Neuromonitoring in the elderly.
Curr Opin Anaesthesiol, 32(1):101-107, 01 Feb 2019
Cited by: 7 articles | PMID: 30507680
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: UH2 HL125119
NIA NIH HHS (3)
Grant ID: UH3 AG050312
Grant ID: R01 AG057901
Grant ID: R24 AG054259
NIGMS NIH HHS (1)
Grant ID: T32 GM108539
 1, for the ENGAGES Research Group
1, for the ENGAGES Research Group




