Abstract
Objectives
This study sought to determine the rate of use of target doses of foundational guideline-directed medical therapy (GDMT) in a contemporary cohort of patients with heart failure with reduced ejection fraction (HFrEF) across systolic blood pressure (SBP) categories.Background
Patients with HFrEF are infrequently titrated to recommended doses of GDMT. The relationship between SBP and achieving GDMT target doses is not well studied.Methods
Patients enrolled in the CHAMP-HF (Change the Management of Patients With Heart Failure) registry without documented intolerance to angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), angiotensin receptor-neprilysin inhibitors (ARNIs), and beta blockers (BBs) were assessed at enrollment. We estimated the proportion receiving target doses (% of target dose [95% confidence interval (CI)]) based on the most recent American College of Cardiology/American Heart Association/Heart Failure Society of America heart failure guidelines at baseline in all patients, and by SBP category (≥110 vs. <110 mm Hg).Results
Of the 3,095 patients eligible for analysis, 2,421 (78.2%) had SBP ≥110 mm Hg. The proportion of patients receiving target doses were 18.7% (95% CI: 17.3% to 20.0%; BB), 10.8% (95% CI: 9.7% to 11.9%; ACEI/ARB), and 2.0% (95% CI: 1.5% to 2.5%; ARNI). Among those with SBP <110 mm Hg (n = 674), 17.5% (95% CI: 14.6% to 20.4%; BB), 6.2% (95% CI: 4.4% to 8.1%; ACEI/ARB), and 1.8% (95% CI: 0.8% to 2.8%; ARNI) were receiving target doses. Among those with SBP ≥110 mm Hg (n = 2,421), 19.0% (95% CI: 17.4% to 20.6%; BB), 12.1% (95% CI: 10.8% to 13.4%; ACEI/ARB), and 2.0% (95% CI: 1.5% to 2.6%; ARNI) were receiving target doses.Conclusions
In a large, contemporary registry of outpatients with chronic HFrEF eligible for treatment with BBs and ACEI/ARB/ARNI, <20% of patients were receiving target doses, even among those with SBP ≥110 mm Hg.Free full text

Target Doses of Heart Failure Medical Therapy and Blood Pressure: Insights from the CHAMP-HF Registry
Associated Data
Abstract
Background:
Patients with heart failure and reduced ejection fraction (HFrEF) are infrequently titrated to recommended doses of guideline-directed medical therapy (GDMT). The relationship between systolic blood pressure (SBP) and achieving GDMT target doses is not well studied.
Objective:
To determine the rate of use of target doses of foundational GDMT in a contemporary cohort of patients with HFrEF across SBP categories.
Methods:
Patients enrolled in the CHAMP-HF registry, without documented intolerance to ACE Inhibitors, (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor- neprilysin inhibitors (ARNI), and beta-blockers (BB) were assessed at enrollment. We estimated the proportion receiving target doses [% of target dose (95% confidence interval)] based on the most recent ACC/AHA/HFSA heart failure guidelines at baseline in all patients, and by SBP category (≥110 versus <110 mmHg).
Results:
Of the 3095 patients eligible for analysis, 2421, (78.2%) had SBP ≥110 mmHg. The proportion of patients receiving target doses were 18.7% (17.3–20.0; BB), 10.8% (9.7–11.9; ACEI/ARB), and 2.0% (1.5–2.5; ARNI). Among those with SBP <110 mmHg (n= 674), 17.5% (14.6–20.4; BB), 6.2 % (4.4–8.1 ACEI/ARB), and 1.8% (0.8–2.8; ARNI) were receiving target doses. Among those with SBP ≥110 mmHg (n=2421), 19.0% (17.4–20.6; BB), 12.1% (10.8–13.4; ACEI/ARB), and 2.0% (1.5–2.6; ARNI) were receiving target doses.
Conclusions:
In a large, contemporary registry of outpatients with chronic HFrEF eligible for treatment with BB and ACEI/ARB/ARNI, less than 20% of patients were receiving target doses, even among those with SBP ≥110 mmHg.
CONDENSED ABSTRACT
Beta blockers (BB), angiotensin receptor inhibitors/angiotensin receptor blockers (ACEI/ARB) and angiotensin-neprilysin inhibitor (ARNI) reduce mortality in patients with HFrEF, with the evidence suggesting a dose related benefit. Guidelines suggest titration to target doses among patients without intolerances. Systolic blood pressure (SBP) can be a limiting factor to intensifying HFrEF therapy, but the relationship between SBP and achieving target doses is unclear. The present study demonstrates that the prevalence those receiving target doses of ACEI/ARB/ARNI and BB was <20% across different SBP categories. This calls for increased efforts to optimize medication dosing in patients with HFrEF.
Heart failure with reduced ejection fraction (HFrEF) is associated with significant morbidity and mortality (1). Over the past few decades, advances in medical therapy have resulted in a substantial reduction in poor cardiovascular outcomes (2-4). Although inhibition of the beta adrenergic system with beta-blockers (BB) and inhibition of the angiotensin pathway with ACE inhibitors (ACEI)/ angiotensin receptor blockers (ARBs) have been the cornerstone of HFrEF treatment(5), sacubitril/valsartan, which belongs to the class of angiotensin II receptor blocker-neprilysin inhibitors (ARNI), has recently been shown to provide additional morbidity and mortality reduction over enalapril alone among patients on background BB therapy (5,6).
In patients with HFrEF, target medication doses established in clinical trials are encouraged in national guidelines (5). This stems from evidence showing dose-related relationships between outcomes and ACEI (7) and BB doses (8,9). Despite this robust evidence, target doses of these important therapies are often underused in clinical practice (10,11). One potential barrier to intensifying guideline directed medical therapy (GDMT) may be concerns regarding low blood pressure, which can cause symptomatic hypotension and fatigue (12). Low blood pressure, as a barrier to achieving targeted dosing of GDMT in routine clinical care has not been described. Understanding whether blood pressure is a barrier to achieving targeted doses of HFrEF therapy can help guide how to achieve more aggressive dosing of evidence-based therapies. To address this gap, we used a large, multi-center, contemporary outpatient registry of patients with HFrEF to conduct an analysis to describe the intensity of HF medication dosing and to determine the proportion of patients in whom lower blood pressures might be a barrier to the intensification of ACEI/ARB/ARNIs or BBs.
Methods
Study Design
For this cross sectional analysis, we used data at enrolment from the CHAnge the Management of Patients with Heart Failure (CHAMP-HF) registry: a prospective, observational study of outpatients with HFrEF at 151 US practice sites (13). Patients eligible for enrollment met the following criteria: (1) age ≥ 18 years, (2) primary diagnosis of HFrEF (LVEF ≤ 40% within twelve months of enrollment), (3) prescribed ≥ 1 oral pharmacotherapy for HF at the time of enrollment, and (4) willingness to complete protocol requirements for study visits, procedures, and questionnaires. Patients were excluded if they were ineligible (actively participating in an interventional clinical research study, receiving comfort care measures only or enrolled in a hospice program, had a life expectancy of less than one year, and had a history of, or planned heart transplant, left ventricular assist device implantation, or dialysis). Additionally, we excluded those who reported HF medication-related side effects (new/worsening cough, worsening renal dysfunction, renal failure, angioedema, dizziness/lightheadedness/hypotension, clinically significant increase in serum potassium), had a known contraindication to ACEI/ARB, ARNI or BB, or were missing SBP and demographic information at enrollment.
Additional data collected at enrollment included patient-level demographics and clinical characteristics, medical history, laboratory results, use of HF medications and devices, and patient-reported health status. Eligible sites were identified based upon the completion of a feasibility survey, which provided investigators with the opportunity to ensure broad geographic and provider specialty representation. Study coordinators at each site were responsible for identification and enrollment of subjects during the course of a scheduled outpatient visit, with this analysis being limited to only those patients enrolled between December 2015 and August 2017. CHAMP-HF was sponsored by Novartis Pharmaceuticals Corporation, and all participating sites obtained local or central institutional review board approval prior to patient enrollment as well as informed consent from each participant.
Data Collection
Site coordinators interviewed patients to collect their sociodemographic characteristics and health status, and abstracted information from the medical record regarding medical history and medications at enrollment. The primary outcome for this analysis was the achievement of target dose beta blocker (≥100% target dose (yes/no)) and ACEI/ARB/ARNI (≥100% target dose (yes/no)), based on each patient’s daily dose at enrollment compared with the guideline-recommended target daily doses (Supplemental Table 1)(5,14). We limited our analysis to these medication classes because, barring any intolerance, guidelines recommend all patients with HFrEF be placed on maximally tolerated (target) doses of these medications before considering additional therapy(14). Medication daily dose was calculated based on dose per administration and frequency. Systolic blood pressure readings were obtained from the patients’ baseline clinic visit as was routine for each participating site.
Statistical Analysis
The enrollment characteristics of the CHAMP-HF cohort were stratified by SBP categories (<110 versus ≥ 110 mmHg). We used proportions for categorical variables and medians with quartiles for continuous variables. We calculated the proportion of patients receiving ACEI/ARB/ARNI and BBs in the overall cohort and by SBP groups. The proportion of patients achieving ≥100% of the target dose are presented along with 95% confidence intervals. The proportion of patients on <50% and 50 to <100% were also calculated, χ2 test was used to compare the proportion of medication classes across SBP groups.
Distribution of SBP was plotted for patients who were not on GDMT defined as fulfilling any of the following; 1) target dose of BB but not target dose of ACEI/ARB/ARNI; 2) target dose of ACEI/ARB/ARNI but not target dose of BB; 3) neither target dose of BB nor target dose of ACEI/ARB/ARNI, so that the percent of patients not receiving guideline-recommended dosing across different SBP thresholds could be described. A sensitivity analysis was conducted using the following SBP cut points (100, 120 and 130 mmHg) to examine the effects on the results. An additional sensitivity analysis excluded those with HR <60 beats per minute, because bradycardia may be a reason for non-intensification of BB.
All analyses were performed using SAS software (version 9.4 SAS Institute, Cary, NC). Analyses were performed independently by the Duke Clinical Research Institute, and the lead author takes responsibility for guiding data analysis and interpretation.
Results:
There were 4,493 patients enrolled across 151 sites in the CHAMP-HF registry between Dec 2015 and Aug 2017. After exclusion of patients ineligible for the registry (n = 42), those with any documented medication-related side effects that could limit use or dosing (n =1105), specific contraindications to ACEI/ARB/ARNI or BB (n = 84), missing SBP (n = 161), and missing demographic information (n= 6), 3,095 patients across 148 sites were included in the final study cohort (Online Figure 1). The median SBP was 120 mmHg [IQR110 - 130] and median diastolic blood pressure (DBP) was 72 mmHg [IQR 64 – 80]. The majority of patients were male (70.6%), older than 64 years (59.6%), white (73.8%) and had SBP ≥110 mmHg (n = 2421, 78.2%) (Table 1). The median Kansas City Cardiomyopathy Questionnaire-Short Form overall summary score (KCCQ-OS) was 69 (IQR 49 – 85.4) and most patients (84%, n = 2601) had either New York Heart Association (NYHA) II or III symptoms. The median left ventricular ejection fraction (LVEF) was 30% (IQR 23 -36).
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; BB = beta blockers. SBP = systolic blood pressure
Table 1.
Demographic and Clinical Characteristics of the Study Population overall and by SBP Categories
| Systolic Blood Pressure | |||
|---|---|---|---|
| Overall N=3095 | <110 mmHg N=674 | ≥110 mmHg N=2421 | |
| Demographics | |||
 Age (y) Age (y) | |||
 Median (Q1-Q3) Median (Q1-Q3) | 68.0 (59.0-75.0) | 66.0 (57.0-74.0) | 68.0 (59.0-76.0) |
 <40 <40 | 100 (3.2%) | 35 (5.2%) | 65 (2.7%) |
 40-64 40-64 | 1,150 (37.2%) | 275 (40.8%) | 875 (36.1%) |
 65-80 65-80 | 1,448 (46.8%) | 288 (42.7%) | 1,160 (47.9%) |
 80+ 80+ | 397 (12.8%) | 76 (11.3%) | 321 (13.3%) |
 Gender Gender | |||
 Male Male | 2,184 (70.6%) | 477 (70.8%) | 1,707 (70.5%) |
 Female Female | 911 (29.4%) | 197 (29.2%) | 714 (29.5%) |
 Race Race | |||
 White White | 2,283 (73.8%) | 513 (76.1%) | 1,770 (73.1%) |
 Black Black | 519 (16.8%) | 111 (16.5%) | 408 (16.9%) |
 Other/unknown Other/unknown | 293 (9.5%) | 50 (7.4%) | 243 (10.0%) |
| Medical history | |||
 Chronic renal insufficiency Chronic renal insufficiency | 552 (17.8%) | 127 (18.8%) | 425 (17.6%) |
 COPD COPD | 943 (30.5%) | 196 (29.1%) | 747 (30.9%) |
 Dementia Dementia | 69 (2.2%) | 11 (1.6%) | 58 (2.4%) |
 Diabetes mellitus Diabetes mellitus | 1,285 (41.5%) | 251 (37.2%) | 1,034 (42.7%) |
 Hepatic dysfunction Hepatic dysfunction | 64 (2.1%) | 19 (2.8%) | 45 (1.9%) |
 Peripheral artery disease Peripheral artery disease | 422 (13.6%) | 75 (11.1%) | 347 (14.3%) |
 Stroke/TIA Stroke/TIA | 325 (10.5%) | 69 (10.2%) | 256 (10.6%) |
 Ischemic heart disease Ischemic heart disease | 1,195 (38.6%) | 258 (38.3%) | 937 (38.7%) |
 Myocardial infarction Myocardial infarction | 1,039 (33.6%) | 244 (36.2%) | 795 (32.8%) |
 Hypertrophic cardiomyopathy Hypertrophic cardiomyopathy | 214 (6.9%) | 22 (3.3%) | 192 (7.9%) |
 Restrictive cardiomyopathy Restrictive cardiomyopathy | 43 (1.4%) | 9 (1.3%) | 34 (1.4%) |
| Vital signs | |||
 Systolic Blood Pressure (mmHg) Systolic Blood Pressure (mmHg) | 120 (110 – 130) | 100 (96 – 105) | 124 (118 – 136) |
 Diastolic blood pressure (mmHg) Diastolic blood pressure (mmHg) | 72 (64-80) | 62 (60-69) | 75 (70-80) |
 Heart rate (bpm) Heart rate (bpm) | 72 (66-81) | 73 (66-82) | 72 (65-80) |
| Clinical measures/Labs | |||
 NYHA Classification NYHA Classification | |||
 I I | 339 (11.0%) | 64 (9.5%) | 275 (11.4%) |
 II II | 1,787 (57.7%) | 366 (54.3%) | 1,421 (58.7%) |
 III III | 814 (26.3%) | 206 (30.6%) | 608 (25.1%) |
 IV IV | 69 (2.2%) | 20 (3.0%) | 49 (2.0%) |
 LVEF (%)) LVEF (%)) | 30 (22-36) | 28 (20-33) | 32 (25-37) |
 <20 <20 | 324 (10.5%) | 131 (19.4%) | 193 (8.0%) |
 20-25 20-25 | 722 (23.3%) | 174 (25.8%) | 548 (22.6%) |
 26-35 26-35 | 1,257 (40.6%) | 255 (37.8%) | 1,002 (41.4%) |
 36-40 36-40 | 772 (24.9%) | 106 (15.7%) | 666 (27.5%) |
 eGFR (mL/min/1.73 m2) eGFR (mL/min/1.73 m2) | 60 (50-70) | 60 (47-61) | 60 (51-73) |
 <30 <30 | 94 (3.0%) | 31 (4.6%) | 63 (2.6%) |
 30-45 30-45 | 259 (8.4%) | 67 (9.9%) | 192 (7.9%) |
 45-60 45-60 | 425 (13.7%) | 116 (17.2%) | 309 (12.8%) |
 >=60 >=60 | 1,205 (38.9%) | 239 (35.5%) | 966 (39.9%) |
| Medication at enrollment | |||
 ACEI ACEI | 1,279 (41.3%) | 286 (42.4%) | 993 (41.0%) |
 ARB ARB | 646 (20.9%) | 99 (14.7%) | 547 (22.6%) |
 MRA MRA | 1,006 (32.5%) | 298 (44.2%) | 708 (29.2%) |
 Beta blocker Beta blocker | 2,560 (82.7%) | 577 (85.6%) | 1,983 (81.9%) |
 ARNI ARNI | 400 (12.9%) | 118 (17.5%) | 282 (11.6%) |
| Quality of life assessment | |||
 KCCQ12 KCCQ12 | 68.8 (49.0-85.4) | 65.4 (45.8-84.0) | 69.3 (50.0-85.4) |
Data are median (interquartile range) or n (%). ACEI = angiotensin converting enzyme inhibitor ; ARNI =angiotensin receptor- neprilysin inhibitor ;ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; KCCQ = Kansas City Cardiomyopathy Questionnaire ; LVEF= left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; TIA = transient ischemic attack
Among those with SBP <110, the median SBP /DBP was 100 (IQR 96 – 105)/62 (IQR 60 -69), compared with 124 (IQR 118 – 136)/75 (IQR 70 – 80) among those with SBP ≥110 mmHg. The median heart rate was 73 (IQR 66 – 82) among those with SBP <110 and 72 (IQR 65 -80) among those with SBP≥110 mmHg (Table 1). BB use was lower (81.9% vs 85.6%; p=0.03), ARB use was higher (22.6% vs 14.7 %; p<0.0001), ARNI use was lower (11.6% vs 17.5%; p <0.0001) and ACEI use was similar (41.0% vs 42.4%; p = 0.5) in patients with SBP ≥110 mmHg, compared with those with SBP <110 mmHg. The prevalence of comorbidities and other baseline characteristics by SBP categories are presented in Table 1. The distribution of SBP among patients not receiving target doses had a mean of 120 mmHg and a standard deviation of 17 (Online Figure 2).
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; BB = beta blockers. SBP = systolic blood pressure
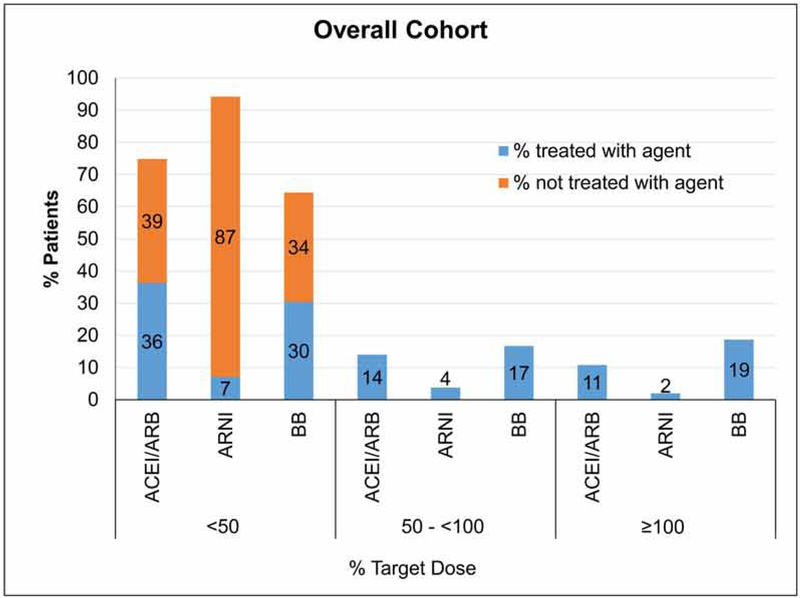
Proportion Receiving Target Doses of ACEI/ARB/ARNI or BBs
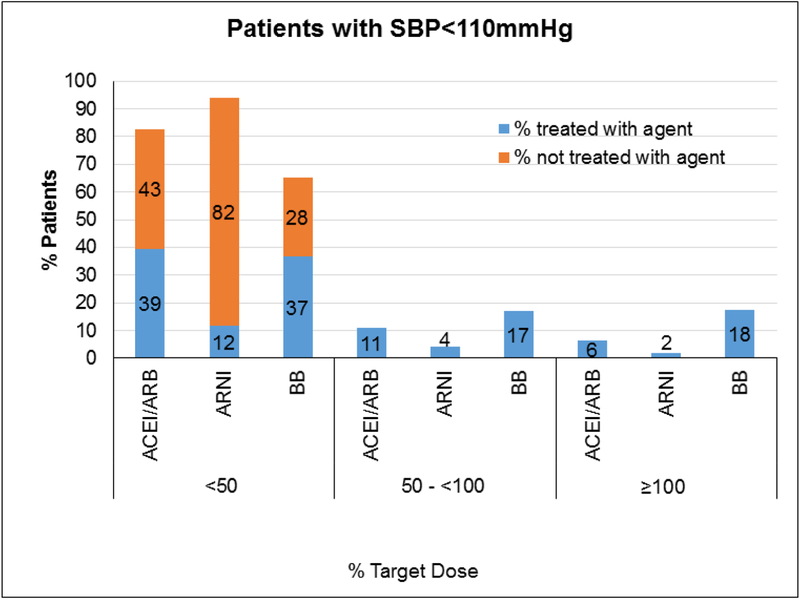
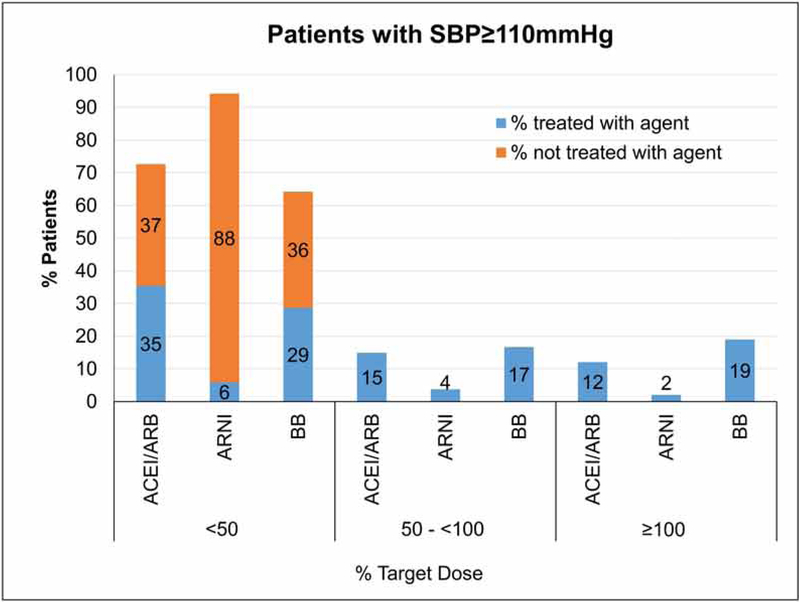
Among the 3,095 patients who were eligible for ACE/ARB/ARNI and BB, 61% were receiving ACEI/ARB, 12.9% were receiving ARNI, and 82.7% were receiving a BB (Figure 1). The proportion of patients receiving target doses for these medication classes were 10.8% (95% CI 9.7– 1.9) for ACEI/ARB, 2.0% (95% CI 1.5–2.5) for ARNI, and 18.7 % (95% CI 17.3–20.0) for BBs (Table 2). Among those with SBP <110 mmHg and were eligible for both ACEI/ARB/ARNI and BB (n= 674), 6.2 % (95% CI 4.4–8.1), 1.8% (95% CI 0.8–2.8) and 17.5% (95% CI 14.6–20.4), were receiving target doses of ACEI/ARB, ARNI and BBs, respectively. A greater proportion of patients were receiving <50% of target doses than were receiving > 50% (Table 2, Figure 2). Among those with SBP ≥110 mmHg who were eligible for both ACE/ARB/ARNI and BB (n=2421), the proportion of patients receiving target doses were 12.1% (95% CI 10.8–13.4) for ACEI/ARB, 2.0% (95% CI 1.5–2.6) for ARNI, and 19.0% (95% CI 17.4–20.6) for BBs. Similarly, a greater proportion of patients were receiving < 50% than were receiving > 50% of target doses for each drug class despite having SBP ≥110mm Hg. (Table 2, Figure 3).
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; BB = beta blockers. SBP = systolic blood pressure
Table 2:
Baseline medication in the overall cohort (n = 3095), among patients with SBP<110 mmHg (N=674), and SBP≥110 mmHg (N=2421)
| Treated (n) | Not Treated (n) | <50% TD (n) | 50% to <100% TD (n) | ≥100% TD (n) | %TD (95% CI) | |
|---|---|---|---|---|---|---|
| Overall Cohort | ||||||
| ACEI/ARB | 1901 | 1194 | 1122 | 434 | 334 | 10.8 (9.7, 11.9) |
| ARNI | 400 | 2695 | 220 | 118 | 61 | 2.0 (1.48, 2.46) |
| Beta Blocker | 2043 | 1052 | 942 | 518 | 578 | 18.7 (17.3, 20.0) |
| SBP<110 mmHg | ||||||
| ACEI/ARB | 382 | 292 | 265 | 73 | 42 | 6.2 (4.41, 8.06) |
| ARNI | 118 | 556 | 78 | 28 | 12 | 1.8 (0.78, 2.78) |
| Beta Blocker | 483 | 191 | 248 | 114 | 118 | 17.5 (14.6, 20.4) |
| SBP ≥100 mmHg | ||||||
| ACEI/ARB | 1519 | 902 | 857 | 361 | 292 | 12.1 (10.8, 13.4) |
| ARNI | 282 | 2139 | 142 | 90 | 49 | 2.0 (1.46, 2.58) |
| Beta-Blocker | 1560 | 861 | 694 | 404 | 460 | 19.0 (17.4, 20.6) |
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; TD = Target dose.
%TD = n(≥100%TD)/n(Treated)
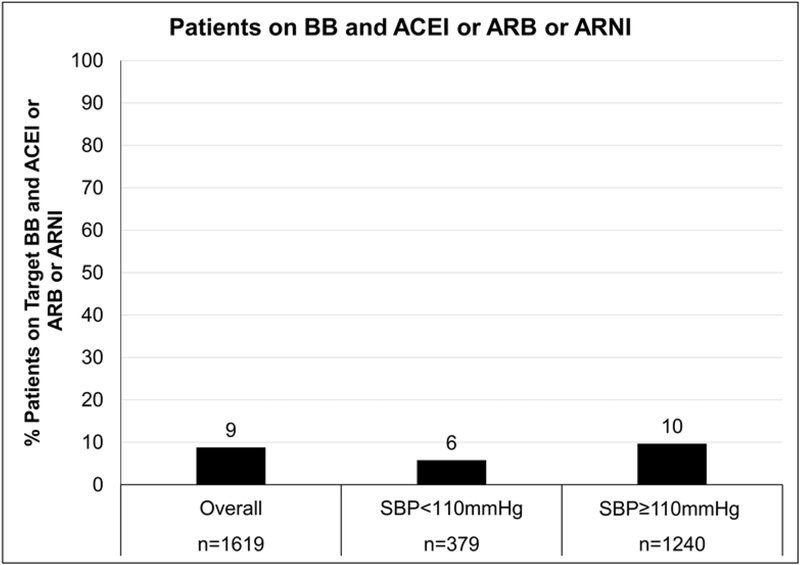
Finally, among patients who were already prescribed two classes of medications including a BB (i.e an ACEI/ARB/ARNI and a BB, (n= 1,619)), only 8.8% (n= 142) were receiving target doses of both (Table 3, Figure 4). Among those with SBP <110 mmHg, 5.8% (n =22) were receiving target doses of both an ACEI/ARB/ARNI and a BB (Table 3). For those with SBP ≥110 mmHg, 9.7% (n = 120) were receiving target doses of both an ACEI/ARB/ARNI and a BB (Table 3). A sensitivity analysis excluding patients with heart rate < 60 bpm produced qualitatively similar results (Supplemental Tables 2a-c). In an additional sensitivity analysis, the proportion of patients receiving target doses of ACEI/ARB increased slightly with increase in SBP cutoff (11.2%, 13.6%, 17.3% among patients with SBP ≥ 100, ≥ 120, and ≥ 130 mmHg respectively; Supplemental Tables 3a, 4a and 5a). The results were otherwise similar to the primary analysis (Supplemental Tables 3-5).
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; BB = beta blockers. SBP = systolic blood pressure
Table 3.
Summary of patients receiving target BB and ACEI/ARB/ARNI doses among those on both a BB and one of ACEI/ARB/ARNI (N=1619)
| Target BB | Target ACEI/ARB/ARNI SBP<110 mmHg | Target ACEI/ARB/ARNI SBP≥110 mmHg | ||||
|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | |
| No | 263 69.4% | 16 4.2% | 279 73.6% | 755 60.9% | 116 9.4% | 871 70.2% |
| Yes | 78 20.6% | 22 5.8% | 100 26.4% | 249 20.1% | 120 9.7% | 369 29.8% |
| Total | 341 90.0% | 38 10.0% | 379 100% | 1004 81.0% | 236 19.0% | 1240 100% |
ACEI = angiotensin converting enzyme inhibitor; ARNI =angiotensin receptor- neprilysin inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker
Discussion:
Translating the benefits of medical therapies that were previously observed in clinical trials to clinical practice partly depends on using therapies in a manner similar to that tested in the clinical trials and endorsed by guidelines. In fact, in an explicit test of the intensity of ACEI dose, the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial found lower rates of mortality and hospitalization with higher vs lower dose lisinopril(7). Similar dose dependent benefit has been demonstrated with angiotensin blockers and beta blockers (8,15). We analyzed a contemporary cohort of patients with HFrEF enrolled in the CHAMP-HF registry because the prescription patterns of dosing intensity according to blood pressure in patients with HFrEF in contemporary US practice is not known. We found an overwhelming majority of patients eligible for either BBs or ACEI/ARB/ARNI were not receiving target doses and that most patients were on <50% of guideline recommended target doses, even after excluding those with medication-specific intolerance or contraindications. Importantly, SBP did not appear to be a barrier to intensifying treatment overall, as marked under treatment was noted in those with a SBP ≥110 mmHg, with an overall similar pattern noticed when we considered SBP ≥120 and SBP≥130 mmHg. Despite a majority of our cohort (52.3%) receiving both a BB and an ACEI/ARB/ARNI, the proportion of patients receiving target doses of both types of medication (beta blockade and angiotensin inhibition) was only slightly higher among those with SBP ≥110 mmHg (9.7%) compared with those having a SBP <110 mmHg (5.8%). Finding that less than 1 in 10 patients in both cohorts were optimally treated with GDMT highlights an important opportunity to intensify foundational HF therapies to potentially further improve morbidity and mortality (9,16).
We used an SBP cutoff of 110 mmHg in our primary analyses because prior analyses utilizing an SBP threshold of 110 showed that while overall risk of death and hospitalizations increase below this threshold in HFrEF, ARB(17), ARNI(18) and BB(19) were associated with reduced mortality and hospitalizations even at SBP < 110 mmHg. We also selected this threshold to reflect a range above which (i) clinicians will likely be more comfortable initiating or intensifying HF medications that affect systemic blood pressure and (ii) further reductions in SBP documented in clinical trials with up-titration were unlikely to result in symptomatic hypotension (17,18). Yet, even with higher SBP levels in sensitivity analyses, the proportion of patients receiving suboptimal medication doses remained high.
Our study adds to the body of evidence showing that despite the dose-response relationship for GDMT with outcomes (20-22), sub-optimal dosing of HF medications remain highly prevalent, and provides new insight that SBP may not be a primary barrier to dosing intensification. Sub-optimal dosing has previously been observed in a population of outpatients with HFrEF(23) and in patients who underwent ICD implantation, an indication predicated upon the use of GDMT at appropriate doses (24). Patient factors (e.g. older age, comorbid lung disease or diabetes, intolerance or contraindications medication cost), and physician factors (e.g. physician knowledge/global self-confidence) have been suggested as potential reasons for suboptimal dosing of HF therapy (25,26). Our study prospectively collected some of these data and excluded patients intolerant of ACEI/ARB/ARNIs and BBs while further exploring whether blood pressure and heart rate could have potentially precluded the institution or intensification of these heart failure therapies (12,27).
Clinical hypotension is commonly considered at SBP <90 mmHg (28), and if present, may cause clinicians to defer or de-escalate therapy (especially if patients are symptomatic). Moreover, SBP ranges between 90 – 110 mmHg may still generate considerable provider discomfort with respect to intensifying HF medications that reduce SBP. However, an SBP >110 mmHg is not considered hypotension, and we posit that a greater proportion of clinicians should be comfortable instituting or intensifying HF medical therapy at these levels.
While low SBP has been associated with poor prognosis in HFrEF (29-31), angiotensin inhibition and beta-blockade have demonstrated improved outcomes, independent of baseline blood pressure (18,21). Our findings of lower overall use of target doses of BBs and ACEI/ARB/ARNI in those with SBP <110 mmHg may reflect clinician hesitation to up-titrate medical therapy due to the association of low SBP and worse outcomes. However, blood pressure level should not be a significant factor among those with SBP ≥110 mmHg. There is a need for qualitative studies to further refine our understanding of how clinicians use SBP in decision making on intensification of medication therapy in HF. In the interim, more aggressive efforts to ensure appropriate medication dosing such as the creation of EMR or clinic-based performance measures may be needed.
Clinical inertia, may contribute to suboptimal dosing of effective therapies (10). Clinical inertia is a well described concept that refers to the failure to act on a clinically identified problem(32). Although we posited that blood pressure might be a potential factor contributing to clinical inertia related to intensifying HF therapy, it seemed to have at most a modest impact, suggesting that other factors contributed to the under-dosing of HF medications. There is an urgent need for concurrent efforts aimed at availing patients of the best medical therapy while simultaneously investigating factors related to sub-optimal dosing of important HF medications in patients who can tolerate them. Despite the advances in treatment, mortality rates for HFrEF remain high (33,34), and the previously observed improvements in mortality among patients with HF appears to have slowed down over recent decades (35). Given the multiple observations that sub-optimal dosing of critical HF therapy is highly prevalent, and the potential survival benefit associated with optimal dosing of HF therapy(36,37), there is a critical opportunity for developing and promoting strategies aimed at stimulating wide spread adoption of optimal HF treatments, including treating to effective medication doses. Performance measures are one way of stimulating adoption of best medical practices, but current performance measures rely on the presence or absence of use of specific medication classes and not the intensity of treatment(38). Given the evidence that medication dose is important to achieve better outcomes in HFrEF, performance measures should consider evolving to reflect the achievement of appropriate doses.
Limitations:
Our study is a descriptive analysis and was intended to explore a potential rationale for not intensifying HF medications to guideline-recommended doses. As a cross-sectional analysis, we cannot comment on changes in treatment over time (in subsequent visits), although patients had been under the care of their providers prior to enrollment in CHAMP-HF. We did not include other evidence-based mediations such as mineralocorticoid antagonists (MRA), which also reduce blood pressure. However, MRA use is typically recommended after optimization of ACEI/ARB and BBs. Although we included a diverse cohort from 151 practices, these findings may not be generalizable to other US patients and practices. While careful attention was paid to adjudicating intolerances, there may have been patients with undocumented intolerances or preferences precluding the use of BBs or ACEI/ARB/ARNI. Further, we assessed SBP at one point in time. It is unknown if SBP was labile, whether physicians relied upon home SBP measurements, or if patients had reported symptoms potentially associated with symptomatic hypotension that may have led to a reduction or inability to up-titrate BB or ACEI/ARB therapies at the time of the clinical visit. ARNI use was low in our study and this is likely a reflection of the recent approval of this drug class. There was a high representation of white male participants in our study. Whether our results apply to other demographic groups remain unclear. Finally, the study was not designed to identify why optimal medication doses were not used.
Conclusion:
In a contemporary cohort of patients with HFrEF, we found that over 90% of patients determined to be eligible for BB and ACEI/ARB/ARNI, and with SBP ≥110 mmHg were not receiving target doses of therapies that have been previously shown to reduce morbidity and mortality in HFrEF. Further efforts, which may include intensive education through the electronic medical record, defined protocols, improvements in transition of patients between providers, creation of dosing-based performance measures, patient education and other strategies are needed to support the use of more effective, guideline-recommended doses of HF medications in patients with HFrEF.
Acknowledgments
Funding: CHAMP-HF is funded by the Novartis Pharmaceuticals Corporation. Drs. P. Peri-Okonny, Y. Khariton and K. Patel are supported by the National Heart, Lung, and Blood Institutes of Health Under Aware Number T32HL110837; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
| ACEI | angiotensin converting enzyme inhibitor |
| ARB | angiotensin receptor blocker |
| ARNI | angiotensin receptor- neprilysin inhibitor |
| BB | beta-blocker |
| CHAMP-HF | CHAnge the Management of Patients with Heart Failure |
| GDMT | guideline directed medical therapy |
| LVEF | left ventricular ejection fraction |
| MRA | mineralocorticoid receptor antagonist |
| TIA | transient ischemic attack |
| TD | target dose |
Footnotes
Disclosures: Dr. John A. Spertus discloses grant funding from NIH, Bayer, and Abbott Vascular. He serves on Scientific Advisory Boards for United Healthcare, Novartis, Janssen and Bayer. He has intellectual property rights for the Kansas City Cardiomyopathy Questionnaire and an equity interest in Health Outcomes Sciences. Dr. Laine Thomas reports research funding from Novartis Pharmaceuticals Corporation. Dr. Gregg C. Fonarow reports research support from the NIH, consulting for Abbott, Amgen, Janssen, Medtronic, and Novartis and serving on the Get With The Guidelines Steering Committee. Dr. Adam D. DeVore receives research support from the American Heart Association, Amgen, NIH, and Novartis; he provides consulting services for Novartis. Dr. Adrian F. Hernandez reports research support from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Luitpold Pharmaceuticals, Merck, and Novartis as well as honoraria from Bayer, Boston Scientific, and Novartis. Dr. Javed Butler has received research support from the National Institutes of Health and the European Union and serves as a consultant for Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, BerlinCures, Boehringer Ingelheim, Bristol Myers Squib, Cardiocell, CVRx, G3 Pharmaceutical, Innolife, Janssen, Lantheus, LinaNova, Luitpold, Medscape, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, StealthPeptide, SC Pharma, V-Wave Limited, Vifor, and ZS Pharma. J. Herbert Patterson reports research funding from Amgen, Bristol-Myers Squibb, Merck, and Novartis and serves as a consultant to Amgen and Novartis. Dr. Puza P. Sharma, Kevin McCague, and Dr. Carol I. Duffy report employment by Novartis Pharmaceuticals Corporation. Dr. Nancy M. Albert serves as a consultant to Novartis Pharmaceuticals Corporation. All other authors have reported no relationships to disclose relevant to the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jchf.2018.11.011
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6440823?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.jchf.2018.11.011
Article citations
Use and Benefit of Sacubitril/Valsartan in Elderly Patients with Heart Failure with Reduced Ejection Fraction.
J Clin Med, 13(16):4772, 14 Aug 2024
Cited by: 0 articles | PMID: 39200914 | PMCID: PMC11355447
Association of Medication Adherence and Health Status in Heart Failure With Reduced Ejection Fraction: Insights From the CHAMP-HF Registry.
Circ Cardiovasc Qual Outcomes, 17(9):e010211, 24 Jul 2024
Cited by: 0 articles | PMID: 39045701
Generalizable Approach to Quantifying Guideline-Directed Medical Therapy.
Circ Heart Fail, 17(5):e011164, 14 May 2024
Cited by: 0 articles | PMID: 38742418
Review
Initiation and continuation of pharmacological therapies in patients hospitalized for heart failure in Japan.
Sci Rep, 14(1):9095, 20 Apr 2024
Cited by: 0 articles | PMID: 38643208 | PMCID: PMC11032365
Adherence to Treatment Guidelines in Ambulatory Heart Failure Patients with Reduced Ejection Fraction in a Latin-American Country: Observational Study of the Colombian Heart Failure Registry (RECOLFACA).
Cardiology, 149(3):228-236, 15 Feb 2024
Cited by: 0 articles | PMID: 38359813 | PMCID: PMC11152016
Go to all (41) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry.
J Am Coll Cardiol, 72(4):351-366, 01 Jul 2018
Cited by: 382 articles | PMID: 30025570
Guideline-directed medical therapy in severe heart failure with reduced ejection fraction: An analysis from the HELP-HF registry.
Eur J Heart Fail, 26(2):327-337, 22 Nov 2023
Cited by: 4 articles | PMID: 37933210
Comparative Effectiveness of Dosing of Medical Therapy for Heart Failure: From the CHAMP-HF Registry.
J Card Fail, 28(3):370-384, 15 Nov 2021
Cited by: 9 articles | PMID: 34793971
Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction.
Cochrane Database Syst Rev, (12):CD009889, 21 Dec 2015
Cited by: 22 articles | PMID: 26689943 | PMCID: PMC8407457
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: T32 HL110837
NIGMS NIH HHS (1)
Grant ID: U54 GM115428





