Abstract
Free full text

The Neuroscience of Natural Rewards: Relevance to Addictive Drugs
Addictive drugs act on brain reward systems, although the brain evolved to respond not to drugs but to natural rewards, such as food and sex. Appropriate responses to natural rewards were evolutionarily important for survival, reproduction, and fitness. In a quirk of evolutionary fate, humans discovered how to stimulate this system artificially with drugs. Many molecular features of neural systems instantiating reward, and of those systems affected by addictive drugs, are conserved across species fromDrosophilae to rats to humans and include dopamine (DA), G-proteins, protein kinases, amine transporters, and transcription factors such as cAMP response element-binding protein (CREB). A better understanding of natural brain reward systems will therefore enhance understanding of the neural causation of addiction.
Reinforcers, drives, and incentive systems
It is first helpful to consider how the field has moved conceptually in recent decades. Although emotions are unobservable, many objective expressions and behavioral, physiological, and neural responses to emotional stimuli have been selected by evolution. Studies of these objective responses in animals and humans provide valuable windows into brain reward function. Early drive theories held that hunger and thirst states motivated behavior directly as aversive drive states and that reinforcers simply reduced those states, strengthening preceding stimulus–response (S–R) habits or increasing the probability of operant response emission. Rewards are recognized now to act at least as importantly as hedonic incentives, causing neural representations that elicit motivation and goal pursuit, rather than as mere habit reinforcers. Physiological drive states nevertheless play important roles in incentive motivation, but primarily by increasing the perceived hedonic and incentive value of the corresponding reward; for example, food tastes better when hungry, drink when thirsty, and so on. Perhaps surprisingly, even drug reward and withdrawal appear to motivate drug-taking behavior primarily via incentive modulation principles rather than directly via simple aversive drives (Stewart and Wise, 1992). Accordingly, it behooves affective neuroscientists to understand the neural basis of incentive properties of rewards.
Mesocorticolimbic dopamine: pleasure, reinforcement, reward prediction, incentive salience, or what?
It has long been recognized that reward processing depends on mesocorticolimbic DA systems, comprising DA neurons in the ventral tegmental area (VTA) and their projections to nucleus accumbens (NAc), amygdala, prefrontal cortex (PFC), and other forebrain regions. Major efforts have attempted to specify what function this system contributes. Does mesocorticolimbic DA mediate the pleasure of reward stimuli? This was originally suggested because mesocorticolimbic systems are activated by many natural and drug rewards, and their blockade impairs the behavioral effectiveness of most reinforcers (Wise, 1985). Do mesocorticolimbic projections instead learn and predict the occurrence of rewards? That influential associative hypothesis was based on evidence that DA neurons fire to cues that predict rewards but not to already predicted hedonic rewards (Schultz, 2000). Do mesocorticolimbic DA systems mediate the incentive salience attributed to neural representations of rewards and cues, causing them to become perceived as “wanted” goals? That incentive “wanting” hypothesis was based originally on evidence that mesolimbic DA is not needed to mediate the hedonic impact or “liking” for sweet rewards, or new learning about them, despite its importance for motivated behavior to obtain the same rewards (Berridge and Robinson, 1998). Or finally, does mesocorticolimbic DA involvement in reward pursuit reflect broader functions, such as attention, complex sensorimotor integration, effort, or switching among behavioral programs? Those functions were proposed on the basis of various observations that do not readily fit a pure reward framework (Salamone, 1994; Gray et al., 1999; Ikemoto and Panksepp, 1999; Redgrave et al., 1999; Horvitz, 2000). Each hypothesis has its adherents, although there is recognition that they share important commonalities, and a consensus on motivational incentive function may now be forming.
Gaining a more correct answer to the question of “what does DA do in reward” is of great importance to understanding addiction, because addictive drugs are widely agreed to act primarily, although not exclusively, on brain mesocorticolimbic systems. For example, hedonic theories of addiction assume that mesocorticolimbic DA systems chiefly mediate the intense pleasure of addictive drugs and anhedonia during withdrawal (Volkow et al., 1999; Koob and Le Moal, 2001). Learning-based addiction theories assume sensitized or altered cellular mechanisms of associative S-R learning, and reward predictions cause ingrained drug-taking habits (Di Chiara, 1998; Kelley, 1999;Berke and Hyman, 2000; Everitt et al., 2001). The incentive-sensitization theory of addiction assumes that neural sensitization causes excessive attribution of incentive salience to drug-associated stimuli and acts, which makes addicts compulsively “want” to take drugs again (Robinson and Berridge, 1993,2000; Hyman and Malenka, 2001).
Regarding natural reward contributions to addiction neuroscience, it is notable that all the major hypotheses of mesocorticolimbic DA function studies were proposed originally on the basis of studies of natural reward. Therefore, a better understanding of what DA does for natural rewards will clarify brain mechanisms of drug addiction.
Mesocorticolimbic dopamine: appetitive versus aversive motivation
Beyond having a role in reward, mesocorticolimbic systems also participate in negative emotional states and aversive motivation. What relation could negative motivation (other than withdrawal) have to addiction? Aversive symptoms of psychosis, paranoia, or anxiety are sometimes precipitated in human addicts and in animal models by drugs such as amphetamine or cocaine (Ettenberg and Geist, 1993), but how can a brain “reward system” also mediate negative motivation and emotion? Some hypotheses suggest that mesocorticolimbic systems mediate general functions, such as attention or sensorimotor integration, and not reward or aversion specifically (Salamone, 1994; Gray et al., 1999;Horvitz, 2000). Another hypothesis is that DA responses to aversive motivation reflect hidden incentive mechanisms involved in the pursuit of safety (Rada et al., 1998; Ikemoto and Panksepp, 1999), drawing on psychological theories of avoidance learning. In other words, active pursuit of food when hungry or of safety when in danger could involve similar mesocorticolimbic incentive processes. However, most researchers probably support a third hypothesis that certain mesocorticolimbic systems play an active role in aversive motivation itself, distinct from DA mediation of reward (Salamone, 1994; Berridge and Robinson, 1998; Gray et al., 1999).
Several lines of evidence indicate direct mesocorticolimbic mediation of aversive motivation. Mesocorticolimbic brain systems are activated in animals and humans by aversive stimuli such as stress, electric shocks, etc. (Piazza et al., 1996; Becerra et al., 2001). Amphetamine administration enhances aversive associative conditioning of behavioral responses (Gray et al., 1999), whereas lesions of the NAc core disrupt conditioning of aversive responses to Pavlovian cues (Parkinson et al., 1999). Negative motivation versus reward may be mediated by different mesocorticolimbic channels of information processing. Neuroanatomical and neurochemical segregation of valence are indicated by observations that GABAergic microinjections in the NAc shell can elicit either intense positive motivation or negative motivation, depending on the shell subregion. GABA agonist microinjections in the anterior medial shell elicit appetitive eating behavior, but the same microinjections in the posterior medial shell elicit fearful defensive treading (Stratford and Kelley, 1999; Reynolds and Berridge, 2001), a behavior normally reserved by rodents in the wild for noxious stimuli such as threatening rattlesnakes (Treit et al., 1981; Coss and Owings, 1989; Owings and Morton, 1998). Further clarification of how mesocorticolimbic subsystems code positive versus negative motivational states should be a high priority as a means to shed light on why drugs of abuse sometimes produce mixed motivational effects, including anxiety and susceptibility to psychosis.
Natural rewards as windows into reward “liking” versus reward “wanting”
Although drug addicts want to take drugs more than other people, they may not proportionately like those drugs more, especially if neuropharmacological tolerance grows to their pleasurable impact; however, distinctions between neural systems of “wanting” reward and “liking” reward have emerged most clearly from studies of natural rewards, especially sweet taste reward, where it is possible to use affective facial expressions to measure immediate “liking” or hedonic impact. In human infants (Fig. (Fig.1),1), sucrose taste elicits a set of facial “liking” expressions (tongue protrusions, smile, etc), whereas quinine taste elicits facial “disliking” expressions (gape, etc.) (Steiner et al., 2001). Comparisons of human infant expressions with those of at least 11 great ape and monkey species indicate that primate expression patterns for “liking” and “disliking” are characterized by strong taxonomic continuity across species and by homology of microstructure features, such as allometric control of component speed (Steiner et al., 2001). Even rats display these reactions to tastes that reflect core affective processes and hedonic neural mechanisms homologous to those of humans (Grill and Norgren, 1978; Berridge, 2000).
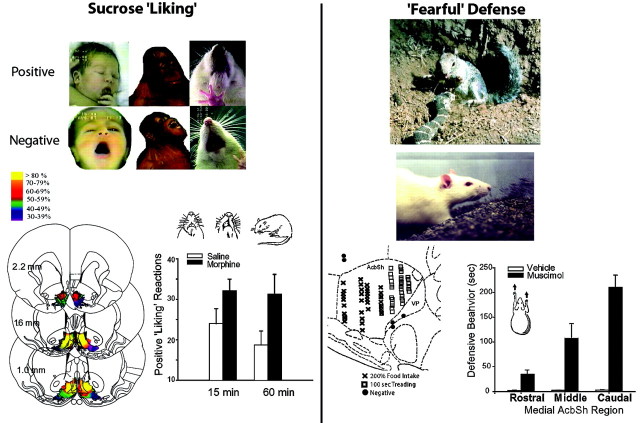
Naturalistic behavior assays of reward liking and negative fearful defense. Liking facial expressions are elicited by the taste of sucrose from newborn human infants, orangutans, and rats [top left, facial photographs fromSteiner et al. (2001) and Berridge (2000)]. Disliking expressions are elicited by the taste of quinine. NAc coronal map of opioid liking and wanting sites for food reward shows intensity of food wanting produced by morphine microinjections in the shell [bottom left, Peciña and Berridge (2000)]. Accompanying graph shows the increase in sucrose liking reactions caused by morphine microinjections in the accumbens shell. Conversely, anxiogenic and psychotic effects of addictive drugs may be related to natural fearful active defense reactions (right). Fearful defensive treading is elicited naturally from rodents by rattlesnake predators and centrally by GABA agonist microinjections in the caudal accumbens shell [California ground squirrel photograph by John Cooke from Coss and Owings (1989); rat photograph from Reynolds and Berridge (2001)]. Bar graph shows elicitation of fearful defensive treading along a rostrocaudal gradient in the NAc shell after GABA agonist microinjections (Reynolds and Berridge, 2001). Separate mesocorticolimbic channels for appetitive and aversive motivational functions is suggested by sagittal map of NAc shell rostrocaudal segregation of GABA-elicited positive feeding behavior (anteriorx symbols) versus fearful defensive behavior (posterior squares).
Opioid peptide neurotransmission within the NAc modulates the hedonic impact of food reward (Glass et al., 1999; Peciña and Berridge, 2000; Kelley et al., 2002), providing further support that drugs of abuse act on systems evolved to mediate such natural pleasures as sweetness “liking.” For example, microinjection of morphine into NAc shell directly increases rat “liking” orofacial expressions elicited by sucrose (Peciña and Berridge, 2000) and alters intake consistent with enhanced food palatability (Zhang and Kelley, 2000). Such findings demonstrate the importance of neurochemical systems other than dopamine in the hedonic impact of rewards.
Originally surprising were findings that mesocorticolimbic DA manipulations do not change “liking” for the taste of sucrose (Peciña et al., 1997; Wyvell and Berridge, 2000), despite their role in incentive “wanting” for these and other rewards. The neurochemical dissociation of “liking” from “wanting” has obvious relevance to addiction. The incentive–sensitization theory suggests that addiction may be characterized by increased “wanting” of drugs caused by sensitized DA-related systems, even in the absence of drug “liking” (Robinson and Berridge, 2000; Hyman and Malenka, 2001).
From nodes to dynamic networks
Reward-related behavior emerges from the dynamic activity of entire neural networks rather than from any single brain structure. The functions of NAc, amygdala, etc., in natural reward or addiction can be understood only in terms of the extended neural system within which they reside (Fig. (Fig.2).2). Although we now have a working knowledge of key brain structures of reward, deeper understanding will require examination of network interactions between subregions of amygdala, PFC, NAc, and other structures in reward and motivation (Kalivas and Nakamura, 1999; Rolls, 1999; Everitt et al., 2000; Schultz, 2000;Jackson and Moghaddam, 2001). For example, amygdala and orbital prefrontal cortex may play complementary roles in reward learning regarding acquisition of cue incentive value versus response selection (Schoenbaum et al., 1999; Baxter et al., 2000).
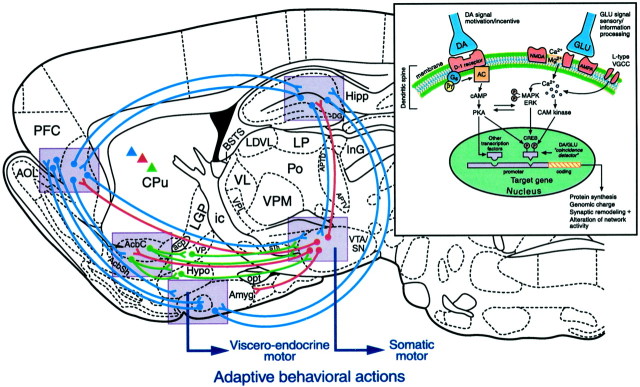
Schematic representation of rat brain sagittal section depicting pathways involved in processing of natural rewards and in neural plasticity underlying reward-related learning. Circuitry represented in blue indicates long glutamatergic pathways between prefrontal cortex (PFC), amygdala (Amyg), hippocampus (Hipp), ventral striatum (nucleus accumbens), and ventral tegmental area (VTA). Red circuitry represents principal ascending mesocorticolimbic dopamine systems. Greendescending pathways indicate primarily GABAergic descending systems.Triangles in corresponding colors indicate similar DA, glutamate, and GABAergic coding in dorsal striatum.Violet-shaded boxes represent important nodes within this distributed network where NMDA/D1 receptor-mediated plasticity is proposed to be a critical substrate for behavioral adaptation and learning. For purposes of simplicity, not all relevant circuitry is shown; for example, there are important connections between hypothalamus and amygdala, and glutamatergic thalamic inputs are not shown. Drawing of section is based on the atlas of Paxinos and Watson (1998). Large arrows indicate flow of effector pathways converging on viscero–endocrine and autonomic systems (emerging from hypothalamus and amygdala) and somatic voluntary motor systems (emerging from basal ganglia and ventral midbrain).Inset reflects intracellular and genomic mechanisms hypothesized to govern DA- and glutamate-dependent plasticity within the indicated (violet shaded) nodes. Such plasticity, which may result in altered network activity, is hypothesized to mediate normal learning and memory related to natural rewards but is also a key component of addiction. AcbC, Accumbens core;Acb shell, accumbens shell; Cpu, caudate–putamen; VP, ventral pallidum;Hypo, hypothalamus; SN, substantia nigra. Other abbreviations can be found in Paxinos and Watson (1998).
A further network feature concerns the efferent projections of NAc to target structures such as lateral hypothalamus and ventral pallidum. This outflow appears crucial to NAc mediation of natural appetitive behavior (Kalivas and Nakamura, 1999; Stratford and Kelley, 1999; Zahm, 2000). Elicitation of eating behavior by inhibition of spiny neurons in the NAc shell depends on signals to the lateral hypothalamus, which activates lateral hypothalamic neurons via disinhibition (Rada et al., 1997; Stratford and Kelley, 1999). Thus, the NAc shell may gate corticolimbic information to the lateral hypothalamus and exert executive control over brain circuits controlling feeding behavior and related motivation (Kelley, 1999; Petrovich et al., 2001). This corticostriatal–hypothalamic–brainstem network deserves to be the focus of further study, in the contexts of both natural reward and addiction (Swanson, 2000).
Neural ensembles and behavioral selection
Dynamic modulation of incentive value emerges from afferent network signals that cause variation in the states of individual medium spiny NAc neurons. For example, these neurons exhibit “bistable” membrane potential states, which depend on phasic excitatory glutamatergic input from afferent structures such as hippocampus (O'Donnell and Grace, 1995). NAc neurons are depolarized by PFC input when they are in the hippocampal-gated “up” state, and thus network synchrony arises between NAc and hippocampus (Goto and O'Donnell, 2001). Similar gating of NAc neurons may occur between amygdala and hippocampal inputs (Mulder et al., 1998; Floresco et al., 2001b). DA input also plays a critical role in the NAc switching and is influenced in turn by hippocampal glutamatergic input to VTA (Legault and Wise, 2001). Thus, dynamic modulation by incoming network signals can control which NAc motivational ensembles predominate to guide behavior toward natural or drug rewards.
Network plasticity mediated by DA–glutamate interactions
Addictive drugs induce long-term neuroadaptations at the structural, cellular, molecular, and genomic levels (Hyman and Malenka, 2001), but how does such plasticity relate to natural reward and motivation? An exciting synthesis is emerging from studies of glutamate–DA-mediated plasticity and its transcriptional consequences. Coincident activation of DA D1 receptors and glutamate NMDA receptors plays a critical role in shaping synaptic configurations and neural ensembles involved in motivation and learning.
In both striatum and PFC, D1 activation potentiates NMDA responses (Seamans et al., 2001; Wang and O'Donnell, 2001), and long-term potentiation at hippocampal–prefrontal cortex synapses is dependent on coactivation of NMDA and D1 receptors and on intracellular cascades involving protein kinase A (Gurden et al., 2000). Sensitization by drugs of abuse is facilitated by a related glutamate–dopamine interaction caused when drugs are administered in a novel distinct environment (Uslaner et al., 2001). In accumbens neurons, cooperative action of both D1 and NMDA receptors mediates hippocampal-evoked spiking activity (Floresco et al., 2001b), and a similar synergism is observed for the amygdalo-accumbens pathway (Floresco et al., 2001a). Molecular studies complement these findings, showing NMDA-receptor dependence of D1-mediated phosphorylation of CREB (Konradi et al., 1996; Das et al., 1997), a transcription factor thought to be an evolutionarily conserved modulator of memory processes. Transcriptional consequences of NMDA and D1 coactivation in the NAc core and PFC are necessary for appetitive learning about cues, rewards, and behavioral actions, particularly at early acquisition stages (Baldwin et al., 2000, 2002a,b; Smith-Roe and Kelley, 2000). In sum, coordinated activation of DA D1 and NMDA systems within corticolimbic–striatal circuits is an important feature of adaptive reward learning.
This story suggests that drugs of abuse that target DA and glutamate synapses should enduringly modify basic cellular and molecular functions. Such long-lasting plasticity in reward neurons induced by drugs may contribute to abnormal information processing and behavior, resulting in poor decision making, loss of control, and the compulsivity that characterizes addiction. That drugs of abuse induce D1- and NMDA-mediated neuronal cascades shared with normal reward learning is an important insight regarding addiction that has emerged in the past decade.
Reward outside traditional limbic network?
Although little studied, reward may also be significantly processed in brain structures not traditionally considered mesocorticolimbic, motivational, or related to addiction. For example, “motor” regions of caudate–putamen contain neurons that respond to food and drink reward stimuli, in a manner similar to DAergic or ventral striatal neurons (Aosaki et al., 1994; Schultz, 2000). Eating can be elicited in rats directly by microinjections of opioid agonists into these same motor regions of dorsal striatum (Zhang and Kelley, 2000). Eating is disrupted by DA receptor blockade or lesions in the same dorsal striatal regions (Cousins and Salamone, 1996). Sensorimotor regions of striatum undergo dynamic changes during rewarded “habit” learning (Jog et al., 1999), and their damage impairs learning (Packard and White, 1990). Such evidence suggests that “sensorimotor” structures may participate in natural reward functions to a surprising degree (White, 1989). If so, such extended neural reward processing has implications for addiction as well.
Conclusion
Drugs can impact natural brain reward systems to produce addiction in only three ways. (1) Drug rewards might activate the same brain systems as intense natural rewards. Addiction theories based on pleasurable drug hedonia or positive reinforcement suppose that drugs act as natural rewards. (2) Addictive drug rewards might also change the quantitative scaling of some reward components, fragmenting and distorting normal reward processes to cause compulsive behavior. Addiction theories based on sensitization of incentive salience propose that drugs sensitize mesocorticolimbic substrates of incentive salience, fractionating natural reward by intensifying “wanting” disproportionately to cause compulsive drug taking behavior (Robinson and Berridge, 2000; Hyman and Malenka, 2001). Addiction theories based on associative long-term potentiation or alterations in learning systems propose unusually strong drug-taking S-R habits (O'Brien et al., 1992; Di Chiara, 1998; Robbins and Everitt, 1999; Berke and Hyman, 2000; Everitt et al., 2001). (3) Addictive drugs could induce new brain processes, such as aversive withdrawal states, which may play larger opponent-process roles for addiction than for normal rewards (Solomon and Corbit, 1974; Koob and Le Moal, 2001).
These three possibilities are exhaustive but not mutually exclusive. Many intriguing facts have been discovered that illuminate their interaction. Future studies will further clarify how drugs interact with brain reward systems to produce the compulsive motivation and relapse that characterize addiction.
Footnotes
This work was supported by Grants DA09311, DA04788, and DA13780 from the National Institute on Drug Abuse (A.E.K.) and IBN 0091611 from the National Science Foundation (K.C.B.). We thank Terry Robinson, Sheila Reynolds, Matthew Andrzejewski, and Susana Peciña for helpful suggestions on this manuscript.
Correspondence should be addressed to A. E. Kelley, Department of Psychiatry, University of Wisconsin–Madison Medical School, 6001 Research Park Boulevard, Madison, WI 53719. E-mail:ude.csiw.ffatscaf@yellekea.
REFERENCES
Articles from The Journal of Neuroscience are provided here courtesy of Society for Neuroscience
Full text links
Read article at publisher's site: https://doi.org/20026361
Read article for free, from open access legal sources, via Unpaywall:
https://www.jneurosci.org/content/jneuro/22/9/3306.full.pdf
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/content/full/22/9/3306
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/reprint/22/9/3306.pdf
Free to read at www.jneurosci.org
http://www.jneurosci.org/cgi/content/abstract/22/9/3306
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/20026361
Article citations
The potential effect of α7 nicotinic receptors modulation on palatable food-induced dependence-like behaviors.
Saudi Pharm J, 32(8):102138, 04 Jul 2024
Cited by: 0 articles | PMID: 39109164 | PMCID: PMC11301368
Neurofunctional changes related to methamphetamine and sexual cues in methamphetamine dependence from short- to long-term abstinence.
Addict Biol, 29(6):e13405, 01 Jun 2024
Cited by: 1 article | PMID: 38837586
Navigating the complex terrain of motivated behavior: a bibliometric and neuroscientific perspective.
Front Behav Neurosci, 18:1363856, 26 Apr 2024
Cited by: 0 articles | PMID: 38737489 | PMCID: PMC11082395
Review Free full text in Europe PMC
Synthetic exendin-4 disrupts responding to reward predictive incentive cues in male rats.
Front Behav Neurosci, 18:1363497, 14 Mar 2024
Cited by: 0 articles | PMID: 38549620 | PMCID: PMC10976559
How do lateral septum projections to the ventral CA1 influence sociability?
Neural Regen Res, 19(8):1789-1801, 08 Nov 2023
Cited by: 1 article | PMID: 38103246 | PMCID: PMC10960288
Go to all (776) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Brain reward circuitry: insights from unsensed incentives.
Neuron, 36(2):229-240, 01 Oct 2002
Cited by: 540 articles | PMID: 12383779
Review
Illicit dopamine transients: reconciling actions of abused drugs.
Trends Neurosci, 37(4):200-210, 20 Mar 2014
Cited by: 55 articles | PMID: 24656971 | PMCID: PMC4064368
Review Free full text in Europe PMC
Dopaminergic control of motivation and reinforcement learning: a closed-circuit account for reward-oriented behavior.
J Neurosci, 33(20):8866-8890, 01 May 2013
Cited by: 31 articles | PMID: 23678129 | PMCID: PMC6618820
A neural network model with dopamine-like reinforcement signal that learns a spatial delayed response task.
Neuroscience, 91(3):871-890, 01 Jan 1999
Cited by: 164 articles | PMID: 10391468
Funding
Funders who supported this work.
NIDA NIH HHS (6)
Grant ID: R37 DA004788
Grant ID: R01 DA004788
Grant ID: DA04788
Grant ID: DA13780
Grant ID: R01 DA009311
Grant ID: DA09311





