Abstract
Free full text

Prenatal Exposure to Cocaine Disrupts D1A Dopamine Receptor Function Via Selective Inhibition of Protein Phosphatase 1 Pathway in Rabbit Frontal Cortex
Abstract
Previous work has demonstrated that in utero cocaine exposure induces an uncoupling of brain D1A dopamine receptors (D1ADARs) from Gs-protein. The present work is an attempt to define the mechanism underlying the uncoupling. We detected a significant elevation of phosphoserine in frontal cortical D1ADARs of rabbits that were exposed prenatally to cocaine compared with saline controls. This increase in phosphorylation is observed at gestational day 22 and persists to postnatal day 20. The hyperphosphorylation of the D1ADAR is accompanied by a 45% inhibition in frontal cortex (FCX) protein phsphatase-1 (PP1) activity that appears to be mediated via DARPP-32 (dopamine and cAMP-regulated phosphoprotein) as indicated by elevated FCX phospho-DARPP-32 (Thr34). Furthermore, we demonstrated in both FCX and in PC2 cells that express D1ADARs that PP1 is physically associated with D1ADARs. We also observed a dramatic decrease in D1ADAR-associated PP1 activity in FCX of prenatal cocaine-exposed rabbits, indicating that the reduction in PP1 activity may be responsible for the hyperphosphorylation of the receptor. Furthermore, pretreatment of cortical membranes obtained from cocaine-exposed animals with exogenous PP1 dephosphorylated the phosphorylated D1ADAR and significantly reversed the impaired receptor–Gαs coupling. This work indicates (1) that D1ADAR dephosphorylation via PP1 is essential for receptor resensitization or reactivation and (2) an alteration in the DARPP-32/PP1 cascade appears to be a primary event responsible for D1ADAR dysfunction in in uterococaine-exposed rabbit progeny. The present finding of an altered DARPP-32/PP1 cascade in association with a dysfunction in D1ADAR signal transmission in the prenatal cocaine-exposed rabbit brain may implicate novel strategies for the prevention and treatment for in utero cocaine-induced developmental and behavioral abnormalities.
Accumulated evidence suggests that usage of cocaine during pregnancy may cause long-lasting behavioral abnormalities in newborn humans and animals (Spear et al., 1989;Henderson and McMillan, 1990; Dow-Edwards, 1991; Volpe, 1992; Murphy et al., 1997; Richardson, 1998). Recent reports suggest that many of the effects of cocaine on the fetus and on postnatal brain development are the result of changes in dopamine (DA) receptor transmembrane signaling (Friedman et al., 1996; Levitt et al., 1997;Friedman and Wang, 1998; Lidow, 1998; Simansky et al., 1998; Harvey et al., 2001). We reported that in utero cocaine results in sustained impairment in coupling of brain D1Adopamine receptors (D1ADARs) to Gα-protein (Friedman et al., 1996; Friedman and Wang, 1998) and that this effect parallels the emergence of abnormal dendritic development in corticolimbic neurons that receive dopaminergic innervation (Levitt et al., 1997; Jones et al., 2000; Harvey et al., 2001). The disruption in D1ADAR signaling has also been related to cognitive abnormalities in the offspring (Levitt, 1998; Harvey et al., 2001).
The impaired transmembrane signaling via D1ADAR occurs without changes in receptor number, Gαs-protein, or nerve terminal DA transporter sites (Friedman et al., 1996, 1998; Lidow, 1998), indicating that the effects of in utero cocaine exposure may be attributable to differences in posttranslational modifications, such as phosphorylation, that determine the efficiency of receptor–G-protein coupling. Phosphorylation and dephosphorylation play essential roles in the regulation of desensitization and resensitization of G-protein-coupled receptor (GPCR) signaling (Freedman and Lefkowitz, 1996; Bunemann and Hosey, 1999). Protein kinase A (PKA), protein kinase C, and G-protein receptor kinases (GRKs) have been reported to phosphorylate GPCRs and consequently induce receptor desensitization (Lefkowitz, 1998). PKA-mediated phosphorylation was shown to mediate desensitization of the D1ADAR (Bates et al., 1991; Zhou et al., 1991; Black et al., 1994; Jiang and Sibley, 1999). It is generally believed that a balance between receptor desensitization and resensitization mediates the precise response level of the receptor (Yu et al., 1993; Krueger et al., 1997; Bunemann and Hosey, 1999). The reactivation of the phosphorylated β2 adrenergic receptor via dephosphorylation by a member of the 2A protein phosphatase family was demonstrated previously (Pitcher et al., 1995). Moreover, regulation of NMDA receptor activity was shown to be modulated by both PP2A and protein phosphatase 1 (PP1), a major multifunctional serine/threonine protein phosphatase (PSP) in brain (Wang et al., 1994). However, a role of specific protein phosphatases in modulating D1ADAR sensitivity has not been identified.
DARPP-32 (dopamine and cAMP-regulated phosphoprotein) was shown to play a central role in the biology of neurons that express D1DARs (Greengard et al., 1999), and receptor stimulation was shown to induce phosphorylation of DARPP-32 on Thr34, converting this phosphoprotein into a powerful inhibitor of PP1. PP1 dephosphorylates numerous cellular substrates, including neurotransmitter receptors and transporters (Hemmings et al., 1990; Greengard et al., 1999). Most relevant in the present context is the demonstration of increased phospho-DARPP-32 (Thr34) after acute cocaine administration in mice, suggesting that the DARPP-32/PP1 system may be involved in the psychopharmacology of cocaine (Nishi et al., 1997). In the present communication, we investigated the mechanism through which prenatal cocaine exposure elicits transmission dysfunction in the D1A DAR system.
MATERIALS AND METHODS
Materials. Dibutyryl-cAMP was from BioMol (Plymouth Meeting, PA), and dopamine, SKF81297, and antibodies to Gα subunits were obtained from Research Biochemicals (Natick, MA). Tautomycin, inhibitor-2 (I-2), and okadaic acid were obtained from Calbiochem (La Jolla, CA). Monoclonal rat anti-D1A dopamine receptor antibody and myelin basic protein (MBP) were from Sigma (St. Louis, MO). Anti-phospho(Thr34) DARPP-32 and anti-DARPP-32 were a kind gift from Dr. P. Greengard and G. L. Snyder (Laboratory of Molecular and Cellular Neuroscience, The Rockefeller University, New York, NY). Electrophoresis reagents were obtained from Bio-Rad (Richmond, CA). Anti-PP1 and horseradish peroxidase-linked secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). [γ-32P]ATP (3000 Ci/mmol) and [35S]GTPγS were purchased from NEN (Boston, MA). Other reagents were purchased from standard laboratory suppliers.
Animal preparation. Dutch belted male and female rabbits were obtained from Myrtle's Rabbitry (Thompson Station, TN). Rabbits were housed individually with access to rabbit chow and water ad libitum under a 12 hr light/dark cycle and maintained at 22 ± 1°C. Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile saline at a concentration of 4 mg/ml and injected via the marginal ear vein at a dose of 4 mg/kg. Control animals received an equal volume of saline injection. Cocaine or saline were administrated twice per day on gestational day 8 (G8) through G29. After birth, rabbit pups were kept with their mother until they were killed. This rabbit model of prenatal cocaine exposure has been reported in detail previously and has been proven to reproducibly induce behavioral and dendritic developmental abnormalities (Murphy et al., 1997; Jones et al., 2000). This treatment did not affect body weight gain of the pregnant rabbit nor did it change the number of live births per litter or survival rate of the kits (Harvey et al., 2001). Animals were killed by decapitation; brains were removed, and frontal cortices (FCXs) were dissected at 4°C. For the study of phosphorylation of D1ADRs during development, tissues were rapidly dissected from fetuses on G15, G22, and G25 and postnatal day 1 (P1) and P20; for all other studies unless specific indicated, FCXs from rabbits of P20 were used.
Membrane preparation, [32P]phosphate labeling, and analysis of [32P]phosphate-labeled D1ADAR in frontal cerebrocortical slices.Rabbit brain FCX was cut into 300 × 300 × 300 μm prisms using a McIllwain tissue chopper (Brinkmann Instruments, Westbury, NY). The prisms were washed in oxygenated phosphate-free Krebs'–Ringer's buffer (KRB), and 20 mg of tissue was incubated at 37°C for 30 min with 500 μCi/ml 32Pi in phosphate-free KRB (buffer A), which had the following composition: 25 mm HEPES, pH 7.4, 143 mmNaCl, 4.8 mm KCl, 20 mmNaHCO3, 1.3 mmCaCl2, 1.2 mmMgSO4, 10 mm glucose, 100 μm ascorbic acid, 0.2% 2-mercaptoethanol, 50 μm pargyline, and protease inhibitors (20 μg/ml leupeptin, 25 μg/ml pepstatin A, 0.01 U/ml soybean trypsin inhibitor, and 0.04 mm PMSF), oxygenated for 10 min (total incubation volume is 500 μl). Tissues were subsequently incubated with 10 μm dibutyryl-cAMP (PKA activator) or DA for an additional 30 min, and reactions were terminated by adding 1.5 ml of ice-cold KRB containing 10 mm EDTA, followed by centrifugation. The tissues were sonicated and lysed in lysis buffer B containing 20 mm Tris, pH 7.8, 150 mmNaCl, 50 M NaF, 2 mm EGTA, 1 mm EDTA, 0.5 mmβ-glycerophosphotate, 1 mm vanadate, 1% Triton X-100, 1 mm PMSF, and proteinase inhibitor cocktail. The protein content of the supernatant was determined by the method of Bradford (Bio-Rad). Aliquots of supernatants (400 μg) were immunoprecipitated with D1ADAR antibody using a previously published method with minor modification (Wang et al., 1995). The immunoprecipitates were separated on SDS-PAGE, and phosphorylated proteins were then assessed by autoradiography. To assess the effect of PP1 on phosphorylated D1ADARs, membranes were prepared from FCX slices of control and cocaine-exposed rabbits that were preincubated with dibutyryl-cAMP or DA (to induce D1ADAR phosphorylation). The slices were homogenized in a glass–glass homogenizer in 10 vol of buffer containing 25 mmHEPES, pH 7.4, 100 mm sucrose, 1 mm EGTA, 0.2% 2-mercaptoethanol, and protease inhibitors. The homogenates were centrifuged for 5 min at 750 ×g, and the supernatants were centrifuged for 10 min at 48,200 × g (4°C). The membrane pellets were washed three times with KRB, and protein content was determined. Five hundred micrograms of membrane protein were incubated with either vehicle or PP1 for 30 min. Tissues were then solubilized, the D1ADAR was immunoprecipitated with an anti-D1ADAR antibody, and the extent of PP1-mediated dephosphorylation of labeled D1ADARs was determined.
[35S]GTPγS-binding to Gα-proteins. Membrane fractions were prepared as described above. Membrane protein (200 μg) was incubated at 30°C in KRB containing 2 nm[35S]GTPγS for 5 min, followed by 5 min stimulation with DA or vehicle. The reactions were stopped, and tissues were solubilized in buffer B. The [35S]GTPγS bound to Gα-proteins were immunoprecipitated with specific anti-Gαs or Gαq antibodies (Wang et al., 1995; Friedman et al., 1996). The radioactivity precipitated by normal rabbit serum was subtracted from the amount of antibody-precipitated radioactivity for each of the Gα antisera, and percentage of stimulation was calculated.
Phosphorylated proteins and immunoblot analysis. Frozen brain tissues were lysed for 30 min at 4°C in buffer B and centrifuged for 15 min at 12,000 × g, and protein content in the supernatant was determined. The supernatant was stored at −80°C before use in immunoprecipitation or immunoblot assays. For immunoprecipitation, aliquots of protein were incubated with anti-D1ADAR rat monoclonal antibody (mAb) for 4 hr at 4°C with shaking, followed by the addition of agarose-conjugated protein A/G PLUS beads (Santa Cruz Biotechnology) and further incubating for 1 hr. The immunocomplex was then collected by centrifugation, washed with lysis buffer, boiled in sample preparation buffer, and loaded onto 12% SDS-PAGE. The proteins were separated electrophoretically and transferred to nitrocellulose membranes. The membranes were blocked with 10% (w/v) fat-free dry milk in 0.1% Tween 20–PBS (TBS) overnight at 4°C, followed by incubation with monoclonal anti-phosphoserine or anti-phosphothreonine antibody (Sigma) or with an anti-PP1 antibody (Santa Cruz Biotechnology) for 2 hr. The membranes were washed and then incubated for 1 hr with species-specific HRP-conjugated secondary IgG antibody (1:5000–1:10,000 dilution) in 0.1% TBS. The membranes were washed once with 0.3% TBS for 20 min, followed by four washes for 10 min each with 0.1% TBS, and the signals were visualized by the ECL/HRP method (Supersignal; Pierce, Rockford, IL). For the analysis of DARPP-32, the brain tissues were sonicated and prepared by boiling in 1% SDS-contained sample preparation buffer as described previously (Nishi et al., 1997). Equal amounts of homogenate protein were loaded onto 12% SDS-PAGE, and the membranes were then probed with anti-phospho-DARPP-32 (1:750) or anti ARPP-32 antibody (Snyder et al., 1998).
Protein phosphatase activity assay. FCX tissues were homogenized in buffer containing 10 mm Tris, pH 7.4, 150 mm NaCl, 1 mmEDTA, 1% Triton X-100, 1 mmNa3VO4, and proteinase inhibitors. After centrifugation, the PSP activity assay was performed in 50 μl of buffer (Dunigan and Madlener, 1995) by incubating 5 μg of protein of brain extracts or anti-D1A receptor or anti-PP1 precipitates with [32P]-labeled MBP. A preliminary titration assay indicated linear release of labeled [32P] by protein phosphatase at 2–10 μg of lysate protein when tested for 5 min at 30°C. In some experiments, 1 nm tautomycin, 2 nm I-2, or 2 nm okadaic acid was included in the assay to assess PP1 or PP2A activities (Bennecib et al., 2000; Spurney, 2001). The reaction was terminated by adding 150 μl of ice-cold 20% trichloroacetic acid. The mixture was vortexed, incubated on ice for 15 min and centrifuged at 16,000 ×g for 5 min at 4°C, and the supernatant was subjected to liquid scintillation counting. The background phosphate released was determined by incubating [32P]-labeled MBP with buffer or with normal anti-IgG, respectively.
Data analysis. Data are expressed as mean ± SEM and analyzed by ANOVA, followed by Newman–Keuls test unless otherwise indicated. Statistical significance was considered p < 0.05.
RESULTS
Prenatal cocaine exposure results in hyperphosphorylation of serine residues in brain D1ADARs
The effect of prenatal cocaine exposure on the phosphorylation state of brain D1ADARs was assessed during development. The levels of phosphoserine in D1ADAR immunoprecipitates were analyzed in FCX obtained from, in utero, control saline- and cocaine-treated rabbits on G15 through P20. Phosphorylated serine residues in D1ADAR was evident as early as G15; however, elevated phosphoserine levels were observed in cortical D1ADAR of G22 in tissue of cocaine-exposed compared with saline control animals (Fig.(Fig.1).1). This increase in phospho-D1ADAR persisted in tissues obtained from cocaine-exposed offspring assessed at P1 and P20, suggesting that prenatal cocaine-induced alteration in D1ADAR phosphorylation state is developmental stage dependent. This increase in phospho-D1ADAR is not related to a change in receptor protein expression because we demonstrated previously that prenatal cocaine exposure did not alter D1ADAR expression and binding activity (Friedman et al., 1996; Jones et al., 2000), implicating posttranslational modification in mediating the increase in phosphoserine levels in D1ADAR. Prenatal cocaine exposure did not change phosphothreonine levels of frontal cortical D1ADARs (Fig. (Fig.1,1, bottom panel), suggesting that cocaine exposure, during early fetal development, results in a selective increase in serine phosphorylation in the D1ADAR.
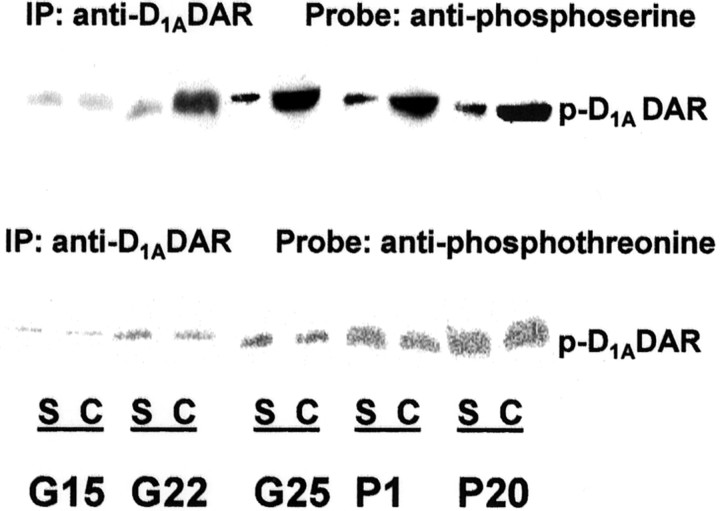
Increased D1ADAR phosphoserine levels in FCX of in utero cocaine-exposed rabbit brains. FCX extracts were prepared at different developmental stages from offspring of rabbit dams that were injected with cocaine or saline during pregnancy. The lysates (400 μg) were immunoprecipitated with 5 μg of anti-D1ADAR mAb, and the precipitates were blotted and probed with anti-phosphoserine or anti-phosphothreonine antibody (1:1000). S, Saline control; C, cocaine-exposed; G, gestational day; P, postnatal day; IP, immunoprecipitation,p-D1ADAR, phospo-D1Adopamine receptor.
Hyperphosphorylation of D1ADAR is associated with receptor dysfunction in FCX of prenatal cocaine-exposed rabbits
It has been shown that PKA mediates D1ADAR desensitization by phosphorylation of the serine residues in the receptor (Jiang and Sibley, 1999). Moreover, the fact that prenatal cocaine induces D1ADAR dysfunction by uncoupling the receptor from its G-protein, and the finding of increased receptor phosphoserine levels in FCX of progeny of rabbits administered cocaine, led us to speculate that PKA-mediated serine phosphorylation may underlie the loss of function in D1ADAR in cocaine rabbits. Indeed, compared with slices of control animals, there was a remarkable reduction in dibutyryl-cAMP-stimulated D1ADAR serine phosphorylation when cortical slices from prenatal cocaine-exposed rabbits were [32P]-labeled in the presence of a PKA activator or DA (Fig.(Fig.22A). Thus, suggesting that cAMP/PKA- and/or GRK-targeted sites in the D1ADAR are occupied and therefore unavailable for additional phosphorylation in brains from cocaine-treated rabbit kits. Furthermore, in agreement with previous observations in cultured cells, phosphorylation of D1ADAR results in functional disruption of the receptor (Fig. (Fig.22B) (Jiang and Sibley, 1999). The present data indicated that cAMP/PKA- or DA-mediated receptor phosphorylation also reduced the coupling of the receptor to Gαs-protein in brain tissue (Fig.(Fig.22B), thus, mimicking the functional deficit in FCX D1ADAR of the in utero cocaine-exposed rabbit. The results, therefore, suggest that the enhanced phosphorylation of serine residue(s), presumably via PKA or GRK, may contribute to D1ADAR dysfunction in FCX of prenatal cocaine-exposed rabbits.
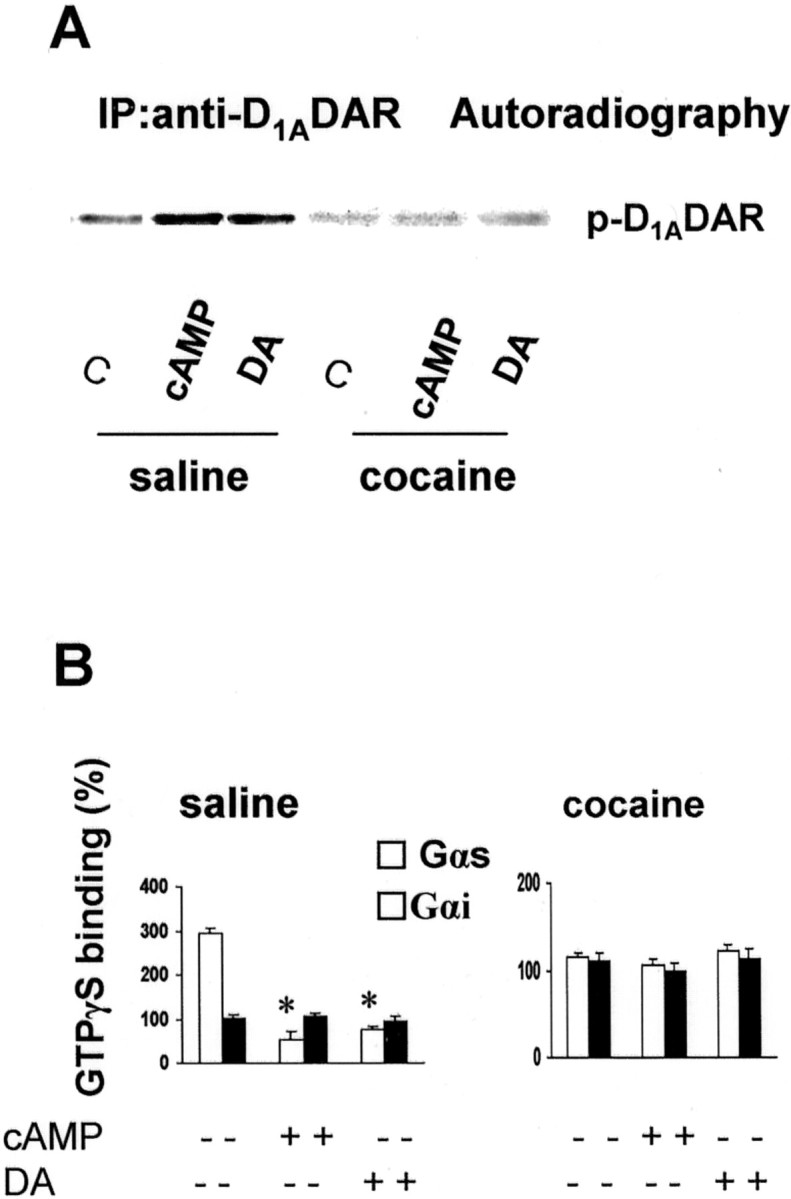
Phosphorylation of D1ADAR disassociates Gαs-protein from the receptor. Phosphorylation of D1ADAR induced by PKA or DA is shown inA. Brain FCX slices were incubated with [32P]orthophosphate for 30 min at 37°C in the presence of 10 μm dibutyryl-cAMP (PKA activator) or 10 μm DA. Reactions were terminated by addition of 1.5 ml of ice-cold phosphate-free Krebs'–Ringer's solution containing 10 mm EDTA. Tissues were homogenized in buffer B and allowed to stand for 1 hr at 4°C. Protein content in the supernatant was determined, and aliquots of extracts were incubated with anti-D1ADAR mAb, followed by incubation with 15 μl of protein A/G PLUS (Santa Cruz Biotechnology) for 2 hr. The precipitates were washed extensively and boiled in 2× sample buffer and subsequently separated on 10% SDS-PAGE. Proteins were stained with Coomassie blue, gels were dried at 80°C under vacuum, and32P incorporation into D1ADAR was monitored by autoradiography. B, PKA- and DA-mediated phosphorylation of D1ADAR uncouples the receptor from Gαs-protein but not from Gαi-protein. FCX slices were incubated for 30 min at 37°C with 10 μmdibutyryl-cAMP (PKA activator) or 10 μm DA to induce D1ADAR phosphorylation as described inA. Membranes were prepared, and aliquots were incubated with 1 μm DA for 5 min in the presence of [35S]GTPγS. [35S]GTPγS binding to Gα-proteins was performed (Wang et al., 1995), and data are expressed as percentage of increase in GTPγS binding to Gαs (white bars) or Gαi(black bars) over unstimulated basal control and are depicted as mean ± SEM obtained from at least four animals in each group. *p < 0.01, compared with dopamine stimulation without previous exposure to cAMP or DA. C, Control.
Prenatal cocaine exposure induces selective inhibition of PP1 in FCX
PSP was shown to regulate the phosphorylation state of receptors and their signaling (Wang et al., 1994; Pitcher et al., 1995; Bunemann and Hosey, 1999; Westphal et al., 1999). We, therefore, examined the possible role of PSP in mediating D1ADAR hyperphosphorylation and impaired D1ADAR signal transduction in rabbit progeny exposed to cocaine in utero. PSP activity in whole FCX lysates of rabbits exposed to cocainein utero was markedly decreased (Fig.(Fig.33A). Because PP1 constitutes approximately half of total brain PSP activity, we determined whether the change in total PSP activity represents a change in PP1 activity. As shown in Figure Figure33A, the PP1 inhibitors tautamycin or I-2 inhibited PSP activity by 45% in whole control lysate. However, the PP1 inhibitors exhibited no significant inhibition in PSP activity in whole FCX lysates of cocaine-exposed animals, suggesting that the reduction in total PSP activity in FCX of prenatal cocaine-exposed rabbits may result from a reduction of PP1 activity. PP2A activity, as monitored by measuring okadaic acid (2 nm)-sensitive protein phosphatase activity, was unchanged in FCX of cocaine-exposed animals relative to saline controls (Fig. (Fig.33B), suggesting that prenatal exposure to cocaine selectively diminishes PP1 activity in FCX. This was directly confirmed when PP1 activity was assessed in PP1 immunoprecipitates (Fig.(Fig.33C). However, PP1 protein expression was not altered in rabbit brains exposed to cocaine in utero (Fig.(Fig.33D).

Prenatal cocaine exposure selectively inhibits protein phosphatase (PP1) activity. A, Prenatal cocaine-exposed rabbits exhibit decreased PP1 activity in FCX. Total protein phosphatase activity and protein phosphatase I-2 (I-2; 2 nm)- and tautomycin (taut; 3 nm)-insensitive PSP activities were measured as described in FCX lysate from both saline control (Con.) and prenatal cocaine-exposed rabbits.B, PP2A activity, calculated as the okadaic acid (2 nm)-sensitive PSP activity. Data are expressed as mean ± SEM released 32P from four to five determinations in counts per minute. C, Dephosphorylation activity in PP1 immunoprecipitates. PP1 was precipitated from FCX lysates with 3 μg of anti-PP1 mAb. PP1 activity was assessed in PP1 precipitates, and data expressed in fold activity relative to saline control is summarized and presented as mean ± SEM of at least four animals in each group. *p < 0.01, compared with saline control; Student's t test. D, PP1 protein expression. Aliquots (20 μg) of FCX lysate protein were separated on 10% SDS-PAGE, transferred to nitrocellulose, and blotted with anti-PP1 mAb (1:1500). The experiments were repeated in FCX of four animals in each group, and the same results were obtained.
Prenatal cocaine increases phosphorylation of DARPP-32 in FCX
The DARPP-32/PP1 signaling pathway was shown to regulate phosphorylation of neurotransmitter receptors, and cocaine stimulated the phosphorylation of DARPP-32 (Svenningsson et al., 2000). We therefore tested whether the DARPP-32 signaling pathway changes in brains of prenatal cocaine-exposed rabbits. As depicted in FigureFigure4,4, a robust increase in phospho-DARPP-32 was detected in FCX and in striata of cocaine-exposed rabbits using a monoclonal anti-phospho-(Thr34) DARPP-32 antibody without a change in the expression level of DARPP-32 protein, indicating that the reduction in FCX PP1 activity of in utero cocaine-exposed animals may be the consequence of a sustained activation of DARPP-32, as indicated by the increase in phospho-(Thr34) DARPP-32.
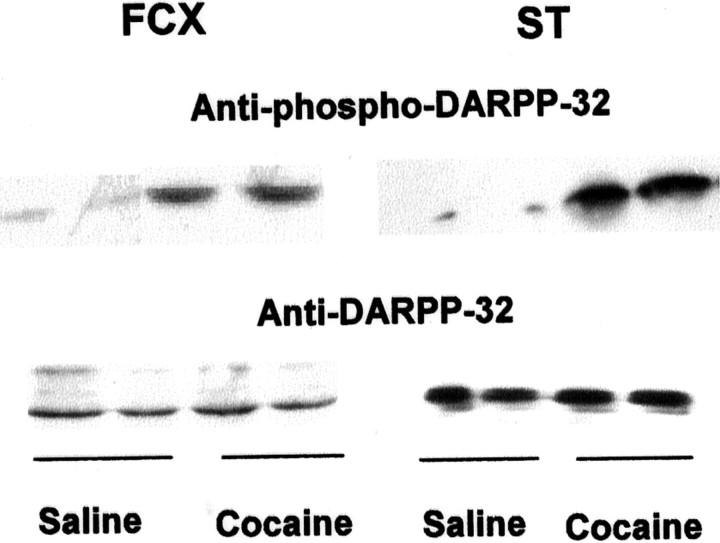
Prenatal cocaine exposure-enhanced phosphorylation of DARPP-32 on Thr34 in FCX and striatum (ST). FCX and striatum from saline and prenatal cocaine-exposed rabbits were lysed, and 100 (for phospho-DARPP-32) or 20 μg (for total DARPP-32) of supernatant protein was loaded on SDS-PAGE. The proteins were separated, transferred to a nitrocellulose membrane, and probed with anti-phospho-Thr34DARPP-32 (1:750) or anti-DARPP-32 (1:10,000) mAb, respectively. Representative blots are shown. The experiments were repeated in four animals for each group with similar results.
PP1 is physically associated with the D1ADAR, and prenatal cocaine reduces receptor-associated PP1 activity
DARPP-32/PP1 was shown to regulate the function of a number of neurotransmitter receptors or transporters by modulating their phosphorylation (Greengard et al., 1999). To determine whether the DARPP-32/PP1 signaling cascade is involved in regulating D1ADAR phosphorylation, we first examined whether PP1 and the receptor are physically associated. The D1ADAR in FCX lysates was immunoprecipitated and the precipitated proteins were electrophoretically separated and blotted with anti-PP1 antibody. The data depicted in FigureFigure55A shows that PP1 coimmunoprecipitates with the D1ADAR in both saline control- and cocaine-treated animals. Furthermore, the detection of PP1 inhibitor (I-2)-sensitive protein phosphatase activity in D1ADAR immunoprecipitates of control FCX clearly indicates the presence of PP1 activity in association with the receptor (Fig. (Fig.55B). In agreement with a reduction of PP1 activity in whole FCX lysates (Fig. (Fig.3),3), we also observed a dramatic decline in D1ADAR-associated PP1 activity in FCX of progeny of rabbits treated with cocaine (Fig. (Fig.55B), although the amount of PP1 protein detected in D1ADAR immunoprecipitation did not change (Fig. (Fig.55A). This association of PP1 with D1ADAR was further confirmed in PC2 cells that stably express D1ADARs (Fig.(Fig.66A,B). Interestingly, stimulation of D1ADARs with a selective receptor agonist exhibited no significant effect on total PSP or PP1 (with I-2) activity (Fig. (Fig.66C). Together, the results demonstrate that PP1 is physically associated with the D1ADAR and prenatal cocaine exposure decreases PP1 activity. This decrease in PP1 parallels the hyperphosphorylation of the receptor. The data strongly implicate that an alteration in the DARPP-32/PP1 signaling pathway is associated with D1ADAR hyperphosphorylation in FCX of in utero cocaine-exposed rabbits.
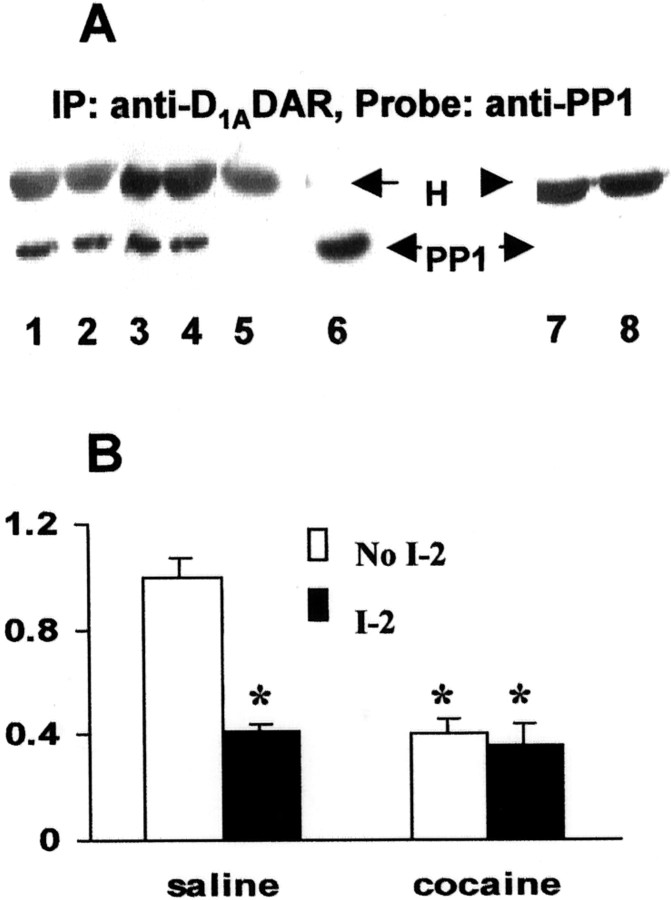
The D1ADAR is associated with PP1, and prenatal cocaine reduces PP1 activity in D1ADAR immunoprecipitates. A, PP1 is associated with D1ADAR. Equal amounts (400 μg) of FCX lysates from saline control or prenatal cocaine-exposed rabbits were incubated with 5 μg of anti-rat D1ADAR mAb or 10 μl of normal rat IgG overnight at 4°C with constant shaking, and the immunocomplexes were separated by SDS-PAGE, blotted, and probed with antibody to PP1.Lanes 1 and 2, Saline control;lanes 3 and 4, prenatal cocaine;lane 5, normal preimmune-IgG; lane 6, PP1 immunoblot using whole FCX tissue lysate; lanes 7 and8, second immunoprecipitation with anti-D1ADAR antibody using the supernatant that was precipitated previously with excess anti-D1A receptor antibody. H, IgG heavy chain. A representative blot is shown. The experiment was repeated with tissue from at least four animals, and each exhibited similar results. B, PP1 activity is present in D1ADAR immunoprecipitates. Equal amount of lysate protein is incubated with 5 μg of anti-rat D1ADAR mAb for 2 hr, followed by adding protein A/G PLUS for 1 hr. Protein phosphatase activity was determined by measuring the [32P] released from pre-[32P]-labeled MBP in D1ADAR immunoprecipitates in the presence (open bars) or absence (filled bars) of 2 nm of the PP1 inhibitor I-2. Results are the summary of data obtained from at least four animals for each group and are expressed as mean ± SEM in fold increases over saline control. *p < 0.01, compared with saline control.
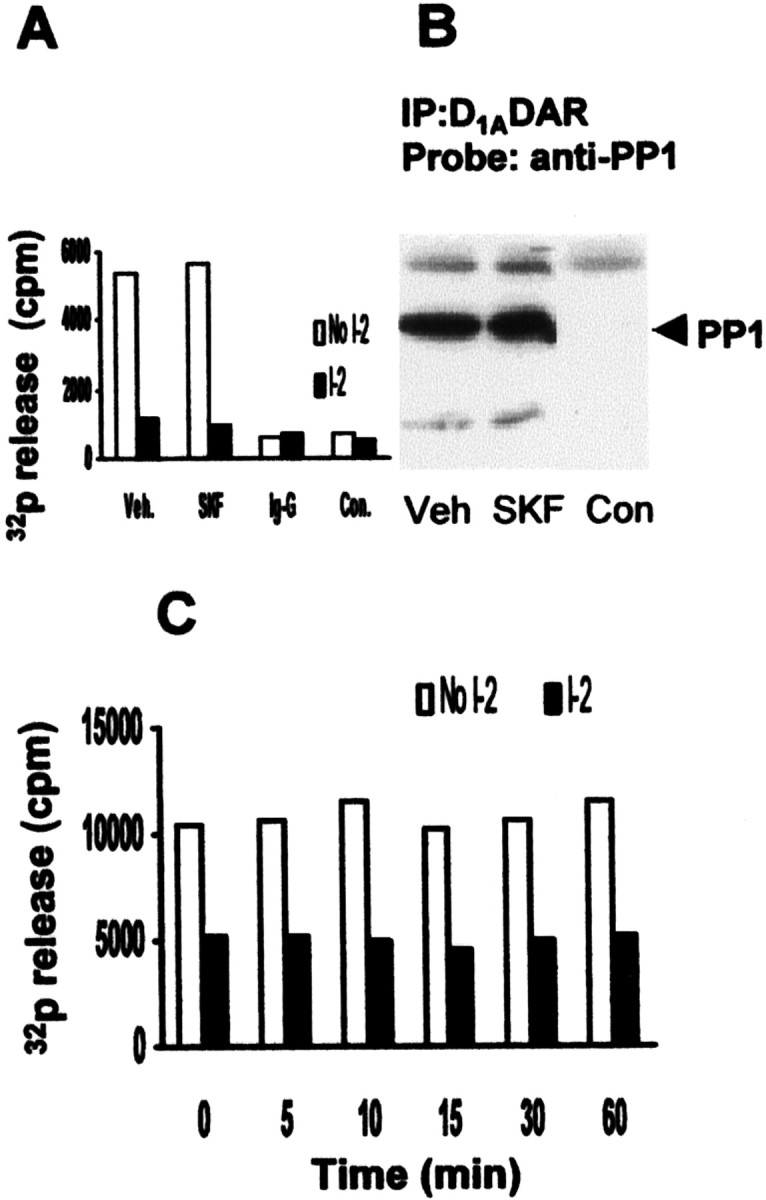
PP1 is associated with D1ADAR, and receptor stimulation does not alter PP1 activity in D1ADAR-expressing PC2 cells. Control and D1ADAR-expressing PC2 cells were cultured as described in Materials and Methods. Cells were stimulated with 5 μmSKF81297 for the indicated times, and protein phosphatase activity was assayed in cell lysates. Equal amount of lysates (500 μg/each) were immunoprecipitated with anti-D1ADAR antibody or with normal rat IgG for 2 hr, and the immunocomplex was collected for protein phosphatase assay (A) or for immunoblotting for PP1 (B) as described in Figure Figure5.5. Total PSP activity, measured in whole lysates, is shown in C.Veh, Vehicle; SKF, 5 μm SKF 81297; Con, control cells transfected with empty vector;I-2, inhibitor-2.
Decreased PP1 activity is associated with a dysfunctional brain D1ADAR in the cocaine-exposed rabbit
To further test the role of the DARPP-32/PP1 pathway in prenatal cocaine-induced dysfunction of FCX D1ADAR, we first tested whether PP1 can directly dephosphorylate the hyperphosphorylated D1ADAR in FCX of prenatal cocaine-exposed rabbits. As shown in FigureFigure77A, treatment of FCX membranes with exogenous PP1 resulted in a dramatic decline in D1ADAR phosphoserine level. Moreover, the dephosphorylation of FCX membrane D1ADARs was accompanied by a significant reversal in D1ADAR–Gs-protein uncoupling, as indicated by the partial restoration of dopamine-stimulated GTPγS binding to Gαs-protein (Fig. (Fig.77B). In contrast, dopamine-stimulated GTPγS binding to Gαi-protein was not affected by the treatment of membranes with PP1 (data not shown). Thus, PP1 is able to selectively regulate receptor–Gs-protein coupling by modulating the phosphorylation state of the D1ADAR. Together, our results indicate that the reduction in PP1-mediated dephosphorylation of the receptor, as a consequence of an activation of the DARPP-32/PP1 cascade in the brains of rabbits exposed to cocaine in utero, is responsible for the impaired resensitization of the D1ADAR and, therefore, results in its dysfunction.

Treatment of FCX membrane preparations with purified PP1 reverses the uncoupling of D1ADAR from Gαs-protein in prenatal cocaine-exposed rabbit.A, Membranes prepared from FCX were pretreated with 0.5 U/ml rabbit muscle PP1 or vehicle for 30 min in buffer containing 50 mm imidazole, pH 7.4, 1 mm EDTA, 2 mm DTT, 2 mm MnCl2, and 0.2 mg/ml BSA at 37°C with shaking. The PP1 was then washed out with ice-cold homogenization buffer, and the tissues were lysed in buffer B. Aliquots of lysate protein were incubated with anti-D1ADAR mAb, and the precipitates were blotted and probed with anti-phosphoserine antibody to detect phosphoserine in D1ADAR. B, Treatment of brain membranes with PP1 increased the coupling of D1ADAR with Gαs-protein in in utero cocaine-exposed tissue. PP1-treated FCX membranes from saline control and prenatal cocaine-exposed rabbits were stimulated with 1 μm DA. The binding of [35S]GTPγS to Gαs was determined as described. Data are expressed as percentage of saline basal control and are summarized as mean ± SEM obtained from at least four independent experiments. White bars, Saline; black bars, cocaine; *p < 0.01, compared with the respective control by Student'st test.
DISCUSSION
The present paper demonstrates that in utero exposure to cocaine elicits an elevation in the level of phosphoserine residues in brain D1ADARs. We also detected a marked decline in PP1 activity in FCX of prenatal cocaine-exposed rabbits. Moreover, we provide direct evidence that PP1 is physically associated with the D1ADAR and that a decrease in PP1 activity is involved in the hyperphosphorylation of brain D1ADARs in cocaine-exposed animals. Furthermore, we demonstrated that the alteration in the DARPP-32/PP1 cascade is responsible for the dysfunction in D1ADARs in brains of rabbits subjected to cocaine in utero.
In previous investigations, we showed that D1ADAR-mediated stimulation of GTP binding to Gαs is markedly reduced in frontal cortical membranes prepared from brains of prenatal cocaine-exposed rabbits. This change occurs without alteration in receptor density or in Gαs-protein expression (Friedman et al., 1996), suggesting that in utero exposure to cocaine results in an apparent desensitization of the D1ADAR. Moreover, it has been suggested that the behavioral, biochemical, and anatomical changes observed in prenatal cocaine-exposed animals are related to this defect in D1ADAR function (Simansky and Kachelries, 1996; Levitt et al., 1997; Jones et al., 2000). However, the molecular mechanism that underlies the D1ADAR signaling dysfunction is unknown. The present work demonstrates that (1) PP1 activity is dramatically reduced in whole FCX lysates and in D1ADAR immunoprecipitates obtained from prenatal cocaine-exposed rabbits, (2) PP1 is able to dephosphorylate the phosphorylated D1ADAR in vitro, and (3) dephosphorylation of the hyperphosphorylated D1ADAR by treating cortical membranes prepared from brains of prenatal cocaine-treated rabbits with PP1 partially reverses the impaired coupling between the D1ADAR and Gαs. These findings clearly indicate that reduced PP1 activity plays an essential role in the prenatal cocaine-mediated impairment of D1ADAR function.
It is known that PP1 is an important protein phosphatase in CNS. It not only regulates multiple cellular substrates, including a number of neurotransmitter receptors, but it is also involved in the modulation of nuclear transcriptional factors such as cAMP response element-binding protein (CREB) in brain (Greengard et al., 1999;Sala et al., 2000). In parallel with the reduction in frontal cortical PP1 activity, we also observed a reduction in stimulation-induced CREB activation in the treated rabbits (our unpublished data), indicating that alteration in PP1 activity by early exposure to cocaine may play an essential role in the sequellae to cocaine abuse. A reduction in protein phosphatase activity has been associated with hyperphosphorylated tau in Alzheimer's disease (Bennecib et al., 2000), suggesting that altered PP1 activity may have far-reaching consequences on brain function.
DARPP-32 plays a central role in the functional regulation of dopaminoceptive neurons. Dopamine and other neurotransmitters have been shown to regulate the phosphorylation of DARPP-32 by PKA. The phosphorylated form of DARPP-32 (Thr34), a potential inhibitor of PP1, in turn, regulates the phosphorylation state and activity of many cellular proteins and neurotransmitter receptors. On the other hand, the phosphorylation state of DARPP-32 (Thr34) is subject to regulation by PP2B and PP2A; both phosphatases dephosphorylate phospho-DARPP-32 (Thr34), leading to an increase PP1 activity (Greengard et al., 1999). In agreement with the previous demonstration of increased phospho (Thr34)-DARPP-32 after acute cocaine administration in mice (Svenningsson et al., 2000), we also observed an elevation in phosphorylated DARPP-32 (Thr34) in FCX of rabbits exposed prenatally to cocaine, suggesting that modulation of the DARPP-32/PP1 pathway may be a common neurobiological target of cocaine. At present, we do not know the precise molecular mechanism underlying cocaine-induced PP1 inhibition and enhanced phospho (Thr34)-DARPP-32. One possible mechanism is that cocaine regulates the PKA/DARPP-32 pathway by increasing synaptic DA, leading to receptor activation and thus enhancing DARPP-32 phosphorylation (Greengard et al., 1999). Alternatively, cocaine may regulate the phosphorylation state of DARPP-32 through the PP2B pathway. In addition, it was shown recently that DARPP-32 can be phosphorylated at the threonine(75) residue by cyclin-dependent kinase-5, and phospho-Thr-75 DARPP-32 is an inhibitor of PKA. Also, cocaine was shown to regulate striatal DARPP-32 phosphorylation at Thr-75 (Nishi et al., 2000; Bibb et al., 2001). Therefore, DARPP-32 is a bifunctional signaling molecule regulating both protein kinase and protein phosphatase (Bibb et al., 1999). It will be of interest to investigate whether prenatal cocaine regulates phospho-Thr-75 DARPP-32 and the functional implication of this action in the cocaine-induced functional defect of D1ADARs.
Receptor phosphorylation is believed to play an essential role in the regulation of desensitization and internalization of GPCRs. Receptor desensitization is the loss in functional response to receptor stimulation that is accompanied by a reversible loss of agonist affinity for the receptor that results from the uncoupling of the receptor from its G-protein. Receptor phosphorylation by GRKs or by second-messenger-sensitive kinases was shown to mediate desensitization of GPCRs (Bunemann and Hosey, 1999; Jian and Sibley, 1999). In concordance with one model of GPCR desensitization, the phosphorylated GPCR is internalized into intracellular endosomes, and the agonist-bound receptor can then recycle back to the membrane after dephosphorylation by protein phosphatases (Yu et al., 1993; Bunemann and Hosey, 1999). Although protein kinase-mediated desensitization has been widely studied, the regulatory mechanism involved in resensitization of the desensitized receptor is less clear. Both protein phosphatase 2A and PP1 have been shown to be involved in this process. For example, a PP2A-like enzyme dephosphorylates the β2 adrenergic receptor and both PP1 and PP2A can form complexes with the receptor (Pitcher et al., 1995; Shih et al., 1999). A similar role for PP1 and PP2A is also reported in the functional regulation of the thromboxane receptor (Spurney, 2001). However, in the case of D1ADAR, the mechanism and the protein phosphatase involved in receptor resensitization have not been identified. The present work provides the first evidence that PP1 is physically associated with the D1ADAR (Figs. (Figs.5,5, ,6)6) and that protein phosphatase is able to dephosphorylate and reactivate the D1ADAR (Fig. (Fig.77)
It is interesting to point out that stimulation of D1ADARs by the selective agonist SKF81297 in D1AR-transfected PC2 cells exhibited no effect on total PP1 activity (Fig. (Fig.55C), although the receptor is physically associated with PP1 (Fig. (Fig.55B). This may be attributable to the absence of DARPP-32 in these cells. The functional implication of this association in cells that do not express DARPP-32 is unclear at the present time. However, because receptor phosphorylation–desensitization, internalization, and dephosphorylation–resensitization are dynamic processes, the absence of a change in total PP1 activity may not necessarily reflect real change of receptor-associated PP1 activity. A recent report indicates that the association of β2 adrenergic receptor with PP2B is dynamic and that it parallels the change in PP2B activity (Shih et al., 1999). Whether a similar pattern exists with regard to the interaction of the D1ADAR with PP1 is not presently known. In addition, it has been noted in previous studies that anchoring and/or scaffolding proteins may be required for receptor desensitization or resensitization (Lefkowitz, 1998; Shih et al., 1999). The detailed mechanism involved in the interaction between PP1 and the receptor and its signal regulation, especially in cells that lack DARPP-32, remains to be investigated.
Prenatal cocaine exposure produces behavioral, neurochemical, and anatomical changes in brains of both animal and human offspring (Romano and Harvey, 1996, 1998; Levitt, 1998; Simansky et al., 1998). Impaired D1ADAR signaling has been demonstrated previously to contribute to the behavioral and morphological abnormalities observed in the developing brain as a result of cocaine exposure (Levitt, 1998; Simansky et al., 1998; Jones et al., 2000). The present work describes a novel mechanism in the regulation of D1ADAR function. We also demonstrated the involvement of an altered DARPP-32/PP1 pathway in mediating a D1ADAR resensitization defect in the developing brain of in utero cocaine-exposed rabbit. The results may provide insights into new strategies aimed at the prevention and treatment of the manifestations of maternal cocaine abuse.
Footnotes
This work was supported by National Institutes of Health Grant DA11029 and a March of Dimes grant.
Correspondence should be addressed to either Eitan Friedman or Xuechu Zhen, Department of Pharmacology and Physiology, City of University of New York Medical School, 138th Street and Convent Avenue, New York, NY 10031. E-mails: ude.ynuc.dem@namdeirf orude.ynuc.dem@uhceux.
REFERENCES
Articles from The Journal of Neuroscience are provided here courtesy of Society for Neuroscience
Full text links
Read article at publisher's site: https://doi.org/10.1523/jneurosci.21-23-09160.2001
Read article for free, from open access legal sources, via Unpaywall:
http://www.jneurosci.org/content/jneuro/21/23/9160.full.pdf
Free to read at www.jneurosci.org
http://www.jneurosci.org/cgi/content/abstract/21/23/9160
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/reprint/21/23/9160.pdf
Free after 6 months at www.jneurosci.org
http://www.jneurosci.org/cgi/content/full/21/23/9160
Citations & impact
Impact metrics
Citations of article over time
Article citations
α7nAChR-mediated recruitment of PP1γ promotes TRAF6/NF-κB cascade to facilitate the progression of Hepatocellular Carcinoma.
Mol Carcinog, 57(11):1626-1639, 26 Aug 2018
Cited by: 11 articles | PMID: 30074282
GSK-3β Interacts with Dopamine D1 Receptor to Regulate Receptor Function: Implication for Prefrontal Cortical D1 Receptor Dysfunction in Schizophrenia.
CNS Neurosci Ther, 23(2):174-187, 20 Dec 2016
Cited by: 9 articles | PMID: 27996211 | PMCID: PMC6492711
Cocaine-induced neurodevelopmental deficits and underlying mechanisms.
Birth Defects Res C Embryo Today, 108(2):147-173, 01 Jun 2016
Cited by: 11 articles | PMID: 27345015 | PMCID: PMC5538582
Review Free full text in Europe PMC
Prenatal Cocaine Exposure Upregulates BDNF-TrkB Signaling.
PLoS One, 11(8):e0160585, 05 Aug 2016
Cited by: 14 articles | PMID: 27494324 | PMCID: PMC4975466
Developmental consequences of fetal exposure to drugs: what we know and what we still must learn.
Neuropsychopharmacology, 40(1):61-87, 18 Jun 2014
Cited by: 184 articles | PMID: 24938210 | PMCID: PMC4262892
Review Free full text in Europe PMC
Go to all (48) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system.
Ann N Y Acad Sci, 846:238-247, 01 Jun 1998
Cited by: 29 articles | PMID: 9668411
Review
A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor.
J Neurosci, 18(24):10297-10303, 01 Dec 1998
Cited by: 213 articles | PMID: 9852567 | PMCID: PMC6793330
Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons.
Neuropsychopharmacology, 30(5):916-926, 01 May 2005
Cited by: 36 articles | PMID: 15726118
Prenatal cocaine exposure alters glycogen synthase kinase-3beta (GSK3beta) pathway in select rabbit brain areas.
Neurosci Lett, 349(3):143-146, 01 Oct 2003
Cited by: 12 articles | PMID: 12951189




