Abstract
Purpose
To investigate the efficacy of the combination of the FLT3 inhibitors midostaurin or gilteritinib with the Bcl-2 inhibitor venetoclax in FLT3-internal tandem duplication (ITD) acute myeloid leukemia (AML) and the underlying molecular mechanism.Experimental design
Using both FLT3-ITD cell lines and primary patient samples, Annexin V-FITC/propidium iodide staining and flow cytometry analysis were used to quantify cell death induced by midostaurin or gilteritinib, alone or in combination with venetoclax. Western blot analysis was performed to assess changes in protein expression levels of members of the JAK/STAT, MAPK/ERK, and PI3K/AKT pathways, and members of the Bcl-2 family of proteins. The MV4-11-derived xenograft mouse model was used to assess in vivo efficacy of the combination of gilteritinib and venetoclax. Lentiviral overexpression of Mcl-1 was used to confirm its role in cell death induced by midostaurin or gilteritinib with venetoclax. Changes of Mcl-1 transcript levels were assessed by RT-PCR.Results
The combination of midostaurin or gilteritinib with venetoclax potently and synergistically induces apoptosis in FLT3-ITD AML cell lines and primary patient samples. The FLT3 inhibitors induced downregulation of Mcl-1, enhancing venetoclax activity. Phosphorylated-ERK expression is induced by venetoclax but abolished by the combination of venetoclax with midostaurin or gilteritinib. Simultaneous downregulation of Mcl-1 by midostaurin or gilteritinib and inhibition of Bcl-2 by venetoclax results in "free" Bim, leading to synergistic induction of apoptosis. In vivo results show that gilteritinib in combination with venetoclax has therapeutic potential.Conclusions
Inhibition of Bcl-2 via venetoclax synergistically enhances the efficacy of midostaurin and gilteritinib in FLT3-mutated AML.See related commentary by Perl, p. 6567.Free full text

Inhibition of Bcl-2 Synergistically Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Preclinical Models of FLT3-mutated Acute Myeloid Leukemia
Abstract
Purpose:
To investigate the efficacy of the combination of the FLT3 inhibitors midostaurin or gilteritinib with the Bcl-2 inhibitor venetoclax in FLT3-ITD acute myeloid leukemia (AML) and the underlying molecular mechanism.
Experimental Design:
Using both FLT3-ITD cell lines and primary patient samples, annexin V-FITC/propidium iodide staining and flow cytometry analysis were used to quantify cell death induced by midostaurin or gilteritinib, alone or in combination with venetoclax. Western blot analysis was performed to assess changes in protein expression levels of members of the JAK/STAT, MAPK/ERK, and PI3K/AKT pathways, and members of the Bcl-2 family of proteins. The MV4–11-derived xenograft mouse model was used to assess in vivo efficacy of the combination of gilteritinib and venetoclax. Lentiviral overexpression of Mcl-1 was used to confirm its role in cell death induced by midostaurin or gilteritinib with venetoclax. Changes of Mcl-1 transcript levels were assessed by real-time RT-PCR.
Results:
The combination of midostaurin or gilteritinib with venetoclax potently and synergistically induces apoptosis in FLT3-ITD AML cell lines and primary patient samples. The FLT3 inhibitors induced downregulation of Mcl-1, enhancing venetoclax activity. Phosphorylated-ERK expression is induced by venetoclax but abolished by the combination of venetoclax with midostaurin or gilteritinib. Simultaneous downregulation of Mcl-1 by midostaurin or gilteritinib and inhibition of Bcl-2 by venetoclax results in “free” Bim, leading to synergistic induction of apoptosis. In vivo results show that gilteritinib in combination with venetoclax has therapeutic potential.
Conclusion:
Inhibition of Bcl-2 via venetoclax synergistically enhances the efficacy of midostaurin and gilteritinib in FLT3-mutated AML.
INTRODUCTION
Acute myeloid leukemia (AML) is defined by the clonal proliferation of immature myeloid elements and survival rates have remained low in both pediatric and adult patients, with 5-year survival rates approximating 61.5–65.1% and 25%, respectively (1). FMS-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase and important early regulator of hematopoiesis, in which mutations are seen in approximately one third of AML patients (2). FLT3-internal tandem duplications (ITD) mutations, seen in approximately 25% of patients with AML, result in constitutive activation of downstream pathways which promote cell survival and proliferation, including the MAPK/ERK, PI3K/AKT, and JAK/STAT pathways; understandably, it confers a poor prognosis (2, 3).
Direct inhibition of FLT3 is therefore a promising therapeutic avenue, with some agents already available for immediate use. Midostaurin, a first generation multi-kinase inhibitor was approved by the US Food and Drug Administration (FDA) in April 2017 for use in newly diagnosed FLT3-mutated AML patients, in combination with standard 7+3 cytarabine and daunorubicin induction and cytarabine consolidation, on the basis of results of the RATIFY trial (4). In patients over the age of 60 however (e.g. the largest population of AML patients), and those with other comorbidities, 7+3 chemotherapy is not well tolerated due to its extensive toxicities and severe side effects (5). Gilteritinib was approved by the US FDA in November 2018 for use in adult patients with relapsed or refractory FLT3-mutated AML, following the ADMIRAL trial () (6). As such, treatment options for FLT3-mutated AML are beginning to exist, but carry with them certain limitations – particularly in relation to monotherapy, with efficacious but short-lived responses. As such, new therapies to combine with FLT3 inhibitors are an unmet clinical need.
Venetoclax (ABT-199) is a selective inhibitor of Bcl-2, and was granted approval by the US FDA in November 2018 for first-line use in newly diagnosed AML in adults over 75 or those with comorbidities precluding use of intensive induction chemotherapy, in combination with low-dose cytarabine or the hypomethylating agents azacitidine or decitabine (7). Importantly, patients with FLT3 mutations did not fare worse than their peers while receiving venetoclax, despite the usual poor prognosis associated with this mutation (8, 9). Again however, while encouraging, there is room to improve upon these results; these studies show median complete remission times of 4–6 months, across all arms, complete remission rates of 21–54% (8, 9).
Our previous work demonstrated that venetoclax reduces the association of Bcl-2 with Bim; however, once freed, a compensatory increase in Bim/Mcl-1 binding occurs, particularly in venetoclax-resistant cell lines, preventing apoptosis (10). Mcl-1 inhibition is capable of halting this association and abrogating venetoclax resistance via a reduction in Bim/Mcl-1, freeing Bim (11–14). It therefore appears that joint inhibition of both Mcl-1 and Bcl-2 is required for effective induction of apoptosis via this mechanism. We therefore hypothesized that combining FLT3 inhibition via gilteritinib or midostaurin with selective Bcl-2 inhibition via venetoclax would result in synergistic antileukemic activity against FLT3-mutated AML. We theorized that FLT3 inhibition, via downstream downregulation of MAPK/ERK, JAK/STAT, and PI3K/AKT pathways, would result in decreased Mcl-1 expression and that the addition of the selective Bcl-2 inhibitor, venetoclax, would prevent free Bim from being sequestered by Bcl-2, thereby freeing it and allowing induction of apoptosis.
METHODS
Drugs
Midostaurin (PKC-412), gilteritinib (ASP-2215), venetoclax (ABT-199), and SCH772984 (an ERK-selective inhibitor) were purchased from Selleck Chemicals (Houston, TX, USA).
Cell Cultures
MOLM-13 cells were purchased from AddexBio (2012; San Diego, CA, USA). MV4–11, THP-1, and U937 cell lines were purchased from the American Type Culture Collection (2006, 2014, 2002, respectively; Manassas, VA, USA). Cell lines were cultured using RPMI 1640 media plus 10–20% fetal bovine serum (CLARK Bioscience, Claymont, DE, USA), 2 mM L-glutamine, and 100 U/ml penicillin and 100 μg/ml streptomycin and incubated in a humidified, 5%CO2/95% air environment at 37 °C. All lines were tested for the presence of mycoplasma using the PCR method described by Uphoff and Drexler (15) on a monthly basis. The cell lines were authenticated in 2017 at the Genomics Core at Karmanos Cancer Institute using the PowerPlex® 16 System from Promega (Madison, WI, USA).
Clinical Samples
Primary patient samples were obtained from the First Hospital of Jilin University, following obtainment of informed consent according to the Declaration of Helsinki. The study itself and the obtainment of patient samples was first approved by the Human Ethics Committee of The First Hospital of Jilin University. All patient samples were screened for FLT3-ITD, NPM1, C-kit, CEBPA, IDH1, IDH2, N-RAS and DNMT3A mutations by PCR amplification and automated DNA sequencing, cytogenetics, and fusion genes utilizing real-time RT-PCR, as described previously (16, 17). Individual patient characteristics are provided in Supplementary Table S1. Patient samples were purified using Ficoll-Hypaque density centrifugation and cultured in RPMI 1640 media plus 20% fetal bovine serum, ITS Solution (Sigma-Aldrich, St Louis, MO, USA), and 20% supernatant of the 5637 bladder cancer cell line (to provide sources of granulocyte–macrophage colony-stimulating factor, granulocyte colony-stimulating factor, interleukin-1 beta, macrophage colony-stimulating factor, and stem cell factor) (16, 18, 19).
Annexin V-fluorescein isothiocyanate (FITC) / Propidium Iodide (PI) Staining and Flow Cytometry Analyses
Cells were treated with either midostaurin, gilteritinib, SCH772984, or venetoclax, alone or in combination, for up to 24 hours. Flow cytometry analysis using the annexin V-FITC/PI Apoptosis Kit (Beckman Coulter; Brea, CA, USA) was performed as previously described (20, 21). Results are displayed as percentage of annexin V positive cells. All experiments were repeated in triplicate independently; displayed cell line data are from one experiment representative of findings of all three, and patient sample experiments were performed once in triplicate due to limited sample. The extent and direction of the antileukemic interaction was identified via calculation of the combination index (CI) using CompuSyn software (Combosyn Inc., Paramus, NJ, USA), where CI<1, CI=1 and CI>1 are indicative of synergistic, additive, and antagonistic effects, respectively (20, 22).
Western Blots
Cell lysis was performed in the presence of protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Whole-cell lysates underwent SDS-polyacrylamide gel electrophoresis, electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific, Rockford, IL, USA), and subsequently immunoblotted utilizing anti-Mcl-1, -Bcl-2, -Bcl-xL, -Bax, -β-actin, -ERK (Proteintech, Rosemont, IL, USA), -p-AKT (T308), -p-AKT (S473) (Affinity Biosciences, Zhenjiang, Jiangsu, China), -Bim, -cf-Caspase 3, -p-S6 (S240/244), -p-STAT5(Y694), -p-Mcl-1 (T163) (Cell Signaling Technologies, Danvers, MA, USA), -Bak, -p-ERK, -AKT (Abcam, Cambridge, MA, USA) as previously described (23, 24). Visualization of immunoreactive proteins was performed via the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE, USA), as described by the manufacturer. Western blots were repeated in triplicate (at minimum), with one representative blot displayed. Densitometry measurements were performed using Odyssey V3.0 (Li-Cor) and normalized to β-actin, calculated as fold-change compared to the corresponding control no-drug treatment specimen.
Co-immunoprecipitation
AML cell lines were treated for 4 hours with either midostaurin or venetoclax, alone or in combination, then lysed with 1% CHAPS, 5 mM MgCl2, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM Tris and 0.05% Tween-20 in the presence of protease inhibitors. Co-immunoprecipitation of Bim was carried out as described previously (25). 2 μg of anti-Bim antibody (Cell Signaling Technology, Danvers, MA, USA), 1 mg protein lysate, and Protein A agarose beads (Roche Diagnostics). Proteins were then eluted with 50 mM glycine at pH 2.0, and then analyzed via Western blotting as described above.
hours with either midostaurin or venetoclax, alone or in combination, then lysed with 1% CHAPS, 5 mM MgCl2, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM Tris and 0.05% Tween-20 in the presence of protease inhibitors. Co-immunoprecipitation of Bim was carried out as described previously (25). 2 μg of anti-Bim antibody (Cell Signaling Technology, Danvers, MA, USA), 1 mg protein lysate, and Protein A agarose beads (Roche Diagnostics). Proteins were then eluted with 50 mM glycine at pH 2.0, and then analyzed via Western blotting as described above.
Ectopic overexpression of Mcl-1 and shRNA knockdown of Bak and Bax
The pMD-VSV-G and delta 8.2 plasmids were gifts from Dr. Dong at Tulane University. Red fluorescent protein (RFP), Mcl-1 cDNA constructs were purchased from Thermo Fisher Scientific Biosciences (Lafayette, CO, USA). Bak and Bax shRNA constructs were purchased from Sigma Aldrich. Lentivirus production and transduction were carried out as previously described (26).
Quantification of gene expression by real-time RT-PCR
Total RNA was extracted using TRIzol (Thermo Fisher Scientific) and cDNAs were prepared from 2 μg total RNA using random hexamer primers and a RT-PCR Kit (Thermo Fisher Scientific), and then purified using the QIAquick PCR Purification Kit (Qiagen), as described previously (16, 22). Mcl-1 transcripts were quantitated using the TaqMan probe Hs01050896_m1 (Thermo Fisher Scientific) and a LightCycler 480 real-time PCR machine (Roche Diagnostics), based on the manufacturer’s instructions. Real-time PCR results were expressed as means from three independent experiments and were normalized to GAPDH transcripts measured by TaqMan probe (Hs02786624_g1). Fold changes were calculated using the comparative Ct method (27).
Leukemia xenograft model
MV4–11 cells (1 x 106 cells/mouse; 0.2ml/injection) were injected intravenously (day 0) into immunocompromised triple transgenic NSG-SGM3 female mice at eight weeks of age (NSGS, JAX#0103062; non-obese diabetic scid gamma (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3, CSF2, KITLG)1Eav/MloySzJ) Jackson Laboratory, Bar Harbor, ME, USA). Mice were randomly placed (5 mice/group; day 4) into vehicle control, 40 mg/kg gilteritinib (administered orally, p.o.), 85 mg/kg ABT-199 (p.o.), or 40 mg/kg gilteritinib + 85 mg/kg ABT-199 cohorts [p.o.; 3% ethanol (200 proof), 1% Tween-80 (polyoxyethylene (20) sorbitan monooleate) and sterile water; all USP grade; v/v]. For combined treatment, gilteritinib was administered first, followed by venetoclax within 1 h. Mice were treated daily for 27 days (treatment was stopped when one mouse in the control group showed leukemic symptoms). Mice were assessed 2x/day minimum for the duration of the study and body weights recorded daily. Mice were humanely euthanized when they presented with: >20% weight loss, decreased mobility limiting food and water access, lymph node metastases, progressive anemia, or lateral recumbency. % increase in lifespan (%ILS) was calculated: % ILS = [T-C/C] x 100 where “T” = treated and “C” = control median day of death. In vivo experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University.
Statistical Analysis
Differences were compared utilizing two-sample t-test. Statistical analyses were performed utilizing GraphPad Prism 5.0. Error bars represent ± standard error of the mean (s.e.m.); significance level was set at p<0.05. Overall survival probability was estimated (Kaplan-Meier method) and statistical analysis was performed using the log-rank test.
RESULTS
Midostaurin and venetoclax synergistically induce apoptosis in FLT3-ITD AML cell lines and primary patient samples
To determine if midostaurin synergizes with venetoclax in inducing cell death in AML, we treated the FLT3-ITD positive cell lines MOLM-13 and MV4–11 as well as 9 primary patient samples [8 harbor FLT3-ITD and 1 harbors FLT3-TKD (tyrosine kinase domain) mutations] with midostaurin and venetoclax, alone or in combination, for 24 hours. Synergistic cell death induction was observed in both cell lines (MOLM-13 CI<0.20, MV4–11 CI<0.18; Figure 1A), and in all nine primary patient samples (CI <0.001 to < 0.39; Figure 1B). Increase in annexin V positive cells was accompanied by increased cleaved caspase 3, demonstrating induction of apoptosis (Figure 1C). We also assessed the combination in the FLT3 wild-type (FLT3-wt) cell lines U937 and THP-1 and three FLT3-wt primary patient samples, and found CI values ranging from 0.002 to 2.12 (Figure 1D). While the CI values indicate a synergistic interaction, the magnitude of cell death induced by the combination was substantially lower than the FLT3-ITD cells, suggesting a minor contribution of a non-FLT3 mechanism of action. Evaluation of the combination using normal peripheral blood mononuclear cells revealed statistically significant but biologically minimal increase of apoptosis in drug treated compared to vehicle control treated cells (Figure 1E).
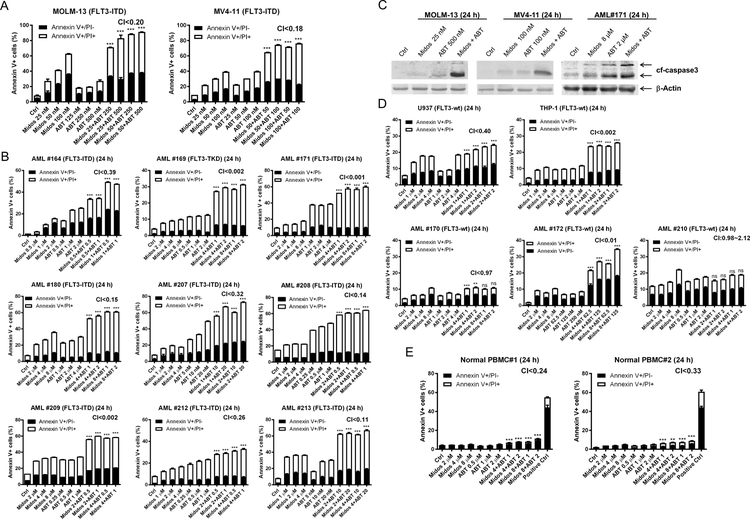
(A-B) FLT3-ITD AML cell lines MOLM-13 and MV4–11 (panel A) and FLT3-mutated (8 FLT3-ITD and 1 FLT3-TKD) primary patient samples (panel B) were treated with midostaurin and/or venetoclax for 24 hours, stained with annexin V-FITC/PI, and analyzed by flow cytometry. Flow cytometry results from one representative experiment is shown. Combination index (CI) values calculated using CompuSyn software are shown. ***indicates p<0.001. (C) AML cells were treated with the indicated drugs for 24 hours. Whole cell lysates were subject to Western blot analysis of cleaved-caspase3 (cf-caspase3). (D&E) FLT3-wt AML cell lines and primary patient samples (panel D) and normal peripheral blood mononuclear cells (PBMC; panel E) were treated with midostaurin and/or venetoclax for 24 hours, then stained with annexin V-FITC/PI and analyzed by flow cytometry. Flow cytometry results from one representative experiment is shown. CI values are shown. **indicates p<0.01, ***indicates p<0.001, and ns indicates not significant.
Midostaurin prevents venetoclax-induced upregulation of p-ERK
To begin to examine the mechanism of action in FLT-ITD AML, we assessed protein levels across the MAPK/ERK, PI3K/AKT, and JAK/STAT pathways via western blot in two FLT3-ITD positive AML cell lines and a primary patient sample 24 h post drug treatment. Midostaurin treatment resulted in downregulation of p-S6 and p-STAT5, which was preserved in cells treated with midostaurin in combination with venetoclax in all three samples tested (Figure 2A). Downregulation of p-AKT (both S473 and T308) was detected after venetoclax treatment and was further decreased in the combination treatment; total AKT levels decreased to a similar extent as p-AKT, while the effect of midostaurin was cell-specific (Figure 2A). The level of p-ERK decreased following midostaurin treatment in the primary AML patient sample, however it remained unchanged in MV4–11 cells and increased in MOLM-13 cells. Interestingly, venetoclax treatment also resulted in substantial increase of p-ERK in all three samples, which was abrogated by combination with midostaurin (Figure 2A). To better understand this phenomenon, we conducted a time-course analysis in both MOLM-13 and MV4–11 cell lines. Increased p-ERK was detected within 12–24 hours of venetoclax exposure. Midostaurin treatment downregulated p-ERK as early as 4 hours, unlike at the 24 h endpoint (Figure 2B&C). In the combined treatment, p-ERK levels were nearly undetectable after just 4 hours of treatment and remained below baseline up to 24 h during treatment. Next, we performed a time-course annexin V-FITC/PI flow cytometry experiment and found that the combination induced apoptosis as early as 4 hours (Figure 2D), which coincided with the early enhanced downregulation of p-ERK.
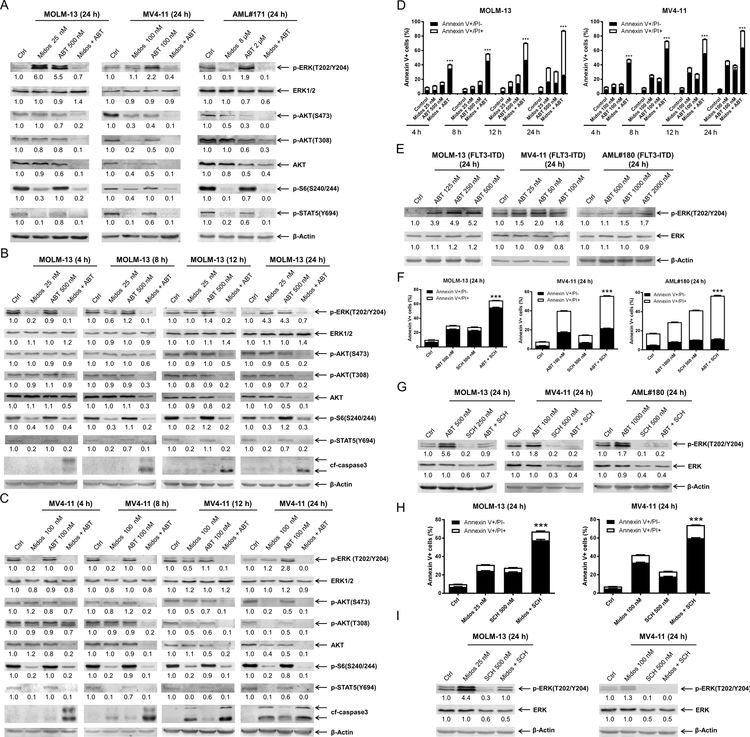
(A-C) FLT3-ITD AML cell lines MOLM-13, MV4–11, and primary patient sample AML#171 were treated with midostaurin alone or in combination with venetoclax for up to 24 hours. Representative Western blots generated using whole cell lysates are shown. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (D) MOLM-13 and MV4–11 cell lines were treated for 4–24 hours with vehicle control, midostaurin, venetoclax, or in combination. Annexin V-FITC/PI staining and flow cytometry results are shown. ***indicates p<0.001. (E) MOLM-13, MV4–11, and primary patient sample AML#180 were treated with variable concentrations of venetoclax. Representative Western blots are shown. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (F-G) AML cells were treated with venetoclax alone or in combination with the ERK- selective inhibitor SCH772984 for 24 hours. Flow cytometry and Western blot analyses are shown. ***indicates p<0.001. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (H-I) AML cells were treated with midostaurin alone or in combination with the ERK selective inhibitor SCH772984 for 24 hours. Flow cytometry and Western blot analyses are shown. ***indicates p<0.001. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control.
To begin to determine if p-ERK is a mechanism of resistance to venetoclax, we treated AML cells with variable concentrations of venetoclax and found that there was a concentration-dependent increase of p-ERK (Figure 2E). Further, treatment with the ERK-selective inhibitor SCH772984 completely abolished venetoclax-induced expression of p-ERK and significantly enhanced venetoclax-induced apoptosis (Figure 2F&G). Taken together, these results suggest that induction of p-ERK is a mechanism of resistance to venetoclax.
To assess the role of p-ERK in the antileukemic activity of midostaurin, we treated AML cells with midostaurin alone or in combination with SCH772984 and found that SCH772984 significantly enhanced cell death induced by midostaurin (Figure 2H). Western blots confirmed downregulation of p-ERK by SCH772984 alone and reduction of midostaurin-induced upregulation of p-ERK (Figure 2I). These results suggest that upregulation of p-ERK is also a mechanism of resistance to midostaurin.
Midostaurin downregulates Mcl-1 expression
Next, we examined the expression of the Bcl-2 family of proteins across two AML cell lines and a primary AML patient sample since their expression can be modulated by the FLT3 downstream signaling pathways. We identified consistent downregulation of Mcl-1 across the samples when treated with midostaurin alone and in combination with venetoclax for 24 hours (Figure 3A). Bcl-2, Bcl-xL, and Bim protein levels were not notably affected by either agent, alone or in combination. While reduced levels of Bak and Bax were detected, the decrease was not consistent across all three samples. Time-course evaluation showed that Mcl-1 downregulation occurs within 4 hours of exposure to midostaurin in both MOLM-13 and MV4–11 (Figure 3B); these results relate to the previously noted flow cytometric time-point analysis of enhanced cell death at 4 hours (Figure 2D). Co-immunoprecipitation revealed that midostaurin treatment reduced Mcl-1 binding to Bim, but increased Bim binding to Bcl-2, in both MOLM-13 and MV4–11 cells. Consistent with our published work (10, 11, 28), venetoclax treatment reduced Bcl-2 binding to Bim, but increased binding of Mcl-1 to Bim (Figure 3C). In the combined treatment, both Mcl-1 and Bcl-2 binding to Bim were reduced. To further confirm the role Mcl-1 plays in the combined treatment, we overexpressed Mcl-1 in MV4–11 cells. Its overexpression significantly reduced apoptosis induced by venetoclax alone and in combination with midostaurin (Figure 3D), suggesting that Mcl-1 plays an important role in the mechanism of action of combined midostaurin and venetoclax. To determine if the combination induces intrinsic apoptosis, Bak and Bax shRNA knockdown were performed. Knockdown of Bak and Bax significantly reduced annexin V positivity (Figure 3E). While the reduction of annexin V positive cells was only partial, potentially due to the redundancy of Bak and Bax, these results show that the combination induces apoptosis, at least partially through the intrinsic apoptosis pathway.
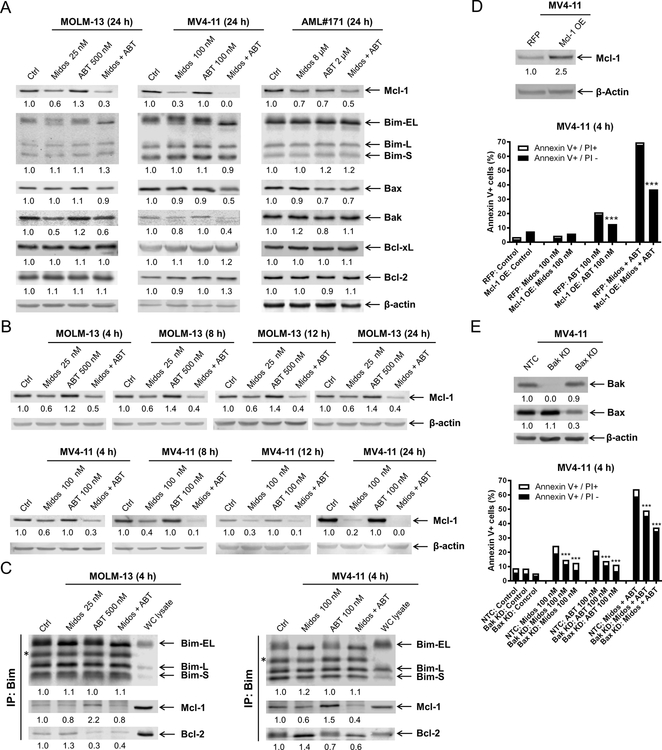
(A) Western blot analysis of members of the Bcl-2 protein family following 24 hour treatment with midostaurin alone and in combination with venetoclax in FLT3-ITD AML cell lines MOLM-13 and MV4–11 and primary patient sample AML#171. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (B) Western blot analyses of MOLM-13 and MV4–11 cells treated with midostaurin alone and in combination for up to 24 hours are shown. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (C) MOLM-13 and MV4–11 cells were treated with the indicated drugs for 4 hours. Bim was immunoprecipitated and subjected to Western blot analysis. *indicates light chain of the Bim antibody. (D&E) Lentiviral overexpression (OE) of red fluorescent protein (RFP) control and Mcl-1 (panel D) and shRNA knockdown of Bak and Bax (panel E) were performed as described in the ‘Methods’ section. Whole cell lysates were subjected to Western blot (upper panel). Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. RFP and Mcl-1 overexpressing and Bak and Bax knockdown cells were treated with midostaurin and venetoclax, alone and in combination, for 4 hours. Flow cytometry analyses results are shown (lower panel). ***indicates p<0.001.
To begin to determine how midostaurin treatment reduces Mcl-1 protein level, we first performed real-time RT-PCR. Midostaurin treatment significantly reduced Mcl-1 transcript levels 4 hours after drug treatment. Reduced levels were maintained in the combined drug-treated cells (Figure 4A), indicating that midostaurin transcriptionally downregulates Mcl-1. To determine if midostaurin treatment affects Mcl-1 protein stability, we treated MOLM-13 and MV4–11 cells with midostaurin and venetoclax, alone or in combination, for 4 hours. Cycloheximide treatment (10 μg/mL) revealed that Mcl-1 levels decreased significantly faster in MV4–11 cells treated with midostaurin alone and in combination with venetoclax (p<0.01; Figure 4B&C), while we did not detect a significant change in MOLM-13 cells. Since phosphorylation of Mcl-1 at T163 has been shown to stabilize Mcl-1 by prolonging its half-life (29), we looked at phosphorylation of Mcl-1 post-midostaurin treatment. Consistent with our cycloheximide results, we detected significantly reduced phosphorylation of Mcl-1 at T163 in MV4–11 cells treated with midostaurin alone and in combination with venetoclax, while we did not detect obvious change for MOLM-13 cells (Figure 4D&E). Taken together, these results show that midostaurin treatment reduces Mcl-1 transcript levels and decreases expression of Mcl-1 protein. While midostaurin can affect Mcl-1 stability, it may be a cell line-specific response. Nonetheless, downregulation of Mcl-1 transcript levels occurred in both cell lines, suggesting that downregulation of Mcl-1 by midostaurin is predominantly due to alteration of transcripts.
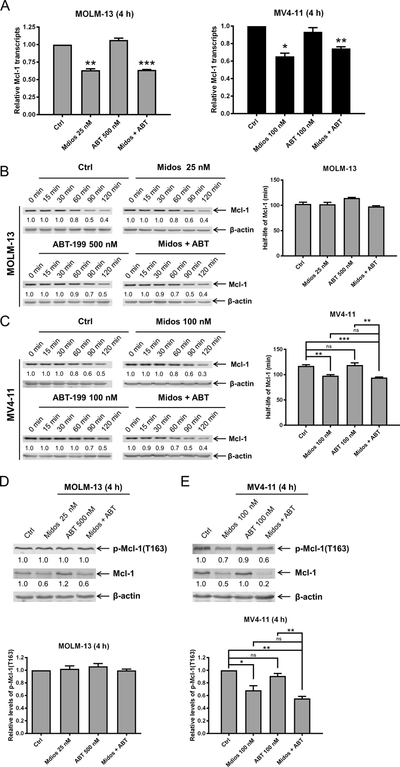
(A) MOLM-13 and MV4–11 cells were treated with midostaurin and venetoclax for 4 hours. Total RNA was isolated and Mcl-1 transcripts were determined by real-time RT-PCR using TaqMan probes. *indicates p<0.05, **indicates p<0.01, and ***indicates p<0.001. (B-C) MOLM-13 and MV4–11 cells were treated with midostaurin and/or venetoclax for 4 h, washed, and then treated with cycloheximide for up to 2 hours. Whole cell lysates were subjected to Western blotting. The fold changes for the Mcl-1 densitometry measurements, normalized to β-actin and then compared with no drug treatment control, are shown. Representative blots are shown on the left side, while densitometry measurements are graphed on the right. **indicates p<0.01 and ***indicates p<0.001 (D-E) MOLM-13 and MV4–11 cells were treated with midostaurin and venetoclax for 4 h. Western blot analyses of p-Mcl-1 and total Mcl-1 are shown. Representative blots are shown in the upper panels and the quantification results are graphed in the lower panels. **indicates p<0.01 and ***indicates p<0.001
Gilteritinib enhances the antileukemic effects of venetoclax in vitro and in vivo
As midostaurin is a first generation FLT3 inhibitor and displays low selectivity, we sought to confirm that the efficacy and mechanism demonstrated were indeed due to FLT3-inhibition and not due to off-target effects. We therefore used the second-generation FLT3-inhibtor gilteritinib. Consistent with midostaurin, gilteritinib synergized with venetoclax to induce annexin V positivity in MOLM-13 and MV4–11 cells (Figure 5A) accompanied by cleavage of caspase 3 (Figure 5B), demonstrating that the two agents synergize in inducing apoptosis in FLT3-ITD AML cells. Synergy was confirmed in 8 primary FLT3-ITD AML patient samples (Figure 5C). Similar to midostaurin, the combination of venetoclax and gilteritinib also demonstrated synergy against FLT3-wt cell lines and patient samples (Figure 5D), though the magnitude of cell death was substantially less than in the FLT3-ITD samples. The combination treatment induced apoptosis in normal PBMCs, though the magnitude was minimal compared to vehicle control treated cells (PBMC#1: 4.4% vs 7.4% and PBMC#2: 2.2% vs 8.8%; Figure 5E).
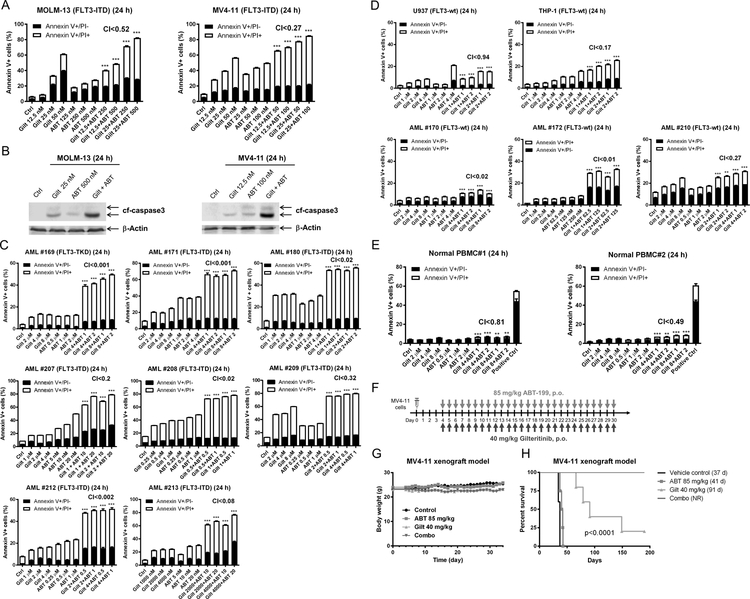
(A) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone or in combination, for 24 hours. Annexin V-FITC/PI staining and flow cytometry analyses results are shown. ***indicates p<0.001. (B) MOLM-13 and MV4–11 cells were treated as indicated for 24 hours. Whole cell lysates were subjected to Western blotting. (C-E) Primary AML patient samples (panels C&D) and normal peripheral blood mononuclear cells (PBMC; panel E) were treated with gilteritinib and venetoclax, alone or in combination, for 24 hours. Annexin V-FITC/PI staining and flow cytometry analyses results are shown. ***indicates p<0.001. (F-H) MV4–11 cells (1 x 106 cells/mouse) were injected through the tail vein of immunocompromised NSGS mice. Four days post cell injection the mice were randomized (5 mice/group) and treated daily with vehicle control (3% 200 proof ethanol, 1% polyoxyethylene (20) sorbitan monooleate, and USP water), 40 mg/kg gilteritinib (p.o.), 85 mg/kg venetoclax (p.o.), or 40 mg/kg gilteritinib + 85 mg/kg venetoclax (p.o.) for 27 consecutive days (treatments were stopped when one mouse in the vehicle control group showed leukemic symptoms; panel F). Body weights were measured on a daily basis and are graphed as mean ± SEM (panel G). Overall survival probability, estimated with the Kaplan-Meier method, is shown (panel H).
In vivo efficacy potential of gilteritinib in combination with venetoclax was evaluated in an early stage MV4–11-derived xenograft mouse model. We first conducted a toxicity/preliminary efficacy study with 2 mice/group. The mice were treated with vehicle control, 40 mg/kg gilteritinib, 85 mg/kg venetoclax, or gilteritinib + venetoclax on a daily basis for 13 days (Figure S1A). Moderate body weight loss (3% or less) was observed during treatment, though this was completely reversible (Figure S1B). Median survival for vehicle control and venetoclax treated mice was 41.5 days and 43.5 days, respectively, while gilteritinib alone was 61.5 days and the combination was 95.5 days, an ILS of 130% was achieved (Figure S1C). Based on these encouraging results, we conducted a full trial using 5 mice/group and extending treatment to 27 consecutive days (Figure 5F). Moderate body weight loss (6% or less) was observed during treatment, though it was completely reversible (Figure 5G). One mouse from both the venetoclax group and the combination group was excluded due to technical issues. Median survival for vehicle control, venetoclax, and gilteritinib treated mice was 37 days, 41 days, and 91 days, respectively (Figure 5H). On day 190, the one remaining gilteritinib treated mouse and the four combination treated mice were asymptomatic. At this point, we rechallenged the mice with tumor to rule-out a leaky system. Two weeks after implanting tumors, the tumors were significantly larger, demonstrating the survival of these mice was not due to regain of immunity.
Gilteritinib treatment abolishes the induction of p-ERK by venetoclax and downregulates Mcl-1 protein
Early induction of cell death in both FLT3-ITD cell lines was observed, with significantly enhanced killing demonstrated within 4 hours of combined exposure (Figure 6A). Next, we evaluated the downstream effects of gilteritinib upon the MAPK/ERK, PI3K/AKT, and JAK/STAT pathways via western blot and observed downregulation of p-ERK, p-S6, and p-STAT5, which were maintained when combined with venetoclax (Figure 6B). Combined gilteritinib and venetoclax treatment reduced p-AKT (T308 in both cell lines, while S473 in MV4–11 only) and total AKT levels. In accordance with midostaurin treatment, gilteritinib treatment resulted in downregulation of Mcl-l protein levels. Similar to midostaurin, treatment with single agent gilteritinib or venetoclax for 24 hours resulted in increased p-ERK, while the combination resulted in an overall decrease of p-ERK (Figure 6C). To determine if upregulation of p-ERK may be a mechanism of resistance to gilteritinib, we treated AML cells with gilteritinib alone and in combination with the ERK-selective inhibitor SCH772984 and found that SCH772984 significantly enhanced apoptosis induced by gilteritinib and prevented gilteritinib or venetoclax upregulation of p-ERK (Figure 6D&E). To determine if Mcl-1 plays a role in the mechanism of action, we overexpressed Mcl-1 in MV4–11 cells. Overexpression of Mcl-1 significantly reduced apoptosis induced by venetoclax treatment alone or in combination with gilteritinib (Figure 6F). Consistent with midostaurin treatment, gilteritinib treatment caused downregulation of Mcl-1 transcript levels (Figure 6G). In agreement with midostaurin treatment, gilteritinib combined with venetoclax was at least partially dependent on Bak and Bax (Figure 6H), showing that the combination induces apoptosis, at least partially through the intrinsic apoptosis pathway. In summary, upregulation of p-ERK plays a role in resistance to gilteritinib treatment and the addition of venetoclax prevents induction of ERK phosphorylation. Further, downregulation of Mcl-1 by gilteritinib enhances cell death induced by venetoclax.
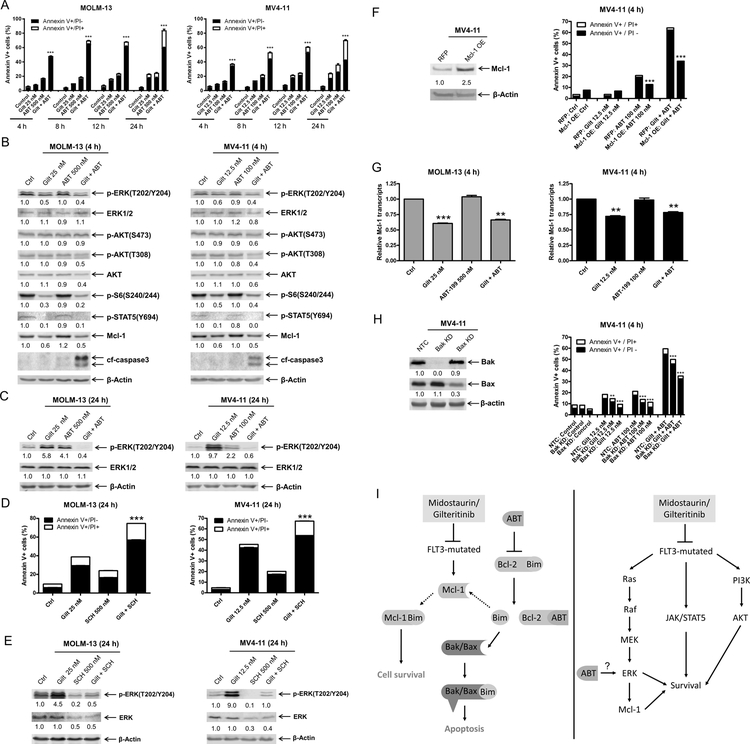
(A) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone or in combination, for up to 24 hours. Annexin V-FITC/PI staining and flow cytometry analyses results are shown. ***indicates p<0.001. (B) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone or in combination, for 4 hours. Whole cell lysates were subjected to Western blot analyses. Representative blots are shown; densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (C) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone or in combination, for 24 hours. Representative Western blots are shown; densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (D&E) MOLM-13 and MV4–11 cells were treated with gilteritinib alone or in combination with the ERK-selective inhibitor SCH772984 for 24 hours. Flow cytometry (panel D) and Western blot analyses (panel E) are shown. ***indicates p<0.001. Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. (F) Lentiviral OE of RFP control and Mcl-1 were performed as described in the ‘>Methods’ section. Whole cell lysates were subjected to Western blot (upper panel). Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. Overexpression cells were treated with gilteritinib and venetoclax, alone and in combination, for 4 hours. Flow cytometry analyses results are shown (lower panel). ***indicates p<0.001. (G) MOLM-13 and MV4–11 cells were treated with gilteritinib and venetoclax, alone and in combination, for 4 hours. Total RNA was isolated and Mcl-1 transcripts were determined by real-time RT-PCR by using TaqMan probes. **indicates p<0.01 and ***indicates p<0.001. (H) Lentiviral shRNA knockdown of Bak and Bax were performed as described in the ‘>Methods’ section. Whole cell lysates were subjected to Western blot (left panel). Densitometry measurements, shown below the corresponding blot, were normalized to β-actin and expressed as fold change compared to vehicle control. shRNA knockdown cells were treated with gilteritinib and venetoclax, alone and in combination, for 4 hours. Flow cytometry analyses results are shown (right panel). ***indicates p<0.001. (I) Inhibition of FLT3 synergizes with venetoclax via two proposed mechanisms in FLT3-mutated AML cells. Inhibition of FLT3 downregulates Mcl-1. Venetoclax frees Bim from Bcl-2, but also that this Bim can then be sequestered by Mcl-1, and therefore that joint Bcl-2 inhibition and Mcl-1 downregulation/inhibition is required for effective induction of apoptosis (left panel). Venetoclax potently induced p-ERK, which is abrogated by the addition of midostaurin or gilteritinib, leading to enhanced cell death.
DISCUSSION
In combining the FDA-approved FLT3 inhibitors midostaurin or gilteritinib with an additional FDA-approved anti-AML agent, venetoclax, we identified notable antileukemic synergy in FLT3-ITD AML cell lines and primary patient samples. The synergy observed between midostaurin/gilteritinib and venetoclax appears to be mechanistically based around two key points (Figure 6I). Firstly, Mcl-1 is downregulated by midostaurin/gilteritinib, though the precise molecular mechanism still needs to be determined – we have previously established that venetoclax frees Bim from Bcl-2, but also that this Bim can then be sequestered by Mcl-1, and therefore that joint Bcl-2 inhibition and Mcl-1 downregulation/inhibition is required for effective induction of apoptosis (10–14). Secondly, we expected to see suppression of multiple cell survival pathways due to multi-kinase inhibition with the FLT3 inhibitors, but we were surprised to observe the potent induction of p-ERK by venetoclax monotherapy. This induction was entirely abrogated by the addition of midostaurin or gilteritinib, leading us to theorize that this is another potential mechanism responsible for synergistic induction of apoptosis by the two classes of drugs (Figure 6I). By using the selective ERK inhibitor SCH772984, we were able to confirm that resistance to venetoclax was indeed at least partially due to p-ERK induction.
In agreement with Bruner et al (30), we found that midostaurin/gilteritinib treatment downregulates p-ERK as early as 4 hours, though by 24 hours p-ERK levels were at least back to baseline, if not substantially increased. This is consistent with previous reports that FLT3 inhibitor treatment can cause persistent ERK activation in the presence of bone marrow stroma (31–33), though our results were obtained in the absence of bone marrow stroma. We also found that this rebound of p-ERK was a mechanism of resistance to both midostaurin and gilteritinib. Based on these findings, we speculate that the timing of drug administration could potentially affect the efficacy. Administration of midostaurin or gilteritinib too far in advance of venetoclax could potentially reduce the activity of the combined treatment due to upregulation of p-ERK, a mechanism of resistance to venetoclax treatment. While administration of midostaurin or gilteritinib for a period of time long enough to downregulate Mcl-1 but prior to upregulation of p-ERK would likely afford venetoclax the opportunity to further reduce the amount of Bim bound to Bcl-2/Mcl-1 and induce apoptosis; on the other hand, this will also prevent the rebound of p-ERK during FLT3 inhibitor treatment. Our in vivo study was designed based on our in vitro findings; significantly prolonged survival was realized in mice treated with the combination of gilteritinib and venetoclax. Additional studies are warranted to further optimize the schedule of drug administration, which should take into consideration the pharmacokinetic and pharmacodynamic profiles of these agents.
Although clearly showing marked synergy in FLT3-ITD AML cell lines and patient samples, we additionally identified synergy between venetoclax and midostaurin/gilteritinib in FLT3-wt cell lines and primary patient samples. We postulate that this is reflective of the existence of non-FLT3-related mechanisms. Specifically, both gilteritinib and midostaurin have known efficacy against FLT3-wt AML in the clinical setting (34, 35), so it is perhaps unsurprising to see evidence of such activity here. This synergy likely arises due to the multi-kinase inhibitory activity of midostaurin and the AXL-targeting of gilteritinib, which do not depend on the presence of FLT3-ITD/TKD mutations or constitutive activation of FLT3.
In summary, we found that combining midostaurin or gilteritinib with venetoclax potently induces cell death in FLT3-ITD AML cell lines and primary patient samples. Our study demonstrates that p-ERK expression is induced by venetoclax but abolished by the combination with midostaurin or gilteritinib and that downregulation of Mcl-1 by midostaurin or gilteritinib enhances venetoclax-induced cell death. Results from our MV4–11-derived xenograft mouse model show that the combination has potent antileukemic activity against FLT3-ITD AML in vivo, supporting the clinical translation of midostaurin/gilteritinib plus venetoclax. Fittingly, clinical investigations of FLT3-ITD inhibitors in combination with venetoclax were recently initiated in relapsed/refractory AML ( and ).
GRANT SUPPORT
This study was supported by Jilin University, Changchun, China, the Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, and by grants from the National Natural Science Foundation of China, NSFC 31671438 and NSFC 31471295, Graduate Innovation Fund of Jilin University, Hyundai Hope on Wheels, LaFontaine Family/U Can-Cer Vive Foundation, Kids Without Cancer, Children’s Hospital of Michigan Foundation, Decerchio/Guisewite Family, Justin’s Gift, Elana Fund, Ginopolis/Karmanos Endowment and the Ring Screw Textron Endowed Chair for Pediatric Cancer Research. The Animal Model and Therapeutics Evaluation Core and Genomics Core are supported, in part, by NIH Center Grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University. The funders had no role in study design, data collection, analysis and interpretation of data, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest disclosure: The authors declare no potential conflicts of interest.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1158/1078-0432.ccr-19-0832
Read article for free, from open access legal sources, via Unpaywall:
https://clincancerres.aacrjournals.org/content/clincanres/25/22/6815.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1158/1078-0432.ccr-19-0832
Article citations
NL101 synergizes with the BCL-2 inhibitor venetoclax through PI3K-dependent suppression of c-Myc in acute myeloid leukaemia.
J Transl Med, 22(1):867, 27 Sep 2024
Cited by: 0 articles | PMID: 39334157 | PMCID: PMC11429391
Recent Developments and Evolving Therapeutic Strategies in KMT2A-Rearranged Acute Leukemia.
Cancer Med, 13(20):e70326, 01 Oct 2024
Cited by: 0 articles | PMID: 39428967 | PMCID: PMC11491690
Review Free full text in Europe PMC
Venetoclax: a new player in the treatment of children with high-risk myeloid malignancies?
Blood Adv, 8(13):3583-3595, 01 Jul 2024
Cited by: 0 articles | PMID: 38701350 | PMCID: PMC11319833
Review Free full text in Europe PMC
Activation of non-classical Wnt signaling pathway effectively enhances HLA-A presentation in acute myeloid leukemia.
Front Oncol, 14:1336106, 19 Jun 2024
Cited by: 0 articles | PMID: 38962268 | PMCID: PMC11219938
ITGAM sustains MAPK signaling and serves as an adverse prognostic factor and therapeutic target in acute myeloid leukemia.
Transl Cancer Res, 13(6):3062-3074, 27 Jun 2024
Cited by: 0 articles | PMID: 38988941
Go to all (85) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combining the novel FLT3 and MERTK dual inhibitor MRX-2843 with venetoclax results in promising antileukemic activity against FLT3-ITD AML.
Leuk Res, 144:107547, 24 Jun 2024
Cited by: 0 articles | PMID: 38968731
FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation.
Signal Transduct Target Ther, 6(1):186, 24 May 2021
Cited by: 41 articles | PMID: 34024909 | PMCID: PMC8141515
FLT3-selective PROTAC: Enhanced safety and increased synergy with Venetoclax in FLT3-ITD mutated acute myeloid leukemia.
Cancer Lett, 592:216933, 04 May 2024
Cited by: 0 articles | PMID: 38705564
Gilteritinib for the treatment of relapsed and/or refractory FLT3-mutated acute myeloid leukemia.
Expert Rev Clin Pharmacol, 12(9):841-849, 27 Aug 2019
Cited by: 8 articles | PMID: 31454267
Review
Funding
Funders who supported this work.
Karmanos Cancer Institute at Wayne State University (1)
Grant ID: P30 CA022453
NCI NIH HHS (1)
Grant ID: P30 CA022453
National Natural Science Foundation of China (2)
Grant ID: NSFC 31471295
Grant ID: NSFC 31671438
U Can-Cer Vive Foundation (1)
Grant ID: R2-2017-53





