Abstract
Free full text

Fungal biomass is a key factor affecting polymorphonuclear leucocyte-induced hyphal damage of filamentous fungi
Summary
Previous studies have not systematically assessed the effect of fungal biomass on polymorphonuclear leucocyte (PMN)-induced hyphal damage (HD) of filamentous fungi. We hypothesised that fungal biomass is a significant factor affecting PMN-induced HD. One isolate each consisting of a volume of 2 × 104 conidia ml−1 of Aspergillus fumigatus, Aspergillus flavus, Aspergillus terreus, Rhizopus oryzae, Rhizopus microsporus, Cunninghamella bertholletiae, Scedosporium prolificans and Fusarium solani were incubated for six different time periods yielding biomass values between 0.01 and 0.1 optical density (OD, 405 nm). Polymorphonuclear leucocyte were added at effector–target (E : T) ratios of 5 : 1, 10 : 1, 20 : 1, 50 : 1 and 100 : 1, and HD was assessed by XTT [2,3-bis-(2-methoxy-4-nitro-5-sulphophenyl)-2H-tetrazolium-5-carboxanilide] metabolic assay. Hyphal damage decreased with increasing biomass following the sigmoid (Emax) model (median R2: 0.87). Hyphal damage at 0.01 OD exceeded HD at 0.1 OD (P < 0.01) by >twofold in 64 out of 80 comparisons. The sigmoid curves were shifted to the right with higher E : T ratios; the EC50 values (OD values showing HD halfway between maximal and minimal HD) obtained for 50 : 1 or 100 : 1 were higher than for 5 : 1 (P < 0.01). Using the same E : T ratio, interspecies differences were observed; for 5 : 1, lower EC50 values were obtained for A. flavus and the zygomycete species. In conclusion, PMN-induced HD decreases with increasing biomass. This effect is both species-dependent and E : T ratio-dependent.
Introduction
Invasive infections caused by filamentous fungi are a major cause of morbidity and mortality among immunocompromised patients. Although Aspergillus fumigatus is more frequently isolated from clinical specimens, the non-A. fumigatus Aspergillus species as well as other less common filamentous fungi also have emerged as important pathogens.1–4 Innate immunity, including oxidative and non-oxidative mechanisms of hyphal damage (HD) by polymorphonuclear leucocytes (PMNs), is an important constituent of host response to fungal pathogens.5,6 Studies of HD caused by human PMNs against filamentous fungi have greatly contributed in our understanding of the pathogenesis of invasive mycoses, the relative virulence of implicated species as well as the immunomodulatory effects of cytokines.
Previous studies of HD by human PMNs have used standardised approaches of studying effector–target (E : T) ratio of PMNs : conidia as the principal variable for assessment or comparison of results. The hyphae used as stimuli in these studies are generated after incubation of conidia for various time periods in order to generate ‘short’ hyphae (subjectively characterised),7 hyphae of prespecified length (160–200 μm8 or ≥30 μm,9 microscopically assessed) or a presumably appropriate hyphal mat without objective assessment of hyphal growth.10 In some of these studies including different species, the same amount of conidia was incubated for the same period for all species.10 In other studies, the concentration of the conidial suspension varied among species based on differences in their metabolic activities.11 However, we considered that the overall hyphal biomass of organism may also be an important determinant in host–pathogen interaction.
It becomes clear from the above that in the majority of studies of PMN-induced HD there was no systematic and objective assessment of the fungal biomass to which the neutrophil suspension was added. Assessment of hyphal length is an objective criterion that, however, does not take into account the hyphal width. Consequently, the same hyphal length may, for example, correspond to a significantly higher biomass for Zygomycetes (whose hyphae are broad) than for Aspergillus spp. Furthermore, assessment of hyphal length is a cumbersome procedure, requiring microscopic examination of multiple hyphal elements.
We hypothesised that fungal biomass is a significant factor affecting PMN-induced HD of filamentous fungi. We therefore sought to investigate the relationship between biomass and HD for three Aspergillus species and other, medically important, mould species using spectrophotometry as an easy and objective method for biomass quantification. We subsequently analysed the data using non-linear regression analysis in order to generate quantitative parameters for description of the biomass–HD-relationship and comparisons between different species or E : T ratios.
Materials and methods
Isolates
Eight clinical isolates of filamentous fungi were studied, one each of Aspergillus fumigatus, Aspergillus terreus, Aspergillus flavus, Rhizopus oryzae, Rhizopus microsporus, Cunninghamella bertholletiae, Scedosporium prolificans and Fusarium solani. Conidia were harvested after isolates were subcultured on potato dextrose agar at 35 °C for 1 day and then at room temperature for 4–6 days, and were suspended in normal saline containing 0.025% Tween 20. Conidial suspensions were counted with a haemocytometer and kept at 4 °C for no longer than 2 weeks.
Fungal growth
From each of the above isolates, an inoculum of 2 × 104 conidia ml−1 of RPMI 1640 medium with l-glutamine but without bicarbonate, buffered to pH 7.0 with 0.165 mol l−1 3-(N-morpholino) propane sulfonic acid (Cambrex Bio Science Walkersville, Inc., Walkersville, MD, USA), was placed in flat-bottom 96-well microtitration plates (Costar 3596; Corning Inc., Corning, NY, USA) at a volume of 200 μl per well and incubated at 37 °C, with the exception of S. prolificans and F. solani, which were incubated at 32 °C.
Each organism was incubated for 6 one- to two-hourly different time periods, in order to yield biomass values ranging approximately between 0.01 (0.005–0.015) and 0.1 (0.09–0.11) optical density (OD) as measured by spectrophotometer (Elx808; Bio-Tek Instruments, Winooski, VT, USA) at 405 nm. Biomass determination was based on spectrophotometric reading of 24 replicate wells for each isolate and incubation period, of which the average OD value was recorded. The high number (24) of replicate wells was chosen in order to increase the precision of biomass determination and achieve narrow 95% confidence intervals (CI) of the mean biomass throughout the whole range of OD values. Indeed, preliminary studies showed that the 95% CI of the mean tended to increase with lower OD values. Using a smaller number (6) of replicate wells, the ratio of standard error (SE) vs. the mean was as high as 30% with OD of 0.005–0.015. Using 24 replicate wells the ratio SE/mean was always less than 15%.
Following preliminary growth studies, the six different incubation periods required to yield biomass values between 0.01 and 0.1 OD ranged from 11 to 20 h for A. fumigatus, 18–28 h for A. terreus, 11–24 h for A. flavus, 7–15 h for R. oryzae and R. microsporus, 4–10 h for C. bertholletiae, 15–24 h for S. prolificans and 12–20 h for F. solani.
Preparation of human PMNs
Blood from healthy adult volunteers was heparinised and 3% dextran 500 (Amersham Biosciences, Uppsala, Sweden) was added at a 1 : 2 volume ratio of 3% dextran to blood. The dextran/blood solution was kept at room temperature for 20 min in order to allow the red blood cells to settle to the bottom of the tube. The supernatant was carefully removed, slowly layered over 10 ml of Ficoll [Lymphocyte Separation Medium (LSM); MP Biomedicals, LLC, Irvine, CA, USA] and centrifuged at 500 g, 25 °C for 30 min with slow break. Following centrifugation, the dextran/plasma, mononuclear cell and LSM layers were discarded, leaving the neutrophil/red blood cell pellet, which subsequently was disrupted with gentle swirling or tapping of the tube. A volume of 5.8 ml of sterile water was then added to the pellet and gently swirled for 20 s in order to hypotonically lyse the red blood cells. At the end of this 20-s period, tonicity was brought back to the isotonic state with the addition of 2 ml of 3.5% hypertonic saline solution. The suspension of PMNs was then brought to a total volume of 30 ml after addition of Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+ (Quality Biological, Inc. Gaithersburg, MD, USA) and centrifuged at 500 g, 4–10 °C for 10 min with slow break. The supernatant was discarded and PMNs were resuspended in 2–3 ml of HBSS without Ca2+ and Mg2+ and counted with a haemocytometer. Cell viability was confirmed using trypan blue; only viable cells were counted.
Hyphal damage assay
Polymorphonuclear leucocyte-induced HD was studied using a previously described assay12 with modifications. In particular, the tetrazolium salt XTT (Sigma-Aldrich, Saint Louis, MO, USA) and the electron transfer agent menadione (Sigma-Aldrich) were used. XTT was dissolved in phosphate-buffered saline (PBS) (Quality Biological, Inc.) at 0.25 mg ml−1. Menadione was first dissolved in absolute ethanol at 10 mg ml−1 (58 × 10−3 mol l−1) and subsequently added to the PBS/XTT solution at a concentration of 25 μmol l−1.The PBS/XTT/menadione solution was prepared fresh for every experiment and kept in dark at 4–8 °C until its use.
At the end of the six different incubation periods for each isolate, the microtitration plates were removed from the incubator and fungal biomass was measured spectrophotometrically as already described. The plates were then centrifuged at 800 g for 30 min at ambient temperature (25 °C); subsequently, the medium (RPMI 1640) from each row of wells was aspirated and immediately replaced by equal volume (200 μl per well) of HBSS with Ca2+ and Mg2+ (Quality Biological, Inc.), in which the human PMNs had been added at E : T ratios of 5 : 1, 10 : 1, 20 : 1, 50 : 1 and 100 : 1. In some wells, the RPMI 1640 was replaced by 200 μl per well of HBSS with Ca2+ and Mg2+ but without PMNs; these would serve as control wells. The plates were then incubated at 37 °C, 5% CO2 for 2 h and subsequently centrifuged at 800 g for 30 min at 15 °C. The supernatant was then carefully aspirated from each well, in order to preserve the lawn of hyphae covering the bottom of the well, and replaced by 200 μl of sterile water. The plates were centrifuged again at 800 g for 30 min at 15 °C. This ‘washing’ step was performed twice in order to achieve complete lysis of the PMNs.10 For F. solani, whose hyphae became very fragile and easily detachable following the 2 h incubation with the PMNs, the supernatant during the washing process was discarded not by aspiration but by turning the plate upside-down and gently tapping it over an absorbing paper sheet.
Following the washing step, the 200 μl of sterile water in each well was replaced by 150 μl of the above PBS solution containing 0.25 mg ml−1 XTT and 25 μmol l−1 menadione. In each experiment, a row of wells was filled with 150 μl of the same PBS/XTT/menadione solution and served to provide background absorbance values in subsequent spectrophotometric measurements of XTT conversion. The plates were subsequently incubated at 37 °C, 5% CO2 for 2 h, for A. fumigatus, A. terreus and S. prolificans, or 1 h, for the three species of Zygomycetes, A. flavus and F. solani, which showed greater metabolic activity. The plates were then shaken for 1–2 min (Wallac Plate Shake 1296–004; Wallac OY, Turku, Finland) for further dissolution of the formazan derivatives, and colour absorbance was measured at dual wave-length (450 nm with reference 630 nm, in order to correct for the absorbance of hyphae) with a microtitration plate spectrophotometric reader (Elx808; Bio-Tek Instruments, Winooski, VT, USA). After subtraction of background absorbance values, the percentage of HD for each isolate, biomass value and E : T ratio, was calculated as: percentage of HD = (1−X/C) × 100, where X is the absorbance of the well with the corresponding E : T ratio and C is the average absorbance of control wells, where hyphae had not been exposed to PMNs.
In each experiment, four replicate wells were tested for every species, incubation period (corresponding to different biomass value) and E : T ratio or control. The experiments were repeated in duplicate.
Modelling of relationship between hyphal damage and fungal biomass
For each experiment, species and E : T ratio, the relationship between HD and fungal biomass (OD) was analysed with the Emax model (sigmoid curve with variable slope), described with the equation:
Statistical analysis
Comparisons of percentage of HD between different biomass values were performed for each E : T ratio using the non-parametric Kruskal–Wallis test followed by Dunn’s test for multiple comparisons. Comparisons of the EC50 values generated with the Emax (sigmoid) model between different E : T ratios was performed after log10 transformation of values and passing the Bartlett’s test for equal variances by one-way analysis of variance (anova) followed by Bonferroni’s post-test. Statistical analysis also was performed using the graphpad prism software (4.0b).
Results
Hyphal damage decreased gradually with increasing biomass for all species and E : T ratios. When the percentage of HD obtained with the lowest biomass studied (0.005–0.015 OD) was compared with that obtained with the highest biomass (0.09–0.11 OD), for all isolates and E : T ratios, it exceeded the latter by more than twofold in 64 out of 80 comparisons (eight isolates × five E : T ratios × two replicate experiments) and by more than 1.5-fold in 71 out of 80 comparisons. The difference in percentage of HD between the lowest and highest biomass values was statistically significant (P < 0.01) for all E : T ratios (Fig. 1).
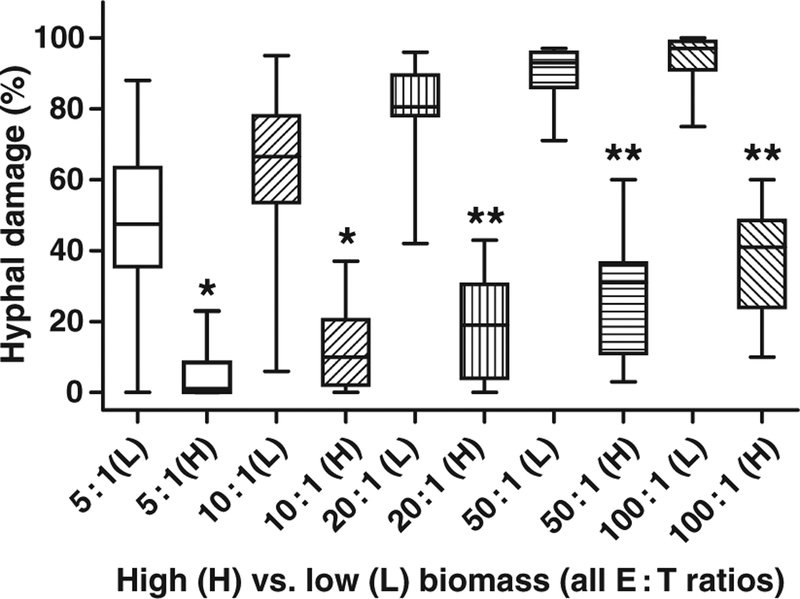
Box and whiskers plot of median percentage of hyphal damage for all species comparing the lowest (L, 0.01 OD) and highest (H, 0.1 OD) biomass values for E : T ratios from 5 : 1 to 100 : 1. For each E : T ratio, the percentage of hyphal damage obtained with the lowest and highest biomass were compared. *P < 0.01; **P < 0.001.
The Emax (sigmoid) model was able to describe the gradual decrease of percentage of HD in relation to increasing fungal biomass (for every isolate and E : T ratio), as manifested by the relatively high R2 values (median R2: 0.87, range: 0.49–0.98) and significant deviation from the model in only five out of 80 data sets to which it was applied, based on the runs test and visual inspection (Fig. 2). As indicated in Fig. 2, however, the sigmoid curves describing the decrease of percentage of HD with increasing fungal biomass were shifted to the right with higher E : T ratios. This shifting to the right was associated with higher EC50 values, generated by the Emax model, in the presence of higher E : T ratios. Indeed, the EC50 values obtained with E : T ratios of 100 : 1 were significantly higher than those obtained with E : T ratios of 5 : 1 (P < 0.001) and 10 : 1 (P < 0.05); the EC50 values obtained with E : T ratios of 50 : 1 were significantly higher than those obtained with 5 : 1 (P < 0.01) (Fig. 3).
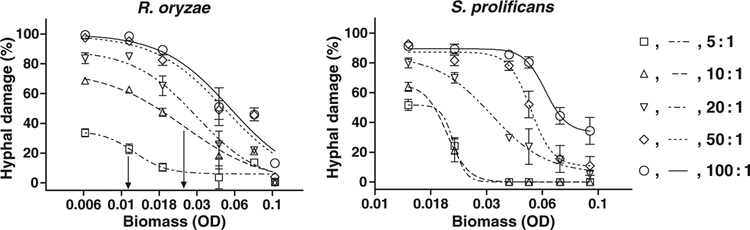
Representative sigmoid curves, generated with the Emax model, describing the relationship between fungal biomass and percentage of hyphal damage for Rhizopus oryzae and Scedosporium prolificans. The EC50 value is the biomass at which 50% reduction of hyphal damage occurs. In the presence of higher E : T ratios, the curves are shifted to the right and the EC50 values are higher. The two vertical arrows indicate the EC50 values corresponding to E : T ratios of 5 : 1 and 10 : 1 for R. oryzae. The error bars indicate standard errors of the means.
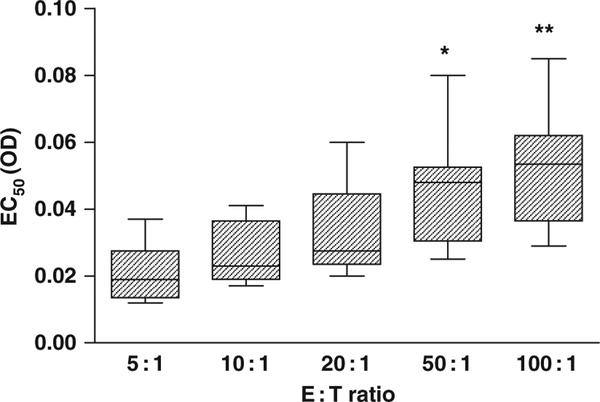
Box and whiskers plot of EC50 values obtained for all species in the presence of increasing E : T ratios. *P < 0.01 for comparison of EC50 values obtained with E : T ratio of 50 : 1 vs. those obtained with 5 : 1; **P < 0.001 and P < 0.05 for comparison of EC50 values obtained with 100 : 1 vs. those obtained with 5 : 1 and 10 : 1 respectively.
While the EC50 values increased with higher E : T ratios for all species, differences were also observed among species using the same E : T ratio. For E : T ratio 5 : 1, for example, lower EC50 values were obtained for A. flavus and the three zygomycete species (R. oryzae, R. microsporus, C. bertholletiae) (Fig. 4, panels a, b). This suggests that for A. flavus and the zygomycete species a 50% reduction in HD was observed with smaller increases in biomass compared with the other species studied. Given the different growth rates in RPMI 1640 of the species studied, the differences in EC50 values corresponded to even greater interspecies differences in the time of incubation required to achieve biomass equal to these EC50 values. For example, using a 20 : 1 E : T ratio, a 50% reduction in HD (compared with that obtained with the lowest biomass values) would be obtained by incubating A. terreus for 22 h but C. bertholletiae for only 6.5 h. The interspecies differences in the incubation times required to reach the EC50 values ranged from 5.5 to 19.5 h for 5 : 1 E : T ratio, and from 7.5 to 26 h for 100 : 1 E : T ratio (Fig. 4, panels c, d).
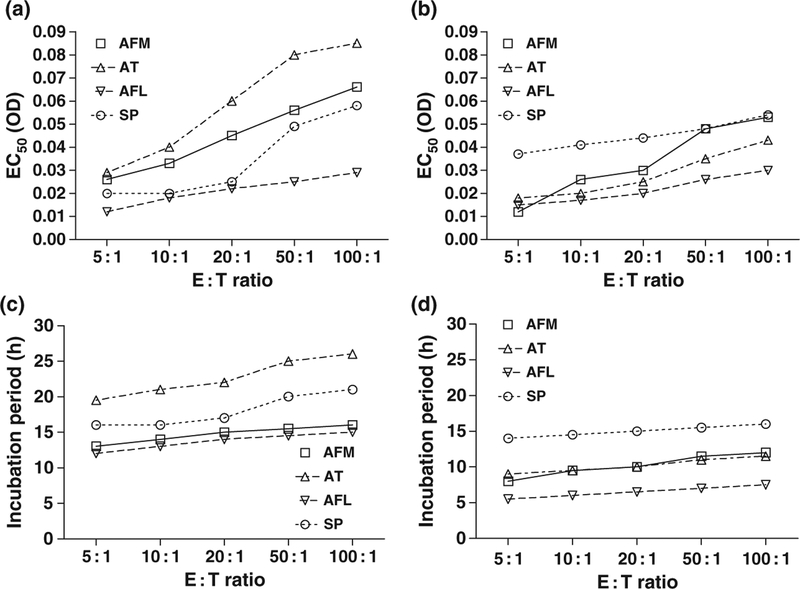
Panels a, b: EC50 values (OD, mean of two replicate experiments) generated by the Emax (sigmoid) model describing the reduction of percentage of hyphal damage with increasing fungal biomass for dierent species and E : T ratios. Panels c, d: incubation periods (h) required to achieve biomass equal to these EC50 values. Interspecies dierences in EC50 values and corresponding incubation periods can be appreciated. For clarity, up to four species per graph were presented. AFM: A. fumigatus, AT: A. terreus, AFL: A. flavus, SP: S. prolificans, RO: R. oryzae, RM: R. microsporus, CB: C. bertholletiae, FS: F. solani.
Discussion
This study demonstrated that fungal biomass is a key factor affecting PMN-induced HD of medically important filamentous fungi. This is the first study, to our knowledge, that systematically assesses the effect of biomass on HD. For this purpose, spectrophotometry was used for biomass determination, while modelling of data revealed a sigmoid pattern of HD decrease in response to increasing biomass and generated quantitative parameters (EC50 values) for comparisons between different E : T ratios or species.
Spectrophotometric determination of fungal biomass has already been used in studies of in vitro susceptibility of filamentous fungi to single or combinations of antifungal agents.14–16 This simple and objective method of biomass assessment demonstrated high inter-experimental reproducibility and yielded comparable results with visual determination or metabolic assays of fungal growth.17,18 Besides filamentous fungi, in the recently published definitive document EDef 7.1 of the European Committee for Antimicrobial Susceptibility Testing (EUCAST) regarding antifungal susceptibility testing of yeasts, spectrophotometric reading is the suggested method for determination of fungal growth.19 In this study, spectrophotometric determination of fungal biomass was easy to perform between the steps of the already established XTT HD methodology, without interfering with the whole assay. A possible limitation of this method is that the presence of non-homogeneous growth (for example, clumps of hyphae) in the wells may increase the interexperimental variability of biomass assessment.17 This is more likely to occur with smaller fungal biomass and was consistent with the higher SE/mean ratios of spectrophotometric measurements observed with lower OD values in our preliminary studies. However, increasing the number of replicate wells will result in precise determination of biomass. In this study, the number of 24 replicate wells was associated with a SE/mean ratio that was less than 15% for all biomass values. It should be emphasised at this point that the higher number of replicate wells does not really signify complexity of the spectrophotometric method, as these wells can be very easily filled with fungal inoculum using a multi-channel pipette, while their average OD value, following incubation and spectrophotometric reading, can be quickly calculated in an Excel worksheet.
The reduction of PMN-induced percentage of HD with increasing fungal biomass, for a given E : T ratio, suggests that the same amount of PMNs that can cause significant damage against small hyphae at the early phases of fungal growth, is not sufficient to inflict a proportionally similar damage against the long, well developed and branched hyphae that are observed after longer incubation periods. It is also likely that hyphae incubated for longer incubation periods undergo maturational changes compared with ‘younger’ hyphae that may additionally influence their susceptibility to neutrophil damage. The findings of this study therefore corroborate our initial hypothesis that fungal biomass is a significant factor affecting PMN-induced HD of filamentous fungi. Furthermore, this study provided valuable insights on (i) the magnitude of the effect of fungal biomass on PMN-induced HD as well as the relationship between biomass and HD, and (ii) the biomass values at which a substantial decrease of PMN-induced HD occurs for different fungal species or E : T ratios. Modelling of data was very helpful for answering the above questions as it allowed the generation of quantitative parameters for description of the biomass-HD relationship and comparisons between different species or E : T ratios. In particular, using one of the parameters estimated by the model, the EC50, we first were able to determine the biomass value at which a 50% reduction of HD occurs for every isolate and E : T ratio. Subsequently, we showed that with increasing E : T ratios higher EC50 values are obtained for each isolate; i.e. the 50% reduction of HD occurs with greater biomass. Finally, we demonstrated notable interspecies differences among the biomass values at which HD is reduced by 50%.
The precise mechanisms explaining the interspecies differences in EC50 values are currently unknown, but they may be related to the virulence of these mould species. For example, our finding that the three zygomycete species were among those with the lower EC50 values is consistent with the fact that infections caused by members of the order Mucorales in immunocompromised hosts are characterised by rapid progression and very poor prognosis compared with other mould infections in this population.20
The results of this study may have practical implications. First, in comparative studies of PMN-induced HD against different mould species, the biomass effect may be a confounding factor, if not taken into account. For example, if, prior to the addition of PMNs the same incubation period of 12 h in RPMI 1640 is used for both A. flavus and R. oryzae isolates of this study, then the average OD will be 0.012 for A. flavus and 0.053 for R. oryzae (Fig. 4). Consequently, if a lower value of percentage of HD is then found for R. oryzae compared with A. flavus, it would be impossible to clarify whether this finding represents a real interspecies difference in susceptibility to PMN antifungal activity or simply reflects the fact that the biomass value of R. oryzae was more than fourfold higher than that of A. flavus at the time of addition of PMNs. Based on the findings herein, it seems now reasonable that if different species are compared they would be incubated for appropriate time periods in order to achieve similar biomass values prior to the addition of PMNs. Given the possibility of interspecies differences in growth rates, these periods of incubation may need to differ considerably. For example, a biomass value of 0.026 OD would be achieved by incubating A. fumigatus for 13 h but C. bertholletiae for only 7 h (Fig. 4).
A second implication of the present findings would be the use of biomass in a manner similar to adjustment of E : T ratio, for optimisation of immunomodulatory studies of cytokines or other compounds, such as corticosteroids and antifungal agents, on PMN activity against different filamentous fungi. For example, if the compound studied is expected to inhibit PMN-induced HD, then the expected inhibitory effect could be better demonstrated using a lower fungal biomass value that would allow a relatively high percentage of HD in the control wells. Conversely, if the compound is expected to prime PMN-mediated HD, then using a higher biomass would result in a relatively small percentage of HD in the control wells and allow a better demonstration of the enhancing effect on PMN antifungal activity.
As the relationship between fungal biomass and PMN-induced HD was only investigated in vitro in this study, firm conclusions pertaining to clinical practice cannot be drawn. Our findings may imply, however, that when the fungal load in vivo is high, host defences may not be adequate to control the infection. Thus, in a neutropenic patient with invasive filamentous fungal infection, recovery from neutropenia may not be that efficient in eradicating the organism if the fungal burden is high. This hypothesis needs to be confirmed by appropriately designed in vivo and clinical studies.
In conclusion, PMN-induced HD of medically important filamentous fungi decreases with increasing biomass. This effect is both species dependent and E : T ratio dependent. These findings may be a useful factor in the design and interpretation of studies of PMN-mediated HD of filamentous fungi. Spectrophotometry can be used for biomass determination in these studies.
Acknowledgment
This work was supported by the Intramural Research Program of the National Cancer Institute.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1439-0507.2009.01725.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6999700?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Hyphae of Rhizopus arrhizus and Lichtheimia corymbifera Are More Virulent and Resistant to Antifungal Agents Than Sporangiospores In Vitro and in Galleria mellonella.
J Fungi (Basel), 9(10):958, 23 Sep 2023
Cited by: 2 articles | PMID: 37888214 | PMCID: PMC10607466
A bacterial endosymbiont of the fungus Rhizopus microsporus drives phagocyte evasion and opportunistic virulence.
Curr Biol, 32(5):1115-1130.e6, 07 Feb 2022
Cited by: 17 articles | PMID: 35134329 | PMCID: PMC8926845
Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World.
Mycopathologia, 186(6):739-754, 19 Aug 2021
Cited by: 113 articles | PMID: 34414555 | PMCID: PMC8375614
Review Free full text in Europe PMC
Is there an association between zinc and COVID-19-associated mucormycosis? Results of an experimental and clinical study.
Mycoses, 64(10):1291-1297, 01 Sep 2021
Cited by: 27 articles | PMID: 34420245 | PMCID: PMC8661931
Natural Killer Cell Line NK-92-Mediated Damage of Medically Important Fungi.
J Fungi (Basel), 7(2):144, 17 Feb 2021
Cited by: 4 articles | PMID: 33671240 | PMCID: PMC7922546
Go to all (11) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cunninghamella bertholletiae exhibits increased resistance to human neutrophils with or without antifungal agents as compared to Rhizopus spp.
Med Mycol, 48(5):720-724, 01 Aug 2010
Cited by: 13 articles | PMID: 20100138
Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae.
J Infect Dis, 198(2):186-192, 01 Jul 2008
Cited by: 120 articles | PMID: 18500936 | PMCID: PMC7185301
Amphotericin B formulations variably enhance antifungal activity of human neutrophils and monocytes against Fusarium solani: comparison with Aspergillus fumigatus.
J Antimicrob Chemother, 61(4):810-817, 13 Feb 2008
Cited by: 16 articles | PMID: 18272514
Pulmonary defense mechanisms against opportunistic fungal pathogens.
Immunol Ser, 47:243-271, 01 Jan 1989
Cited by: 62 articles | PMID: 2490078
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z01 SC006830




