Abstract
Free full text

Safety and efficacy of oral panobinostat plus chemotherapy in patients aged 65 years or younger with high-risk acute myeloid leukemia
Abstract
The role of histone deacetylase inhibitors in the treatment of acute myeloid leukemia (AML) is not well characterized. The current study evaluated the safety and efficacy of panobinostat in combination with idarubicin and cytarabine in newly diagnosed patients aged ≤65 years with primary or secondary high-risk AML based on cytogenetic classification. Treatment included fixed dose idarubicin (12 mg/m2/d, IV; day 1–3) and cytarabine (100 mg/m2/d, continuous IV infusion; day 1–7) and escalating oral doses of panobinostat at 15 mg, 20 mg, and 25 mg, thrice weekly starting at week 2 of a 28-day cycle. Forty-six patients were enrolled (primary AML [n=36], secondary AML [n=10]). The median age was 55 years. The most common all-grade AEs were diarrhea (54.3%), nausea (39.1%), vomiting, and decreased appetite (each, 21.7%), stomatitis (19.6%), and fatigue (17.4%). The overall response rate was 60.9%, 43.5% achieved a complete remission (CR), and 17.4% achieved CR with incomplete count recovery. The event-free survival at 1-year was 78.3%. Panobinostat in combination with idarubicin and cytarabine demonstrated tolerable safety and efficacy in younger patients with high-risk AML. The recommended phase 2 dose of panobinostat in this combination was 20 mg. ClinicalTrials.gov registry no: NCT01242774, and European Trial Registry EudraCT no: 2009-016809-42.
1. Introduction
Acute myeloid leukemia (AML) is characterized by proliferation of abnormal blast cells of myeloid origin in the bone marrow, blood, and other tissues [1]. The response rates to standard chemotherapy in newly diagnosed younger patients (aged < 60 years) range from 60% to 80%, [2,3], but it is significantly lower in older patients. A majority of patients relapse, and 5-year survival for all adult patients varies between 20% and 30% [4–6]. The presence of intrinsic AML resistance results in markedly worse outcome in older patients [5].
Treatment of younger patients with AML includes intensive induction and consolidation regimens using chemotherapy and hematopoietic stem cell transplantation (HSCT). The combination of anthracyclines including daunorubicin or idarubicin plus cytarabine (ara-C) (“3 + 7” therapy), has been the standard induction regimen for patients with newly diagnosed AML for the past 30 years [7]. Although many significant advances have been made in the identification of mutations in AML and in understanding the mechanisms of resistance to chemotherapy, [8,9], relapsed or refractory disease continue to be common clinical challenges [10,11].
Preclinical studies have shown that decreasing the activity of histone deacetylases (HDAC) can potentiate the effect of standard chemotherapy in AML. The pan-HDAC inhibitor panobinostat was shown to reduce the expression of BRCA1, CHK1, and RAD51 in AML cell lines by inhibition of both HDAC1 and HDAC2 enzymes [12]. This helps potentiate the apoptotic effect of cytarabine and duanorubicin in AML cells. The combination of panobinostat and doxorubicin induces cell death by increasing mitochondrial permeability, release of cytochrome c, and caspase-dependent apoptosis in AML cell lines and primary AML cells from patients [13]. In a phase 1a/2 trial of single-agent panobinostat in patients with AML whose disease had progressed on standard therapy, complete remission (CR) was seen in 2 of 86 patients at 60-mg dose.14 Panobinostat in combination with standard chemotherapies may prove useful in AML and a recent phase 1b/2 study of panobinostat combined with idarubicin and ara-C in elderly patients (> 65 years) with newly diagnosed AML showed CR rates of 64% and the median time to relapse of 17.0 months (range, 12.8–21.1 months) [15]. Another phase 1b study of patients with first relapse or primary refractory AML tested panobinostat plus ara-C and mitoxantrone and showed CR/CR with incomplete count recovery (CRi) rates of 46% [16]. Based on these encouraging results, the current phase 1b study assessed the safety and efficacy of panobinostat in combination with idarubicin and ara-C in younger patients (< 65 years) with newly diagnosed high-risk AML.
2. Methods
2.1. Study design
Patients aged 18 to ≤ 65 years with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤ 2 were included if they had newly diagnosed primary or secondary high-risk AML (defined as intermediate, intermediate II, or adverse cytogenetic subsets, by Dohner et al. [3]). Secondary AML was defined as treatment-related AML or AML arising from previously diagnosed myelodysplasia (MDS) or other antecedent hematologic disorders (AHDs) including aplastic anemia, polycythemia vera, essential thrombocytopenia, myelofibrosis, paroxysmal nocturnal hemoglobinuria, or other hematopoietic disorders. Patients could be treatment naïve or who received prior conventional care therapies for MDS/AHD. Patients were excluded if they belonged to “favorable” or “better risk” cytogenetic subsets of AML (defined as t(15;17), t(8;21), inv(16) or t(16;16)) [3], however, normal karyotype patients with mutated NPM1 and wild type FLT3 or CEBPA+ remained eligible. Other exclusion criteria included prior histone deacetylase inhibitor (HDACi) therapy, and concurrent, severe, and/or uncontrolled medical conditions. The study was conducted according to the Declaration of Helsinki guidelines and written informed consent was obtained from all the patients.
The primary objective of this study was to determine the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D) of panobinostat given 3 times a week in combination with a fixed dose of standard idarubicin and ara-C chemotherapy in younger patients (< 65 years) with newly diagnosed, high-risk AML. Other objectives were to determine the safety and tolerability of panobinostat, assess pharma-cokinetic (PK) profile of panobinostat, and evaluate preliminary anti-leukemic activity of this combination in this high-risk population.
The treatment was divided into 2 phases, an induction phase consisting of dose escalation to determine the RP2D with additional patients enrolled (dose expansion) at RP2D, and a consolidation phase for patients who achieved a CR, or CRi during induction, who were not candidates for stem-cell transplantation (SCT), and exhibited no persistent adverse events (AEs) to protocol therapy (Fig. 1).
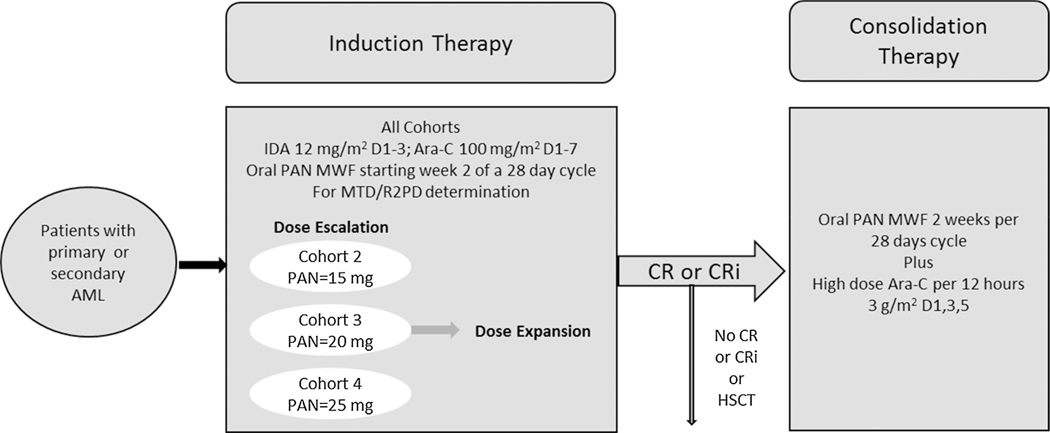
Study design.
Abbreviations: PAN, panobinostat; IDA, idarubicin; Ara-C, cytarabine; D, day; MWF, Monday Wednesday and Friday; MTD, maximum tolerated dose; R2PD, recommended dose for phase 2 studies; CR, complete remission; CRi, complete remission with incomplete blood count recovery.
Primary AML was defined as newly diagnosed disease according to World Health Organization criteria of with ≥ 20% of bone marrow blasts by bone marrow aspiration or biopsy and the patient had not been treated for AML.
Secondary AML was defined as treatment-related AML, or AML arising from previously diagnosed myelodysplasia (MDS) or other antecedent hematologic disorders (AHDs).
Induction phase treatment consisted of fixed dose idarubicin (12 mg/m2/d, IV; day 1–3) and ara-C (100 mg/m2/d, continuous IV infusion; day 1–7) and escalating oral doses of panobinostat at 15 mg, 20 mg, and 25 mg, thrice weekly starting at week 2 of a 28-day cycle (on days 8, 10, 12, 15, 17, and 19). Treatment was given for a maximum of 2 cycles. In the consolidation phase, patients received up to 4 courses of high-dose ara-C, (3 g/m2 q12 h, IV over 3 h on days 1, 3, and 5) with a panobinostat dose that was equal to or 1 dose level lower than their induction phase dose levels.
2.2. Safety and MTD determination
Adverse events were assessed according to the Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0 [17]. A dose limiting toxicity (DLT) was defined as a clinically relevant grade > 3 AE (or grade > 2 neurotoxicity) assessed as study drug related, but unrelated to disease progression, which occurred after the first study cycle’s dose of panobinostat and up to the first dose of the next study cycle. The MTD was determined using an adaptive Bayesian Logistic Regression Model (BLRM) incorporating escalation with overdose control (EWOC) principles [18–20]. MTD was defined as the highest dose of panobinostat, which when given together with chemotherapy in the first induction treatment cycle had a ≤ 25% probability of causing DLTs in > 33.3% of the patients. The RP2D was defined as the panobinostat dose that was ≤ MTD/last dose level and evaluated after 22 patients had been treated at that dose.
2.3. Pharmacokinetics (PK)
At specified time points of pre-dose, 0.5, 1, 2, 3, 5, 7, 24, 28 h, 3 mL of blood samples were collected on cycle 1 day 8 during the dose escalation and expansion in induction phase, to characterize oral panobinostat PK. The PK parameters for panobinostat alone (none of the other 2 drugs) were determined in plasma using non-compartmental methods. These parameters included maximum or peak plasma concentration after a single dose (Cmax), time to reach maximum plasma concentration (Tmax), the area under the curve from time zero to last measurable concentration time (AUC0-tlast), area under the curve from time zero to 24 h (AUC0–24), the elimination half-life (T1/2), the total body clearance (Cl/F), and the apparent volume of distribution (Vz/F).
2.4. Efficacy
The rate of CR, CRi, and partial remission (PR) were estimated according to Cheson et al. [21] International Working Group (IWG) criteria for AML, during the induction phase [21]. The rates of treatment failure, relapse and/or death, duration of response (CR or CRi), and 1-year event-free survival were also assessed.
3. Results
3.1. Patient disposition and baseline demographics and disease characteristics
In total, 46 patients were enrolled and evaluated in the induction phase (dose escalation and dose expansion) and 19 patients (41%) continued on to the consolidation phase. The median age of all 46 patients was 55.5 years (range, 19–65 years) (Table 1). There was a balance of male and female patients, the majority were Caucasians (37 patients, 80.4%) and 87% of patients had an ECOG PS ≤ 1 (Table 1). Median time since initial diagnosis of AML to first dose of drug was 5 days (range, 1–28 days). Status of AML at the time of initial diagnosis for majority of the patients was de novo (36 patients, 78.3%). A total of 7 patients had AML secondary to either MDS (5 patients) or AHD (3 patients), and 1 patient had therapy-related AML (Table 1). A summary of World Health Organization (WHO) classification of AML at the time of initial diagnosis by panobinostat dose group for all enrolled patients is given in Table 2.
Table 1
Baseline demographics and disease characteristics by dose group of panobinostat (PAN).
| Patient characteristics | PAN 15 mg n = 11 | PAN 20 mg n =15 | PAN 25mg n =8 | PAN 20 mg (dose expansion) n = 12 | All patients n = 46 |
|---|---|---|---|---|---|
| Median age, year (range) | 46.0 (19–65) | 56.0 (21–64) | 52.5 (21–59) | 59.5 (38–64) | 55.5 (19–65) |
| Male gender, n (%) | 9 (81.8) | 7 (46.7) | 5 (62.5) | 4 (33.3) | 25 (54.3) |
| ECOG status, n (%) | |||||
| 0 | 6 (54.5) | 6 (40.0) | 1 (12.5) | 4 (33.3) | 17 (37.0) |
| 1 | 4 (36.4) | 7 (46.7) | 6 (75.0) | 6 (50.0) | 23 (50.0) |
| 2 | 1 (9.1) | 2 (13.3) | 1 (12.5) | 2 (16.7) | 6 (13.0) |
| AML status at initial diagnosis - n (%) | |||||
| De novo | 10 (90.9) | 12 (80) | 5 (62.5) | 9 (75) | 36 (78.3) |
| Secondary MDS | 0.0 | 2 (13.3) | 2 (25) | 1 (8.3) | 5 (10.9) |
| Secondary to AHDf | (9.1) | 0.0 | 0.0 | 2 (16.7) | 3 (6.5) |
| Therapy-related AML | 0.0 | 0.0 | 1 (12.5) | 0.0 | 1 (2.2) |
| Unknown | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) |
| Median blasts in bone marrow at baselineg, % (range) | 66.0 | 49.0 | 82.0 | 47.0 | 55.5 |
| (20.5–89) | (26–69) | (4–94) | (15–74.5) | (4–94) | |
| Median blasts in peripheral blood at baseline, % (range) | 37.5 | 8.0 | 41.0 | 3.0 | 17.0 |
| (10–67) | (0–78) | (0–80) | (0–48) | (0–80) | |
| Extramedullary disease at baseline, n (%) | |||||
 Yes Yes | 3 (27.3) | 1 (6.7) | 3 (37.5) | 0 (0.0) | 7 (15.2) |
 No No | 8 (72.7) | 14 (93.3) | 4 (50.0) | 12 (100.0) | 38 (82.6) |
| Entered consolidation phase n (%) | 8 (72.7) | 5 (33.3) | 2 (25) | 4 (33.3) | 19 (41.3) |
| Discontinued treatment n (%) | 3 (27.3) | 10 (66.7) | 6 (75) | 8 (66.7) | 27 (58.7) |
| Primary reason for discontinuation | |||||
 Disease progressiona Disease progressiona | 1 (9.1) | 5 (33.3) | 3 (37.5) | 2 (16.7) | 11 (23.9) |
 Adverse eventsb Adverse eventsb | 1 (9.1) | 3 (20.0) | 3 (37.5) | 1 (8.3) | 8 (17.4) |
 Withdrawal of consent Withdrawal of consent | – | – | – | 1 (8.3) | 1 (2.2) |
 Deathc Deathc | 1 (9.1) | 1 (6.7) | – | 3 (25) | 5 (10.9) |
 Othersd Othersd | – | – | – | 3 (33.3) | 3 (6.5) |
 Entered posttreatment evaluatione Entered posttreatment evaluatione | 2 (18.2) | 7 (46.7) | 5 (62.5) | 1 (8.3) | 15 (32.6) |
Abbreviations: AHD antecedent hematologic disorders, AML acute myeloid leukemia, ECOG Eastern Cooperative Oncology Group, MDS myelodysplasia, PAN panobinostat.
Table 2
Patient Disease history of AML – WHO classification by dose group of panobinostat (PAN).
 Disease history of AML Disease history of AML WHO class (initial diagnosis), n (%) WHO class (initial diagnosis), n (%) | PAN 15 mg n = 11 | PAN 20 mg n = 15 | PAN 25 mg n = 8 | PAN 20 mg (expansion phase) n = 12 | All patients n = 46 |
|---|---|---|---|---|---|
| AML with recurrent genetic abnormalities | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (2.2) |
 AML with t(9;11)(p22;q23);(MLLT3-MLL) AML with t(9;11)(p22;q23);(MLLT3-MLL) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (2.2) |
| AML with multi-lineage dysplasia | 2 (18.2) | 4 (26.7) | 3 (37.5) | 3 (25.0) | 12 (26.1) |
 Without antecedent MDS or MDS/MPD, but with dysplasia in at least 50% of cells Without antecedent MDS or MDS/MPD, but with dysplasia in at least 50% of cells | 1 (9.1) | 3 (20.0) | 1 (12.5) | 3 (25.0) | 8 (17.4) |
 Evolving from MDS or MDS/MPD Evolving from MDS or MDS/MPD | 1 (9.1) | 1 (6.7) | 2 (25.0) | 0 (0.0) | 4 (8.7) |
| AML, not otherwise specified | 7 (63.6) | 10 (66.7) | 4 (50.0) | 9 (75.0) | 30 (65.2) |
 AML with minimal differentiation AML with minimal differentiation | 1 (9.1) | 1 (6.7) | 1 (12.5) | 2 (16.7) | 5 (10.9) |
 AML with maturation AML with maturation | 2 (18.2) | 5 (33.3) | 1 (12.5) | 2 (16.7) | 10 (21.7) |
 Acute myelomonocytic leukemia Acute myelomonocytic leukemia | 1 (9.1) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 3 (6.5) |
 Acute monoblastic/monocytic leukemia Acute monoblastic/monocytic leukemia | 2 (18.2) | 0 (0.0) | 1 (12.5) | 1 (8.3) | 4 (8.7) |
 Acute erythroid leukemia Acute erythroid leukemia | 1 (9.1) | 1 (6.7) | 0 (0.0) | 3 (25.0) | 5 (10.9) |
 Others Others | 0 (0.0) | 1 (6.7) | 1 (12.5) | 1 (8.3) | 3 (6.5) |
 Unknown Unknown | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) |
Abbreviations: AHD antecedent hematologic disorders, AML acute myeloid leukemia, MDS myelodysplasia, MLL mixed lineage leukemia, MPD myeloproliferative disorders, WHO World Health Organization.
Primary reason for treatment discontinuation was disease progression in 11 patients (23.9%) and AEs in 8 patients (17.4%) (Table 1). Of the 19 patients who entered consolidation phase, 8 patients (42.1%) completed treatment per protocol, 5 patients (26.3%) withdrew consent, and 3 patients (15.8%) discontinued due to AEs. Overall, 5 deaths were reported, 3 in the induction and 2 in the consolidation phase. One additional death occurred 28 days after the last therapy.
3.2. Determination of MTD
Patients needed to have received sufficient study treatment defined as 5 doses of panobinostat and 1 dose (day 1–7) of ara-C and 1 dose (day 1–3) of idarubicin during cycle 1 of the induction for eligibility of MTD determination. Of the 29 patients eligible for MTD determination, 7 patients experienced DLTs during induction; 4 in the 20 mg dose group and 3 in the 25 mg dose group. In the 20 mg group, 1 patient had grade 3 hepatosplenic candidiasis, 1 patient had increased QTcF of > 480 ms and 2 patients had left ventricular systolic dysfunction. In the 25 mg group, 2 patients had grade 3 febrile neutropenia and 1 patient had grade 3 diarrhea.
The BLRM estimated the probability of excessive toxicity to be 0.7% for 15 mg of panobinostat, 1.3% for 20 mg of panobinostat, and 2.9% for 25 mg of panobinostat. Based on considerations for MTD estimation along with overall assessment of safety and tolerability data, the RP2D was determined to be 20 mg of panobinostat when administered with fixed dose combination of idarubicin and ara-C. The analysis for the determination of MTD is described in Table 3.
Table 3
Dose-limiting toxicities (DLTs) during cycle 1 by dose group of panobinostat (PAN).
| Panobinostat dose (mg) | Patients with DLT (observed) | Posterior probability of DLT | Underdosing1 [%] | Probability of target toxicity2[%] | Unacceptable or excessive toxicity3[%] | |
|---|---|---|---|---|---|---|
| Total | n | Mean (SD) | ||||
| 15 | 10 | 0 | 18.2 (5.80) | 38.7 | 60.6 | 0.7 |
| 20 | 12 | 4 | 19.5 (6.10) | 30.7 | 68 | 1.3 |
| 25 | 7 | 3 | 21.0 (6.60) | 23.6 | 73.5 | 2.9 |
Total: number of evaluable patients (included in dose determining set).
n: Number of patients with at least 1 DLT.
%: percentage is based on total.
The maximum next dose level is determined if the probability of unacceptable or excessive toxicity is not exceeding 25%.
3.3. Description of AEs
In the induction phase, 84.8% of patients had AEs; of these, 73.9% were grade 3 or 4 AEs suspected to be treatment-related (Table 4). All-grade hematologic AEs regardless of study drug relationship included thrombocytopenia (45.7%), anemia (37%), febrile neutropenia (37%), neutropenia (19.6%), and leukopenia (4.3%). All-grade non-hematologic AEs regardless of study drug relationship included diarrhea (54.3%), nausea (39.1%), vomiting (21.7%), decreased appetite (21.7%), stomatitis (19.6%), and fatigue (17.4%). Adverse events (mostly hematologic) lead to dose adjustments for 4 patients (8.7%) in the induction phase and 5 patients (26.3%) in the consolidation phase.
Table 4
Adverse events (AEs) suspected be related to panobinostant (PAN) during inducation phase. All grades and grade 3 or 4 (≥ 15% in any dose group).
| PAN 15 mg N = 11 | PAN 20 mg N = 15 | PAN 25 mg N = 8 | PAN 20 mg (expansion phase) N = 12 | All patients N = 46 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preferred term | Any grade, n (%) | Grade 3 or 4, n (%) | Any grade, n (%) | Grade 3 or 4, n (%) | Any grade, n (%) | Grade 3 or 4, n (%) | Any grade, n (%) | Grade 3 or 4, n (%) | Any grade, n (%) | Grade 3 or 4, n (%) |
| Hematologic | ||||||||||
| Thrombocytopenia | 7 (63.6) | 7 (63.6) | 9 (60.0) | 9 (60.0) | 1 (12.5) | 1 (12.5) | 4 (33.3) | 4 (33.3) | 21 (45.7) | 21 (45.7) |
| Anemia | 7 (63.6) | 5 (45.5) | 4 (26.7) | 4 (26.7) | 3 (37.5) | 3 (37.5) | 3 (25.0) | 3 (25.0) | 17 (37.0) | 15 (32.6) |
| Febrile neutropenia | 5 (45.5) | 4 (36.4) | 6 (40.0) | 6 (40.0) | 2 (25.0) | 2 (25.0) | 4 (33.3) | 4 (33.3) | 17 (37.0) | 16 (34.8) |
| Neutropenia | 3 (27.3) | 3 (27.3) | 3 (20.0) | 3 (20.0) | 1 (12.5) | 1 (12.5) | 2 (16.7) | 2 (16.7) | 9 (19.6) | 9 (19.6) |
| Leukopenia | 2 (18.2) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) | 2 (4.3) |
| Cardiac disorders | ||||||||||
| Left ventricular dysfunction | 0 (0.0) | 0 (0.0) | 3 (20.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (6.5) | 2 (4.3) |
| Gastrointestinal disorders | ||||||||||
| Diarrhea | 7 (63.6) | 0 (0.0) | 8 (53.3) | 1 (6.7) | 5 (62.5) | 1 (12.5) | 5 (41.7) | 1 (8.3) | 25 (54.3) | 3 (6.5) |
| Nausea | 3 (27.3) | 0 (0.0) | 7 (46.7) | 0 (0.0) | 2 (25.0) | 0 (0.0) | 6 (50.0) | 0 (0.0) | 18 (39.1) | 0 (0.0) |
| Vomiting | 1 (9.1) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 5 (41.7) | 0 (0.0) | 10 (21.7) | 0 (0.0) |
| Stomatitis | 2 (18.2) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 4 (33.3) | 0 (0.0) | 9 (19.6) | 0 (0.0) |
| Abdominal pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 3 (6.5) | 0 (0.0) |
| Dyspepsia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| General disorders and administration site conditions | ||||||||||
| Fatigue | 1 (9.1) | 0 (0.0) | 5 (33.3) | 1 (6.7) | 1 (12.5) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 8 (17.4) | 1 (2.2) |
| Pyrexia | 2 (18.2) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 6 (13.0) | 0 (0.0) |
| Metabolism and nutrition disorders | ||||||||||
| Decreased appetite | 4 (36.4) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 1 (8.3) | 10 (21.7) | 1 (2.2) |
| Hypokalemia | 1 (9.1) | 0 (0.0) | 3 (20.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 1 (8.3) | 7 (15.2) | 3 (6.5) |
| Skin and subcutaneous tissue disorders | ||||||||||
| Alopecia | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 3 (6.5) | 0 (0.0) |
A patient with multiple occurrences of an AE under one treatment is counted only once in the AE category for that treatment. A patient with multiple AEs within a primary system organ class is counted only once in the total row. Adverse events occurring more than 28 days after the discontinuation of study treatment are not summarized.
3.3.1. Serious adverse events
Nineteen patients (41.3%) experienced at least 1 serious AE (SAE) that was suspected to be study drug related. The most frequent SAE was grade ≥ 3 febrile neutropenia (10 patients) with 4 patients in 15 mg, 3 patients in 20 mg, 1 patient in 25 mg, and 2 patients in the 20 mg expansion dose groups. Infections were the second most frequent SAE (5 patients) with sepsis in 2 patients, 1 in 25 mg and 1 in 20 mg expansion groups, pneumonia in 1 patient in the 15 mg group, lung infection in 1 patient in the 25 mg and hepatosplenic candidiasis (also a DLT) in 1 patient in the 15 mg dose groups. Cardiac disorders were the next most frequent SAEs (5 patients) with QTcF prolongation in 1 patient, left ventricular dysfunction in 3 patients, all in the 20 mg dose groups, and hypertension and cardiac tamponade in 1 patient in the 20 mg expansion dose group. Two patients in the 20 mg dose group had SAEs of hematochezia.
3.3.2. Deaths
A total of 6 deaths were reported during the study period or within 28 days after the end of treatment. Three on-treatment deaths (6.5%) were reported during the induction phase; all in the 20 mg expansion dose group. One patient died due to sepsis, 1 patient died due to disease progression of AML and 1 due to ischemic stroke. One patient’s death (sepsis) was suspected to be study drug related. Two patients died in the consolidation phase, 1 due to sepsis in the 15 mg dose group and 1 due to cerebral hemorrhage in the 20 mg expansion group. Investigator did not suspect relationship to panobinostat dose for these 2 deaths. In addition to on-treatment deaths, 1 patient in the 20 mg dose group died due to infection (pneumonia) 28 days after the end of treatment.
3.4. Panobinostat PK
The PK parameters for panobinostat are summarized in Table 5. The plasma concentration time profile for each panobinostat dose group is given in Fig. 2. The AUC0–24 and the Cmax increased with increase in panobinostat doses in escalation cohorts across 15–25 mg levels. The median Tmax ranged between 1.05 h and 3.0 h for the all dose groups (15 mg, 20 mg, 25 mg, and 20 mg expansion phase). However, exposure in the expansion phase of 20 mg was higher than any dose in escalation phase, probably due to the large variability in panobinostat PK and small sample size. Panobinostat AUC0–24 and Cmax generally over-lapped between patients who responded to the combination therapy (CR, CRi, and PR) and patients who did not (treatment failure).
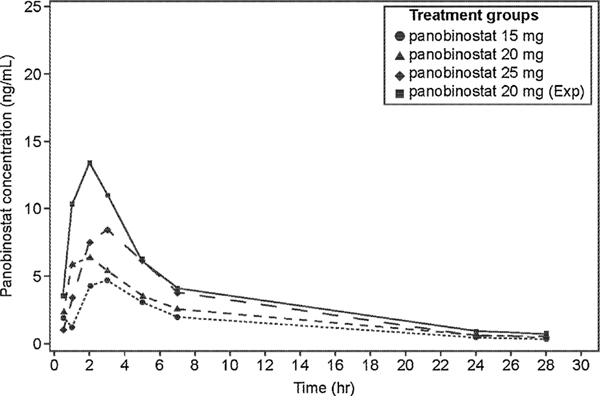
Geometric mean of panobinostat plasma concentrations versus time by dose groups.
Zero concentrations at individual time-points are excluded from geometric mean computation
Table 5
Summary of panobinostat PK parameter by dose group of panobinostat (PAN).
| PK Parameter | PAN 15 mg N = 10 | PAN 20 mg N= 11 | PAN 25 mg N= 8 | PAN 20 mg (expansion phase) N= 10 |
|---|---|---|---|---|
| AUC0inf (ng*h/mL) | 50.8 (54.7) | 74.3 (68.2) | 85.0 (44.3) | 103 (32.3) |
| AUC0–24 (ng*h/mL) | 42.5 (54.6) | 62.6 (68.0) | 74.6 (45.5) | 92.7 (32.3) |
| CL/F (L/h) | 295 (54.7) | 269 (68.2) | 294 (44.3) | 193 (32.3) |
| Vz/F (L) | 3920 (58.6) | 3790 (62.8) | 3360 (48.8) | 1810 (63.2) |
| Cmax (ng/mL) | 6.03 (40.2) | 10.8 (121.1) | 11.2 (67.8) | 15.0 (47.3) |
| Tmax (h) Median (range) | 3.0 (0.5–3.05) | 1.05 [0.98 –7.00] | 2.02 [1.25–4.90] | 1.98 [1.00–5.00] |
| T1/2 (h) Median (range) | 8.74 (6.84–12.6) | 9.70 [7.98–11.8] | 7.81 [6.21–10.7] | 7.94 [2.19–11.1] |
All values are given as geometric mean (%Coefficient of Variation [CV]) except Tmax and T1/2 (given as median and range).
n: number of subjects with nonmissing values.
AUC0inf: The area under the curve from time zero to infinity (ng*hr/mL).
AUC0–24: The area under curve from time zero to 24 h (ng*hr/mL).
CL/F: The total body clearance of drug from the plasma (volume x time-1).
Vz/F: The apparent volume of distribution during terminal phase (associated with λz) (volume).
Cmax: The maximum (peak) observed plasma concentration after single dose (ng/mL).
Tmax: The time to reach maximum (peak) plasma, blood, serum, or other body fluid drug concentration after single dose administration (hr).
T1/2: The elimination half-life associated with the terminal slope (λz) of a semi logarithmic on concentration-time curve (hr) when feasible.
CV = coefficient of variation (%)=sd/mean*100.
CV% geo-mean= sqrt (exp (variance for log transformed data)-1)*100.
3.5. Efficacy
The overall response rate (ORR) (CR or CRi) was 60.9% (95% CI: 45.4, 74.9); CR was achieved in 20 patients (43.5%) and 8 patients (17.4%) had a CRi. The ORR was 60% (9 patients) in the escalation phase and 50% (6 patients) in the expansion phase for the 20-mg dose level (Table 6). The highest response rate (81.8%) was observed in the 15-mg dose group with CR observed in 6 patients (54.5%) and CRi observed in 3 patients (27.3%). Ten of 46 patients underwent a second induction cycle to give a re-induction rate of 22%. The 1 year EFS was 78.3%. The median duration of response was not estimable due to inadequate number of events at the time of last censoring.
Table 6
Best overall response for all dose groups of panobinostat (PAN).
| Best response | PAN 15 mg N =11 n (%) | PAN 20 mg N= 15 n (%) | PAN 25 mg N= 8 n (%) | PAN 20 mg (expansion phase) N= 12 n (%) | All patients N =46 n (%) |
|---|---|---|---|---|---|
| Response rate (CR or CRi) | 9 (81.8) | 9 (60.0) | 4 (50.0) | 6 (50.0) | 28 (60.9) |
| 95% confidence interval1 | [48.2, 97.7] | [32.3, 83.7] | [15.7, 84.3] | [21.1, 78.9] | [45.4, 74.9] |
 Complete remission (CR) Complete remission (CR) | 6 (54.5) | 7 (46.7) | 2 (25.0) | 5 (41.7) | 20 (43.5) |
 Complete remission with incomplete blood count recovery (CRi) Complete remission with incomplete blood count recovery (CRi) | 3 (27.3) | 2 (13.3) | 2 (25.0) | 1 (8.3) | 8 (17.4) |
| Partial remission (PR) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (8.3) | 2 (4.3) |
| Treatment failure | 1 (9.1) | 6 (40.0) | 3 (37.5) | 3 (25.0) | 13 (28.3) |
| Unknown | 1 (9.1) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 3 (6.5) |
4. Discussion
In leukemic cells, panobinostat decreases the levels of key proteins BRCA1, CHK1, and RAD51 that control both DNA repair and cell cycle checkpoint activation [12]. Its activity is synergistic with standard chemotherapeutic agents in AML and panobinostat enhances cytarabine or daunorubicin induced DNA damage and apoptosis and further abolishes the chemotherapy induced cell checkpoint activation [12].
Early trials of single-agent panobinostat in patients with advanced hematologic malignancies showed few CR responses [14], and the MTD of panobinostat alone was determined to be 60 mg [14]. Thus it was thought that panobinostat may have better efficacy when combined with standard chemotherapeutic agents for AML. This was known to be true for other HDAC inhibitors such as vorinostat and valproic acid when used as single agent in patients with AML [22,23]. However when vorinostat was combined with idarubicin and ara-C in patients with de novo AML (age: 18–65 years), CR was seen in 76% of patients and after a median follow-up of 82 weeks, the median overall survival was 82 weeks [24]. This is similar to response rates seen in the current study in which the median overall survival was not reached. Adverse events reported with vorinostat are similar to those reported in our trial; vorinostat versus panobinostat AEs: all-grade cardiac events, 15% versus 6.5%; diarrhea, 72% versus 54%, and nausea, 65% versus 40% [24]. However, a more recent large scale 3-arm randomized study in 738 patients compared vorinostat plus idarubicin/cytarabine with idarubicin/cytarabine alone or cytarabine/daunorubicin did not find any significant differences in overall survival rates for all 3 arms [25]. Therefore it is important to find the right chemotherapy combination for a specific HDAC inhibitor in order to optimize efficacy.
Panobinostat at 30 mg dose when combined with azacitidine had a higher toxicity (grade ≥ 3 AEs of 97%) than azacitidine alone (grade ≥ 3 AEs of 81%) in patients with AML [26]. In our study, 3 lower doses of panobinostat (15 mg, 20 mg, and 25 mg) were tested in combination with idarubicin and ara-C. Additionally, idarubicin and ara-C were administered on week 1 of a 28-day cycle while panobinostat was administered separately on week 2/3. This may have helped achieve a manageable safety profile in our study.
In the current study, the principle DLTs were left ventricular systolic dysfunction and febrile neutropenia in 2 patients each. Another study [16], using oral panobinostat (thrice weekly for 2 weeks) in combination with fixed dose mitoxantrone 5 mg/day on day 1–5 and fixed dose ara-C 1 g/day on days 1–6 as salvage therapy, no DLTs occurred in the first 2 cohorts with 5 patients treated at 20 mg and 4 patients treated at 30 mg thrice weekly oral panobinostat; the RP2D of panobinostat in this combination was 50 mg. The dose range of 15 mg, 20 mg, and 25 mg of panobinostat used in our study showed manageable tolerability and the recommended dose for expansion was fixed at 20 mg of panobinostat in combination with fixed dose idarubicin and ara-C. A study by Ocio et al. assessed escalating oral doses of panobinostat (20 mg, 30 mg, 40 mg, and a −1 dose level of 10 mg) with idarubicin and ara-C in elderly patients (> 65 years) with newly diagnosed AML [15]. In this trial, the MTD was defined as 10 mg of panobinostat with 8 mg/m2 idarubicin (day 1–3) and 100 mg/m2 ara-C (day 1–7). However, in our study younger patients (18–65 years of age) were better able to tolerate panobinostat in the staggered schedule with idarubicin and ara-C, and the MTD was not reached. The current study established the recommended dose for panobinostat with standard chemotherapy in patients with newly diagnosed AML, and this dose can be used for expansion studies to further assess the efficacy of this combination.
Our study included only previously untreated patients with intermediate or high-risk AML based on cytogenetic classification, which was different from other trials involving HDAC inhibitors. Since panobinostat modifies epigenetic pathways, it was expected that patients with adverse cytogenetics may benefit from the addition of panobinostat to standard chemotherapy.
A study limitation was the lack of characterization of biomarkers before and after panobinostat therapy. Evolving trends in AML therapy suggest the use of specific treatment regimen for patients based upon their mutational burden. For example, addition of tyrosine kinase inhibitors to standard chemotherapy benefited patients with FLT3-ITD–mutated AML [27]. The combination of panobinostat with 3 + 7 induction chemotherapy was stopped due to limited efficacy. However, future studies should assess biomarkers that are affected by panobinostat therapy and identify patient subpopulations that would benefit with the addition of panobinostat in combination with standard chemotherapy.
Acknowledgments
The study was sponsored by Novartis Pharmaceuticals Corporation. We thank the investigators of the study. We thank Shefalee Bhavsar and Bhavani Yamsani of Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance and writing support for this manuscript.
Dr. Strickland received Honoraria for advisory board role from Alexion, Baxalta, Boehringer-Ingelheim, CTI BioPharma, Sunesis and Tolero. Dr. Strickland also received consulting fees from Astellas and Daiichi-Sankyo and research funding from Sunesis. Dr. Rӧllig received fees from Amgen, BMS, Celgene, Janssen, Novartis, Roche, and Takeda. Dr. Rӧllig also received grant from Bayer and non-financial support from Gilead. Dr. Medeiros and Dr. Schlenk received fees and grant from Novartis. Dr. Sierra received grants from Amgen and Celgene and fees from Pfizer, Amgen, Celgene, Janssen, Seattle Genetics and Novartis. Dr. Thol and Dr. DeAngelo received Honoraria for advisory board role at Novartis. Dr. Stuart reports grants from Sunesis, Novartis, and Celator. Dr. Walker received research funding from Gilead. Dr. Ocio received honoraria and research support from Novartis. Dr. Noah Berkowitz, and Sue-zette Valera are employees of Novartis Pharmaceutical Corporation, East Hanover, NJ, USA and Kohinoor Dasgupta is an employee of Novartis healthcare Private Limited, Hyderabad, India.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.leukres.2019.106197
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7108400?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Histone deacetylase inhibitors for leukemia treatment: current status and future directions.
Eur J Med Res, 29(1):514, 26 Oct 2024
Cited by: 0 articles | PMID: 39456044 | PMCID: PMC11515273
Review Free full text in Europe PMC
Current treatment strategies targeting histone deacetylase inhibitors in acute lymphocytic leukemia: a systematic review.
Front Oncol, 14:1324859, 21 Feb 2024
Cited by: 2 articles | PMID: 38450195
Review
A Review of Childhood Acute Myeloid Leukemia: Diagnosis and Novel Treatment.
Pharmaceuticals (Basel), 16(11):1614, 15 Nov 2023
Cited by: 3 articles | PMID: 38004478 | PMCID: PMC10674205
Review Free full text in Europe PMC
Epigenetic Activation of Plasmacytoid DCs Drives IFNAR-Dependent Therapeutic Differentiation of AML.
Cancer Discov, 12(6):1560-1579, 01 Jun 2022
Cited by: 8 articles | PMID: 35311997 | PMCID: PMC9355625
Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia.
Molecules, 27(12):3911, 18 Jun 2022
Cited by: 10 articles | PMID: 35745032 | PMCID: PMC9230975
Review Free full text in Europe PMC
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT01242774
European Clinical Trials
- (1 citation) EU Clinical Trials Register - 2009-016809-42
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Histone Deacetylase Inhibition with Panobinostat Combined with Intensive Induction Chemotherapy in Older Patients with Acute Myeloid Leukemia: Phase I Study Results.
Clin Cancer Res, 25(16):4917-4923, 31 May 2019
Cited by: 16 articles | PMID: 31152020
Panobinostat as part of induction and maintenance for elderly patients with newly diagnosed acute myeloid leukemia: phase Ib/II panobidara study.
Haematologica, 100(10):1294-1300, 09 Jul 2015
Cited by: 19 articles | PMID: 26160880 | PMCID: PMC4591761
Idarubicin, cytarabine, and topotecan in patients with refractory or relapsed acute myelogenous leukemia and high-risk myelodysplastic syndrome.
Am J Hematol, 68(4):237-245, 01 Dec 2001
Cited by: 16 articles | PMID: 11754412
Gemtuzumab-ozogamicin in combination with fludarabine, cytarabine, idarubicin (FLAI-GO) as induction therapy in CD33-positive AML patients younger than 65 years.
Leuk Res, 32(12):1800-1808, 14 Jul 2008
Cited by: 16 articles | PMID: 18621416
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA016058




