Abstract
Background and objectives
Blinatumomab (BLINCYTO®) is a novel bispecific T cell engager (BiTE®) approved in the USA for the treatment of relapsed or refractory B cell precursor acute lymphoblastic leukemia (ALL) in children and adults, as well as minimal residual disease ALL in adults. This analysis characterized the population pharmacokinetics of intravenous blinatumomab in pediatric and adult patients.Methods
A total of 2417 serum concentrations of blinatumomab from 674 patients, including adult (n = 628) and pediatric patients (n = 46), from eight clinical studies were analyzed. The impact of covariates on pharmacokinetic parameters were explored, and significant covariates were further evaluated using a simulation approach.Results
Blinatumomab pharmacokinetics were described by a one-compartment linear model with first-order elimination, a clearance (CL) of 2.22 L/h, and a central volume of 5.98 L. A statistically significant effect of body surface area (BSA) on CL was observed. The smallest BSA of 0.37 m2 in the pediatric population was associated with a 63% reduction in blinatumomab systemic CL, relative to an adult patient with the median BSA (1.88 m2), supporting the use of BSA-based dosing in patients of lower bodyweight. The BSA effect was minimal, with a ≤ 25% change in CL over the range of BSA in adults, supporting no need for BSA-based dosing.Conclusions
Blinatumomab pharmacokinetics were adequately described by a one-compartment linear model with first-order elimination. No covariates other than BSA on CL were identified as significant. BSA-based dosing should be considered for lightweight patients to minimize inter-subject variability in blinatumomab exposure.Free full text
Population Pharmacokinetics of Blinatumomab in Pediatric and Adult Patients with Hematological Malignancies
Abstract
Background and objectives
Blinatumomab (BLINCYTO®) is a novel bispecific T cell engager (BiTE®) approved in the USA for the treatment of relapsed or refractory B cell precursor acute lymphoblastic leukemia (ALL) in children and adults, as well as minimal residual disease ALL in adults. This analysis characterized the population pharmacokinetics of intravenous blinatumomab in pediatric and adult patients.
Methods
A total of 2417 serum concentrations of blinatumomab from 674 patients, including adult (n =
= 628) and pediatric patients (n
628) and pediatric patients (n =
= 46), from eight clinical studies were analyzed. The impact of covariates on pharmacokinetic parameters were explored, and significant covariates were further evaluated using a simulation approach.
46), from eight clinical studies were analyzed. The impact of covariates on pharmacokinetic parameters were explored, and significant covariates were further evaluated using a simulation approach.
Results
Blinatumomab pharmacokinetics were described by a one-compartment linear model with first-order elimination, a clearance (CL) of 2.22 L/h, and a central volume of 5.98 L. A statistically significant effect of body surface area (BSA) on CL was observed. The smallest BSA of 0.37 m2 in the pediatric population was associated with a 63% reduction in blinatumomab systemic CL, relative to an adult patient with the median BSA (1.88 m2), supporting the use of BSA-based dosing in patients of lower bodyweight. The BSA effect was minimal, with a ≤
≤ 25% change in CL over the range of BSA in adults, supporting no need for BSA-based dosing.
25% change in CL over the range of BSA in adults, supporting no need for BSA-based dosing.
Conclusions
Blinatumomab pharmacokinetics were adequately described by a one-compartment linear model with first-order elimination. No covariates other than BSA on CL were identified as significant. BSA-based dosing should be considered for lightweight patients to minimize inter-subject variability in blinatumomab exposure.
Electronic supplementary material
The online version of this article (10.1007/s40262-019-00823-8) contains supplementary material, which is available to authorized users.
Key Points
| A population pharmacokinetic model suitably described blinatumomab concentrations after continuous intravenous infusions in children and adults with hematologic malignancies, including relapsed/refractory (Philadelphia chromosome-positive or -negative) acute lymphoblastic leukemia (ALL), minimal residual disease-positive B cell precursor ALL, and non-Hodgkin’s lymphoma. |
| Dose adjustments based on body surface area (BSA) are not justifiable in pediatric and adult patients weighing at least 45 kg. |
| BSA-based dosing is warranted in children and adults weighing less than 45 kg. |
Introduction
B cell precursor acute lymphoblastic leukemia (ALL), minimal residual disease (MRD)-positive B cell precursor ALL, non-Hodgkin’s lymphoma (NHL), Philadelphia chromosome-positive (Ph +) ALL, and childhood ALL represent the majority of difficult-to-treat lymphoid cancers. Despite different combinations of aggressive multidrug chemotherapies, many patients with these leukemias or lymphomas either fail to achieve complete remission or end up with relapsed or refractory (R/R) diseases, with only 20–40% of the adult population achieving long-term remission [1] and only 85–90% in the pediatric population being cured, albeit with relapse rates of 10–15% [2]. Thus, there remains a need for novel therapeutic approaches. For cancers of lymphoid origin, CD19 is an attractive target due to its high expression in most NHLs and many leukemias, including ALL, chronic lymphocytic leukemia, and hairy cell leukemia. As the CD19 marker is not lost during neoplastic transformation of a normal cell into a cancer cell, CD19 is an ideal immunological target for the treatment of clonal B cell diseases [3–6].
+) ALL, and childhood ALL represent the majority of difficult-to-treat lymphoid cancers. Despite different combinations of aggressive multidrug chemotherapies, many patients with these leukemias or lymphomas either fail to achieve complete remission or end up with relapsed or refractory (R/R) diseases, with only 20–40% of the adult population achieving long-term remission [1] and only 85–90% in the pediatric population being cured, albeit with relapse rates of 10–15% [2]. Thus, there remains a need for novel therapeutic approaches. For cancers of lymphoid origin, CD19 is an attractive target due to its high expression in most NHLs and many leukemias, including ALL, chronic lymphocytic leukemia, and hairy cell leukemia. As the CD19 marker is not lost during neoplastic transformation of a normal cell into a cancer cell, CD19 is an ideal immunological target for the treatment of clonal B cell diseases [3–6].
Blinatumomab (BLINCYTO®, Amgen Inc., Thousand Oaks, CA, USA) is a novel single-chain antibody construct in the class of the bispecific T cell engager (BiTE®) against CD19/CD3 [7]. Blinatumomab mediates the formation of a synapse between the CD3+ T cell and the CD19+ target cell, transiently connecting malignant cells with T cells to induce T cell-mediated killing of the bound malignant cell. Consequently, granules containing granzymes and the pore-forming protein perforin fuse with the T cell membrane and discharge their toxic content. Released perforin forms pores in the presence of extracellular calcium that are inserted into the cancer cell membrane. These pores can then serve as entry sites for granzymes and are responsible for release of cytosolic content, leading to lysis of the CD19+ target cells [7]. Blinatumomab is approved in the USA for the treatment of R/R B cell precursor ALL in adult and pediatric populations [8, 9], as well as ALL with MRD [10]. It is currently under investigation for the treatment of NHL and its subtypes in adults [11].
Previously, the pharmacokinetics of blinatumomab were evaluated in several phase I and II clinical trials [11–16] in adults with NHL, MRD-positive ALL, R/R ALL, and R/R NHL. In these studies, blinatumomab exhibited linear and time-independent pharmacokinetics and fast clearance (CL) under continuous intravenous (cIV) infusion for 4–8 weeks per cycle over a dose range of either 5–90 µg/m2/day or 9–112 µg/day. Moreover, a population pharmacokinetic model in adult patients with Philadelphia chromosome-negative (Ph−) R/R ALL was previously reported (n =
= 365), in which blinatumomab concentration data were well-described by an open one-compartment linear pharmacokinetic model [17]. The typical value (geometric mean) of blinatumomab volume of distribution (Vd) in adult patients was estimated to be 3.40 L. CL was multimodal, where 90% of patients had a typical CL value of 1.36 L/h, and 10% had a typical CL value of 5.49 L/h with high inter-individual variability (IIV) (coefficient of variation 60%) [17]. Pharmacokinetics were similar in patients with ALL and NHL and were not affected by patient demographics. Creatinine clearance (CrCL) was estimated using the Cockcroft-Gault formula [18] and was a significant covariate for drug CL; however, no dose adjustment was required in adult patients with mild or moderate renal impairment. The incidence of neutralizing anti-blinatumomab antibodies was reported as less than 1% [17].
365), in which blinatumomab concentration data were well-described by an open one-compartment linear pharmacokinetic model [17]. The typical value (geometric mean) of blinatumomab volume of distribution (Vd) in adult patients was estimated to be 3.40 L. CL was multimodal, where 90% of patients had a typical CL value of 1.36 L/h, and 10% had a typical CL value of 5.49 L/h with high inter-individual variability (IIV) (coefficient of variation 60%) [17]. Pharmacokinetics were similar in patients with ALL and NHL and were not affected by patient demographics. Creatinine clearance (CrCL) was estimated using the Cockcroft-Gault formula [18] and was a significant covariate for drug CL; however, no dose adjustment was required in adult patients with mild or moderate renal impairment. The incidence of neutralizing anti-blinatumomab antibodies was reported as less than 1% [17].
In the present population pharmacokinetic analysis, the pharmacokinetics of blinatumomab were evaluated simultaneously in adult and pediatric patients with ALL for the first time (n =
= 674). Pharmacokinetic data from phase I, II, and III studies in patients with MRD-positive ALL and with Ph+ and Ph- R/R ALL were included. The objectives of this analysis were to characterize the pharmacokinetics of blinatumomab in patients following cIV administration over the age range of 7 months to 80 years; quantify the degree of IIV in pharmacokinetic parameters and residual variability; evaluate patient-related covariates as potential sources of variability in the pharmacokinetics of blinatumomab and its relationship with selected dosing regimens; and conduct model-based simulations to support dose conversion between body surface area (BSA)-based dosing and fixed doses.
674). Pharmacokinetic data from phase I, II, and III studies in patients with MRD-positive ALL and with Ph+ and Ph- R/R ALL were included. The objectives of this analysis were to characterize the pharmacokinetics of blinatumomab in patients following cIV administration over the age range of 7 months to 80 years; quantify the degree of IIV in pharmacokinetic parameters and residual variability; evaluate patient-related covariates as potential sources of variability in the pharmacokinetics of blinatumomab and its relationship with selected dosing regimens; and conduct model-based simulations to support dose conversion between body surface area (BSA)-based dosing and fixed doses.
Methods
Clinical Data
Data from eight clinical studies (one phase I, one phase I/II, five phase II, and one phase III) in 674 patients were included in this analysis. Study populations included adult patients with relapsed NHL (n =
= 67), MRD-positive B-lineage ALL (n
67), MRD-positive B-lineage ALL (n =
= 52), R/R Ph– ALL (n
52), R/R Ph– ALL (n =
= 472), R/R Ph+ ALL (n
472), R/R Ph+ ALL (n =
= 37), and pediatric patients with R/R Ph– ALL (n
37), and pediatric patients with R/R Ph– ALL (n =
= 46). Data were pooled for this analysis (Table 1). Patients received blinatumomab as a cIV infusion over 4–8 weeks (multiple cycles) at dose ranges 0.5–90 μg/m2/day and 9–28 μg/day. Blinatumomab pharmacokinetics were assessed for up to ten cycles across studies. The relevant characteristics of each clinical study used for the current analyses are summarized in Table 1; additional details of these studies have been previously reported [11–15, 19–21].
46). Data were pooled for this analysis (Table 1). Patients received blinatumomab as a cIV infusion over 4–8 weeks (multiple cycles) at dose ranges 0.5–90 μg/m2/day and 9–28 μg/day. Blinatumomab pharmacokinetics were assessed for up to ten cycles across studies. The relevant characteristics of each clinical study used for the current analyses are summarized in Table 1; additional details of these studies have been previously reported [11–15, 19–21].
Table 1
Overview of blinatumomab clinical studies and data included in the analyses
| Study: ClinicalTrials.gov identifier (Amgen study number) [trial phase] | Blinatumomab dosing regimen | PK sampling scheme frequency | Number of patients (median blinatumomab samples per patient) | Population |
|---|---|---|---|---|
| NCT00274742 (MT103-104) [I] | 0.5–90 µg/m2/day over 4–8 weeks per cycle, with varying number of cycles | Dependent on duration Typical 4-week cycle: predose, 45 min, 2 h, 6 h, 12 h, 24 h, 2 days, 3 days, 8 days, 15 days, 22 days, 29 days after infusion start and at infusion stop, and at 1, 2, 4, 6, and 24 h after stopping infusion | 67 (12) | Relapsing NHL |
| NCT00560794 (MT103-202) [II] | 15 or 30 µg/m2/day over 4 weeks followed by 2 weeks off-treatment per cycle for up to 10 cycles | Cycle 1: predose, 2 h, 6 h, 12 h, 7 days, 14 days, 21 days, 28 days Post infusion stop at 1, 2, 4, 6, 8, and 24 h Cycle 2 and beyond: predose, 7, 14, 21, and 28 days | 20 (10.5) | MRD-positive B lineage ALL |
| NCT01207388 (MT103-203) [II] | 15 µg/m2/day for 28 days per treatment cycle, followed by 14-day infusion-free interval. Every patient received at least 1 and up to 4 cycles of treatment. In the case of neurologic events, the dose of blinatumomab may have been reduced to 5 µg/m2/day | Cycle 1: days 3 (at least 48 h after start of infusion), 15, and 29 | 32 (3) | MRD-positive B lineage ALL |
| NCT01471782 (MT103-205) [I/II] | 3.75–30 µg/m2/day over 4 weeks followed by a 14-day treatment-free interval per cycle for up to 5 cycles | Sparse PK samples were collected at predose, 48 h after the start of the infusion, and then weekly at steady state. At day 29, PK samples were collected 2, 4, and 8 h after EOI for age groups 2–7 and 7–16 years | 46 (7) | R/R ALL (pediatric) |
| NCT01209286 (MT103-206) [II] | 5 µg/m2/day for 1 week followed by 15 µg/m2/day for weeks 2–4 followed by 2 weeks off treatment, or 15 or 30 µg/m2/day over 4 weeks followed by 2 weeks off treatment per cycle for up to 8 cycles | Cycles 1 and 2: predose, days 3 (at least 48 h following start of infusion/dose step), 8, 15, 22, and 29 (at EOI). In case of dose step on day 8 or 15, a sample will be taken just before dose step and on day 10 or 17 (at least 48 h following dose step), respectively | 36 (9) | R/R ALL |
| NCT01466179 (MT103-211) [II] | 9 µg/day during week 1 of cycle 1 and 28 µg/day for the remainder of the 4-week treatment followed by 2 weeks off treatment, with up to 4 additional cycles of 28 µg/day | Cycles 1 and 2: predose, days 3 (at least 48 h following start of infusion/dose step), 8, 15, 22, and 29 (at EOI). In case of dose step on day 8 or 15, a sample will be taken just before dose step and on day 10 or 17 (at least 48 h following dose step), respectively | 213 (6) | R/R ALL |
| NCT02000427 (20120216) [II] | First cycle was 9 µg/day for the first 7 days, then escalated to 28 µg/day starting at day 8 (week 2) through day 29 (week 4). Subsequent cycles were 28 µg/day for all 4 weeks of continuous treatment for up to 5 cycles | Two PK samples: (1) cycle 1, day 8, 6–8 h after the dose step to 28 μg/day; and (2) cycle 2, day 1, 6–8 h after blinatumomab infusion of 28 μg/day | 37 (2) | R/R Ph+ ALL |
| NCT02013167 (00103311) [III] | 9 μg/day for the first 7 days of treatment followed by 28 μg/day starting on day 8 (week 2) through day 29 (week 4). For all subsequent induction and consolidation cycles, 28 μg/day for the entire 4 weeks of continuous treatment. For maintenance therapy, treatment was administered every 12 weeks (4 weeks of continuous infusion with an 8-week treatment-free interval) at the dose last received following the completion of the last consolidation cycle for up to 5 cycles | Two PK samples: (1) cycle 1, day 2; and (2) cycle 1, day 15. Both PK samples taken during the infusion | 223 (2) | R/R ALL |
ALL acute lymphoblastic leukemia, EOI end of infusion, MRD minimal residual disease, NHL non-Hodgkin’s lymphoma, Ph+ Philadelphia chromosome-positive, PK pharmacokinetic, R/R relapsed or refractory
Bioanalysis
Blinatumomab serum concentrations were assessed using a CD69 activation bioassay [22] with an upper limit of quantification of 1000 pg/mL and lower limit of quantification of 50–100 pg/mL (100 pg/mL in earlier studies: MT103-104 and MT103-202; and 50 pg/mL in later studies: MT103-203, MT103-205, MT103- 206, MT103-211, 20120216, and 00103311) [17]. The accuracy of the assay ranged from 73 to 123%, and the precision of the assay, including at the lower limit of quantification, ranged from 72 to 120% within the linear range [17]. The basis of the assay is that during the blinatumomab-mediated cytotoxicity reaction, the CD69 activation marker is expressed on T cells in a blinatumomab concentration-dependent manner. In the assay, HPB-ALL cells (T cell line) were incubated with Raji cells (B cell line) in the presence of serial blinatumomab dilutions in serum, ranging from 200 ng/mL to 3 pg/mL, as previously published [17]. After incubation, T cells were labelled with mouse anti-CD69-FITC monoclonal antibody and measured in a fluorescence-activated cell sorting Canto unit. The forward scatter/side scatter mode was used for data analysis.
Non-Compartmental and Exploratory Analyses
Definitive non-compartmental analyses (NCAs) were conducted for clinical study report generation and are presented as stand-alone results but also used as an exploratory tool to better understand the data and any relationship between demographic and treatment-related factors with blinatumomab pharmacokinetic parameters. In this analysis, individual blinatumomab CL values, calculated as R0/Css, where R0 is the infusion rate and Css is the average of Css (steady-state concentration) values available for an individual patient, were utilized to graphically evaluate any relationships between blinatumomab CL and BSA, age, race, and disease. Individual blinatumomab CL values, calculated as R0/Css where Css is the individual Css, were utilized to graphically evaluate any relationships between blinatumomab CL and dose and treatment cycle. One pediatric patient for the NCA analysis only, and not the population pharmacokinetic analysis, was excluded from the pharmacokinetic parameter reporting because the pharmacokinetic sample was from a retreatment cycle.
Software for Population Pharmacokinetic Analysis
Serum concentration versus time profiles were used for non-linear mixed-effect modelling [23] using NONMEM® version 7.2 (ICON Development Solutions, Ellicott City, MD, USA) [24]. First-order conditional estimation (FOCE), FOCE interaction, and Laplacian methods were evaluated [25]. Graphical and all other statistical analyses, including evaluation of NONMEM® outputs, were performed using R version 3.0.1 or higher. In the exploratory analysis, blinatumomab pharmacokinetic parameters were also assessed using NCA with Phoenix WinNonlin® version 6.3 (Certara, Princeton, NJ, USA).
Pharmacostatistical and Covariate Modeling
Pharmacokinetic modeling was conducted on eight pediatric/adult clinical studies as outlined in Table 1. Full details regarding pharmacostatistical and covariate modeling methodologies are found in the Electronic Supplementary Materials.
Internal Model Evaluation
The final population pharmacokinetic model was internally evaluated using prediction-corrected visual predictive check (pcVPC) using 100 replicates [26] and non-parametric bootstrap methodologies using 500 replicates [27, 28]. Replication of the original dataset (a bootstrap sample) was obtained by random draws of individual data (with replacement) from the dataset. The population pharmacokinetic model was refitted to each new dataset. The stability of the model was evaluated by visual inspection of the distribution of the model parameter estimates obtained from the newly replicated datasets, and was compared with that obtained from the fit of the original dataset. The parameter estimates of the model were compared to the mean and 95% confidence interval (CI) of the model parameter obtained from the non-parametric bootstrap replicates that had been successfully minimized. If the parameter estimates were within the 95% CI obtained from the bootstrap analysis, the model was considered acceptable.
Simulations
As recommended in the prescribing information [8], blinatumomab is to be administered as a fixed dose for patients ≥
≥ 45 kg and as a BSA-based dose for patients
45 kg and as a BSA-based dose for patients <
< 45 kg. To compare fixed dosing (28 μg/day), BSA-based dosing (15 μg/m2/day), and per label-based (15 μg/m2/day for patients
45 kg. To compare fixed dosing (28 μg/day), BSA-based dosing (15 μg/m2/day), and per label-based (15 μg/m2/day for patients <
< 45 kg and 28 μg/day for patients
45 kg and 28 μg/day for patients ≥
≥ 45 kg) dosing strategies, 10,000 virtual patients were simulated from the final model using the multivariate distribution between bodyweight and BSA. Each simulated patient received each of the three dosing strategies. There were 1000 simulations for each 10 kg interval between 5 and 105 kg to ensure an equal number of patients across the bodyweight bins. The final model was then used to generate blinatumomab Css, which was summarized in 10 kg bins.
45 kg) dosing strategies, 10,000 virtual patients were simulated from the final model using the multivariate distribution between bodyweight and BSA. Each simulated patient received each of the three dosing strategies. There were 1000 simulations for each 10 kg interval between 5 and 105 kg to ensure an equal number of patients across the bodyweight bins. The final model was then used to generate blinatumomab Css, which was summarized in 10 kg bins.
Results
Summary of Data for Population Pharmacokinetic Analysis
A total of 674 patients in the combined population pharmacokinetic dataset received blinatumomab as a cIV infusion over 4–8 weeks for up to ten cycles at doses ranging from 0.5 to 90 µg/m2/day and from 9 to 28 µg/day.
There were 4841 serum samples, 13 (0.27%) had missing time, 287 (5.93%) were collected more than 90 days after the start of infusion, and 248 (5.12%) were collected more than 1 day after the end of the infusion, leaving 4293 samples. Of these 4293 samples, 664 (15.5%) were below the lower limit of quantification, leaving 3629 serum samples in the analysis dataset. MRD patients constituted 7.7% of all patients available for analysis. The number of samples per patient, by study, is presented in Table 1. Individual concentration versus time profiles are shown in Electronic Supplementary Material Fig. 1.
One patient each had missing aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values, two were missing total bilirubin, eight were missing serum albumin, and three were missing CrCL. Summary statistics for continuous and categorical covariates are shown in Electronic Supplementary Material Tables 1 and 2. Median age was 41.0 years (range 0.6–80). Median bodyweight was 70.7 kg (range 7.5–149), and median BSA was 1.8 m2 (range 0.4–2.70). In total, 406 men and 268 women were included in the analysis; 86% were white. Median CrCL was 121 mL/min (range 36–150) calculated using the Cockcroft-Gault equation for adult patients and the Schwartz equation for pediatric patients. Liver function was characterized by median values of 28.2 U/L for AST, 41.0 U/L for ALT, 37.1 g/L for serum albumin, and 8.2 μmol/L of total bilirubin. Median lactate dehydrogenase (LDH) and hemoglobin were 297 IU/L and 10.1 g/dL, respectively.
Non-Compartmental and Exploratory Analyses
Graphical presentations for exploring relationships between blinatumomab CL and BSA, age, race, dose, treatment cycle, and disease are depicted in Fig. 1. Figures showing BSA-normalized CL by race, treatment cycle, dose, and disease are depicted in Electronic Supplementary Material Fig. 2. NCA results showed that blinatumomab CL (L/h) increased with BSA (Fig. 1a). Once the CL values were normalized by BSA in pediatric patients (Fig. 1b), they were similar across the age range, supporting BSA-based dosing in patients with lower BSA values. No apparent differences in the CL ranges across races were identified from this dataset (Fig. 1c). Blinatumomab CL values were consistent across treatment cycles (Fig. 1d) and dose levels (Fig. 1e). Blinatumomab was also similar across disease populations (Fig. 1f).
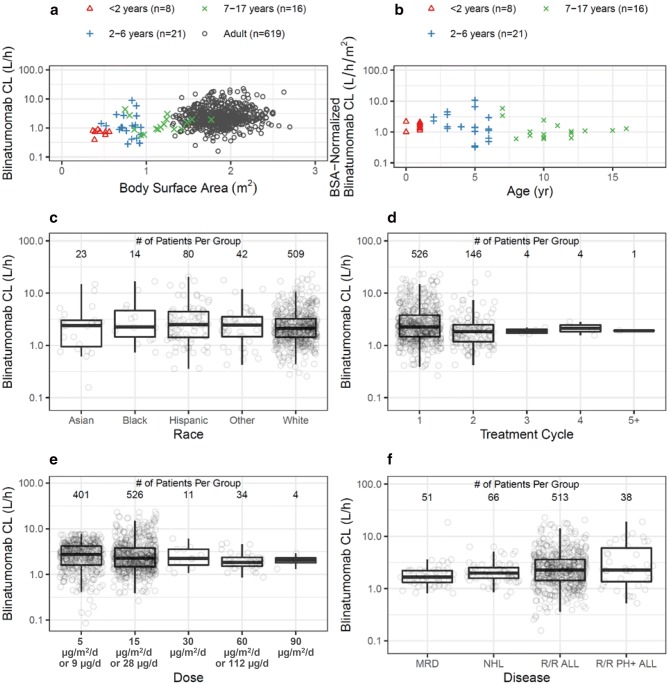
Relationships between blinatumomab clearances and demographic, treatment, and disease-related factors. a Clearance versus body surface area. b Body surface area-normalized clearance versus age in pediatrics. c Clearance by race. d Clearance by treatment cycle after administration of 5 µg/m2/day or 9 µg/day in adults. e Clearance by dose after cycle 1 dose administration in adults. f Clearance by disease. The top, middle, and bottom of the boxes in figures c–f are the third quartile, median, and first quartile, respectively. The whiskers are drawn to the nearest value not beyond 1.5 ×
× (upper hinge—lower hinge). In figures d–e individual blinatumomab clearance values were calculated as R0/Css where Css is the individual Css. Height or weight data was not collected for four subjects in the clinical dataset. ALL acute lymphoblastic leukemia, BSA body surface area, CL clearance, Css steady-state concentration, d day, MRD minimal residual disease, NHL non-Hodgkin’s lymphoma, PH Philadelphia chromosome, R0 infusion rate, R/R relapsed or refractory
(upper hinge—lower hinge). In figures d–e individual blinatumomab clearance values were calculated as R0/Css where Css is the individual Css. Height or weight data was not collected for four subjects in the clinical dataset. ALL acute lymphoblastic leukemia, BSA body surface area, CL clearance, Css steady-state concentration, d day, MRD minimal residual disease, NHL non-Hodgkin’s lymphoma, PH Philadelphia chromosome, R0 infusion rate, R/R relapsed or refractory
The summary of pharmacokinetic parameters generated by the NCA for patient groups weighing ≥
≥ 45 and
45 and <
< 45 kg (Table 2) indicates that with the recommended dosing regimens in the drug label, comparable Css values can be achieved for the two groups. A limited number of patients had the necessary intensive sampling to enable the estimation of volume of distribution based on terminal phase (Vz) and elimination half-life (t½), explaining the difference in the n for the parameters. Based on these observations, the one-compartment model was consistent with the previously published model [17].
45 kg (Table 2) indicates that with the recommended dosing regimens in the drug label, comparable Css values can be achieved for the two groups. A limited number of patients had the necessary intensive sampling to enable the estimation of volume of distribution based on terminal phase (Vz) and elimination half-life (t½), explaining the difference in the n for the parameters. Based on these observations, the one-compartment model was consistent with the previously published model [17].
Table 2
Blinatumomab non-compartmental pharmacokinetic parameter estimates following continuous intravenous infusion in patients with bodyweight ≥
≥ 45 and
45 and <
< 45 kg
45 kg
| Population | Clearance | Vz | t½,Z | Dose | Cycle 1 Css |
|---|---|---|---|---|---|
≥ 45 kg 45 kg | 3.15 (3.03) L/h n | 4.40 (2.53) L n | 2.10 (1.41) h n | 9 µg/day | 229 (360) pg/mL n |
| 28 µg/day | 615 (538) pg/mL n | ||||
| 5 µg/m2/day | 191 (79.8) pg/mL n | ||||
| 15 µg/m2/day | 608 (265) pg/mL n | ||||
< 45 kg 45 kg | 1.87 (1.96) L/h n | 3.48 (2.61) L n | 2.20 (1.56) h n | 9 µg/day | 174 (124) pg/mL n |
| 28 µg/day | 637 (554) pg/mL n | ||||
| 5 µg/m2/day | 157 (188) pg/mL n | ||||
| 15 µg/m2/day | 538 (403) pg/mL n |
Css steady-state concentration after cycle 1 administration of 5/15 µg/m2/day or 9/28 µg/day of blinatumomab, n sample size, t½,Z terminal half-life, Vz volume of distribution based on terminal phase
Model Development
The previously published model [17] reported a bimodal distribution in the predicted CL. When this model was applied to the new analysis, CL was no longer bimodal. Since the previous model was not externally valid for the new data, further model development was necessary to adequately characterize blinatumomab population pharmacokinetics.
Simplification of the model was achieved by removing the mixture model for two CL subpopulations, reducing to one residual error, and removing the IIV on the residual error. Therefore, the model used at the initiation of redevelopment had four parameters: Vd, CL, IIV on CL, and one residual error parameter. Based on assessment of empirical Bayes estimates (EBEs) versus BSA, BSA on CL was added to the model. This resulted in a significant decrease in the minimum value of the objective function of 35.6. Switching to an FOCE approach instead of a Laplacian approach (as previously utilized) further improved fitting. At this point in the model-building phase, the covariance step was not successful. When including IIV on the residual error, the model was stabilized, with a successful covariance step and a low condition number of 7. Inclusion of the effect of BSA on Vd resulted in unrealistic values of Vd (Vd >
> 9) and an unsuccessful covariance step. The model, now having Vd, CL, BSA on CL, IIV on CL, residual variability, and IIV on residual variability, was used as the basis for which other covariates could be investigated.
9) and an unsuccessful covariance step. The model, now having Vd, CL, BSA on CL, IIV on CL, residual variability, and IIV on residual variability, was used as the basis for which other covariates could be investigated.
The NCA and the visual and statistical analyses of the empirical Bayesian post hoc estimates for CL showed flat relationships, with no r2 values higher than 3% (Electronic Supplementary Material Figs. 3 and 4 with statistics in-figure). The lack of any meaningful relationships between CL random effects and covariates, combined with a low shrinkage value of 9%, confirmed that it was unnecessary to test the effect of any covariate formally in NONMEM®, including for dose level and treatment cycle.
With the addition of BSA as a covariate on CL, IIV on CL was decreased from 51.4 to 47.6%. There were minor changes in CL (from 2.13 to 2.22 L/h), Vd (from 5.96 to 5.98 L), IIV for residual error (from 63.9 to 64.3%), and residual variability (from 55.5 to 55.9%). There was no additional benefit to using a BSA covariate effect only in patients <
< 45 kg while keeping a fixed dosing approach in heavier patients, with the IIV on CL increasing from 47.6 to 47.8%. In the final model, the shrinkage value for the random effects on residual variability was 5%. The final model was a one-compartment linear pharmacokinetic model parameterized in terms of systemic CL and Vd. Pharmacokinetic parameters were assumed to be log-normally distributed, and an exponential IIV term was estimated for CL. Residual variability was modeled using an additive error model in the log-domain with IIV.
45 kg while keeping a fixed dosing approach in heavier patients, with the IIV on CL increasing from 47.6 to 47.8%. In the final model, the shrinkage value for the random effects on residual variability was 5%. The final model was a one-compartment linear pharmacokinetic model parameterized in terms of systemic CL and Vd. Pharmacokinetic parameters were assumed to be log-normally distributed, and an exponential IIV term was estimated for CL. Residual variability was modeled using an additive error model in the log-domain with IIV.
Goodness-of-fit plots are shown in Electronic Supplementary Material Fig. 5. Observed versus predicted plots showed random normal scatter around the identity line, indicating the absence of systematic bias and the adequacy of the model to describe the data. In addition, conditional weighted residuals (CWRES) and normalized prediction distribution errors (NPDE) showed random normal scatter around zero, with no specific pattern. The distribution of CWRES and NPDE versus time remained fairly constant, which demonstrated the absence of time-dependent pharmacokinetics. The mean of the NPDE for the serum concentrations was 0.037 (95% CI 0.00862–0.0654) with a standard deviation (SD) of 0.891 (95% CI 0.869–0.912). Although a slight bias of the pharmacokinetic variability was found, these results confirmed the appropriate model accuracy and precision because the 95% CI for the mean and SD of the NPDE for blinatumomab were very close to including 0 and 1, respectively. There were no observations with absolute values of the CWRES >
> 6, and hence there were no identified outliers.
6, and hence there were no identified outliers.
The NONMEM®-based final parameter estimates of the model are found in Table 3. Both fixed and random effects parameters were estimated with good precision [percentage relative standard error (%RSE) ≤
≤ 16.1%], and the condition number of 7 (calculated as the ratio of the highest to the lowest eigenvalue) indicated that the model was not ill conditioned.
16.1%], and the condition number of 7 (calculated as the ratio of the highest to the lowest eigenvalue) indicated that the model was not ill conditioned.
Table 3
Parameter estimates and bootstrap analysis of the blinatumomab population pharmacokinetic parameters (n =
= 674)
674)
| Parameter | Final model (mean (RSE, %) [95% CI]) | Non-parametric bootstrap: 500 successful of 500 replicates (median [95% CI]) |
|---|---|---|
| Pharmacokinetic parameters | ||
| Volume (L) | 5.98 (8.86) [5.14–6.98] | 5.99 [5.14–6.98] |
| CL (L/h) | 2.22 (2.95) [2.08–2.35] | 2.22 [2.08–2.35] |
| Effect of BSA on CL (θ)a | 0.620 (12.7) [0.46–0.76] | 0.625 [0.463–0.756] |
| Inter-individual variability (%CV) | ||
| ωCL | 47.6 (16.1) [38.1–54.2] | 47.1 [38.1–54.1] |
| ωEPS | 64.3 (14.5) [55.0–73.0] | 64.4 [55.0–73.0] |
| Residual variability (%CV) | 55.9 (3.99) [52.6–61.5] | 56.1 [46.3–75.6] |
BSA body surface area, CI confidence interval obtained from NONMEM® asymptotic standard errors, CL clearance, CV coefficient of variation, RSE relative standard error, ωCL inter-subject variability in CL, ωEPS inter-subject variability in residual variability
aCLindividual =
= CL·(BSA/1.876)Effect of BSA on CL
CL·(BSA/1.876)Effect of BSA on CL
Internal Model Evaluation
The pcVPC performed using the final model (Fig. 2) suggested that the developed model was appropriate in describing the time course of blinatumomab and its variability in a heterogeneous patient population across age groups. Since the pediatric pharmacokinetic parameters were supported by a relatively small number of patients who had sparse pharmacokinetic sampling, an additional pediatric-only pcVPC showing adequate model performance in pediatrics is presented in Electronic Supplementary Material Fig. 6.
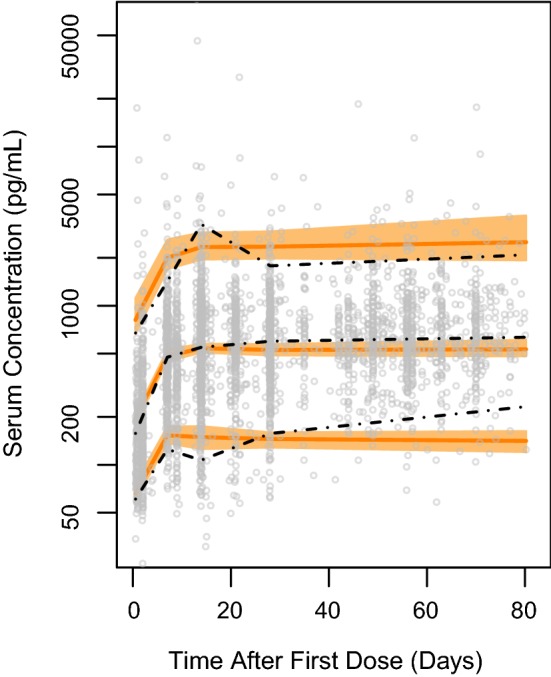
Prediction-corrected visual predictive check, using 100 replicates, for all studies using the final model. Dashed black lines are the medians and 90% prediction intervals of observed data, which are shown as grey open circles. The shaded regions represent the 95% confidence intervals of the orange solid lines (median and 90% prediction intervals of simulated data)
The non-parametric bootstrap distributions are summarized in Electronic Supplementary Material Fig. 7. The bootstrap tabulations of the model parameters are displayed in Table 3. Overall, the population estimates for the final model were very similar to the median of the bootstrap replicates, and were contained within the 95% CI obtained from the bootstrap analysis. The precision of the NONMEM® parameter estimates was good since the %RSE for the fixed and random effects were lower than 17%. This finding confirms the robustness of the NONMEM®-predicted parameter estimates.
Simulations of Fixed, Body Surface Area-Based, and Per-Label Dosing Strategies
During blinatumomab clinical development, BSA-based doses were converted to fixed doses in adult patients with a bodyweight of at least 45 kg to reduce dosing error rates, reduce drug waste, and improve dosing convenience. As illustrated in Fig. 3, the per-label dosing strategy resulted in similar exposures across the broad range of bodyweight. The median Css ranges across the 10 kg bins were as follows: (1) fixed dosing: 484–873 pg/mL; (2) BSA-based dosing: 391–567 pg/mL; and (3) per-label dosing: 391–603 pg/mL. The interquartile ranges of blinatumomab Css for all patients <
< 45 kg for fixed, BSA-based, and per-label dosing were 819, 453, and 453 pg/mL, respectively, and for all patients
45 kg for fixed, BSA-based, and per-label dosing were 819, 453, and 453 pg/mL, respectively, and for all patients ≥
≥ 45 kg were 567, 564, and 567 pg/mL, respectively. Note the broader range when using a fixed dosing approach for patients
45 kg were 567, 564, and 567 pg/mL, respectively. Note the broader range when using a fixed dosing approach for patients <
< 45 kg.
45 kg.
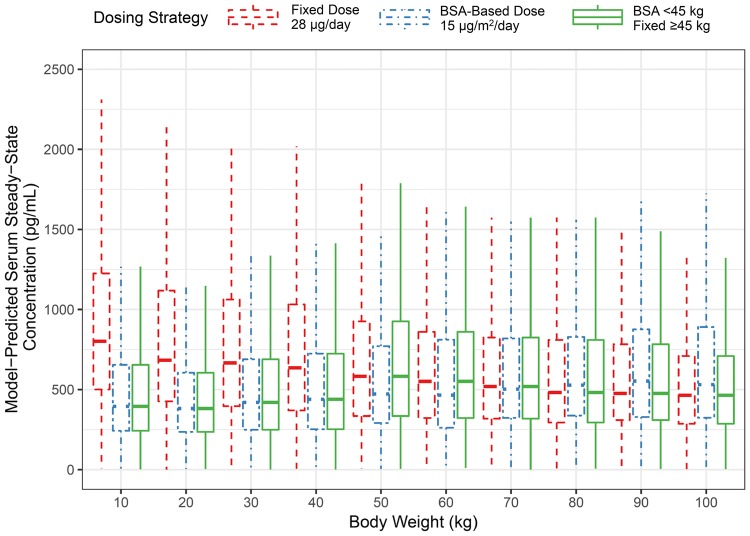
Simulated serum steady-state concentration for fixed dosing (28 μg/day, red dashed line), body surface area-based dosing (15 μg/m2/day, blue dot-dashed line), and per label-based (15 μg/m2/day for patients <
< 45 kg, and 28 μg/day for patients
45 kg, and 28 μg/day for patients ≥
≥ 45 kg, green solid line) regimens in virtual patients having a broad range of bodyweights using a weight/body surface area multivariate distribution. Body weights were binned for each 10 kg interval between 5 and 105 kg, with 1000 patients simulated for each bin resulting in a total of 10,000 simulated patients. The top, middle, and bottom of the boxes are the third quartile, median, and first quartile, respectively. The whiskers are drawn to the nearest value not beyond 1.5
45 kg, green solid line) regimens in virtual patients having a broad range of bodyweights using a weight/body surface area multivariate distribution. Body weights were binned for each 10 kg interval between 5 and 105 kg, with 1000 patients simulated for each bin resulting in a total of 10,000 simulated patients. The top, middle, and bottom of the boxes are the third quartile, median, and first quartile, respectively. The whiskers are drawn to the nearest value not beyond 1.5 ×
× (upper hinge—lower hinge). BSA body surface area
(upper hinge—lower hinge). BSA body surface area
Discussion
Blinatumomab showed linear kinetics across dose levels and over time based on population pharmacokinetic modeling. These results are consistent with the NCA and previous population pharmacokinetic modeling results [17]. In the population pharmacokinetic analysis, an open one-compartment linear pharmacokinetic model was suitable to describe the time course of blinatumomab concentrations, in different age groups, after cIV administrations of different doses in patients with hematologic malignancies, including R/R ALL, MRD-positive B cell precursor ALL, NHL, Ph+ ALL, and childhood R/R ALL.
While full-length immunoglobulin G antibodies with neonatal Fc receptor (FcRn) domains typically have a terminal t½ of approximately 23 days in humans [29], small fusion proteins such as BiTE® antibody constructs, including blinatumomab, typically have shorter t½ values, measurable in hours—comparable to many small molecules [30]. Blinatumomab, a BiTE® against CD19/CD3, does not directly kill B cells, but rather it activates T cells and triggers an immune response which leads to B cell depletion—one activated T cell can kill more than one B cell—contributing to significant cytotoxicity at low pg/mL concentrations [7]. Linear drug disposition, and not target-mediated drug disposition (TMDD), has been observed for blinatumomab within the range of doses studied, which contrasts with non-linear pharmacokinetics that may otherwise be associated with TMDD. Like monoclonal antibodies, BiTE® antibody constructs have limited volumes of distribution, similar to plasma volume, and an elimination pathway consisting of rapid catabolism into simple amino acids [30]. According to the population pharmacokinetic model, the typical blinatumomab Vd value was estimated to be 5.98 L, which is very close to plasma volume and similar to the values reported for other therapeutic fusion proteins [30]. The typical CL value was 2.22 L/h. Of note, the previous mixture model was no longer appropriate since the bimodal distribution filled into a single distribution with the additional data from the newly included studies.
The correlation coefficients for all covariates against CL were below 0.05, suggesting that no clinically relevant covariates existed. However, the goodness-of-fit plots showed pediatric patients were not well-described by the model. With the inclusion of BSA on CL there was a significant improvement in the model fit for pediatric patients. Pediatric pharmacokinetics can be influenced by differences in blood flow, pH, skin differences, absorption, body water and fat content, and circulating proteins, among other differences. This can impact (1) the volume of distribution; (2) intestinal, catabolic, liver, and kidney function; and (3) receptor-mediated endocytosis [31, 32]. Pediatric dosage is often established empirically, based on age, bodyweight, BSA, or other differences such as fat composition. Further adjustments are often not necessary since the totality of CL and other differences are often simultaneously addressed [32]. The smallest BSA of 0.37 m2 in the pediatric population was associated with a 63% lower blinatumomab systemic CL relative to a patient with the median BSA (1.88 m2), supporting the use of BSA-based dosing in patients with lower body weight values. In contrast, the smallest and largest BSA values of 1.31 and 2.7 m2 in adults would be associated with a 20% reduction and a 25% increase, respectively, in blinatumomab systemic CL relative to a patient with the median BSA (1.88 m2). The magnitude of this effect is relatively low compared with the 48% unexplained between-subject variability in CL, and the 56% residual variability (which residual variability itself had a 64% between-subject variability) in blinatumomab pharmacokinetics. While dose adjustments based on BSA in patients ≥
≥ 45 kg are not justifiable, it is important to note that the use of 15 µg/m2/day BSA-based dosing in patients
45 kg are not justifiable, it is important to note that the use of 15 µg/m2/day BSA-based dosing in patients <
< 45 kg prevented excessive adverse events as determined in a phase I/II study of blinatumomab in pediatric patients [21], providing a strong incentive to retain this approach in lighter weight patients.
45 kg prevented excessive adverse events as determined in a phase I/II study of blinatumomab in pediatric patients [21], providing a strong incentive to retain this approach in lighter weight patients.
On average, for a patient with a BSA of 0.37, 1.31, 1.88, or 2.7 m2, the blinatumomab t½ was estimated to be 5.11, 2.34, 1.86, or 1.49 h, respectively. Therefore, the vast majority of the patients achieved Css within the first day of a 28-day cycle, regardless of BSA.
Other than BSA, none of the potential covariates (age, CrCL, sex, AST, ALT, total bilirubin, albumin, LDH, hemoglobin, dose level, and treatment cycle) were correlated with the between-patient variability of blinatumomab CL.
Clinical experience and further updates can be made when ongoing phase III studies in pediatric and adult patients are completed. Possible limitations to the analysis include lack of pharmacokinetic data in pediatric patients weighing ≥
≥ 45 kg treated with the fixed-dose regimen, lack of pharmacokinetic data from adult patients weighing
45 kg treated with the fixed-dose regimen, lack of pharmacokinetic data from adult patients weighing <
< 45 kg treated with a BSA-based dosing regimen, having fewer Black, Asian, and other non-White patients than White patients, and the lack of T cell and B cell baseline counts from all studies preventing their testing as a covariates in this analysis. Simulation results (Fig. 3) further indicated that the Css ranges were comparable across bodyweights when patients weighing
45 kg treated with a BSA-based dosing regimen, having fewer Black, Asian, and other non-White patients than White patients, and the lack of T cell and B cell baseline counts from all studies preventing their testing as a covariates in this analysis. Simulation results (Fig. 3) further indicated that the Css ranges were comparable across bodyweights when patients weighing <
< 45 kg were treated with the BSA-based dosing regimen or patients weighing
45 kg were treated with the BSA-based dosing regimen or patients weighing ≥
≥ 45 kg were treated with the fixed-dose regimen. The simulations also provide evidence that slight modifications to the weight cut-off would likely not change the drug exposure significantly. Since BSA-based dosing is not expected to offer benefit over fixed dosing, recommendation of the simpler fixed-dosing strategy for patients weighing at least 45 kg [8] is still reasonable.
45 kg were treated with the fixed-dose regimen. The simulations also provide evidence that slight modifications to the weight cut-off would likely not change the drug exposure significantly. Since BSA-based dosing is not expected to offer benefit over fixed dosing, recommendation of the simpler fixed-dosing strategy for patients weighing at least 45 kg [8] is still reasonable.
Conclusion
An open one-compartment pharmacokinetic model with linear elimination was suitable to describe the time course of the serum blinatumomab concentration following cIV administration of doses ranging from 0.5 to 90 µg/m2/day or two fixed-dose levels of 9 and 28 µg/day in patients with hematologic malignances, including patients with relapsing NHL, MRD-positive B-lineage ALL, R/R ALL (pediatric and adult), and R/R Ph+ ALL. The model has clinical applications in the dose and regimen selection in pediatric and adult patients over multiple indications. No covariates, other than BSA on CL, were identified as significant. While fixed blinatumomab dosing is acceptable in patients ≥
≥ 45 kg, BSA-based dosing should be considered for lower bodyweight patients to avoid excessive serum blinatumomab Css values.
45 kg, BSA-based dosing should be considered for lower bodyweight patients to avoid excessive serum blinatumomab Css values.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank James Ziobro (funded by Amgen Inc.) for medical writing assistance in the preparation of this manuscript. They would also like to thank the reviewers for their helpful comments and suggestions.
Compliance with Ethical Standards
This study was funded by Amgen Inc. Amgen designed the study, and collected, analyzed, and interpreted the data.
John D. Clements, Bianca Terminello, and Sameer Doshi are employees of and shareholders in Amgen Inc. Min Zhu and Mita Kuchimanchi were employees of and shareholders in Amgen Inc. when the analysis was conducted. Min Zhu is currently employed by Regeneron. Mita Kuchimanchi is currently employed by Biogen.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments, or comparable ethical standards.
Informed consent was obtained from all individual participants included in the studies.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40262-019-00823-8
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s40262-019-00823-8.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s40262-019-00823-8
Article citations
Infant Acute Lymphoblastic Leukemia-New Therapeutic Opportunities.
Int J Mol Sci, 25(7):3721, 27 Mar 2024
Cited by: 0 articles | PMID: 38612531 | PMCID: PMC11011884
Review Free full text in Europe PMC
[Chinese consensus for the bispeific T cell engager in the treatment of B-cell acute lymphoblastic leukemia (2022)].
Zhonghua Xue Ye Xue Za Zhi, 43(6):448-455, 01 Jun 2022
Cited by: 1 article | PMID: 35968586 | PMCID: PMC9800224
The New Treatment Methods for Non-Hodgkin Lymphoma in Pediatric Patients.
Cancers (Basel), 14(6):1569, 18 Mar 2022
Cited by: 5 articles | PMID: 35326719 | PMCID: PMC8945992
Review Free full text in Europe PMC
Clinical pharmacology of cytotoxic drugs in neonates and infants: Providing evidence-based dosing guidance.
Eur J Cancer, 164:137-154, 02 Dec 2021
Cited by: 8 articles | PMID: 34865945 | PMCID: PMC8914347
Review Free full text in Europe PMC
Immunotherapy in Pediatric B-Cell Acute Lymphoblastic Leukemia: Advances and Ongoing Challenges.
Paediatr Drugs, 22(5):485-499, 01 Oct 2020
Cited by: 16 articles | PMID: 32860590 | PMCID: PMC7537790
Review Free full text in Europe PMC
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (3)
- (1 citation) ClinicalTrials.gov - NCT00274742
- (1 citation) ClinicalTrials.gov - NCT00560794
- (1 citation) ClinicalTrials.gov - NCT01207388
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Blinatumomab, a Bispecific T-cell Engager (BiTE(®)) for CD-19 Targeted Cancer Immunotherapy: Clinical Pharmacology and Its Implications.
Clin Pharmacokinet, 55(10):1271-1288, 01 Oct 2016
Cited by: 106 articles | PMID: 27209293
Blinatumomab: A First-in-Class Bispecific T-Cell Engager for Precursor B-Cell Acute Lymphoblastic Leukemia.
Ann Pharmacother, 49(9):1057-1067, 03 Jun 2015
Cited by: 30 articles | PMID: 26041811
Review
Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia.
Cancer Sci, 111(4):1314-1323, 11 Feb 2020
Cited by: 7 articles | PMID: 31971321 | PMCID: PMC7156857

 1
1


