Abstract
Free full text

COVID-19-associated acute disseminated encephalomyelitis (ADEM)
Abstract
A 51-year-old woman with COVID-19 infection developed coma and an impaired oculocephalic response to one side. MRI of the brain demonstrated acute multifocal demyelinating lesions, and CSF testing did not identify a direct cerebral infection. High-dose steroids followed by a course of IVIG was administered, and the patient regained consciousness over the course of several weeks. As more patients reach the weeks after initial infection with COVID-19, acute disseminated encephalomyelitis should be considered a potentially treatable cause of profound encephalopathy or multifocal neurological deficits.
Dear Editor,
Neurological symptoms with SARS-CoV-2 infection (COVID-19) have been commonly encountered, but specific syndromes are only beginning to be described. Here, we report a patient with coma, an impaired unilateral oculocephalic response, and left hemiparesis. MRI, CSF, and her clinical course were consistent with acute disseminated encephalomyelitis (ADEM).
A 51-year-old woman presented to a local hospital with dyspnea, fever, and vomiting. She had no pertinent neurological history. She was febrile, tachycardic, and hypoxic. Chest X-ray revealed extensive patchy airspace opacification. SARS-CoV-2 PCR from a nasopharyngeal swab was positive. She was intubated, and was maintained on sedative drips. On hospital day 18, she was transferred to our facility due to persistent fever and failure to progress clinically.
Sedatives were held on arrival. Neurological exam was notable for unresponsiveness (GCS 3). Pupils were equal and reactive to light, corneal responses were intact, and the oculocephalic response to the left was impaired. Muscle tone was flaccid throughout, and the extremities did not move spontaneously or to noxious stimuli. Deep tendon reflexes were depressed, and plantar responses were mute.
An MRI of the brain done with and without gadolinium contrast on hospital day 24 showed scattered hyperintense lesions on FLAIR imaging in deep hemispheric and juxtacortical white matter. These lesions were hyperintense on diffusion weighted imaging (DWI), and a minority showed subtle restricted diffusion on the apparent diffusion coefficient (ADC), indicating acuity, but not consistent with infarction (Figs. 1, ,2).2). A FLAIR hyperintensity in the left frontal juxtacortical white matter showed mild enhancement with gadolinium contrast (Fig. 3). There was a small amount of intraventricular hemorrhage (IVH) layering in the occipital horns of both lateral ventricles. The Gradient Echo sequence did not show evidence of parenchymal hemorrhage.
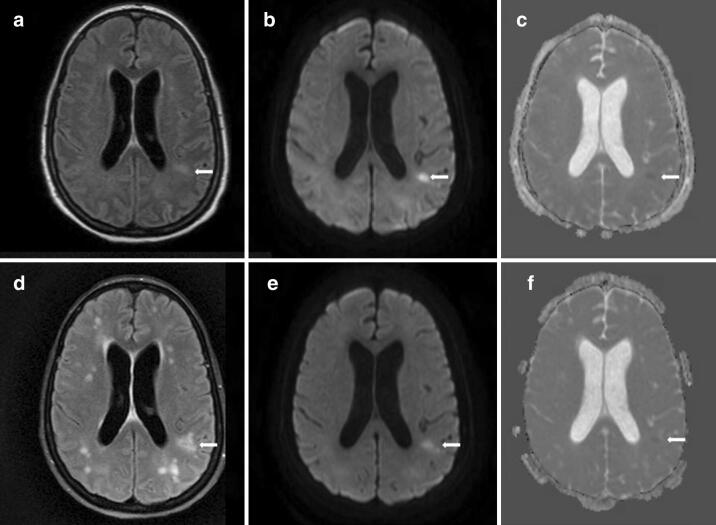
MRI brain on hospital day 29 showed FLAIR hyperintensities in the deep hemispheric, periventricular, and juxtacortical white matter (arrow, a), mostly hyperintense on diffusion weighted imaging (DWI) (arrow, b), and some show subtle restricted diffusion on apparent diffusion coefficient (ADC) imaging (arrow, c). Repeat MRI brain on hospital day 58 showed an increased number and distribution of FLAIR hyperintensities in the hemispheric white matter (arrow, d), with persistence of some previous lesions on DWI (arrow, e) and ADC (arrow, f), but no new lesions on these sequences
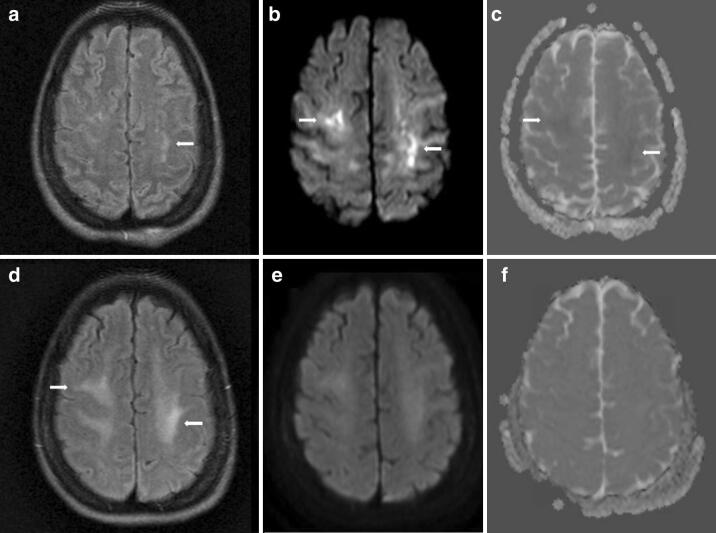
MRI brain on hospital day 24 showed small FLAIR hyperintensities in the juxtacortical white matter (arrow, a), more widespread hyperintensities on diffusion weighted imaging (DWI) (arrows, b), with subtle restricted diffusion on apparent diffusion coefficient (ADC) imaging (arrows, c). Repeat MRI brain on hospital day 58 showed poorly defined FLAIR hyperintensities in the juxtacortical white matter (arrow, d), with resolution of the signal abnormalities on DWI (e) and ADC (f) sequences
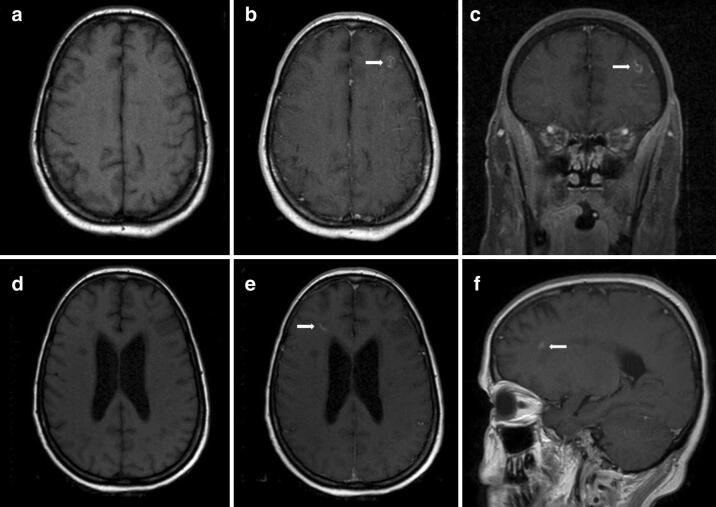
MRI brain on hospital day 24 showed a small contrast enhancing lesion (arrows) in the left frontal lobe at the grey-white interface (a T1 axial. b T1 axial post-gadolinium. c T1 coronal post-gadolinium). MRI brain on hospital day 58 showed a small contrast enhancing lesion (arrows) in the right frontal white matter (d T1 axial, e T1 axial post-gadolinium, f T1 sagittal post-gadolinium.)
Lumbar puncture was atraumatic. CSF analysis demonstrated 1 white blood cell, 2095 red blood cells with xanthochromia, 62 mg/dl protein, 56 mg/dl glucose. Bacterial culture, fungal culture, and a PCR panel (including HSV, VZV, EBV, and CMV) were negative. There were four oligoclonal bands, present in both serum and CSF. SARS-CoV-2 was not detected by qualitative PCR.
Renal function was normal throughout the hospital course. Transaminases were mildly elevated. CT angiogram of the Head and Neck was normal, excluding ruptured aneurysm as a cause of the IVH. Transthoracic echocardiogram and serum tests for ANA, ANCA, Syphilis, HIV, and Aquaporin 4 antibody were negative or normal. EEG demonstrated diffuse slowing.
Methylprednisolone 1 g IV daily for 5 days was administered for presumed ADEM. Exams with sedation held were grossly unchanged. Intravenous Immunoglobulin (IVIG) 0.4 g/kg daily was administered for 5 days starting on hospital day 31. Alertness improved gradually and on hospital day 36 she started to follow simple commands. Left hemiparesis became evident. On hospital day 39, she was extubated and was able to begin speaking, and on hospital day 59, she was fully oriented. MRI of the brain with and without gadolinium contrast was repeated on hospital days 29, 38, and 58 with an increase in the number and distribution of FLAIR lesions (Figs. 1, ,2)2) and a new right frontal enhancing lesion (Fig. 3).
ADEM is a rare demyelinating disease, often post-viral, and more commonly seen in children than adults. Clinically, it is heterogeneous, generally causing encephalopathy and multifocal deficits. MRI typically demonstrates FLAIR hyperintensities in deep white matter and at the grey/white matter interface. Post-contrast enhancement is not always present but is often punctate or rim enhancing. Diffusion restriction can be seen, especially early in the course of the disease [1, 2]. ADEM lesions can be hemorrhagic [3, 4], but the etiology of the intraventricular hemorrhage in this case is unclear. Anticoagulation had not been utilized, blood pressure had been well controlled, there was no laboratory evidence of coagulopathy, and she had not suffered trauma.
The location and evolution of lesions on MRI, sparse contrast enhancement, clinical exam, and CSF are all consistent with an acute demyelinating event, and the clinical situation in this case, weeks after an acute viral infection, is favorable for the development of ADEM. ADEM has been reported in a child following a coronavirus infection [5]. As more patients reach the weeks after initial infection with COVID-19, ADEM should be considered a potentially treatable cause of profound encephalopathy or multifocal neurological deficits.
Author contributions
TP MD, SB MD, CB DO, JG MD, HA MD, MT MD: drafted and revised the manuscript for intellectual content.
Funding
Not applicable.
Availability of data and material
Not applicable.
Compliance with ethical standards
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Not applicable.
Not applicable.
Consent to publish acquired from legal next of kin and the patient.
Not applicable.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00415-020-09951-9
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s00415-020-09951-9.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s00415-020-09951-9
Article citations
Cerebellar encephalitis and peripheral neuropathy with an atypical clinical and neuroimaging signature following Covid-19 vaccine: a report of two cases.
J Neurol, 271(7):4680-4684, 04 May 2024
Cited by: 0 articles | PMID: 38704487
Implementation of Multimodal Stimulation and Physical Therapy in Improving the Level of Consciousness and Recovery in Acute Disseminated Encephalomyelitis.
Cureus, 15(12):e51217, 28 Dec 2023
Cited by: 0 articles | PMID: 38288205 | PMCID: PMC10823210
The neurobiology of SARS-CoV-2 infection.
Nat Rev Neurosci, 25(1):30-42, 04 Dec 2023
Cited by: 9 articles | PMID: 38049610
Review
Radiologic-Pathologic Correlation of COVID-19-Associated Acute Disseminated Encephalomyelitis.
Cureus, 15(7):e42275, 21 Jul 2023
Cited by: 1 article | PMID: 37605696 | PMCID: PMC10440160
The Occurrence of Acute Disseminated Encephalomyelitis in SARS-CoV-2 Infection/Vaccination: Our Experience and a Systematic Review of the Literature.
Vaccines (Basel), 11(7):1225, 10 Jul 2023
Cited by: 7 articles | PMID: 37515041 | PMCID: PMC10385010
Review Free full text in Europe PMC
Go to all (140) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acute Demyelinating Encephalomyelitis (ADEM) in COVID-19 Infection: A Case Series.
Neurol India, 68(5):1192-1195, 01 Sep 2020
Cited by: 43 articles | PMID: 33109874
Spectrum of Neurological Manifestations in Covid-19: A Review.
Neurol India, 68(3):560-572, 01 May 2020
Cited by: 54 articles | PMID: 32643664
Review
Neurological Disorders Identified during Treatment of a SARS-CoV-2 Infection.
Intern Med, 59(17):2187-2189, 21 Jul 2020
Cited by: 11 articles | PMID: 32713924 | PMCID: PMC7516316
Acute disseminated encephalomyelitis after SARS-CoV-2 infection.
Neurol Neuroimmunol Neuroinflamm, 7(5):e797, 01 Jun 2020
Cited by: 115 articles | PMID: 32482781 | PMCID: PMC7286650

 1,2
1,2 



