Abstract
Background
The use of 12-core systematic prostate biopsy is associated with diagnostic inaccuracy that contributes to both overdiagnosis and underdiagnosis of prostate cancer. Biopsies performed with magnetic resonance imaging (MRI) targeting may reduce the misclassification of prostate cancer in men with MRI-visible lesions.Methods
Men with MRI-visible prostate lesions underwent both MRI-targeted and systematic biopsy. The primary outcome was cancer detection according to grade group (i.e., a clustering of Gleason grades). Grade group 1 refers to clinically insignificant disease; grade group 2 or higher, cancer with favorable intermediate risk or worse; and grade group 3 or higher, cancer with unfavorable intermediate risk or worse. Among the men who underwent subsequent radical prostatectomy, upgrading and downgrading of grade group from biopsy to whole-mount histopathological analysis of surgical specimens were recorded. Secondary outcomes were the detection of cancers of grade group 2 or higher and grade group 3 or higher, cancer detection stratified by previous biopsy status, and grade reclassification between biopsy and radical prostatectomy.Results
A total of 2103 men underwent both biopsy methods; cancer was diagnosed in 1312 (62.4%) by a combination of the two methods (combined biopsy), and 404 (19.2%) underwent radical prostatectomy. Cancer detection rates on MRI-targeted biopsy were significantly lower than on systematic biopsy for grade group 1 cancers and significantly higher for grade groups 3 through 5 (P<0.01 for all comparisons). Combined biopsy led to cancer diagnoses in 208 more men (9.9%) than with either method alone and to upgrading to a higher grade group in 458 men (21.8%). However, if only MRI-target biopsies had been performed, 8.8% of clinically significant cancers (grade group ≥3) would have been misclassified. Among the 404 men who underwent subsequent radical prostatectomy, combined biopsy was associated with the fewest upgrades to grade group 3 or higher on histopathological analysis of surgical specimens (3.5%), as compared with MRI-targeted biopsy (8.7%) and systematic biopsy (16.8%).Conclusions
Among patients with MRI-visible lesions, combined biopsy led to more detection of all prostate cancers. However, MRI-targeted biopsy alone underestimated the histologic grade of some tumors. After radical prostatectomy, upgrades to grade group 3 or higher on histopathological analysis were substantially lower after combined biopsy. (Funded by the National Institutes of Health and others; Trio Study ClinicalTrials.gov number, NCT00102544.).Free full text

MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis
Abstract
BACKGROUND
The use of 12-core systematic prostate biopsy is associated with diagnostic inaccuracy that contributes to both overdiagnosis and underdiagnosis of prostate cancer. Biopsies performed with magnetic resonance imaging (MRI) targeting may reduce the misclassification of prostate cancer in men with MRI-visible lesions.
METHODS
Men with MRI-visible prostate lesions underwent both MRI-targeted and systematic biopsy. The primary outcome was cancer detection according to grade group (i.e., a clustering of Gleason grades). Grade group 1 refers to clinically insignificant disease; grade group 2 or higher, cancer with favorable intermediate risk or worse; and grade group 3 or higher, cancer with unfavorable intermediate risk or worse. Among the men who underwent subsequent radical prostatectomy, upgrading and downgrading of grade group from biopsy to whole-mount histopathological analysis of surgical specimens were recorded. Secondary outcomes were the detection of cancers of grade group 2 or higher and grade group 3 or higher, cancer detection stratified by previous biopsy status, and grade reclassification between biopsy and radical prostatectomy.
RESULTS
A total of 2103 men underwent both biopsy methods; cancer was diagnosed in 1312 (62.4%) by a combination of the two methods (combined biopsy), and 404 (19.2%) underwent radical prostatectomy. Cancer detection rates on MRI-targeted biopsy were significantly lower than on systematic biopsy for grade group 1 cancers and significantly higher for grade groups 3 through 5 (P<0.01 for all comparisons). Combined biopsy led to cancer diagnoses in 208 more men (9.9%) than with either method alone and to upgrading to a higher grade group in 458 men (21.8%). However, if only MRI-target biopsies had been performed, 8.8% of clinically significant cancers (grade group ≥3) would have been misclassified. Among the 404 men who underwent subsequent radical prostatectomy, combined biopsy was associated with the fewest upgrades to grade group 3 or higher on histopathological analysis of surgical specimens (3.5%), as compared with MRI-targeted biopsy (8.7%) and systematic biopsy (16.8%).
CONCLUSIONS
Among patients with MRI-visible lesions, combined biopsy led to more detection of all prostate cancers. However, MRI-targeted biopsy alone underestimated the histologic grade of some tumors. After radical prostatectomy, upgrades to grade group 3 or higher on histopathological analysis were substantially lower after combined biopsy.
THE AGGRESSIVENESS OF PROSTATE CAN-cers ranges from indolent to highly lethal. Low-grade prostate cancer (i.e., grade group 1) has been shown in large trials to be associated with a very low risk of cancer-specific death.1–5 In contrast, cancers of grade groups 3 through 5 have significantly higher metastatic potential and were responsible for the majority of the predicted 31,620 deaths from prostate cancer in the United States in 2019.6,7 This variation in lethality of prostate cancer subtypes highlights the importance of accurate prostate cancer diagnosis.
Currently, the transrectal, ultrasonographically guided, 12-core systematic biopsy is the most common method for the initial diagnosis and grading of prostate cancer.8 Whereas in most other cancers diagnostic biopsies target abnormalities detected on imaging or physical examination, systematic prostate biopsy provides a nontargeted, systematically spaced sampling of the prostate gland. This systematic-biopsy method is associated with missed cancer diagnoses and substantial grade misclassification at the time of biopsy. In addition, further upgrading or downgrading of the cancer diagnosis at the time of radical prostatectomy is common.9–12 One consequence of this diagnostic inaccuracy is overtreatment of patients with low-grade disease because of concern that a high-grade cancer may have been missed. These uncertainties contribute to findings that 43% of prostatectomies are performed in men who are subsequently confirmed to have indolent disease on histopathological analysis and that 60% of men who receive radical therapy (i.e., radiation or radical prostatectomy) are found to have grade group 1 cancers on preoperative biopsy.13 Conversely, when aggressive disease is missed on biopsy, patients risk undertreatment.
Advances in prostate multiparametric magnetic resonance imaging (MRI) have allowed for MRI-targeted biopsies of suspicious imaging findings.14–16 Studies have shown that MRI-targeted biopsies result in a higher rate of detection of high-grade cancers than systematic biopsy.14,17–19 However, despite the improved detection of clinically significant cancers with MRI-targeted biopsies, debate persists about whether MRI-targeted biopsy should be used in place of systematic biopsy or in conjunction with it.18,20,21 Specifically, controversy exists regarding whether the systematic biopsy should still be performed and whether previous biopsy status should affect the type of biopsy method that is selected.22–24 The Trio Study was a substudy of a larger clinical trial, called Use of Tracking Devices to Locate Abnormalities During Invasive Procedures. In this substudy, we assessed the use of MRI-targeted, systematic, or combined prostate biopsy in an attempt to define the most effective method for prostate cancer diagnosis.
METHODS
STUDY DESIGN
In 2007, we initiated this clinical study at the National Cancer Institute (NCI) to evaluate the use of electromagnetic tracking devices for MRI-targeted biopsies. The electromagnetic tracking device was developed as part of a cooperative research-and-development agreement between the National Institutes of Health (NIH) and Philips and is commercially available as the UroNav platform.
Adult men (≥18 years of age) who had an elevated serum prostate-specific antigen (PSA) level or an abnormal digital rectal examination were eligible to undergo prostate MRI. Patients who were found to have a prostate lesion on MRI and who consented to undergo a prostate biopsy were eligible for enrollment. Exclusion criteria included previous treatment for prostate cancer, the absence of MRI-visible prostate lesions, or an inability to undergo MRI (i.e., body habitus incompatible with MRI equipment, presence of ferrous metallic implants, or claustrophobia). All the patients provided written informed consent.
MRI PROTOCOL
MRIs were performed with the use of a 3-tesla MRI (Achieva, Philips) with an endorectal coil (BPX-30, Medrad, Bayer) for all initial scans. The use of an endorectal coil was omitted for rare contraindications such as latex allergy, anal fistula, active hemorrhoids, or absence of a rectum.
All MRIs were reviewed by one of two expert genitourinary radiologists with more than a decade of experience in reviewing MRIs of the prostate. T2-weighted, contrast-enhanced, and diffusion-weighted series were obtained, as described previously.25 MRI lesions were given a Prostate Imaging Reporting and Data System (PI-RADS) score of 1 to 5 (with higher scores indicating more clinically suspicious lesions) to stratify the risk of prostate cancer (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).15 Before the adoption of the PI-RADS scoring system in April 2015, we used a 5-point NIH-developed scoring system that has been shown to correlate with PI-RADS scores.26–28 PI-RADS scores were subsequently reported for all patients. Before prostate biopsies, lesions that had been identified were labeled for biopsy by the radiologists with the use of the DynaCAD (Philips) software. A maximum of five targets for biopsy were labeled for each patient. If a lesion extended into a second prostate segment (as defined by prostate segmentation used in systematic biopsy) or crossed the midline, a second target was placed on that lesion, as described previously.29
PROSTATE BIOPSY PROTOCOL
All patients underwent both MRI-targeted and systematic biopsies at a single institution. UroNav Fusion Biopsy System (Philips) was used to superimpose labeled T2-weighted MRI images over real-time prostate ultrasonographic scans, thereby facilitating MRI identification and targeting of lesions. Two biopsy cores of each targeted lesion were obtained with the use of an end-fire transrectal ultrasonographic probe (Philips) and software guidance by the UroNav device. The MRI overlay targets were then removed from the ultrasonographic scan, and a second physician performed a 12-core systematic extended sextant biopsy with only ultrasonographic guidance. Systematic biopsies were performed with the use of standard segmentation to acquire medial and lateral cores from each sextant prostate region.30 In the event that physicians noticed targeting information (i.e., hemorrhage tracts from previous MRI-targeted biopsies) at the time of systematic biopsies, they were instructed to ignore such information during the procedure. Biopsies were performed by urologists, radiologists, or both. The radiologist who interpreted the MRI and assigned lesions for targeting was never the same person who performed biopsies.
If patients underwent multiple biopsies, only the results of the first combined biopsy (i.e., a combination of the MRI-targeted and systematic methods) were included in the biopsy cohorts. All biopsies were classified according to grade group31 on a scale ranging from 1 to 5, with a higher score indicating more severe disease; biopsy results were reported in accordance with the recommendations of the START (Standards of Reporting for MRI-Targeted Biopsy Studies) Consortium.32 A single, highly experienced genitourinary pathologist interpreted all the biopsy specimens and whole-mount histopathological slides. We recorded the highest Gleason score that was detected by each biopsy method and coded it according to grade group.33–35
DEFINITIONS OF TERMS
For the purpose of our study, clinically insignificant disease was defined as grade group 1 (Gleason score, 3 + 3 = 6). Clinically significant cancer was defined as grade group 3 (Gleason score, 4 + 3 = 7; unfavorable intermediate risk) or higher, although the detection of grade group 2 (Gleason score, 3 + 4 = 7; favorable intermediate risk) or higher is also reported in all tables and figures, since some physicians consider this threshold to be more clinically relevant than grade group 3 or higher. Gleason scores range from 6 (lowest grade of cancer) to 10 (highest grade) and are reported as the sum of the primary and secondary Gleason grades, which are defined as the grade of the most common cancer (first number) and the highest grade of cancer (second number) detected within a given biopsy core.
Throughout this article, the use of MRI-targeted biopsy and systematic biopsy in the same clinical setting is referred to as “combined biopsy.” If a grade group 1 cancer was detected by the addition of a second biopsy method in a patient who had otherwise been found to have no cancer, the lesion was defined as a new grade group 1 cancer detection. Additional detection of cancer in grade group 2 or higher or grade group 3 or higher was defined as the number of upgrades to those grade groups detected by the addition of the listed biopsy method.
Among the patients who underwent radical prostatectomy, we assessed upgrading or downgrading by comparing the grade group determined on prostate biopsy with the grade group determined on whole-mount histopathological analysis after surgery. Upgrading of a cancer to grade group 2 or higher was defined as a transition from grade group 0 or 1 on biopsy to grade group 2 through 5 on whole-mount histopathological analysis; upgrading of a cancer to grade group 3 or higher was defined as a transition from grade group 0 through 2 on biopsy to grade group 3 through 5 on whole-mount histopathological analysis.
PROSTATECTOMY COHORT
Among the patients in whom prostate cancer was diagnosed, various treatment options were offered. These options included active surveillance, prostatectomy, external-beam radiation, focal therapy, or enrollment in a separate clinical trial. For the patients who underwent radical prostatectomy, we correlated the grade group as determined on whole-mount histopathological analysis with the biopsy findings. In this analysis, we excluded patients who had undergone biopsy more than 1 year before surgery or who had received treatment (including radiation, focal therapy, hormonal therapy, or treatment administered as part of a clinical trial) before radical prostatectomy.
DATA ANALYSIS
A data manager collected data in a prospective manner as part of a pretrial-designed database. Data collection began in July 2007 and was suspended in January 2019 for data analysis. The highest grade group that was detected by each biopsy method was recorded, and the highest grade group that was detected by either biopsy method was considered to be the grade group detected on combined biopsy. Among the patients who underwent repeat biopsies at the NCI according to the protocol (available at NEJM.org), only data from the initial biopsy were included for the biopsy cohorts. If patients had undergone multiple prostate biopsies, the biopsy information that had been obtained closest to the surgery was used in the data analysis in the prostatectomy cohort.
OUTCOMES
The primary outcomes were cancer detection rates according to grade group (1 through 5) for each biopsy method. Key secondary outcomes were the rates of cancer detection in grade groups 2 and 3 or higher, cancer detection rates stratified according to previous biopsy status, and rates of cancer reclassification between biopsy and whole-mount histopathological analysis after radical prostatectomy.
STATISTICAL ANALYSIS
The primary hypothesis was that MRI-targeted biopsy would lead to a higher rate of detection of prostate cancers in grade groups 2 through 5 and a lower rate of detection of grade group 1 cancers than the use of systematic biopsy. For the primary analysis, we used McNemar’s test to compare the cancer detection rates between MRI-targeted biopsy and systematic biopsy according to cancer grade group. We used the adjusted Wald interval to calculate confidence intervals for cancer detection rates and the differences in cancer detection rates between the two biopsy methods.36,37 A Bonferroni correction was used to adjust for multiple comparisons of the primary and secondary outcomes, with statistical significance indicated by P<0.01 for the primary outcome and P<0.006 for the secondary outcomes. Some additional statistical analyses were performed post hoc during the peer review and revision process. A detailed description of the statistical methods and corrections for multiple comparisons is provided in the Supplementary Appendix and the study protocol. We did not perform statistical tests to compare cancer detection rates for combined biopsy with each of its constituent biopsy techniques, since the cancer detection rate was defined as the highest grade group detected by the two biopsy techniques. Some of the data that are included in this study have been reported previously.14
RESULTS
PATIENTS
From June 2007 through January 2019, a total of 2732 men underwent prostate MRI. Of these patients, 2180 had MRI-visible lesions and underwent combined MRI-targeted and systematic biopsies in the same clinical setting. Among the patients who underwent biopsy, 77 were excluded from the analysis because they had undergone previous treatment. The remaining 2103 men were included in the analysis (Fig. 1). The majority (79.3%) of men who were enrolled in this study had undergone at least one biopsy at an outside institution before study enrollment (Table 1). Of the men who were included in the analysis, prostate cancer was diagnosed in 1312 (62.4%). Of the men who received a cancer diagnosis, 404 subsequently underwent radical prostatectomy at our institution. The median time between prostate biopsy and radical prostatectomy was 98 days (interquartile range, 74 to 134).
All 2103 men who were included in the primary analysis underwent two methods of prostate biopsy: one that targeted lesions with the use of magnetic resonance imaging (MRI) and one that systematically removed 12 biopsy cores with ultrasonographic guidance (systematic biopsy). Rates of cancer detection by each of these methods and in combination (combined biopsy) were included in the primary analysis. Among the patients in whom prostate cancer was diagnosed, various treatment options were offered, including active surveillance, prostatectomy, external-beam radiation, and focal therapy. Among the patients who underwent radical prostatectomy, the investigators correlated the initial biopsy findings with the cancer grade group as determined on whole-mount histopathological analysis after surgery.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | All Patients (N = 2103) | Previous Biopsy (N = 1667) | No Previous Biopsy (N = 436) | Prostatectomy (N = 404) |
|---|---|---|---|---|
| Age — yr | 63.3±7.6 | 63.4±7.4 | 63.0±8.2 | 62.0±7.6 |
| Race or ethnic group — no. (%)† | ||||
 White White | 1653 (78.6) | 1311 (78.6) | 342 (78.4) | 300 (74.3) |
 Black Black | 289 (13.7) | 237 (14.2) | 52 (11.9) | 78 (19.3) |
 Asian Asian | 75 (3.6) | 60 (3.6) | 15 (3.4) | 11 (2.7) |
 Hispanic Hispanic | 23 (1.1) | 14 (0.8) | 9 (2.1) | 3 (0.7) |
 Other Other | 39 (1.9) | 30 (1.8) | 9 (2.1) | 7 (1.7) |
 Unknown Unknown | 24 (1.1) | 15 (0.9) | 9 (2.1) | 5 (1.2) |
| Prostate-specific antigen — ng/ml‡ | ||||
 Median (IQR) Median (IQR) | 6.7 (4.6–10.2) | 7 (4.8–10.8) | 5.5 (3.9–8.1) | 6.9 (4.8–11.6) |
 Maximum Maximum | 231.6 | 231.6 | 113.6 | 101.7 |
| Tumor stage — no. (%)§ | ||||
 No cancer No cancer | 791 (37.6) | 639 (38.3) | 152 (34.9) | NA |
 Any cancer Any cancer | 1312 (62.4) | 1028 (61.7) | 284 (65.1) | 404 (100) |
   T1c T1c | 1127 (53.6) | 907 (54.4) | 220 (50.5) | NA |
   T2 T2 | NA | NA | NA | 334 (82.7) |
   T2a T2a | 155 (7.4) | 104 (6.2) | 51 (11.7) | NA |
   T2b T2b | 13 (0.6) | 8 (0.5) | 5 (1.1) | NA |
   T2c T2c | 16 (0.8) | 9 (0.5) | 7 (1.6) | NA |
   T3a T3a | 0 | 0 | 0 | 43 (10.6) |
   T3b T3b | 0 | 0 | 0 | 22 (5.4) |
   T4 T4 | 1 (<0.1) | 0 | 1 (0.2) | 3 (0.7) |
| Previous biopsy result — no. (%) | ||||
 No previous biopsy No previous biopsy | 436 (20.7) | NA | 436 (100) | 102 (25.2) |
 Negative Negative | 873 (41.5) | 873 (52.4) | NA | 98 (24.3) |
 Positive Positive | 794 (37.8) | 794 (47.6) | NA | 204 (50.5) |
| Prostate volume on MRI — cm3‡ | ||||
 Median (IQR) Median (IQR) | 51 (37–71) | 52 (38–74) | 45 (36–61) | 40 (31–53) |
 Maximum Maximum | 420 | 420 | 155 | 200 |
| No. of visible targets per prostate on MRI | 2.5±1.3 | 2.4±1.2 | 2.7±1.4 | 2.9±1.4 |
| Patients with available PI-RADS score — no. (%)¶ | 723 (34.4) | 538 (32.3) | 185 (42.4) | 149 (36.9) |
 Score on PI-RADS — no./total no. (%) Score on PI-RADS — no./total no. (%) | ||||
   1 1 | 0 | 0 | 0 | 0 |
   2 2 | 51/723 (7.1) | 41/538 (7.6) | 10/185 (5.4) | 4/149 (2.7) |
   3 3 | 87/723 (12) | 66/538 (12.3) | 21/185 (11.4) | 9/149 (6.0) |
   4 4 | 345/723 (47.7) | 257/538 (47.8) | 88/185 (47.6) | 68/149 (45.6) |
   5 5 | 240/723 (33.2) | 174/538 (32.3) | 66/185 (35.7) | 68/149 (45.6) |
| No. of cores on MRI-targeted biopsy | 4.9±2.5 | 4.8±2.4 | 5.4±2.8 | 5.8±2.7 |
| No. of samples on systematic biopsy | 12.1±0.6 | 12.1±0.6 | 12.2±0.8 | 12.1±0.5 |
| No. of positive samples on MRI-targeted biopsy | 1.7±2.3 | 1.5±2.1 | 2.3±2.8 | 3.2±2.3 |
| No. of positive samples on systematic biopsy | 1.8±2.6 | 1.6±2.3 | 2.6±3.1 | 3.2±2.5 |
ADDITIONAL CANCER DETECTION WITH COMBINED BIOPSY
Among all 2103 patients who underwent the two biopsy methods, prostate cancer was diagnosed in 1104 patients (52.5%) with systematic biopsy alone and in 1084 patients (51.5%) with MRI-targeted biopsy alone. The use of MRI-targeted biopsy led to more diagnoses of cancers in grade groups 3, 4, and 5 than systematic biopsy (P = 0.004, P<0.001, and P = 0.003, respectively) and fewer cancers in grade group 1 (P<0.001) (Fig. 2 and Table S2). The addition of MRI-targeted biopsy to systematic biopsy led to 208 (9.9%) more prostate cancer diagnoses (Fig. 3). Of these new diagnoses, 59 (28.4%) were clinically significant (grade group ≥3) disease. The addition of MRI-targeted biopsy led to a reduction of 60 patients (from 454 to 394) who were classified as having clinically insignificant (grade group 1) cancer (Fig. 2). Specifically, 134 men in whom grade group 1 cancer was diagnosed on systematic biopsy were upgraded to grade group 2 or higher on MRI-targeted biopsy (Fig. 3). Simultaneously, MRI-targeted biopsy led to 74 new grade group 1 cancer diagnoses among men in whom no cancer was detected on systematic biopsy, which led to a net reduction of 60 patients with a grade group 1 cancer diagnosis. In total, MRI-targeted biopsy was responsible for upgrading of events in 458 patients (21.8%) when added to systematic biopsy (blue-shaded areas in Fig. 3).
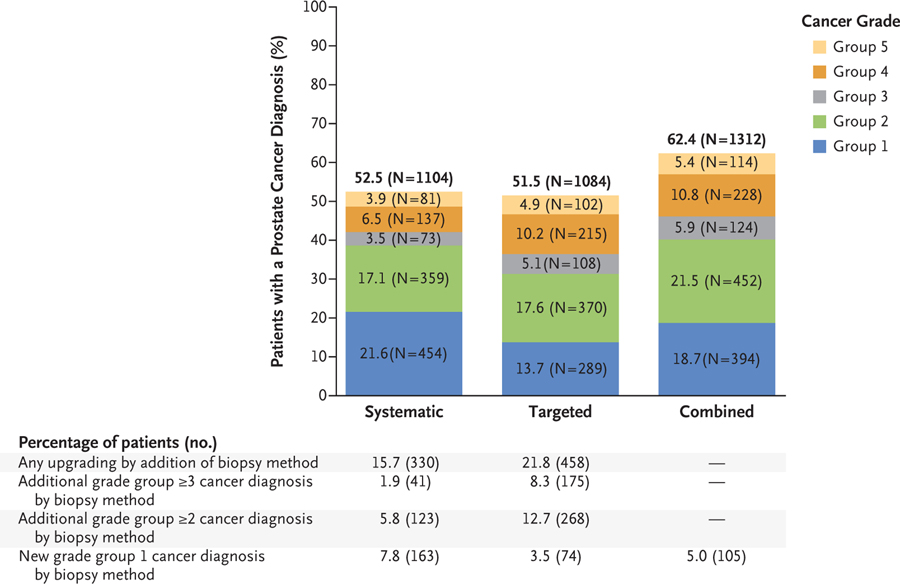
Shown are the total numbers and percentages of cancers that were detected by systematic biopsy, MRI-targeted biopsy, and a combination of the two methods in each of the five grade groups among the 2103 patients who were included in the primary analysis. The difference in the rates of cancer detection between systematic biopsy and MRI-targeted biopsy were significant for grade group 1 (P<0.001), group 3 (P = 0.004), group 4 (P<0.001), and group 5 (P = 0.003). A P value of less than 0.01 was considered to indicate statistical significance after the use of the Bonferroni correction to account for the five primary grade groups. MRI-targeted biopsy led to the detection of significantly more cancers than systematic biopsy in groups 3, 4, and 5. Systematic biopsy led to the detection of significantly more cancers than MRI-targeted biopsy in group 1. Also shown are the numbers and percentages of patients who received an upgraded diagnosis on the basis of the biopsy method that was used. When cancer detection rates were clustered in grade group 2 or higher or in grade group 3 or higher, MRI-targeted biopsy showed a higher rate of cancer detection than systematic biopsy. Newly detected cancers in grade group 1 were seen with the combination of all biopsy methods but were most pronounced with the use of systematic biopsy alone. In the combined-biopsy group, a new diagnosis of grade group 1 cancer (in 105 patients [5.0%]) was defined as the total number of grade group 1 cancer diagnoses on combined biopsy (in 394 patients) minus all grade group 1 diagnoses on targeted biopsy alone (in 289 patients). Details regarding the statistical analysis are provided in the Supplementary Appendix.
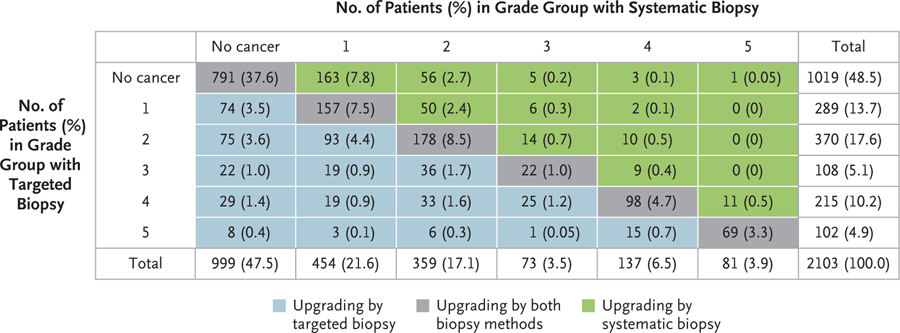
Shown are the numbers and percentages of the 2103 men who were included in the primary analysis in whom no prostate cancer was diagnosed or in whom prostate cancer was diagnosed (grade groups 1 through 5) on systematic biopsy or MRI-targeted biopsy. The areas that are shaded in gray indicate the men in whom systematic biopsy and targeted biopsy detected cancer of the same grade group. The areas that are shaded in blue indicate the men who were found to have a cancer in a higher grade group on MRI-targeted biopsy, and the areas that are shaded in green indicate the men who were found to have cancer in a higher grade group on systematic biopsy.
MRI-targeted biopsy alone detected clinically significant cancers (grade group ≥3) in 425 of 466 patients (91.2%) in whom cancer was detected by combination biopsy. However, MRI-targeted biopsy alone without systematic biopsy would have led to no detection of cancers of grade group 2 or higher in 123 patients (5.8%) and no detection of cancers of grade group 3 or higher in 41 patients (1.9%) (Fig. 2). Similarly, omission of the systematic biopsy would not have resulted in the reclassification to higher-risk disease in 330 patients (15.7%) (green-shaded area in Fig. 3). Of the 466 patients with cancers of grade group 3 or higher that were detected on combined biopsy, 175 (37.6% of patients with grade group 3 or higher or 8.3% of all the patients who underwent biopsy) were detected by MRI-targeted biopsy only, and 41 (8.8% of patients with grade group 3 or higher or 1.9% of all the patients who underwent biopsy) were detected by systematic biopsy only (P<0.001) (Fig. 2).
Small differences were noted between the patients who had undergone previous biopsy and those who had not with respect to rates of detection of additional clinically significant cancers (grade group ≥3) on MRI-targeted biopsy (difference, −0.7 percentage points; 95% confidence interval [CI], −3.4 to 2.5) and on systematic biopsy (difference, −0.4 percentage points; 95% CI, −1.7 to 1.1) (Table S3).
CORRELATION WITH WHOLE-MOUNT HISTOPATHOLOGICAL ANALYSIS
Among the patients who underwent radical prostatectomy, upgrading on histopathological analysis after undergoing combined biopsy occurred in 58 of 404 patients (14.4%). Of these 58 patients, 14 (3.5% of those who underwent radical prostatectomy) were upgraded to clinically significant disease (grade group ≥3). The rates of any upgrading or clinically significant upgrading on whole-mount histopathological analysis were substantially higher for systematic biopsy (41.6% and 16.8%, respectively) and MRI-targeted biopsy (30.9% and 8.7%, respectively) than for combined biopsy (14.4% and 3.5%, respectively) (Fig. 4). Differences in rates of upgrading between systematic and targeted biopsy were significant (P≤0.002 for all comparisons) (Table S5).
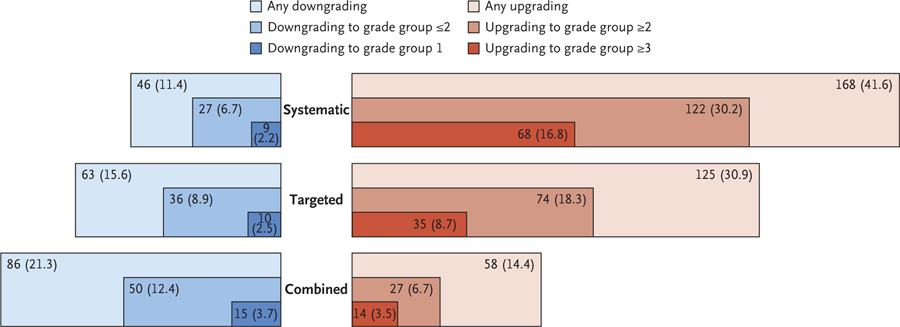
Among the 404 men who underwent radical prostatectomy, shown are the numbers and percentages of those in whom the grade group of prostate cancer was downgraded or upgraded after whole-mount histopathological analysis of surgical specimens according to the biopsy method that was used. The areas that are shaded in blue indicate the downgrading of events from biopsy to whole-mount analysis, and the areas shaded in red indicate the upgrading of events. Darker colors represent more extreme levels of downgrading or upgrading. The lowest percentage of upgrading events was seen with combined biopsy (14.4%) and the highest with systematic biopsy (41.6%). Differences in rates of upgrading between systematic and MRI-targeted biopsy were significant for upgrading of any grade group (P = 0.002), upgrading to grade group 2 or higher (P<0.001), and upgrading to grade group 3 or higher (P<0.001). The differences in rates of downgrading were not significant for any grouping. A P value of less than 0.006 was considered to indicate statistical significance with the use of the Bonferroni correction to account for the eight secondary outcomes. Results of the statistical analyses are shown in Table S5.
Downgrading to clinically insignificant cancer (grade group 1) on whole-mount histopathological analysis was uncommon regardless of biopsy method. However, such downgrading was most common among the patients who underwent combined biopsy (3.7%) (Fig. 4). The differences in downgrading rates to clinically insignificant disease between systematic biopsy and targeted biopsy were not significant (2.2% and 2.5%, respectively; P = 1.00) (Table S5). Cross-tabulations showing rates of cancer detection according to biopsy method against whole-mount histopathological analysis are provided in Table S4.
DISCUSSION
The current approach to prostate cancer diagnosis is characterized by a considerable degree of diagnostic uncertainty. This uncertainty has contributed to both overtreatment and undertreatment and has left the medical community uncertain of the most effective method for diagnosing prostate cancer. With the addition of MRI-targeted biopsy to systematic biopsy, we may have entered an era of increased diagnostic certainty in prostate cancer. In this study, we found that combined biopsy leads to an increase in the number of cancer diagnoses and improves the likelihood that the biopsy findings are predictive of the true pathologic nature of the patient’s disease. Patients who are found to have grade group 2 prostate cancer on combined biopsy have only a small chance of having clinically significant disease of grade group 3 or higher. This knowledge should reduce the risks of both overtreatment and undertreatment out of fear of misdiagnosis.
An alternative perspective may argue for the use of MRI-targeted biopsy alone, since it is responsible for the detection of a majority of clinically significant cancers, requires 12 fewer biopsy cores, and leads to 5% fewer diagnoses of clinically insignificant cancers. However, we found that the omission of systematic biopsy would lead to missing 1.9% more grade group 3 cancers and 5.8% more grade group 2 cancers in our study population. More important, among the patients in whom prostate cancer is diagnosed, the use of MRI-targeted biopsy alone leads to high diagnostic uncertainty, since this method used in isolation is associated with a 30.9% rate of any upgrading of the cancer group and an 8.7% rate of upgrading the cancer to a clinically significant grade group on whole-mount histopathological analysis. Therefore, although combined biopsy resulted in a small net increase in the diagnosis of indolent cancers, its high predictive value for a patient’s true pathological grade group reduces the likelihood of misdiagnosis and should translate into decreased diagnostic uncertainty. With decreased diagnostic uncertainty, both overtreatment and undertreatment should be reduced.
Several earlier studies have shown that MRI-targeted biopsy outperforms systematic biopsy in the diagnosis of clinically significant cancer.17,20,38 However, few of the previous trials have shown whether systematic biopsy can be entirely omitted17,39 and lacked a reference test against which to assess the various biopsy methods. Rouvière et al.18 assessed combined biopsy as a possible improvement in diagnostic method; however, the investigators did not routinely use MRI-targeted biopsy software or 3.0-tesla MRI scanners, which led to some uncertainty regarding their conclusions. Controversy remains regarding whether the use of targeting software results in better cancer detection than cognitive fusion.18,40,41 In addition, Rouvière et al. also lacked a reference test, such as whole-mount histopathological analysis. Our data, which represent a substantially larger population, show improved cancer detection with combined biopsy and correlation with whole-mount histopathological analysis. Potentially, these data may usher in a new era of increased confidence in the selection of prostate cancer treatment on the basis of biopsy results. Future research may define pre-biopsy measures under which selected patients may undergo MRI-targeted biopsy only.
Our study has several strengths, including the enrollment of patients who had undergone previous biopsy and those who had not, which allowed for comparisons between these two subgroups. By design, all the patients underwent all available diagnostic methods, which reduced the possibility of selection bias. In addition, the definition of clinically significant cancer as grade group 3 or higher represents a liberal threshold. If grade group 2 or higher had been used as a threshold for clinically significant disease, combined biopsy would have resulted in the detection of even more clinically significant cancers (Table S2). Finally, this study assessed each diagnostic method against the standard of whole-mount histopathological analysis among the men who underwent radical prostatectomy.
However, our study also has several limitations. It was performed at an institution where many practitioners were experienced in performing and interpreting prostate MRI and prostate histopathological analysis. These results may not be reproducible at institutions with less experienced practitioners.42 In our study, if any MRI scans were incorrectly labeled as normal or abnormal, such errors could have led to bias. Also, since MRI-targeted biopsies were performed before systematic biopsies, it is possible that MRI information, such as hemorrhage tracks, might have influenced the performance of systematic biopsies. Furthermore, the use of one physician to perform the systematic biopsy and another to perform the MRI-directed biopsy is not representative of actual practice patterns. The study focused specifically on patients with MRI-visible lesions, so these findings are not applicable to patients with normal results on prostate MRI. In addition, radical prostatectomy was not performed in all the patients with a prostate cancer diagnosis, which created the possibility of selection bias in the prostatectomy cohort. All our patients were referrals, and the referral patterns of our institution may not reflect the pattern of disease in the community. Finally, our study was conducted in a single institution, a factor that may limit its generalizability.
Among patients with MRI-visible prostate lesions, the addition of MRI-targeted biopsy to systematic biopsy increased the detection of clinically significant cancers (grade group ≥3) and led to a net decrease in the detection of clinically insignificant cancers. Although many of these benefits resulted from MRI-targeted biopsy alone, omission of systematic biopsy would have led to missing the diagnosis of 8.8% of clinically significant cancers. Furthermore, among the patients who underwent subsequent radical prostatectomy, combined biopsy was associated with the lowest rate of upgrading of the cancer grade group between biopsy and whole-mount histopathological analysis. Collectively, these findings suggest that combined biopsy provides improved diagnostic accuracy over either systematic or MRI-targeted biopsy alone and better predicts the results of final histopathological analysis.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the views of the U.S. government nor do they reflect any official recommendation or endorsement of the National Institutes of Health (NIH).
Supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, NIH Clinical Center, and NIH Center for Interventional Oncology; by Philips, which provided materials and technical support as part of a Cooperative Research and Development Agreement; and by a research grant (to Dr. Mehralivand) from the Dr. Mildred Scheel Foundation for Cancer Research.
Dr. Choyke reports participating in a cooperative research agreement with Philips and holding the following patents, licensed to Philips, with royalties paid to Dr. Choyke through the NIH: patent 10,215,830 on computer-aided diagnostics and patent 8731264 on a MRI–ultrasound fusion biopsy system; Dr. Turkbey, participating in a cooperative research-and-development agreement with Nvidia and Philips, with interest held by the National Institutes of Health (NIH), and holding patent 10,215,830 on software-aided detection of prostate cancer, licensed to Philips, with royalties paid to Dr. Turkbey through the NIH; Dr. Wood, receiving grant support, paid to the NIH, from Biocompatibles UK, Boston Scientific, Celsion, Nvidia, Philips, Siemens Medical Solutions, and XACT Robotics, holding a pending patent (20-190-254624) on a robotic-assisted prostate surgery device, holding a pending patent (20-190-175214) on transperineal imaging-guided prostate needle placement, holding patent 9655595 on a system, method, and device for prostate diagnosis and intervention, and holding the following patents, all licensed to Philips with royalties paid to Dr. Wood through the NIH: patent 10-423757 on a system and method for probabilistic ablation planning, patent 10-2238921 on a feedback system for integrating planning and navigation, patent US-9144461-B2 on a feedback system for integrating interventional planning and navigation, patent 8731264 on a system and method for fusing real-time ultrasound images with preacquired medical images, patent CN10-2481115 on a system and method for integrated biopsy and therapy, patent EP-2291136 on a system for performing biopsies, patent CA2647432 on a system, methods, and instrumentation for image-guided prostate treatment, patent EP20-0-1363 on a system and instrumentation for image-guided prostate treatment, patent JP5587993 on a system and method for integrated biopsy and therapy, patent RU2558521 on a system and method for integrated biopsy and therapy, patent 8,447,384 on a method and system for performing biopsies, patent 8948845 on a system, methods, and instrumentation for image-guided prostate analysis, patent 10-290-076 on a system and method for automated initialization and registration of navigation systems, patent US-10,215,830 on automated cancer detection using MRI, patent 1937153 on a device and method for a trackable ultrasound, patent 60200-900-6640.4 on a system for performing biopsies, patent 61/119,464 on a feedback system for integrated interventional planning and navigation, patent 2373241 on a feedback system for integrating interventional planning and navigation, pending patent 12/5164 on a system and method for fusing real-time freehand ultrasound with preacquired medical images, pending patents US20-120-071749 and PCT/IB-20-10/052152 on a system and method for integrated biopsy and therapy, patent 20-120-6592 on a system for performing biopsies, patent 9398892 on a device and method for a trackable ultrasound, and patent 1937153 on a device and method for a trackable ultrasound; and Dr. Pinto, holding the following patents, licensed to Philips, with royalties paid to Dr. Pinto through the NIH: patent US8,447,384 on a method and system for performing biopsies and patent 10,215,830 on automated cancer detection using MRI.
Funded by the National Institutes of Health and others; Trio Study ClinicalTrials.gov number, NCT00102544.
Footnotes
A complete list of members of the Trio Study Group is provided in the Supplementary Appendix, available at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
Citations & impact
Impact metrics
Citations of article over time
Article citations
Prostate cancer lesions in transition zone exhibit a higher propensity for pathological upgrading in radical prostatectomy.
World J Urol, 42(1):608, 30 Oct 2024
Cited by: 0 articles | PMID: 39476187 | PMCID: PMC11525276
Sociodemographic disparities in prostate cancer imaging.
Abdom Radiol (NY), 26 Sep 2024
Cited by: 0 articles | PMID: 39325212
Review
Enhancing spatial domain detection in spatial transcriptomics with EnSDD.
Commun Biol, 7(1):1358, 21 Oct 2024
Cited by: 0 articles | PMID: 39433947 | PMCID: PMC11494180
Prostate Cancer: A Review of Genetics, Current Biomarkers and Personalised Treatments.
Cancer Rep (Hoboken), 7(10):e70016, 01 Oct 2024
Cited by: 0 articles | PMID: 39410867 | PMCID: PMC11480670
Review Free full text in Europe PMC
Trends in pre-biopsy MRI usage for prostate cancer detection, 2007-2022.
Prostate Cancer Prostatic Dis, 21 Sep 2024
Cited by: 0 articles | PMID: 39306635
Go to all (309) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT00102544
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pre-biopsy 3-Tesla MRI and targeted biopsy of the index prostate cancer: correlation with robot-assisted radical prostatectomy.
BJU Int, 119(1):82-90, 03 Jun 2016
Cited by: 5 articles | PMID: 27153933
Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial.
Lancet Oncol, 22(9):1240-1249, 13 Aug 2021
Cited by: 52 articles | PMID: 34391509
Impact of Magnetic Resonance Imaging Targeting on Pathologic Upgrading and Downgrading at Prostatectomy: A Systematic Review and Meta-analysis.
Eur Urol Oncol, 6(4):355-365, 25 May 2023
Cited by: 6 articles | PMID: 37236832
Review
The Key Combined Value of Multiparametric Magnetic Resonance Imaging, and Magnetic Resonance Imaging-targeted and Concomitant Systematic Biopsies for the Prediction of Adverse Pathological Features in Prostate Cancer Patients Undergoing Radical Prostatectomy.
Eur Urol, 77(6):733-741, 21 Sep 2019
Cited by: 40 articles | PMID: 31547938
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z99 CA999999
NCI NIH HHS (1)
Grant ID: Intramural Research Program
National Cancer Institute (1)
Grant ID: Intramural Research Program



