Abstract
Background
Medication adherence is crucial for improving clinical outcomes in the treatment of patients. We evaluate the effect of short message service (SMS) reminder on medication adherence and serum hormones in patients with breast cancer on aromatase inhibitors.Methods
An open-label, multi-centre, prospective randomised controlled trial of SMS versus Standard Care was conducted. Medication adherence was assessed via self-report using the Simplified Medication Adherence Questionnaire at baseline, 6 month, and 1 year. Androstenedione, estradiol, and estrone were measured at baseline and 1 year. The χ2 test and mixed effects logistic regression was performed to compare medication adherence between groups. Difference in androstenedione and estrone levels were assessed using analysis of covariance, whereas χ2 test and logistic regression was used for estradiol. Analysis was based on intention-to-treat.Results
A total of 244 patients were randomised to receive weekly SMS reminder (n = 123) or Standard Care (n = 121) between May 2015 and December 2018. The odds of adherence was higher at 6-month in SMS (OR = 1.78, 95% CI 1.04-3.05, p = 0.034), and not significantly different at 1-year (OR = 1.15, 95% CI: 0.67-1.96 p = 0.617). Mixed effects logistic regression analysis showed higher odds of adherence in SMS over the 1-year period (OR = 2.35, 95% CI: 1.01-5.49, p = 0.048). There was no difference in serum hormone levels between groups.Conclusion
SMS reminder improved medication adherence in the short-term but had no effect on serum hormones levels in the longer term. Future studies could investigate the use of tailored SMS intervention according to patient preference to improve its sustainability.Free full text

Improving medication adherence with adjuvant aromatase inhibitor in women with breast cancer: A randomised controlled trial to evaluate the effect of short message service (SMS) reminder
Abstract
Background
Medication adherence is crucial for improving clinical outcomes in the treatment of patients. We evaluate the effect of short message service (SMS) reminder on medication adherence and serum hormones in patients with breast cancer on aromatase inhibitors.
Methods
An open-label, multi-centre, prospective randomised controlled trial of SMS versus Standard Care was conducted. Medication adherence was assessed via self-report using the Simplified Medication Adherence Questionnaire at baseline, 6 month, and 1 year. Androstenedione, estradiol, and estrone were measured at baseline and 1 year. The χ2 test and mixed effects logistic regression was performed to compare medication adherence between groups. Difference in androstenedione and estrone levels were assessed using analysis of covariance, whereas χ2 test and logistic regression was used for estradiol. Analysis was based on intention-to-treat.
Results
A total of 244 patients were randomised to receive weekly SMS reminder (n = 123) or Standard Care (n = 121) between May 2015 and December 2018. The odds of adherence was higher at 6-month in SMS (OR = 1.78, 95% CI 1.04–3.05, p = 0.034), and not significantly different at 1-year (OR = 1.15, 95% CI: 0.67–1.96 p = 0.617). Mixed effects logistic regression analysis showed higher odds of adherence in SMS over the 1-year period (OR = 2.35, 95% CI: 1.01–5.49, p = 0.048). There was no difference in serum hormone levels between groups.
Conclusion
SMS reminder improved medication adherence in the short-term but had no effect on serum hormones levels in the longer term. Future studies could investigate the use of tailored SMS intervention according to patient preference to improve its sustainability.
Abbreviations
- AET
- Adjuvant endocrine therapy
- AI
- Aromatase inhibitor
- CLIA
- Chemiluminescent immunoassay and
- ECLIA
- Electrochemiluminescence immunoassay
- ELISA
- Enzyme linked immunosorbent assay
- HIV
- Human immunodeficiency virus
- LC-MS/MS
- Liquid Chromatography-Tandem Mass Spectrometry
- RCT
- Randomised controlled trial
- SMAQ
- Simplified Medication Adherence Questionnaire
- SMS
- Short message service
1. Background
Medication adherence is defined by the World Health Organization as the extent to which a person’s medication-taking behaviour corresponds with agreed recommendations from a health care provider [1]. In Singapore, breast cancer is the leading cause of cancer mortality among women [2]. A higher endogenous level of estrogens such as estradiol and estrone is strongly associated with elevated breast cancer risk [3,4]. Hence, deprivation of estrogen signalling via adjuvant endocrine therapy (AET), including tamoxifen or aromatase inhibitors (AI) such as anastrozole, exemestane, or letrozole, is the mainstay treatment for patients with hormone receptor positive breast cancer. AI therapy prevents the pathway of estrogen production via the aromatase enzyme, which is responsible for the conversion of estrogen from its androgenic precursors such as androstenedione and testosterone. In post-menopausal women with early stage breast cancer, AI therapy is superior to tamoxifen in improvement of breast cancer mortality and reduction of recurrence rates [5,6]. Initial guidelines recommended the use of AET for 5 years. Evidence has emerged that extended AET up to 10 years reduced risks of breast cancer recurrence and contralateral breast cancer [7]. Medication adherence is an issue in long term therapy. A systematic review revealed suboptimal adherence to AET, which ranged from 41 to 72% in studies of breast cancer survivors with at least four years of follow up [8]. Non-adherence to AET is associated with increased mortality and higher risk of recurrence [[9], [10], [11]].
To improve medication adherence, mobile phone text message reminders have been implemented in health care services with varying success and high satisfaction among patients [12,13]. The most common groups of targeted patients include those with human immunodeficiency virus (HIV) or diabetes [12]. Previous interventions to increase AET adherence were mainly limited to provision of educational materials [14]. With a mobile phone penetration rate exceeding 100% since the last decade in Singapore [15], there is great potential to utilise a computer automated short message service reminder (SMS) to optimise adherence. Thus, the objectives of this trial are two-fold. The primary objective is to evaluate whether SMS improves medication adherence as compared to Standard Care in women receiving AI therapy. The secondary objective is to examine whether SMS improves the inhibition of the aromatisation process of patients on AI therapy by evaluating its effects on androstenedione, estradiol, and estrone.
2. Methods
This is an open-label, multi-centre prospective randomised controlled trial of SMS versus Standard Care. The clinical trial protocol was described previously [16]. Briefly, the eligible participants comprised women aged at least 21 years with breast cancer, who had been prescribed AET for at least a year and would continue on AI therapy for at least another year. They had to possess a mobile phone that could receive text messages. The participants were recruited from the oncology clinics at the National University Hospital and the Ng Teng Fong General Hospital in Singapore. All eligible patients who had provided informed consent were randomised to receive either SMS or Standard Care in a 1:1 ratio. The SMS reminders were sent weekly on Monday at 9 am and read:
Mdm < PATIENT NAME>, please be reminded to take your anti-cancer medicine as instructed by your doctor. Take one tablet once every day.
The messages were in English, Mandarin, or Malay, according to the patient’s preferred language of communication.
Standard Care consisted of routine clinical follow-up without SMS reminders to take their adjuvant endocrine therapy. Patients with early stage breast cancer on adjuvant endocrine therapy are followed up as per the American Society of Clinical Oncology (ASCO) guidelines [17], with 3 to 6-monthly history and physical examination during the first 2 years, followed by 6-monthly history and physical examination from year 3–5, at our institution. If the patient is on extended endocrine therapy beyond 5 years, then she will continue to be followed up every 6 months with history and physical examination beyond year 5 until endocrine therapy is discontinued, thereafter follow-up is annually. All patients undergo annual surveillance mammogram as per ASCO guidelines.
Enrolled patients were followed up at 6 months and 1 year. Medication adherence was assessed via self-report using the six-item Simplified Medication Adherence Questionnaire (SMAQ) [18]. Medication non-adherence was defined if a patient provided a non-adherence response to any of items 1 to 4 in the SMAQ (which included timeliness, forgetfulness, and omission of dose when not feeling well), had skipped more than two doses during the last week, or had not taken medication for more than two complete days during the last visit.
Hormone assays of androstenedione, estradiol, and estrone were performed at baseline and 1 year. The serum androstenedione, estradiol, and estrone concentration were measured using the methods listed in Table 1.
Table 1
Methods to measure serum androstenedione, estradiol, and estrone.
| Hormone | Period | Manufacturer | Method |
|---|---|---|---|
| Androstenedione | May 2015–Dec 2019 | IBL | ELISA |
| Estradiol | May 2015–Aug 2015 | Beckman Coulter | CLIA |
| Sep 2015–Dec 2019 | Roche | ECLIA | |
| Estrone | May 2015–Aug 2015 | Mayo Clinic Laboratories | LC-MS/MS |
| Sep 2015–Dec 2019 | DRG | ELISA |
CLIA: chemiluminescent immunoassay; ELISA: enzyme linked immunosorbent assay, LC-MS/MS: Liquid Chromatography-Tandem Mass Spectrometry.
From May 2015 to August 2015, estradiol and estrone were measured by the chemiluminescent immunoassay (CLIA) (Beckman Coulter) and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) methods respectively. The initial hormone assay results for estradiol and estrone were below the lowest detection limit of 160 pmol/L for 25 patients and 10 pg/mL for 24 patients respectively. Hence, since September 2015, alternative methods were used. The electrochemiluminescence immunoassay (ECLIA) (Roche) method was implemented for the measurement of estradiol. With ECLIA, most of the estradiol results were still below the lowest detection limit of 18.4 pmol/L. As a more sensitive and commercially available method could not be found, the measurement of estradiol was continued using ECLIA. Concurrently, the enzyme linked immunosorbent assay (ELISA) (DRG) method was used for the measurement of estrone. However, the estrone test kit by DRG was updated using a different method since December 2016, and manufacture of the old kit was discontinued. Thus, summary statistics such as the mean and standard deviation from the reference sample of the respective batches were obtained from DRG to obtain standardised Z–score for estrone to account for batch-to-batch variation (see further details in Statistical Analysis section).
3. Statistical analysis
The assessment of medication adherence at 6-month and 1-year between SMS and Standard Care was performed using the χ [2] test. The treatment effect was quantified based on the odds ratio (OR) and its 95% confidence interval (CI). Medication adherence over the one-year period was evaluated using mixed effects logistic regression, to take into account possible intra-subject correlation in outcomes of medication adherence which were recorded at 6-month and 1-year. The model included intervention, baseline SMAQ, and time of follow-up as fixed effects variables, with the specification of a random intercept. The standard error was estimated via the robust variance estimate. For values of estrone below the detection limit, a value half the lowest detection limit (i.e. 5 pg/mL for LC-MS/MS method and 4 pg/mL for the ELISA method) was assumed for the purpose of analysis. The estrone levels were transformed into a standardised Z-score. The Z-score was calculated as the difference between the estrone level and the reference mean, divided by the reference standard deviation for each batch. Natural log transformation was implemented on the androstenedione levels to normalise the data. The log transformed androstenedione and estrone levels at 1-year were compared between treatment arms using the t-test. To adjust for the respective baseline levels, the analysis of covariance (ANCOVA) was performed. The effect estimate of estrone was quantified based the mean difference of Z-score. The effect estimate of androstenedione was quantified based on the ratio of geometric mean (i.e. relative mean difference, RMD) and its associated 95% CI. The estradiol levels (defined as <18.4 pmol/L versus ≥18.4 pmol/L) was compared between the two arms using the χ [2] test. The logistic regression analysis was implemented to adjust for baseline estradiol level, and its effect estimate was quantified based on odds ratio (OR) and its 95% CI. All analyses were performed according to the principle of intention-to-treat using STATA version 15, assuming a two-sided test at the 5% level of significance.
4. Results
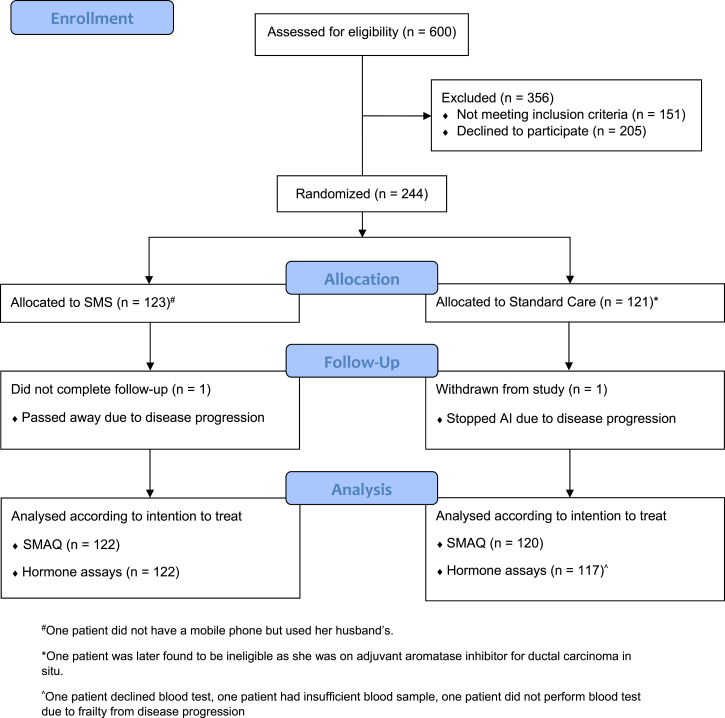
From 27 May 2015 through 27 December 2018, 600 potential patients were screened for eligibility in the two study sites, of whom 151 did not meet the inclusion criteria and 205 declined participation (Fig. 1). In total, 244 patients were enrolled in the trial (123 SMS, 121 Standard Care). The median follow up duration was 11.9 (interquartile range, IQR = 11.5 to 12.4) months. Two patients did not complete the 1 year follow-up due to disease progression, but had nevertheless contributed information to the analysis of medication adherence at 6-month. Overall, the baseline characteristics were comparable between SMS and Standard Care (Table 2). The median age of the trial participants was 61 years (range 32–80). Their ethnicity represented that of the general Singapore population – 74.6% Chinese, 13.9% Malay, and 7.8% Indian. Majority of the patients had at least secondary education (75.8%) and at least one comorbidity (76.2%). The most common comorbidities were hyperlipidemia (57.8%), hypertension (43.0%), and diabetes (24.6%). The median duration of AET prior to trial enrolment was 1.6 (IQR: 1.2–2.6) years. At baseline, 20.9% of the patients had used a medication reminder, such as pill box, alarm, or reminders from their caregivers.
Table 2
Baseline characteristics of 244 trial participants.
| Characteristic | SMS (n = 123) | Standard Care (n = 121) | All patients (n = 244) |
|---|---|---|---|
| Median age (range), years | 60 (32-80) | 62 (39-80) | 61 (32-80) |
| Ethnicity (%) | |||
| Chinese | 91 (74.0) | 91 (75.2) | 182 (74.6) |
| Malay | 17 (13.8) | 17 (14.1) | 34 (13.9) |
| Indian | 10 (8.1) | 9 (7.4) | 19 (7.8) |
| Others | 5 (4.1) | 4 (3.3) | 9 (3.7) |
| Education Level (%) | |||
| Primary and below | 32 (26.0) | 27 (22.3) | 59 (24.2) |
| Secondary | 57 (46.3) | 67 (55.4) | 124 (50.8) |
| Pre-university | 22 (17.9) | 11 (9.1) | 33 (13.5) |
| University | 12 (9.8) | 16 (13.2) | 28 (11.5) |
| Number of comorbidities (%) | |||
| 0 | 28 (22.8) | 30 (24.8) | 58 (23.8) |
| 1 | 22 (17.9) | 17 (14.1) | 39 (16.0) |
| 2 | 24 (19.5) | 30 (24.8) | 54 (22.1) |
| ≥ 3 | 49 (39.8) | 44 (36.4) | 93 (38.1) |
Stage (%) | |||
| 0 | 0 (0) | 1 (0.9) | 1 (0.4) |
| I | 52 (42.6) | 43 (36.8) | 95 (39.8) |
| II | 48 (39.3) | 45 (38.5) | 93 (38.9) |
| III | 22 (18.0) | 28 (23.9) | 50 (20.9) |
| Median duration of breast cancer diagnosis (IQR), years | 2.1 (1.8–3.0) | 2.3 (1.8–3.3) | 2.2 (1.8–3.2) |
| Median duration of adjuvant endocrine therapy (IQR), years | 1.6 (1.2–2.3) | 1.7 (1.3–2.7) | 1.6 (1.2–2.6) |
| Use of medication reminder (%) | 26 (21.1) | 25 (20.7) | 51 (20.9) |
 Five patients who had Nx could not have stage number calculated.
Five patients who had Nx could not have stage number calculated.
According to the SMAQ, 53.3% of patients were adherent to their AET at baseline (Table 3). The median androstenedione level was 2.9 (IQR = 2.1–3.6) nmol/L. All except two patients in Standard Care had baseline estradiol below the detection limit. The median estrone was 31.1 (IQR = 21.7–44.9) pmol/L.
Table 3
Baseline outcome measures of adherence and hormone levels.
| Outcome measure | SMS (n = 123) | Standard Care (n = 121) | All patients (n = 244) |
|---|---|---|---|
| Adherent according to SMAQ (%) | 64 (52.0) | 66 (54.6) | 130 (53.3) |
| Median androstenedione (IQR), nmol/L | 2.8 (2.2–3.5) | 2.9 (2.0–3.7) | 2.9 (2.1–3.6) |
| Mean Z score of estrone (SD) | 2.0 (3.1) | 1.9 (3.1) | 1.9 (3.1) |
| Estradiol, ECLIA | |||
| < 18.4 pmol/L (%) | 111 (100.0) | 105 (98.1) | 216 (99.1) |
| ≥ 18.4 pmol/L (%) | 0 (0.0) | 2 (1.9) | 2 (0.9) |
Note: 1. One patient from Standard Care did not have baseline hormones measured.
2. Estradiol measured using CLIA for the period May to Aug 2015 involving 25 patients (12 SMS and 13 Standard Care) was excluded from the analysis as the results were all below the detection limit of 160 pmol/L, and it could not be determined if their results were below the detection limit of ECLIA.
4.1. Effect of SMS reminders on medication adherence
A total of 242 (122 SMS, 120 Standard Care) patients were available for the primary outcome analysis of medication adherence as defined by the SMAQ. At 6-month, there was a higher percentage of adherence in SMS (72.4%) as compared with Standard Care (59.5%). The unadjusted OR was 1.78 (95% CI 1.04 to 3.05, p = 0.034) (Table 4). At 1-year, the percentage of adherence in SMS (68.9%) was marginally higher than Standard Care (65.8%), and statistical significance was not achieved for this comparison (Unadjusted OR = 1.15, 95% CI: 0.67–1.96 p = 0.617). Based on the mixed effects logistic regression analysis, the odds of adherence over the 1-year period in SMS was 2.35 times that of Standard Care (Adjusted OR = 2.35, 95% CI: 1.01–5.49, p = 0.048).
Table 4
Comparison of medication adherence over time.
| Outcome | SMS (%) | Standard care (%) | OR (95% CI) | p |
|---|---|---|---|---|
| SMAQ at 6-month | 72.4 | 59.5 | 1.78 (1.04–3.05) | 0.034 |
| SMAQ at 1-year | 68.9 | 65.8 | 1.15 (0.67–1.96) | 0.617 |
| SMAQ over the 1-year period | 71.0 | 61.6 | 2.35 (1.01–5.49) | 0.048 |
Note: SMAQ over the 1-year period included the SMAQ information recorded at both 6-month and 1-year. The mixed effects logistic regression model was adjusted for baseline SMAQ and effect of time, with the specification of a random intercept.
4.2. Effect of SMS reminders on serum hormone levels at 1-year
Of the 242 patients who completed the trial, two patients did not perform the blood test and one patient had insufficient blood sample for analysis at 1 year follow up. Consequently, 239 patients (122 SMS, 117 Standard Care) contributed to the analysis of the hormone assays (Table 5).
Table 5
Effect of treatment on hormone assays at 1 year.
| Outcome | SMS | Standard Care | Effect estimate | p-value |
|---|---|---|---|---|
| Androstenedione | ||||
| Model 1 | 2.88 | 2.89 | 1.00 (0.88–1.13) | 0.953 |
| Model 2 | 2.89 | 2.88 | 1.00 (0.91–1.10) | 0.978 |
| Z-score of Estrone | ||||
| Model 1 | 3.14 | 2.18 | 0.96 (−0.43 to 2.35) | 0.174 |
| Model 2 | 3.14 | 2.18 | 0.96 (−0.44 to 2.36) | 0.178 |
| Estradiol | ||||
| Model 1 | 95.9 | 96.6 | 0.83 (0.22–3.16) | 0.783 |
| Model 2 | 94.8 | 97.6 | 0.42 (0.08–2.24) | 0.312 |
Note: 1. The descriptive statistics are presented in terms of geometric mean for androstenedione; mean Z-score for estrone; and proportions of patients with estradiol <18.4 pmol/L for estradiol.
2. In Model 1, the effect estimates were unadjusted. In Model 2, the effect estimates were adjusted for respective baseline hormone assay. The adjusted analysis excluded one patient who did not have baseline hormone assays conducted.
3. Adjusted analysis of estradiol excluded 25 patients (12 SMS, 13 Standard Care) whose baseline estradiol were measured by different methods.
At 1-year, the androstenedione level was similar in both groups (RMD = 1.00, 95% CI: 0.88–1.13, p = 0.953). Although the estrone level at 1-year was higher in SMS as compared with Standard Care, the mean difference of 0.96 (95% CI: −0.43 to 1.35) was not significant (p = 0.174). There was a non-significant reduction in odds of estradiol level <18.4 pmol/L in SMS as compared to Standard Care (OR = 0.83, 95% CI: 0.22–3.16, p = 0.783). In the comparison of these secondary outcomes, the results were not materially altered after adjusting for the respective baseline hormone assays level.
4.3. Acceptability of SMS reminders
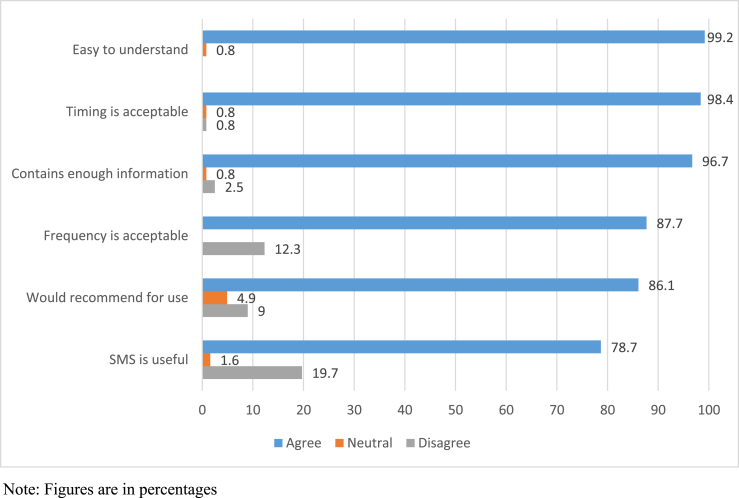
Overall, there was high acceptability of the SMS reminders. Most patients agreed that the SMS was easy to understand (99.2%) with acceptable (98.4%) timing of reminder (Fig. 2). A total of 96.7% of patients agreed that there was enough information provided in the SMS reminder. To improve readability of the message, two patients (1.6%) suggested using less words or emojis, and changing the message template to avoid repetition. The frequency of reminder was also largely acceptable (87.7%). Two patients (1.6%) suggested customising the schedule according to their medication taking routine. Seventeen patients (13.9%) suggested increased frequency of reminders; either daily (9.8%), every alternate day (0.8%), or twice a week (3.3%). Majority of the patients (86.1%) would recommend this service as part of routine care. There were 78.7% of patients who agreed that the SMS reminders were useful. Those who did not find them useful were patients who had other forms of reminders or the ability to remember taking medication because it was already part of their daily routine. However, they recognised that SMS reminder could be useful for patients who were forgetful.
5. Discussion
Medication adherence among breast cancer survivors may decrease over the duration of the recommended length of AET. Unintentional non-adherent behaviour such as forgetfulness could be overcome by the implementation of a SMS reminder service. However, it is uncertain whether the behaviour could be sustained over an extended duration. As such, we have conducted an RCT to investigate the longitudinal effects of SMS reminders in affecting the behaviour of patients on AET.
In a systematic review examining the effectiveness of electronic reminders in HIV patients, Vervloet et al. [19] reported that 8 out of 10 studies with follow up duration of between 3 weeks and 3 months showed significant improvement in medication adherence, whereas only 1 out of 3 studies with follow up duration of longer than 6 months demonstrated significant effects. This was corroborated in our unadjusted analyses of medication adherence, which also showed a significant effect of SMS in improving adherence at 6-month but not 1-year. Nevertheless, fully utilising the information collected at 6-month and 1-year via the mixed effects model, a borderline significant effect of SMS reminder in improving medication adherence over the 1-year period was demonstrated. In a study examining the durability of the effects in the SMS reminder group of young HIV-positive adolescents and adults, Garofalo et al. [20] found a significant effect at 3 months but the effect was attenuated at 6 months from baseline. Interestingly, the improvement in adherence was maintained at 3–6 months post intervention, albeit lower than the proportion of adherence at 3 months during the intervention.
It has been postulated that long term adherence requires sustained behavioural intervention and support. According to a network meta-analysis [21], sustainability of improving medication adherence over time relied on multicomponent interventions that incorporated educational, attitudinal and technical aspects. The educational component involves using knowledge provided by a healthcare professional; the attitudinal component involves modification of behavioural intention based on theory of planned behaviour; whereas the technical component comprise implementation of instruments or systems to facilitate medication taking. As such, an intervention consisting of a simple technical aspect (SMS reminder) might not result in sustained behaviour change.
We did not find any difference between interventions with respect to the secondary outcomes of hormone assays. A limitation is that the hormone levels were not measured at 6-month post intervention, hence we were unable to conclude whether the short-term benefit at 6-month as noted in the primary outcome would be translated to improvements in the hormone levels. The concept of pharmacological forgiveness proposes that therapeutic outcomes may be robust to imperfect adherence when the duration of action of a drug exceeds its dosing interval [22]. The half-lives of anastrozole, exemestane, and letrozole are longer than its daily dosing interval, at 41 h, 27 h, and 4 days respectively [23]. Of those who were non-adherent in our trial, 75% had missed doses only once in the past week and 57% had missed two doses or less in the three months preceding the final follow up. Thus, based on their medication taking behaviour, self-reported non-adherence might not be reflected in the measurement of hormone assays since the lapses in AI doses were temporal. The effect on hormone levels may require a longer period of discontinuation before an increase in estrone and estradiol levels may manifest, as shown by Brier et al. [24] In their cross-sectional study, significantly higher levels of median estrone and estradiol were found in patients who self-reported not taking AI at all in the last month as compared to those who did.
One out of five patients in Standard Care also relied on their own form of medication reminders. As such, there might be possible attenuation of the intervention effect. Moreover, we required patients to fill out logs to monitor daily pill taking. These patient logs may inadvertently serve as reminders for patients to take their medication. In patients randomised to Standard Care, more patients who were non-adherent at 6 months became adherent at 1 year as compared to SMS, thus diminishing the effect of SMS at 1 year. There is no gold standard for measuring medication adherence as each method has its advantages and limitations [25]. The primary outcome of adherence was defined using self-reported adherence via SMAQ, which is a valid and reliable instrument [18,26,27]. Agala et al. reported SMAQ to be measurement invariant across different intervention groups and time points [27]. We have also collected information using patient log and pill count to verify the accuracy of the self-reported items of SMAQ. While patient log and in particular pill count may be thought of as more objective measures of medication adherence, they are susceptible to missing and inaccurate information [25]. In our trial, 36 patients lost their logs at the 6-month and 21 patients at 1-year follow up respectively. Besides, they could forget filling out the log or do so retrospectively [28]. As for pill counting, patients may also not bring the correct number of pills to the clinic for counting. This method is also subject to pill dumping to maintain an appearance of being adherent [25].
Overall, there was high satisfaction with the SMS reminders. This was consistent with previous studies [12] which examined the effect of SMS reminders on medication adherence. Although some participants had suggested increasing the frequency of reminders, there is currently a paucity of evidence suggesting that this would lead to sustained medication adherence in patients on AET. A RCT [29] in HIV patients showed that weekly reminders significantly improved adherence whereas daily reminders did not. On this basis, we have implemented weekly reminders instead of more frequent schedules. Recently, a multicentre RCT conducted in the United States with 3 years of follow up found no significant difference in time-to-adherence failure between patients on AI therapy who received bi-weekly text reminder and those who did not [30].
In another tertiary cancer centre in Singapore, Ali et al. [31] cited forgetfulness as the main reason for non-adherence to AET. Consequently, majority of our patients would recommend SMS reminder service as part of routine care. In addition, they had suggested that the intervention could be improved by customising the timing and content of the SMS. At the 1-year follow up, 58.3% of patients who were randomised to SMS and had forgotten to take their medication did so on a weekend, as their routine tended to be different from weekdays. Huang et al. [32] described that patients preferred to have reminders sent 30 minutes before their scheduled time for medication. Amankwaa et al. [33] reported that studies which varied message content and length demonstrated greater sustained interest and prevented habituation.
Potential strategies to facilitate adherence include the use of a pillbox, patient education, voice calls, SMS reminders, or mobile phone applications. Our intervention was easy to implement but not tailored to the habits of individual patients. Future studies could improve upon this intervention by tailoring the timing, frequency, and message content according to patient preferences, although the logistical issues of a more complex intervention would need to be considered.
6. Conclusion
Our RCT showed a significant short-term effect of SMS in improving medication adherence. Future studies could investigate the use of a tailored SMS intervention according to patient preference.
Authors’ contributions
AW, CCT, PW, SCL and BCT participated in the design of the study and research protocol. AW, CCT, SHT, LEYA, SEL, WQC, JH, SCL significantly contributed to patient recruitment. EHT collected the data. EHT and BCT conducted the statistical analysis. All authors were involved in the writing, editing, and approval of the final manuscript.
Compliance with ethical standards
Funding
This trial is supported by the Singapore Cancer Society Cancer Research Grant 2014; National University Cancer Institute, Singapore (NCIS) Centre Grant Seed Funding Program (Aug 2014 Grant Call); National University Health System Bridging Funds FY17. These funding sources had no role in the design of this study, its execution, analysis, interpretation of the data, or decision to submit the manuscript.
Ethics approval and consent to participate
The study was approved by the National Healthcare Group Domain-Specific Review Board (Reference number 2014/01316). All procedures performed were in accordance with the ethical standards of the institution.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to patient confidentiality and institutional guidelines.
Declaration of competing interest
The authors declare no potential conflicts of interest that is related to this submission.
Acknowledgements
The authors would like to thank the oncologists, nursing staff, research coordinators and patients at the participating sites for their contribution. The following oncologists helped in patient recruitment: Yiqing Huang, Natalie Yan Li Ngoi, Gloria Hui Jia Chan, Anand Jeyasekharan, Hon Lyn Tan, Nesaretnam Barr Kumarakulasinghe, Joan Rou-En Choo, Samuel Guan Wei Ow, Thomas I Peng Soh, Joline Si Jing Lim, Matilda Xinwei Lee, Raghav Sundar, Chee Seng Tan, Boon Cher Goh, Tan Min Chin, Angela Shien Ling Pang, Yi Wan Lim, and Vaishnavi Muthu.
References
Articles from The Breast : Official Journal of the European Society of Mastology are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.breast.2020.06.012
Read article for free, from open access legal sources, via Unpaywall:
http://www.thebreastonline.com/article/S0960977620301429/pdf
Citations & impact
Impact metrics
Article citations
Remote Monitoring App for Endocrine Therapy Adherence Among Patients With Early-Stage Breast Cancer: A Randomized Clinical Trial.
JAMA Netw Open, 7(6):e2417873, 03 Jun 2024
Cited by: 0 articles | PMID: 38935379
Effect of telehealth interventions on adherence to endocrine therapy among patients with breast cancer: a systematic review and meta-analysis.
Support Care Cancer, 32(3):151, 09 Feb 2024
Cited by: 1 article | PMID: 38332357
Review
mHealth App to improve medication adherence among older adult stroke survivors: Development and usability study.
Digit Health, 10:20552076241236291, 01 Jan 2024
Cited by: 0 articles | PMID: 38465293 | PMCID: PMC10921861
Aromatase inhibitors: the journey from the state of the art to clinical open questions.
Front Oncol, 13:1249160, 22 Dec 2023
Cited by: 1 article | PMID: 38188305 | PMCID: PMC10770835
Review Free full text in Europe PMC
Codevelopment of a Text Messaging Intervention to Support Adherence to Adjuvant Endocrine Therapy in Women With Breast Cancer: Mixed Methods Approach.
J Med Internet Res, 25:e38073, 24 May 2023
Cited by: 3 articles | PMID: 37223964 | PMCID: PMC10248768
Go to all (14) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Improving medication adherence with adjuvant aromatase inhibitor in women with breast cancer: study protocol of a randomised controlled trial to evaluate the effect of short message service (SMS) reminder.
BMC Cancer, 18(1):727, 09 Jul 2018
Cited by: 5 articles | PMID: 29986672 | PMCID: PMC6038248
A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored Short Messaging Service (SMS)-SMS4Stroke study.
BMC Neurol, 15:212, 21 Oct 2015
Cited by: 78 articles | PMID: 26486857 | PMCID: PMC4618367
Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial.
J Cancer Surviv, 12(4):431-440, 28 Feb 2018
Cited by: 46 articles | PMID: 29492753 | PMCID: PMC6054536
Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome.
Eur J Cardiovasc Nurs, 14(2):170-179, 02 Feb 2014
Cited by: 63 articles | PMID: 24491349
Funding
Funders who supported this work.