Abstract
Free full text

Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19
Abstract
COVID‐19 may cause pneumonia, acute respiratory distress syndrome, cardiovascular alterations, and multiple organ failure, which have been ascribed to a cytokine storm, a systemic inflammatory response, and an attack by the immune system. Moreover, an oxidative stress imbalance has been demonstrated to occur in COVID‐19 patients. N‐ Acetyl‐L‐cysteine (NAC) is a precursor of reduced glutathione (GSH). Due to its tolerability, this pleiotropic drug has been proposed not only as a mucolytic agent, but also as a preventive/therapeutic agent in a variety of disorders involving GSH depletion and oxidative stress. At very high doses, NAC is also used as an antidote against paracetamol intoxication. Thiols block the angiotensin‐converting enzyme 2 thereby hampering penetration of SARS‐CoV‐2 into cells. Based on a broad range of antioxidant and anti‐inflammatory mechanisms, which are herein reviewed, the oral administration of NAC is likely to attenuate the risk of developing COVID‐19, as it was previously demonstrated for influenza and influenza‐like illnesses. Moreover, high‐dose intravenous NAC may be expected to play an adjuvant role in the treatment of severe COVID‐19 cases and in the control of its lethal complications, also including pulmonary and cardiovascular adverse events.
Abbreviations
- ACE2
- angiotensin‐converting enzyme 2
- ARE
- antioxidant responsive element
- COPD
- chronic obstructive pulmonary diseases
- COVID‐19
- coronavirus disease‐2019
- COX‐2
- cyclooxygenase‐2
- CXCL‐10
- chemokine (C‐X‐C motif) ligand 10
- EGFR
- epidermal growth factor receptor
- GCL
- glutamate‐cysteine ligase
- GPx
- glutathione peroxidase
- GR
- glutathione reductase
- GSH
- reduced glutathione (γ‐glutamylcysteinylglycine)
- GSH‐C4
- N‐butanoyl GSH derivative
- GSSG
- oxidized glutathione
- HIV
- human immunodeficiency virus
- HO‐1
- heme oxygenase‐1
- hs‐CRP
- high‐sensitivity C‐reactive protein
- ICAM‐1
- intercellular adhesion molecule‐1
- ICU
- intensive care units
- IL‐
- interleukin‐
- L‐Cys
- L‐cysteine
- LPS
- lipopolysaccharide
- MAPK p38
- p38 mitogen‐activated protein kinase
- MDA
- malondialdehyde
- MMP‐
- matrix metalloproteinase‐
- MPO
- myeloperoxidase
- NAC
- N‐acetyl‐L‐cysteine
- NF‐κB
- nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NHBECs
- normal human bronchial epithelial cells
- NKA
- Na,K‐ATPase
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- Nrf2
- nuclear factor erythroid 2–related factor 2
- NQO‐1
- NAD(P)H dehydrogenase [quinone]‐1
- O2•−
- superoxide radical anion
- OCl
- hypochlorous acid
- •OH
- hydroxyl radical
- ONOO−
- peroxynitrite
- PDI
- protein disulfide isomerase
- PGE‐2
- prostaglandin E‐2
- RO2•
- peroxyl radical
- ROS
- reactive oxygen species
- RSV
- respiratory syncytial virus
- SARS‐CoV
- severe acute respiratory syndrome coronaviruses
- SARS‐CoV‐2
- coronavirus responsible for COVID‐19
- Th1
- T helper‐1
- TLR3/HA
- toll‐like receptor 3/hemagglutinin
- T TLR4
- toll‐like receptor 4
- TNF
- tumor necrosis factor
- XNIP
- inflammasome activator thioredoxin interacting protein
1. INTRODUCTION
Approximately 15% of coronavirus disease‐2019 (COVID‐19) patients suffer from impairment of gas exchange and pneumonia, and 5% undergo acute respiratory distress syndrome (ARDS), septic shock and/or multiple organ failure that require hospitalization in intensive care units (ICU). 1 ARDS, the leading cause of death in COVID‐19 patients, involves a cytokine storm and a systemic inflammatory response resulting from the release of large amounts of pro‐inflammatory cytokines and chemokines that trigger an attack by the immune system. 2 Besides affecting the respiratory system, COVID‐19 affects other systems, also including the cardiovascular system, and diffuse thrombosis is emerging as an important contributor to adverse outcomes in patients with COVID‐19. Different pathogenic disturbances are involved, such as acute changes in myocardial demand and supply due to tachycardia, hypotension, and hypoxemia resulting in type 2 myocardial infarction; acute coronary syndrome due to acute atherothrombosis in a virally induced thrombotic and inflammatory milieu; microvascular dysfunction due to diffuse microthrombi or vascular injury; stress‐related cardiomyopathy (Takotsubo syndrome); direct viral cardiomyocyte toxicity and myocarditis. 3 All of these complications reflect inflammatory reactions stimulated by the viral infection.
COVID‐19 is particularly severe in individuals who are at risk either because of old age or of pre‐existing pathological conditions. For instance, in Italy the mean age of patients dying of COVID‐19 as of May 28 was 80 years, and 95.9% of deceased persons had important pre‐existing conditions, such as cardiovascular diseases, cerebrovascular diseases, diabetes, dementia, chronic obstructive pulmonary diseases (COPD), cancer, chronic liver disease, chronic renal failure, respiratory failure, human immunodeficiency virus (HIV) infection, autoimmune diseases, and obesity. Specifically, 14.9% had a single comorbidity, 21.5% had 2 comorbidities, and 59.5% had 3 or more comorbidities, with a mean number of 3.3 comorbidities. 4 Especially in patients below the age of 80, the fatal cases were much more frequent in men, 4 who have lower levels of blood reduced glutathione (GSH). 5 Elderly individuals maintain a chronic low level of inflammation that is associated with oxidative stress and inflammatory cytokine production, a condition that increases the severity of viral infections in this population and that could be attenuated by administration of antioxidants. 6
A significant elevation in blood serum glutathione reductase (GR), resulting from oxidative stress imbalance, was detected in COVID‐19 patients, especially when admitted to ICU. 7 From an accumulation of literature data, an endogenous deficiency in GSH may underlie the serious manifestations and death from COVID‐19. 8 As we will discuss below, N‐acetyl‐L‐cysteine (NAC) is used in a broad range of conditions to restore or protect against GSH depletion and has a wide safety margin. It has been of benefit in treating ARDS from other causes and might limit or prevent lung damage in patients with COVID‐19. The evidence for this proposal is reviewed here.
2. INHIBITION BY THIOLS OF SARS‐COV‐2 BINDING TO CELLS
The angiotensin‐converting enzyme 2 (ACE2) is the functional receptor for severe acute respiratory syndrome (SARS) coronaviruses, responsible for entrance into cells of both SARS‐CoV and SARS‐CoV‐2, the etiological agent of COVID‐19. 9 Therefore, the interaction of viral spike proteins with ACE2 is a critical step in the viral replication cycle. The receptor binding domain of the viral spike proteins and ACE2 has several cysteine residues. Molecular dynamic simulations showed that the binding affinity was significantly impaired when all the disulfide bonds of both ACE2 and SARS‐CoV/CoV‐2 spike proteins were reduced to thiol groups. These findings are consistent with the view that the reduction of disulfides into sulfhydryl groups completely impairs the binding of SARS‐CoV/CoV‐2 spike protein to ACE2 (see Figure 1 ) and provide a molecular basis for the severity of COVID‐19 infection due to oxidative stress. 10 Furthermore, both animal studies and clinical studies suggested that supplementation of NAC, which is known to attenuate the tolerance to nitrates, modifies the function of the renin/angiotensin system in vivo. Such an effect is probably mediated by inhibition of ACE activity. 11 By blocking ACE, NAC may provide protection from the deleterious effects of angiotensin II, a potentially useful activity in SARS‐CoV‐2 infection. 12
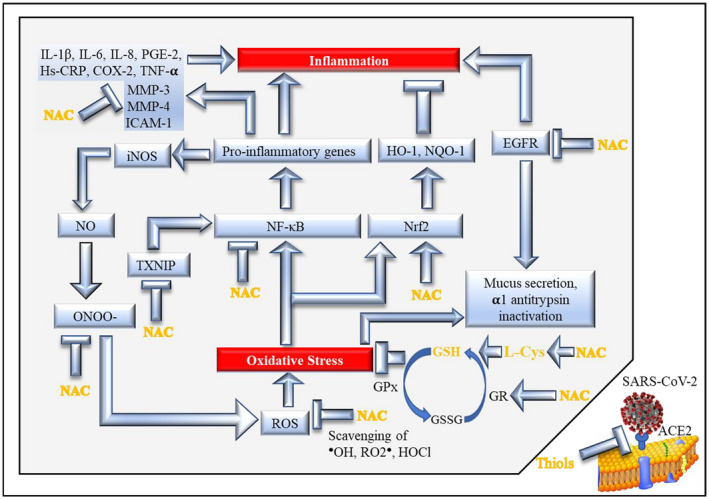
Major mechanisms involved in the antioxidant and anti‐inflammatory action of NAC via GSH (modified and updated from Sadowska, 2012 14 ). The blue arrows indicate stimulation of downstream activities or pathways, whereas the T‐shaped symbols indicate inhibition of downstream activities or pathways. See Text for details, references, and meaning of acronyms
3. GLUTATHIONE AND N‐ACETYL‐L‐CYSTEINE
Reduced glutathione (GSH) is a major defense mechanism of the human body and all living beings. However, being a tripeptide (γ‐glutamylcysteinylglycine), it poorly penetrates cells. L‐cysteine (L‐cys) is the rate‐limiting amino acid in GSH synthesis. NAC easily penetrates cells where it is deacetylated to yield L‐cys thereby promoting GSH synthesis. Therefore, NAC works per se in the extracellular environment and as a precursor of GSH inside cells. Accordingly, all its intracellular effects are mediated by GSH replenishment. Recycling of GSH increases but cannot match the high consumption in COVID‐19 lung disease. This requires new GSH synthesis, which increases enormously, largely through activation and up‐regulated production of glutamate‐cysteine ligase (GCL), the rate‐limiting enzyme for GSH biosynthesis that in turn depends on the availability of intracellular L‐cys, the rate‐limiting substrate for the enzyme. 13 NAC has better oral and topical bioavailability than GSH, and has an excellent safety profile. However, the systemic bioavailability of NAC after oral administration is relatively poor. 14 This thiol has been in clinical use since the 1960s as a mucolytic agent, usually at the oral dose of 600 mg, due to its ability to break the disulfide bonds of mucus and depolymerize mucin. As previously mentioned, breaking disulfide bonds into thiol groups may also reduce the affinity for the virus to attach to ACE2 sites. 9 NAC is also used in nebulized format in patients with acute bronchopulmonary disease, such as pneumonia, bronchitis, and tracheobronchitis. Moreover, NAC is quoted in the WHO Model List of Essential Medicines as an antidote in poisonings. At doses as high as 150 mg/kg intravenously, NAC is extensively used and is in standard practice as a clinically approved antidote against paracetamol (acetaminophen) intoxication, and it is almost 100% effective if given within 8 hours postingestion. 15 Paracetamol overdosage may cause mitochondrial oxidative imbalance and nitrosative stress leading to liver injury, which is the most common cause of acute liver failure in many countries. 16 The intravenous administration of NAC is also used to prevent contrast‐induced nephropathy that, again, is an inflammatory condition. 15
In addition, this pleiotropic drug has been proposed to attenuate the toxicity of various agents that cause the generation of free radicals as well as for the therapy and/or prevention of a variety of diseases involving GSH depletion and redox status alterations, such as heart diseases, diabetes, AIDS, neurodegenerative diseases, neuropsychiatric disorders, and several other conditions. 14 , 17
4. OXIDATIVE STRESS, INFLAMMATION, AND IMMUNE RESPONSE
Oxidative stress and inflammation are strictly inter‐related. Exposure of cells either to the hydroxyl radical (•OH) or the superoxide radical anion (O2•−) induces a dose‐dependent release of pro‐inflammatory cytokines. Lipopolysaccharide (LPS) induces intracellular accumulation of reactive oxygen species (ROS) and augments the release of interleukin (IL)‐1beta, IL‐6, and tumor necrosis factor‐alpha (TNF‐α). A kappa B (κB‐α)/nuclear factor kappa B (NF‐κB)‐independent pathway mediates the redox‐dependent regulation of inflammatory cytokines, and this process is enhanced by GSH depletion. 18
ROS and thiol antioxidants, including GSH, regulate innate immunity at various levels, as documented by a broad literature. GSH is not only important as an antioxidant, but also as a signaling molecule in the redox‐sensitive steps of the cellular mechanisms implicated in inflammation and host defense against infection. 19 It is noteworthy that GSH not only affects certain factors involved in immunological processes, but it also modifies complex immune reactions such as fever, and there are data suggesting that fever induction is associated with oxidative stress. 20 Although the complement system is a key mediator of the innate immune response that protects against infectious agents, it also plays a critical role in promoting the inflammatory process that leads to tissue injury. 21 In particular, complement may be involved in coronavirus pathogenesis, as inferred from the finding that C3 knockout mice infected with SARS‐CoV have less lung disease than wild‐type mice. 22 Preliminary data provide evidence for activation of complement (sC5b‐9 and C5a) in patients with COVID‐19, with significantly higher plasma levels in the patients with severe disease than in those with moderate disease. Hence, complement activation has been suggested as a novel therapeutic target in COVID‐19. 21 In this context, it is noteworthy that administration of NAC (2 × 600 mg/day for 8 weeks) in a placebo‐controlled study has been shown to reduce the plasma levels of inflammatory markers, including complement (C3), in peritoneal dialysis patients. 23
Chronic oxidative stress, causing biomolecular damage, contributes to the age‐related decline in physiological functions, including the immune function. The daily administration of NAC (600 mg) to postmenopausal women strengthened the immune defenses thereby decreasing the probability of immune system‐related diseases in aging, such as infections, as shown by monitoring several lymphocyte functions (adherence, chemotaxis, proliferation, and natural killer activity) and neutrophil functions (adherence, chemotaxis, phagocytosis, and superoxide) as well as cytokine levels (IL‐2, IL‐8, and TNF‐α). 24
5. ANTIOXIDANT AND ANTI‐INFLAMMATORY MECHANISMS OF NAC
NAC works via a broad variety of mechanisms, which inside cells are mediated by GSH replenishment. A major one is its nucleophilicity, which consists of the ability of sulfhydryl groups (–SH) to react with electrophilic metabolites, either directly or via GSH S‐transferases. 17 This property results in binding of DNA reactive metabolites and in blocking reactive intermediates. An example of reactive intermediate is the paracetamol metabolite N‐acetyl‐p‐benzoquinone imine (NAPQI) formed via cytochrome P450 enzymes. In addition, NAC can exert antioxidant activity via p53‐mediated apoptosis. 25 L‐Cys, derived by NAC catabolism, is readily bioconverted to the vasodilator, anti‐inflammatory and readily diffusible hydrogen sulfide. Therefore, NAC should be regarded as a hydrogen sulfide donor. 26
Some major mechanisms responsible for the antioxidant/anti‐inflammatory properties of NAC are summarized in Figure Figure1.1. First of all, it has been established for a long time that NAC is a potent scavenger of ROS and especially of hypochlorous acid (HOCl) and •OH. 27 Inhibition by NAC of the ROS‐producing vascular NAD(P)H oxidases is relevant to prevention of hypertension and to pathological states associated with uncontrolled growth and inflammation, such as atherosclerosis. 28 The SH‐groups within the NAC molecule can also scavenge several reactive nitrogen species (RNS) that play a role in the oxidation of lipids, proteins, and DNA. 29 NAC potentiates the vasodilator and antiaggregatory effects of nitric oxide, which is a very important interaction clinically, and which has been shown to be valuable in the context of acute heart failure and acute myocardial ischemia and infarction. 30
Inhibition by NAC of epidermal growth factor receptor (EGFR), a tyrosine kinase involved in inflammation, 14 , 31 also results in a decreased inactivation of α1‐antitrypsin. In particular, NAC has the ability to improve the efficacy and structural conformational integrity of α1‐antitrypsin via a GSH‐mediated mechanism, and it enhances α1‐antitrypsin transcytosis thus improving its cellular uptake and functions. 32 In experimental studies, the oral administration of NAC to pregnant mice enhanced the expression of the gene encoding for an α1‐antitrypsin precursor in the fetal liver. 33 Together with redox imbalance, α1‐antitrypsin deficiency is involved in the pathogenesis of COPD. NAC was found to reduce IL‐8 levels, normalized C‐reactive protein (CRP) levels, and improved clinical outcomes in patients with COPD exacerbations. 34 A prospective, randomized, controlled trial in the Shandong Province, China enrolling adult bronchiectasis patients with at least two exacerbations in the previous year showed that oral NAC (600 mg twice daily for 12 months) was able to reduce the risk of exacerbations. 35
In addition, NAC has been shown to have protective effects in ARDS. It is well established that ROS play a key role in the pathogenesis of the acute lung injury and that the alveolar epithelial lining fluid of patients with ARDS is deficient in GSH, which may predispose these patients to that disease. 36 A randomized, double‐blind, placebo‐controlled, prospective clinical trial in 5 ICUs in the USA and Canada showed that the intravenous administration of NAC (70 mg/kg body weight), every 8 hours for 10 days, effectively repleted GSH in red blood cells, decreased the number of days of acute lung injury, and significantly increased the cardiac index. 37 NAC (50 mg/kg body weight in 250 mL of 5% dextrose for 6 days) protected the lungs of ARDS patients, as evaluated by measuring the expired ethane and malondialdehyde (MDA) as well as GSSG and GSH in the epithelial lining fluid. 38 In another study, patients hospitalized in ICU who received NAC (150 mg/kg body weight on the first day, followed by 50 mg/kg for 3 days) had a better clinical outcome as compared with patients in the placebo group. Moreover, NAC increased extracellular total antioxidant power and total thiol molecules. 39
A further mechanism is that NAC further enhances the stimulation of nuclear factor erythroid 2‐related factor 2 (Nrf2) by oxidative stress, 40 which favors transcription of phase II enzyme genes and downregulates inflammation. In fact, Nrf‐2 is essential for the antioxidant responsive element (ARE)‐mediated induction of endogenous antioxidant enzymes such as heme oxygenase 1 (HO‐1), NAD(P)H dehydrogenase [quinone] 1 (NQO‐1), and GCL. 41 Interestingly, it has been proposed that targeting the heme‐heme oxygenase system may prevent severe complications following COVID‐19 infection. 42 In parallel, NAC inhibits the oxidative stress‐mediated activation of nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NFκB) and biochemical pathways upregulating pro‐inflammatory genes. 14 NAC also reduced the intracellular hydrogen peroxide concentration and restored the intracellular total thiol contents by inhibiting NFκB translocation to the cellular nucleus and phosphorylation of p38 mitogen‐activated protein kinase (MAPK p38). 43 In influenza infection, NAC inhibits the induction of pro‐inflammatory cytokine response through endosomal toll‐like receptor 3/hemagglutinin (TLR3/HA)‐induced, ROS‐dependent NFκB activation. 44
Furthermore, NAC has anti‐inflammatory activity independently of its antioxidant activity, as shown by the finding that it inhibited the LPS‐mediated neurogenic inflammation by counteracting the release of Na,K‐ATPase (NKA), a marker for cell necrosis, which could explain the fall in IL‐6 with NAC. 45 A multicenter, randomized, placebo‐controlled trial, sequentially testing early use of intravenous NAC, followed by oral ramipril for 12 weeks, is based on the rationale that these agents have the ability to limit nitrosative stress and expression of the inflammasome activator thioredoxin interacting protein (TXNIP). 46
6. ROLE OF GSH AND PROTECTIVE EFFECTS OF NAC IN INFLUENZA AND OTHER VIRAL DISEASES. EXPERIMENTAL FINDINGS AND CLINICAL TRIALS
NAC has been demonstrated to attenuate the incidence and severity of influenza and influenza‐like illnesses. It was tested in a double‐blind trial, involving 20 Italian Centers, which enrolled 262 subjects of both sexes randomized to receive either placebo or NAC tablets (600 mg) twice daily for 6 months. Both local and systemic symptoms were sharply and significantly reduced in the NAC group. Moreover, only 25% of A/H1N1 influenza virus‐infected subjects under NAC treatment developed a symptomatic form vs 79% in the placebo group. 47
Infection by RNA viruses induces oxidative stress in host cells, and growing evidence indicates that viral replication is regulated by the redox state of the host cell and that the GSH content contributes to downregulate influenza virus replication. 48 An experimental study showed that GSH decreased the production of influenza virus particles in canine kidney cells or human small airway epithelial cells, where it inhibited the expression of viral matrix protein, caspase activation, and Fas upregulation. Moreover, administration of GSH with the drinking water to BALB/c mice decreased the viral titer in both lung and trachea 4 days after intranasal inoculation of the mouse‐adapted influenza strain A/X‐31. 49
It has also been demonstrated that NAC inhibits virus replication and expression of pro‐inflammatory molecules in adenocarcinoma human alveolar basal epithelial (A549) cells infected by the highly pathogenic H5N1 influenza virus. 50 NAC inhibited the pulmonary inflammation and edema as well as myeloperoxidase (MPO) activity, total cells, neutrophils, macrophages, TNF‐α, IL‐6, IL‐1β, and chemokine (C‐X‐C motif) ligand‐10 (CXCL‐10) in the bronchoalveolar lavage fluid and reduced the levels of toll‐like receptor 4 (TLR4) protein and mRNA in the lungs of BALB/c mice inoculated intranasally with A/swine/HeBei/012/2008/ H9N2 influenza virus. 51 A study in the same strain of mice showed that old animals had lower GSH concentrations than young animals in spleen, lymph nodes, lungs, and pancreas. The gene expression of endoplasmic reticulum stress markers involved in GSH metabolism and folding of proteins, such as Nrf2 and protein disulfide isomerase (PDI), was reduced in the lung of old mice. Treatment with the N‐butanoyl GSH derivative (GSH‐C4) of old mice infected with influenza A PR8/H1N1 virus increased GSH content in organs, reduced viral replication, and induced an immune response, in particular the Th1 (T helper‐1) cytokine profile. 52
Influenza A and B viruses and respiratory syncytial virus (RSV) are responsible for COPD by increasing inflammatory and apoptosis events through mechanisms involving ROS generation and release of mucins from epithelial cells. NAC inhibited the replication of these viruses and restored the normal functions of alveolar type II A549 cells by modulating MUC5AC overexpression and release and by inhibiting IL‐8, IL‐6, and TNF‐ α as well as NF‐κB translocation to the nucleus and phosphorylation of MAPK p38. 53
Furthermore, it has been known for almost 30 years that GSH plays an important defense role against HIV and that NAC may exert protective effects toward this viral infection. For instance, in agreement with data obtained in cells exposed to herpesvirus type 1 (DNA virus) or to Sendai (an RNA virus belonging to the family of Paramyxoviridae), it was shown that GSH inhibits HIV envelope glycoproteins (gp120) and interferes with late stages of virus replication in chronically infected macrophages, a known reservoir of the virus in the body. 54 Both GSH and NAC inhibited the induction of HIV‐1 expression in a chronically HIV‐1‐infected promonocytic cell line (U1/HIV) and in human primary monocyte/macrophages cultured in vitro, where both thiols decreased HIV‐1 p24 antigen levels as well as reverse transcriptase activity. 55
Interestingly, NAC appears to synergize with antivirals in the protection toward influenza in mouse models, as shown with ribavirin 56 and oseltamivir. 57
7. NAC IN SMOKERS
Smokers are more vulnerable to infectious diseases, as shown in the case of influenza and of the MERS‐CoV outbreak. An analysis of the literature suggests that smoking is most likely associated with the negative progression and adverse outcomes of COVID‐19. 58 Exposure to cigarette smoke increases ROS levels and causes a drop in GSH intracellular concentrations. 59 Aldehydes in cigarette smoke deplete the total available GSH pool by reacting to form nonreducible GSH‐aldehydes derivatives. 60 GSH depletion accelerates cigarette smoke‐induced inflammation and airspace enlargement, and the GSH adaptive response declines with age. 61
By increasing GSH production, NAC has the ability to modulate a large variety of smoking‐related end‐points and cancer in experimental test systems, due to many interconnected mechanisms and properties. 17 , 62 In addition, NAC was shown to attenuate several biomarker alterations in a randomized, double‐blind, placebo‐controlled, Phase II chemoprevention trial in heavy smokers who received NAC tablets (600 mg) twice daily for 6 months. 63
8. CONCLUSIONS
NAC is approved by FDA under various formulations and is popular as a health supplement, this molecule having been proposed as a nutraceutical that might aid the control of RNA viruses including influenza and coronavirus. 64 , 65 , 66 Almost 60 years of experience in the prophylaxis and therapy of a variety of clinical conditions have established the safety of this drug, even at very high doses and for long‐term treatments. Drug repurposing is the fastest strategy toward an effective and accessible treatment against COVID‐19 before a vaccine is available, 67 and molecules working via multiple mechanisms of action, such as NAC, are more likely to be effective as compared with drugs having a single target.
Based on the herein discussed mechanistic premises, NAC may be proposed both in the prevention and in the therapy of COVID‐19. In particular, the oral administration of NAC, at the dose of 600 mg twice daily, may be proposed for preventive purposes aimed at attenuating the risk of developing COVID‐19 and its severity during epidemic periods, as we previously demonstrated in the case of influenza and influenza‐like illnesses, 47 especially in elderly people and in individuals who suffer from chronic conditions that predispose them to this kind of diseases and increase their severity. Persons who have been in proximity of infected SARS‐CoV‐2 carriers, including those detected by means of smartphone contact tracing apps, may be an additional target for taking oral NAC in order to decrease the risk of developing COVID‐19.
In addition, NAC has been shown to exert protective mechanisms against a variety of conditions associated with COVID‐19 and co‐morbidities thereof, also including cardiovascular diseases. 16 Given that cardiac injury and thrombosis are well established as potentially lethal complications in COVID‐19, it is noteworthy that intravenously administered NAC has been shown to potentiate the vasodilator, anti‐inflammatory and antiaggregatory effects of nitroglycerin, and this useful interaction has been translated into improving outcomes, such as in acute myocardial infarction, unstable angina and acute pulmonary edema. 68 , 69 Administration of NAC has been included among the possible strategies aimed at preserving endothelial function and limiting microthrombosis in severe forms of COVID‐19. 70
In case of severe COVID‐19 forms with pulmonary and/or systemic symptoms, intravenously administered NAC at the high doses that are commonly used in case of paracetamol intoxication, given at the first onset of chest symptoms, would be expected to play an adjuvant therapeutic role in combination with antivirals or other drugs. A potential role of NAC and copper in combination with candidate antiviral treatments against SARS‐CoV‐2, such as remdesivir, has been hypothesized based on a systematic literature search. 71 Clearly, the potential anti‐COVID‐19 mechanisms and properties of this thiol have to be substantiated in controlled clinical trials, some of which are now in progress. 12 , 72
CONFLICT OF INTEREST
The authors declare no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
S. De Flora conceived and researched the hypothesis proposed in this manuscript and wrote the manuscript. R. Balansky and S. La Maestra critically reviewed the manuscript and contributed to the hypothesis exploration. All authors read and approved the published version of the manuscript.
Notes
De Flora S, Balansky R, La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. The FASEB Journal. 2020;34:13185–13193. 10.1096/fj.202001807 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1096/fj.202001807
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7436914
Citations & impact
Impact metrics
Article citations
Morphine self-administration is inhibited by the antioxidant N-acetylcysteine and the anti-inflammatory ibudilast; an effect enhanced by their co-administration.
PLoS One, 19(10):e0312828, 29 Oct 2024
Cited by: 0 articles | PMID: 39471200 | PMCID: PMC11521314
Cytokine Storm-Induced Thyroid Dysfunction in COVID-19: Insights into Pathogenesis and Therapeutic Approaches.
Drug Des Devel Ther, 18:4215-4240, 20 Sep 2024
Cited by: 0 articles | PMID: 39319193 | PMCID: PMC11421457
Review Free full text in Europe PMC
Uncovering the Effect of SARS-CoV-2 on Liver Metabolism via Genome-Scale Metabolic Modeling for Reprogramming and Therapeutic Strategies.
ACS Omega, 9(13):15535-15546, 22 Mar 2024
Cited by: 0 articles | PMID: 38585079 | PMCID: PMC10993323
Protective effects of N-acetylcysteine and S-adenosyl-Lmethionine against nephrotoxicity and immunotoxicity induced by ochratoxin A in rats.
Int J Health Sci (Qassim), 18(2):17-24, 01 Mar 2024
Cited by: 0 articles | PMID: 38455596
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.
NPJ Sci Food, 8(1):19, 30 Mar 2024
Cited by: 2 articles | PMID: 38555403 | PMCID: PMC10981760
Review Free full text in Europe PMC
Go to all (100) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Where are we with the use of N-acetylcysteine as a preventive and adjuvant treatment for COVID-19?
Eur Rev Med Pharmacol Sci, 26(2):715-721, 01 Jan 2022
Cited by: 9 articles | PMID: 35113447
Review
Oral high-dose acetylcysteine: Effective against the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)?
Drug Discov Ther, 16(3):139-141, 06 May 2022
Cited by: 1 article | PMID: 35527020
Vitamin D and COVID-19: A review on the role of vitamin D in preventing and reducing the severity of COVID-19 infection.
Protein Sci, 30(11):2206-2220, 04 Oct 2021
Cited by: 11 articles | PMID: 34558135 | PMCID: PMC8521296
Review Free full text in Europe PMC
N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2.
Med Hypotheses, 143:109862, 30 May 2020
Cited by: 66 articles | PMID: 32504923 | PMCID: PMC7261085
 1
1





