Abstract
Free full text

Guillain-Barré Syndrome Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Detection and Coronavirus Disease 2019 in a Child
Abstract
Coronavirus disease (COVID-19) is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Physicians in China reported what is believed to be the first adult case of a SARS-CoV-2 infection associated with acute Guillain-Barré syndrome (GBS), followed by 5 adult Italian patients and another case in the United States. In the current report, we present one of the first descriptions of an association of GBS and SARS-CoV-2 infection in a child. In our facility, an 11-year-old boy presented with typical features of GBS and, after 5 days, a morbilliform skin rash over the palms of both hands. Three weeks before the start of the neurological symptoms, the boy had experienced an episode of mild febrile illness with mild respiratory manifestations and a persistent cough. The diagnosis of SARS-CoV-2 infection was confirmed by oropharyngeal swab on reverse-transcription polymerase chain reaction assay. The disease course of our patient strongly suggests a possible relationship between the development of GBS and SARS-CoV-2 infection. The case is discussed in view of previous case reports regarding the association of GBS and COVID-19.
Coronavirus disease (COVID-19) is caused by the infectious coronavirus strain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The most predominant symptom in adult patients with SARS-CoV-2 infection is pneumonia, sometimes resulting in severe acute respiratory distress syndrome (SARS), necessitating admission to the intensive care unit [1]. In contrast, only limited data are available on the disease course of SARS-CoV-2 infection in children, and most of them only have a mild to moderate respiratory illness, seldom requiring hospital admission [2].
There is increasing evidence that coronaviruses may also affect the central nervous system, inducing neurological disorders [1].Some SARS-CoV-2–infected patients may show neurologic signs (eg, headache, nausea, and vomiting) and may also develop other neurological manifestations such as acute cerebrovascular disease and impaired consciousness. Thus, for clinicians it is important to be aware of these neuroinvasive properties of SARS-CoV-2 when caring for patients with COVID-19 [1].
Guillain-Barré syndrome (GBS) is an acute/subacute-onset polyradiculoneuropathy typically presenting with sensory symptoms and weakness developing over several days, often leading to temporary quadriparesis. Approximately 70% of patients report a recent preceding upper or lower respiratory tract infection or gastrointestinal illness. GBS associated with COVID-19 infection has been described only in 7 adult patients [3–5]. In the current report, we present one of the first descriptions of an association of GBS and SARS-CoV-2 detection in a child.
CASE PRESENTATION
An 11-year-old Palestinian boy presented in the emergency room in our facility on April 10, 2020, with acute onset of unsteady gait and the inability to walk or climb stairs associated with tingling sensation felt in both the legs and feet of 1-day duration. The parents mentioned that their son had developed an acute upper respiratory tract infection with low-grade fever on March 20, 2020, for which he received acetaminophen (paracetamol) and azithromycin for 3 days. Although the fever subsided, the patient had a persistent mild dry cough. The parents denied any exposure to any suspected or confirmed cases of COVID-19. There was no recent history consistent with gastrointestinal illness, nor had he been immunized recently prior to his illness.
The patient was admitted to the pediatric intensive care unit on April 10 for further assessment and management. On physical examination, he appeared to be conscious, attentive, and well oriented with no symptoms or signs suggestive of cranial nerve involvement. Initial neurological assessment showed symmetrical weakness affecting lower limb muscle groups with reduced motor power (3/5), hypotonia, lost ankle and knee reflexes, tingling sensations, and an impaired sensation regarding pain and light touch of both feet up to the mid-legs with impaired proprioception. The neurological examination of the upper limbs revealed normal power and tone, yet reflexes were elicited with reinforcement. The vital signs upon admission showed normal body temperature (37°C), pulse rate 121 beats per minute, respiratory rate 22 breaths per minute, blood pressure 127/82 mm Hg, and oxygen saturation 97% on room air.
On April 10, magnetic resonance imaging of the brain and whole spine was performed and revealed enhancement of the cauda equina nerve roots on postcontrast findings (Figure 1A and and1B),1B), which supported the diagnosis of GBS. Nerve conduction velocity studies of the right median nerve, right tibial nerve, and left peroneal nerve revealed delayed latencies and low amplitude with dispersion of compound muscle action potentials, and with no F-wave response with impaired sensory conduction, which is consistent with demyelinating polyneuropathy, matching the clinical presentation of GBS. The patient was diagnosed as a case of GBS and received intravenous immunoglobulin infusions at a dose of 1 g/kg/day for 2 days.
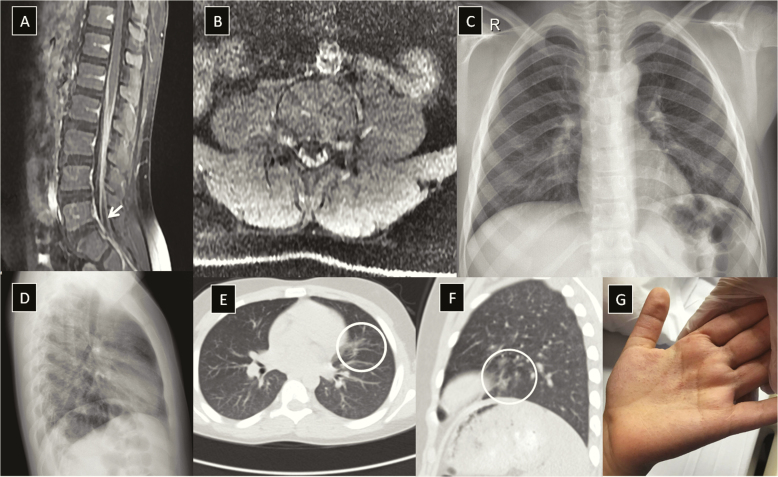
A and B, Sagittal and axial T1 fat saturation postcontrast magnetic resonance imaging showing cauda equina nerve root enhancement (white arrow) indicative of Guillain-Barré syndrome. C and D, Chest radiograph postanterior and left lateral views showing bilateral paracardiac and basal veiling. E and F, Computed tomography of the chest showing small patchy subsegmental faint opacity with atelectasis band in the lingula on the week after admission (white circles). G, Morbilliform skin rash over the palmar aspect of the left hand.
Upon admission (April 10), the following laboratory tests were done: hemoglobin 11.2 g/dL, white blood cell count 5.5 × 103 (neutrophils 64.3% [normal range, 43.0%–65.0%] and lymphocytes 21.6% [normal range, 20.5%–45.5%]), platelets 356 × 103, C-reactive protein 0.5 mg/dL (normal range, 0.0–0.5 mg/dL), creatine kinase 61 ng/mL (normal range, 38–174 ng/mL), aspartate aminotransferase 22 IU/L, alanine aminotransferase 14 IU/L, total protein 10.2 g/dL (normal range, 6.4–8.3 g/dL), normal serum electrolyte levels, serum creatinine 0.5 mg/dL, serum ferritin 87.3 ng/mL (normal range, 12.0–150.0 ng/mL), and elevated D-dimer level (0.72 mg/L [normal, 0.00–0.49 mg/L]). On April 12, diagnostic cerebrospinal fluid (CSF) analysis was performed, which showed clear colorless fluid. The total cell count was 5 cells/μL, with 91% lymphocytes and 9% monocytes; CSF chemistry showed chloride 116 mmol/L (normal range, 120–130 mmol/L), glucose 65 mg/dL (normal range, 40–70 mg/dL), and high protein level (316.7 mg/dL [normal range, 15–45 mg/dL]), which confirmed the presence of albumin cytological dissociation.
In view of the current worldwide pandemic and the patient’s previous history of fever and persisting mild dry cough, a plain chest radiograph was made on April 10, showing bilateral paracardiac and basal veiling opacities (Figure 1C and and1D).1D). Chest computed tomography (CT) was done showing patchy subsegmental faint opacifications with an atelectasis in the lingula (Figure 1E and and1F).1F). Furthermore, on April 11, the boy was investigated for viral-related etiology of GBS including respiratory virus panel by multiplex polymerase chain reaction (PCR) and a fluid microbead–based assay, and all were negative (influenza A and B viruses; influenza A virus subtypes H1, H3, and H5, including subtype H5N1 of the Asian lineage; parainfluenza virus types 1–4; respiratory syncytial virus types A and B; adenovirus; metapneumovirus; rhinovirus; enterovirus; and coronaviruses 229E, HKU1, NL63, and OC43). In view of the findings of the chest radiograph and CT imaging, nasopharyngeal swab for SARS-CoV-2 was done by real-time reverse-transcription PCR (rRT -PCR) on April 11, which was almost 22 days after the initial symptoms and 2 days since the start of the neurological illness; the test result was found to be positive on April 15.
On the morning of April 15, and before starting the treatment for his SARS-CoV-2 infection, the boy developed an unusual dermatological manifestation in the form of nonpruritic morbilliform skin rash over the palmar aspect of both hands (Figure 1G). The same day, the patient was administered acetaminophen when needed (10 mg/kg/dose) for his persisting mild respiratory symptoms and was prescribed hydroxychloroquine twice daily (6.5 mg/kg) for 1 day, and then (3.25 mg/kg) twice daily for 7 days. As thromboprophylaxis, low-molecular-weight heparin (R/enoxaparin) was given subcutaneously 20 IU once daily.
Regarding the neurological manifestations, the child showed gradual improvement as to motor power and ambulation, yet hyporeflexia persisted during the 14 days of admission.
Throughout the admission, the boy was maintaining droplet and contact isolation and was monitored for vital signs and oxygen saturation on room air. On April 20 and 22, 2 nasopharyngeal swabs were taken and yielded negative PCR results for SARS-CoV-2. The patient was discharged home on April 25, with neurologically improved lower limb power, balanced gait, decreased numbness, and normal proprioception and the COVID-19–related respiratory manifestations were resolved. One week after discharge, a follow-up nerve conduction study revealed improvement regarding the previously impaired distal latencies, conduction velocities, and amplitudes with recorded but delayed F-waves.
DISCUSSION
In this report, we describe an 11-year-old boy who presented with acute GBS, 3 weeks after he had an episode of upper respiratory tract infection with low-grade fever; he appeared to recover with only a persistent mild dry cough. Our patient had SARS-CoV-2 detected via nasopharyngeal RT-PCR, with pulmonary CT imaging findings that corresponded with his mild symptomatic respiratory illness. This is in contrast with a recent report that assessed 64 pediatric cases (children aged 0–17 years), showing that most children with SARS-CoV-2 infection had mild to moderate illness and seldom require hospitalization. However, some children with underlying comorbidities or those diagnosed with coinfections have had severe manifestations requiring hospitalization [2].The current case history supports the existing evidence of the association between SARS-CoV-2 infection and the development of GBS, as described among adult patients [3–5].
Of note, some SARS coronavirus strains have the ability to spread via a synapse-connected route to the medullary cardiorespiratory center from the mechanoreceptors and chemoreceptors in the lung and lower respiratory airways [1]. However, there is no current evidence of direct viral invasion by SARS-CoV-2 with inflammation and/or degeneration of motor neurons and peripheral nerves as seen with certain viral infection including poliovirus, enterovirus-68, West Nile virus, herpes zoster, and cytomegalovirus. On the other hand, molecular mimicry was documented between the “prototype” ganglioside GM1 (monosialotetrahexosylganglioside) and the lipo-oligosaccharide component of Campylobacter jejuni isolated from GBS patients [6].
Looking at the disease course in our patient, the clinical and CT findings at admission, and the positive RT-PCR tests, we can be sure that the original upper respiratory infection was a SARS-CoV-2 infection. Doctors are seeing positive PCR results 6 and 8 weeks after first presentation. The PCR test detects pieces of viral RNA, which is critical information for diagnosis, but it is a poor test of cure, as emerging data suggest. It cannot tell whether someone is infectious or not [7].
Our patient developed morbilliform rash on the palms of both hands during the course of the disease; the skin rash appeared before starting hydroxychloroquine treatment, and this excludes a drug-related skin reaction. Recently, in a nationwide consensus study that included 375 SARS-CoV-2–infected patients, classification of the cutaneous manifestations related to COVID-19 disease and their timing in relation to symptoms of the disease, severity, and prognosis were documented [8]. In contrast to the morbilliform skin rash seen in our patient, different patterns of skin lesions associated with COVID-19 were described such as acral areas of erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedo or necrosis (6%) [8].
In summary, our case is considered to be one of the first pediatric patients presenting a possible association between GBS and SARS-CoV-2 infection. Awareness of neuromuscular presentations in children may have a guiding significance for early detection of the combined or preceding infection with SARS-CoV-2.
Notes
Acknowledgments. We thank Ms Anna M. H. St Rose for help with improving the English of our manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Articles from Journal of the Pediatric Infectious Diseases Society are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/jpids/piaa086
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jpids/article-pdf/9/4/510/33761826/piaa086.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/jpids/piaa086
Article citations
Study of the effectiveness of telemedicine-based pulmonary rehabilitation in recovered patients of COVID-19 pneumonitis.
J Family Med Prim Care, 13(6):2237-2241, 14 Jun 2024
Cited by: 0 articles | PMID: 39027852 | PMCID: PMC11254038
The Impact of COVID-19 on the Guillain-Barré Syndrome Incidence.
Biomedicines, 12(6):1248, 04 Jun 2024
Cited by: 0 articles | PMID: 38927455
Review
Neurological complications after COVID-19: A narrative review.
eNeurologicalSci, 33:100485, 15 Nov 2023
Cited by: 6 articles | PMID: 38077923 | PMCID: PMC10700397
Review Free full text in Europe PMC
Guillain-Barré Syndrome Associated with SARS-CoV-2 in Two Pediatric Patients.
Sultan Qaboos Univ Med J, 23(3):400-404, 28 Aug 2023
Cited by: 1 article | PMID: 37655082 | PMCID: PMC10467552
Manifestation of Guillain-Barre Syndrome in a Case of Monkeypox Virus Infection: A Rare Case Report.
Case Rep Infect Dis, 2023:2426659, 11 Sep 2023
Cited by: 0 articles | PMID: 37727329 | PMCID: PMC10506871
Go to all (59) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic.
J Neurol Neurosurg Psychiatry, 91(10):1105-1110, 27 Aug 2020
Cited by: 84 articles | PMID: 32855289
Review
A Rare Axonal Variant of Guillain-Barré Syndrome as a Neurological Complication of COVID-19 Infection.
Arch Iran Med, 23(10):718-721, 01 Oct 2020
Cited by: 8 articles | PMID: 33107316
A Case of Guillain-Barré Syndrome Associated With COVID-19.
J Investig Med High Impact Case Rep, 8:2324709620961198, 01 Jan 2020
Cited by: 19 articles | PMID: 32981333 | PMCID: PMC7545753
Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature.
J Peripher Nerv Syst, 25(2):204-207, 26 May 2020
Cited by: 132 articles | PMID: 32388880 | PMCID: PMC7273104
Review Free full text in Europe PMC





