Abstract
Free full text

COVID-19 is, in the end, an endothelial disease
Abstract
The vascular endothelium provides the crucial interface between the blood compartment and tissues, and displays a series of remarkable properties that normally maintain homeostasis. This tightly regulated palette of functions includes control of haemostasis, fibrinolysis, vasomotion, inflammation, oxidative stress, vascular permeability, and structure. While these functions participate in the moment-to-moment regulation of the circulation and coordinate many host defence mechanisms, they can also contribute to disease when their usually homeostatic and defensive functions over-reach and turn against the host. SARS-CoV-2, the aetiological agent of COVID-19, causes the current pandemic. It produces protean manifestations ranging from head to toe, wreaking seemingly indiscriminate havoc on multiple organ systems including the lungs, heart, brain, kidney, and vasculature. This essay explores the hypothesis that COVID-19, particularly in the later complicated stages, represents an endothelial disease. Cytokines, protein pro-inflammatory mediators, serve as key danger signals that shift endothelial functions from the homeostatic into the defensive mode. The endgame of COVID-19 usually involves a cytokine storm, a phlogistic phenomenon fed by well-understood positive feedback loops that govern cytokine production and overwhelm counter-regulatory mechanisms. The concept of COVID-19 as an endothelial disease provides a unifying pathophysiological picture of this raging infection, and also provides a framework for a rational treatment strategy at a time when we possess an indeed modest evidence base to guide our therapeutic attempts to confront this novel pandemic.
Introduction
The vascular endothelium provides the crucial interface between the blood compartment and tissues. The endothelial monolayer that lines the intima of arteries, veins, and microvessels measures up to 7000 m2 in surface area.1 The endothelium possesses a series of remarkable properties that contribute capitally to homeostasis (Figure 1, left). The endothelium furnishes one of the only surfaces, either natural or synthetic, that under physiological conditions maintains blood in a liquid state during prolonged contact. The endothelium displays a tightly regulated palette of functions that control vasomotion, inflammation, oxidative stress, vascular permeability, and structure.2 The endothelial cells also provide a crucial interface in host defences, forming the front line of encounter with bloodborne pathogens, thus sensing danger threatening the organism in a concerted fashion, sending early warning signals of infection, invasion, or injury.3 While these functions participate in the moment-to-moment regulation of the circulation and coordinate many host defence mechanisms, they can also contribute to disease when their usually homeostatic and defensive functions over-reach and turn against the host (Figure 1, middle and right). 4 , 5
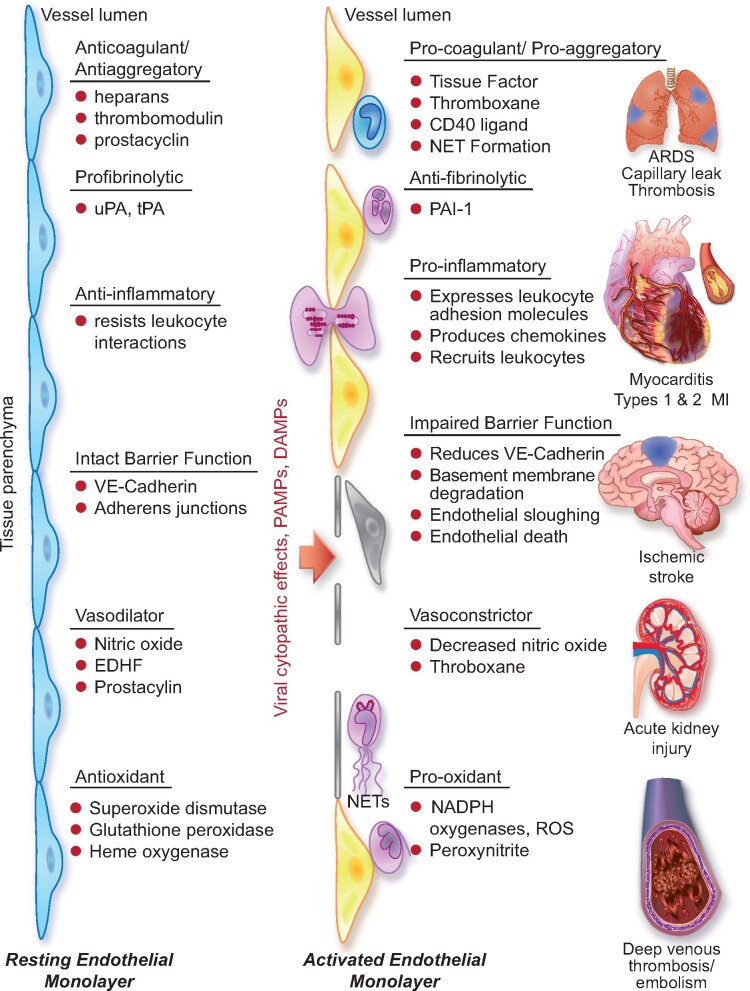
The left side of the diagram depicts a resting endothelial monolayer with the endothelial cells of squamous morphology resting on an intact basement membrane. The homeostatic mechanisms displayed by the resting endothelium include the listed properties as detailed in the text. When the endothelial cells undergo the cytopathic effect of a viral infection such as SARS-CoV-2, or encounter pathogen-associated molecular patterns (PAMPs) derived from viruses or bacteria such as lipopolysaccharide, proinflammatory cytokines such as IL-1 or TNF, or damage-associated molecular patterns (DAMPs) derived from dead or dying cells, the endothelial cells become activated. The endothelial cells display more columnar morphology. They can express adhesion molecules that attract leucocytes and chemokines that direct their migration into the subendothelial space. Sloughing of endothelial cells uncovers the thrombogenic basement membrane. Adherent neutrophils can undergo formation of neutrophil extracellular traps that provide an amplifier for endothelial damage mediated in part by IL-1α. Inflammatory activation of endothelial cells can disrupt VE-cadherin largely responsible for the integrity of the endothelial barrier function.62 Activated endothelial cells can also express matrix metalloproteinases that can degrade the basement membrane and further interrupt endothelial barrier function. In small vessels, such as those that embrace alveoli in the lung, this impaired barrier function can lead to capillary leak. These various disturbances in endothelial function, depicted in the middle part of the diagram, lead to end organ damage including adult respiratory distress syndrome and thrombosis in the lungs, predispose to plaque rupture and thrombosis in coronary arteries, and affect the microvasculature leading to myocardial ischaemia and damage. The thrombotic diathesis provoked by endothelial dysfunction can also predispose towards strokes. Microvascular as well macrovascular injury can potentiate acute renal failure. Hepatic dysfunction can also result from microvascular thrombosis among other mechanisms. Deep venous thrombosis can occur as endothelial disfunction represents an important part of Virchow’s triad, and sets the stage for pulmonary embolism. Thus, loss of the endothelial protective and unleashing of the mechanisms depicted can lead to multiorgan system failure that characterizes the advanced stages of COVID-19.
SARS-CoV-2, the aetiological agent of COVID-19, causes the current pandemic. It produces protean manifestations ranging from head to toe, wreaking seemingly indiscriminate havoc on multiple organ systems, in particular the lungs, heart, brain, kidney, and vasculature. This essay will explore the hypothesis that COVID-19, particularly in the later complicated stages, represents an endothelial disease. This concept not only provides a unifying pathophysiological picture of this raging infection but also furnishes a framework for a rational treatment strategy at a time when we possess an indeed modest evidence base to guide our therapeutic attempts to confront this novel pandemic.
The endothelium participates pivotally in thrombosis and fibrinolysis
The normal endothelial surface owes its remarkable haemocompatibility to a tightly orchestrated set of functions.6 Heparan sulfate proteoglycans decorate the surface of the endothelium. These molecules bind antithrombin III, as do heparinoids that we use daily in practice as an anticoagulant. The endothelial surface bears thrombomodulin, which binds thrombin and stimulates the protein C–protein S anticoagulant axis.1 , 3 The endothelial cell can also express a tissue factor pathway inhibitor that can antagonize triggering of thrombosis by the potent procoagulant protein tissue factor.7
Endothelial cells possess an endogenous mechanism for combatting platelet activation. This function depends on endothelial surface expression of an ecto-ADPase, CD39, as well as the release of nitric oxide and prostacyclin.8 , 9 Together, this array of anticoagulant and antithrombotic properties accounts for much of the ability of the endothelial cell to combat intravascular blood clot formation under normal circumstances. Should a stray thrombus form on the intimal lining of a blood vessel, the endothelial cells can express plasminogen activators that can boost endogenous fibrinolysis.10 Endothelial cells can produce both tissue-type plasminogen activator (tPA) and urokinase plasminogen activator (uPA),11 and, through the release of nitric oxide by platelet-derived substances, inhibit platelet function and increase local blood flow to flush away an evolving clot.
Although the normal endothelium possesses this palette of anticoagulant, antithrombotic, and profibrinolytic attributes, the balance between these salutary functions and an opposite panel of properties that promote thrombus accumulation can change on a dynamically regulated basis. The endothelial cell usually possesses little procoagulant potential. However, when stimulated by proinflammatory cytokines, pathogen-associated molecular patterns such as bacterial endotoxins, or neutrophil extracellular traps (NETs; see below), the endothelial cell can express and in turn exert tissue factor activity.12 , 13 Tissue factor activates the coagulation system by amplifying many-fold the enzymatic capacity of factors VII and X, triggering thrombin generation and clot formation.14 The endothelial cell also stores pre-formed von Willebrand factor (vWf) in intracellular granules called Weibel–Palade bodies. Upon activation, the endothelial cells can release this large protein that in higher molecular weight multimers provides a potent bridge for platelet aggregates and thrombus assembly, favouring formation of an organized clot.15
While under usual circumstances the antiaggregatory arachidonate product prostacyclin (PGI2) dominates endothelial vasoactive prostanoid production, the endothelial cell can also produce thromboxane, a pro-platelet aggregatory and vasoconstrictor prostaglandin.16 The activated endothelial cell can also manufacture plasminogen activator inhibitor-1 (PAI-1), which can antagonize the endogenous fibrinolytic properties conferred upon the endothelial surface by the expression of uPA and tPA, as noted above. Thus, while ordinarily programmed to combat blood clotting and thrombus accumulation, the endothelium—when activated by inflammatory or infectious signals—can exert an opposite battery of functions. While critically important in staunching haemorrhage or other injury, during disease the endothelial surface can promote clotting of arteries, microvessels, and veins, contributing critically to thrombo-embolism.
The endothelial vasodilator/vasoconstrictor balance
Under normal conditions, the endothelial cells promote tonic vasodilatation through the well-known mechanism of production of the vasodilatory gas nitric oxide from l-arginine via the activity of endothelial nitric oxide synthase.17 The endothelial cell can also elaborate diverse hyperpolarizing factors that promote relaxation of smooth muscle and hence dilatation of muscular arteries. As noted above, the normal endothelial cells also secrete PGI2 that, in addition to its antiaggregatory effects on platelets, potently vasodilates.18 This array of vasodilatory actions can also modulate moment-to-moment local blood flow in a paracrine fashion. Numerous mechanisms can interfere with endothelial-dependent vasodilatation. These mechanisms range from impaired nitric oxide synthase expression19 and/or activity to inactivation of nitric oxide or its conversion to highly pro-oxidant compounds by encountering pro-oxidant species such as superoxide anion, yielding the potent pro-oxidant peroxynitrate.20 , 21 Moreover, the endothelial cell can produce one of the most potent vasoconstrictors known, endothelin-1, in response to angiotensin II, thrombin, or oxidized LDL.22 , 23 While key in maintaining normal vascular homeostasis, during disease the salutary endothelium’s functions can give way to inappropriate vasoconstriction contributing to tissue ischaemia.
The endothelial inflammatory balance
Positioned at the key interface between the blood and tissues, the endothelium normally resists prolonged contact with the leucocytes that abound in blood that bathes the intimal surface.3 , 24 Stationed as the sentinel, the endothelium serves as the portal governing the entry of leucocytes into tissues to combat invaders, microbial or viral, and to help repair injury and heal wounds. The interplay of the endothelium with leucocyte mediators of innate and adaptive immunity depends on a series of leucocyte adhesion molecules expressed at negligible levels under physiological circumstances. Members of the selectin class of leucocyte adhesion molecules slow the transit of blood leucocytes past the endothelial surface by mediating rolling of these cells. E-selectin (CD62E) causes polymorphonuclear leucocytes to tarry on the endothelial surface. P-selectin (CD62P) and L-selectin (CD62L) also mediate interaction of the endothelial surface with various classes of blood leucocytes. The elevated expression of these endothelial–leucocyte adhesion molecules depends upon irritative stimuli, principally proinflammatory cytokines such as interleukin-1α (IL-1α) and IL-1β or tumour necrosis factor-α (TNF-α).
The firm binding of leucocytes to the activated endothelial surface depends upon adhesion molecules of the IgG superfamily. These molecules include intercellular adhesion molecule-1 (ICAM-1, CD54) and vascular cell adhesion molecule-1 (VCAM-1, CD106). Integrins associated with the endothelial surface also participate in these adhesive interactions and furnish cognate ligands for the adhesion molecules.25 Once tightly bound to the endothelial surface, chemoattractant cytokines of various classes can beckon the bound cells to traverse the endothelial monolayer and enter tissues where they can combat invaders or contribute to tissue repair.26
Antioxidant/pro-oxidant balance in the endothelium
The endothelial cell possesses a number of defence mechanisms that lower local oxidative stress. When subjected to normal laminar shear stress, the endothelium produces superoxide dismutase that scavenges the important reactive oxygen species
Endothelial barrier function
Under physiological circumstances, the endothelial gateway selectively regulates endothelial permeability and fosters vascular integrity. An intact endothelial barrier depends on myriad mechanisms including vascular endothelial-cadherin (VE-cadherin, CD144).33 A number of derangements can threaten the integrity of this single-cell layer that stands between the blood compartment and tissues. Impaired endothelial viability can promote sloughing of endothelial cells and their death by various mechanisms including pyroptosis and apoptosis.34 , 35 Among the stimuli for these pathways of programmed cell death are proinflammatory cytokines and reactive oxygen species. Endothelial cells can also perish due to accidental cell death or oncosis. Regardless of the mechanism of endothelial injury, breaches in the physical integrity of the monolayer can lead to capillary leak in the microvasculature, overturning the usually semi-permeable properties of the endothelium and contributing to inappropriate leakage of vascular contents into the tissue compartment and extracellular space.36 , 37
Cytokine storm: a perfect storm in COVID-19
As noted in each of the foregoing sections, proinflammatory cytokines conspire to elicit from endothelial cells a change from their homeostatic functions to those that can contribute to thrombosis and local tissue injury. Cytokines such as IL-1α and IL-1β, IL-6, and TNF-α, among others, contribute critically to normal host defences, but when produced inappropriately or in excess they can perturb all of the carefully orchestrated protective functions of the normal endothelium and potentiate pathological processes. The untrammelled production of proinflammatory cytokines contributes to a condition termed a cytokine storm (Figure 2). The pathophysiological mechanisms of a cytokine storm depend on phenomena described in the 1980s that centre on autoinduction of the primordial proinflammatory cytokine IL-1. IL-1 can induce its own gene expression, providing an amplification loop that can instigate a cytokine storm.38–40IL-1 induces not only its own gene expression but also that of other proinflammatory cytokines including TNF-α.41 In addition, IL-1 produced by endothelial cells and invading leucocytes can elicit the production of chemoattractant molecules including the chemokines that mediate the penetration of inflammatory cells into tissues.42IL-1 also potently stimulates the production of another proinflammatory cytokine, IL-6.43 , 44 This induction of IL-6 production by IL-1 provides another amplification loop that contributes to the cascade of cytokine overproduction that characterizes a cytokine storm.
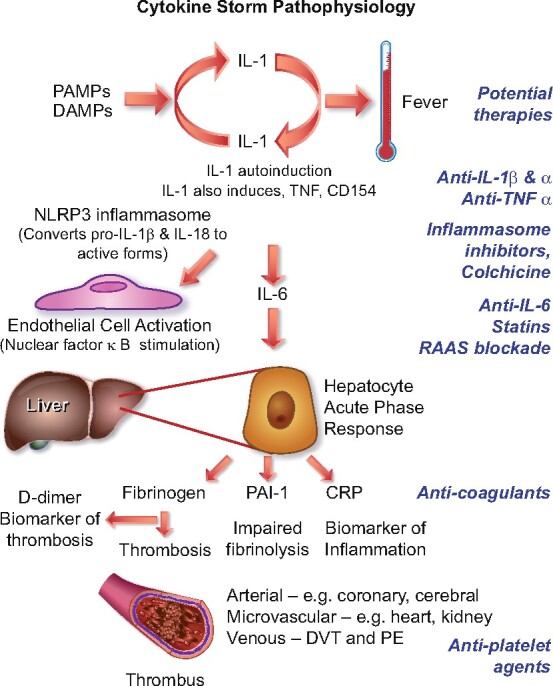
Cytokine storm. Proinflammatory cytokines such as IL-1 and TNF-α induce each other’s gene expression, unleashing an amplification loop that sustains the cytokine storm. The endothelial cell is a key target of cytokines, as they induce action of a central proinflammatory transcriptional hub, nuclear factor-κB. IL-1 also causes substantial increases in production by endothelial and other cells of IL-6, the instigator of the hepatocyte acute phase response. The acute phase reactants include fibrinogen, the precursor of clot, and PAI-1, the major inhibitor of our endogenous fibrinolytic system. C-reactive protein, commonly elevated in COVID-19, provides a readily measured biomarker of inflammatory status. The alterations in the thrombotic/fibrinolytic balance due to the acute phase response promotes thrombosis in arteries, in the microvasculature including that of organs such as the myocardium and kidney, and in veins, causing deep vein thrombosis and predisposing towards pulmonary embolism. Thus, the very same cytokines that elicit abnormal endothelial functions can unleash the acute phase response which together with local endothelial disfunction can conspire to cause the clinical complications of COVID-19. The right side of this diagram aligns therapeutic agents that attack these mechanisms of the cytokine storm and may thus limit its devastating consequences.
In addition to local effects, IL-6 provides a proximal stimulus to the acute phase response.45 This programne of protein synthesis elicited in the hepatocyte by IL-6 boosts the synthesis of fibrinogen, the precursor of clots, of PAI-1, a major inhibitor of our endogenous fibrinolytic mediators, and of C-reactive protein, a biomarker of inflammation that rises consistently in COVID-19.46 The havoc wreaked by the cytokine storm thus not only affects local endothelial function but can also provoke a prothrombotic and antifibrinolytic imbalance in blood that favours thrombus accumulation. The complications of COVID-19 follow very closely the consequences of excessive cytokine actions on endothelial cells outlined above and depicted in Figure 1.
The initial characterization of COVID-19 as a pneumonitis incorporates the notion of disordered endothelial function. While initial infection of type I and II pneumocytes and alveolar macrophages no doubt participates in the initiation of infection, disordered endothelial function certainly contributes to the ongoing ravages of SARS-CoV-2 in the lung as elsewhere. Impaired endothelial barrier function can contribute to protein accumulation in the alveolar space and fluid accumulation and impaired oxygenation of the blood. IL-1 stimulation reduces VE-cadherin, dubbed the guardian of integrity of the endothelium. This finding links a cytokine storm directly to capillary leak, and aggravation of the adult respiratory syndrome (ARDS) picture that advanced COVID-19 presents.33 , 47 The deranged balance in the prothrombotic/antithrombotic properties of the endothelium can certainly contribute to thrombosis in situ in the pulmonary vasculature, as occurs in COVID-19.48 Impaired gateway function of the endothelium for traversal of leucocytes into tissues clearly participates in pneumonitis.
However, we now recognize that SARS-CoV-2’s destructive actions range far and wide beyond the pulmonary parenchyma. Alterations in endothelial thrombotic/fibrinolytic balance can predispose to thrombosis not only in the pulmonary circulation but also in peripheral veins and arteries of the cerebral circulation, causing unheralded strokes in apparently healthy young people and doubtless contributing to the local and patchy embarrassment of blood flow in ‘COVID toes’ that probably represent microvascular dysfunction with tissue ischaemia. In between the brain and distal lower extremities, thromboses can occur in all arterial beds within the microvasculature, including that of the coronary circulation, and that of the kidneys. Venous thrombosis and pulmonary embolism also commonly complicate COVID-19, pathological processes that clearly depend on deranged endothelial functions.49 NETs induced by inflammatory cytokines activate procoagulant functions of endothelial cells, and contribute to coagulation and the formation of the typically tightly organized thrombi in COVID-19. Thus, disordered endothelial homeostasis provoked by cytokines provides a common thread in numerous complications of COVID-19.13 , 50–52
Endothelial functions as a therapeutic target
To combat the adverse balance between thrombotic and fibrinolytic properties of the endothelium, numerous anticoagulant and antiplatelet therapies are under evaluation in ongoing and planned clinical trials in COVID-19. Key questions that require an answer in this domain are which agents to give to whom and in what doses, given the narrow therapeutic window of such agents, and the common concomitant conditions that elevate bleeding risk in many COVID-19 patients.
In view of the central role of inflammatory mediators in the complications of COVID-19, anti-inflammatory therapies merit careful clinical evaluation. Glucocorticoids and colchicine exert generalized anti-inflammatory actions and show promise in the treatment of patients with advanced COVID-19.53 , 54 Statins have direct anti-inflammatory effects beyond their lipid-lowering actions, mediated by inhibition of prenylation of small G proteins or induction of transcription factors such as KLF-2 that promote homeostatic endothelial functions.55Non-randomized treatment with statins yielded preliminary retrospective evidence of improved outcomes in COVID-19, as well as reductions in biomarkers of inflammation.56
Targeted inhibition of cytokines, major effectors of endothelial activation, represents a more focused approach than generalized anti-inflammatory agents. IL-1 not only induces leucocyte adhesion molecules but, by reducing VE-cadherin production, can contribute to impaired endothelial barrier function and thus capillary leak, a major issue that complicates COVID-19 pneumonitis.47 Agents that inhibit the inflammasome–IL-1β–IL-6 pathway may thus comprise a more endothelial-directed approach to treatment of COVID-19. Some clinical trials that use such strategies have already yielded preliminary results; some, but not all, indicate signals of efficacy. Colchicine may act in part as an inhibitor of the assembly of the inflammasome. Small, non-randomized studies of a recombinant form of the endogenous IL-1 receptor antagonist, anakinra, have furnished sufficient encouragement to merit further definitive investigation.57 , 58 Anakinra blocks both IL-1α and IL-1β, and requires daily dosing. Canakinumab, a selective IL-1β antibody, has a much longer biological half-life than anakinra, rendering it less readily reversible. Several studies investigating canakinumab in COVID-19 are underway (NCT04362813 and NCT04365153.)
Downstream of IL-1, antibodies that interfere with IL-6 signalling have also shown signs of benefit in some but not all preliminary studies, although this as well as other anticytokine therapies may entail an increased risk of superinfection.59 , 60 Other anti-IL-6 strategies also warrant consideration.61 Upon inflammatory stimulation, vascular endothelial and smooth muscle cells produce large amounts of IL-6; thus, blocking signalling of this distal mediator can limit local vascular amplification of inflammatory responses, including in the lung. IL-6 also triggers the acute phase response, boosting fibrinogen and PAI-1 production, and thus favours clot formation and persistence (Figure 2).
The pivotal roles of these proinflammatory mediators in host defences render these initial results plausible and promising. Yet, rigorous, controlled, and prospective clinical trials must evaluate the balance between the potential benefits by forestalling the consequences of cytokine storm versus the potential of lowering defences against bacterial superinfections that commonly complicate individuals with impaired pulmonary protective functions and remain subject to the rigours of mechanical ventilation and endotracheal intubation.
In sum, we can envisage COVID-19 as a disease of the endothelium, certainly with respect to its complications. This unifying hypothesis can help to understand the complex pathophysiology of this current plague and may also help to inform our therapeutic approaches to combatting the consequences of SARS-CoV-2 infection.
Funding
P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund.
Conflict of interest: P.L. is an unpaid consultant to, or involved in clinical trials for, Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion, Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Merck, Novartis, Pfizer, and Sanofi-Regeneron. He is a member of the scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. His laboratory has received research funding in the last 2 years from Novartis. He is on the Board of Directors of XBiotech, Inc. and has a financial interest in Xbiotech, a company developing therapeutic human antibodies. His interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. T.L. has no conflicts to declare.
Contributor Information
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Thomas Lüscher, Heart Division, Royal Brompton & Harefield Hospital and National Heart and Lung Institute, Imperial College, London, UK.
References
Articles from European Heart Journal are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/eurheartj/ehaa623
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/eurheartj/article-pdf/41/32/3038/46625915/eurheartj_41_32_3038.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/eurheartj/ehaa623
Article citations
Central artery pulse pressure, not central arterial stiffness impact on all-cause mortality in patients with viral pneumonia infection.
BMC Infect Dis, 24(1):1183, 21 Oct 2024
Cited by: 0 articles | PMID: 39434023 | PMCID: PMC11492499
The home-based breathing and chest mobility exercise improves cardiorespiratory functional capacity in long COVID with cardiovascular comorbidities: a randomized study.
BMC Cardiovasc Disord, 24(1):574, 18 Oct 2024
Cited by: 0 articles | PMID: 39425012 | PMCID: PMC11488120
Comparative Analysis of Heart Rate Variability and Arterial Stiffness in Elite Male Athletes after COVID-19.
J Clin Med, 13(19):5990, 08 Oct 2024
Cited by: 0 articles | PMID: 39408050 | PMCID: PMC11477989
Arterial Thrombosis in Acute Respiratory Infections: An Underestimated but Clinically Relevant Problem.
J Clin Med, 13(19):6007, 09 Oct 2024
Cited by: 0 articles | PMID: 39408067 | PMCID: PMC11477565
Review Free full text in Europe PMC
Risk of COVID-19 infection in patients with NSCLC receiving EGFR-TKI targeted therapy during the first wave in China.
J Int Med Res, 52(10):3000605241281907, 01 Oct 2024
Cited by: 0 articles | PMID: 39387199 | PMCID: PMC11467978
Go to all (541) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy.
Rev Cardiovasc Med, 21(3):315-319, 01 Sep 2020
Cited by: 112 articles | PMID: 33070537
Review
COVID-19 May Predispose to Thrombosis by Affecting Both Vascular Endothelium and Platelets.
Clin Appl Thromb Hemost, 26:1076029620933945, 01 Jan 2020
Cited by: 11 articles | PMID: 32619104 | PMCID: PMC7495934
The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection.
Crit Care, 24(1):353, 16 Jun 2020
Cited by: 289 articles | PMID: 32546188 | PMCID: PMC7296907
Review Free full text in Europe PMC
Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19?
Cancer Metastasis Rev, 39(2):337-340, 01 Jun 2020
Cited by: 129 articles | PMID: 32385712 | PMCID: PMC7207990
Funding
Funders who supported this work.
American Heart Association (1)
Grant ID: 18CSA34080399
NHLBI NIH HHS (1)
Grant ID: R01 HL134892
National Heart, Lung, and Blood Institute (1)
Grant ID: 1R01HL134892

 and
and 



