Abstract
Free full text

Platelet gene expression and function in patients with COVID-19
Key Points
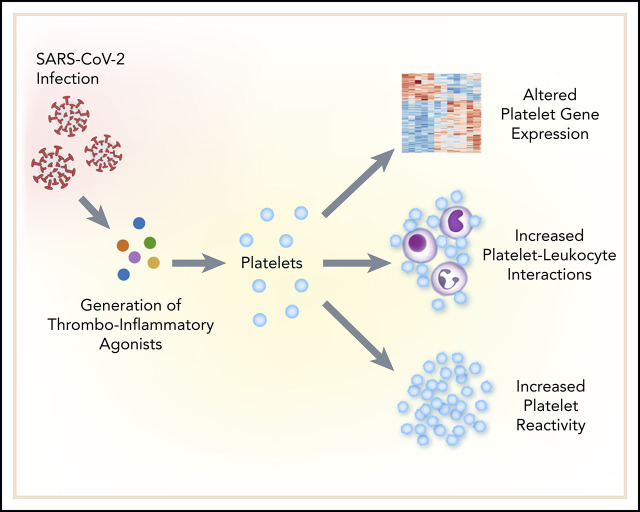
SARS-CoV-2 induces robust gene expression and functional changes in platelets.
Platelet hyperreactivity may contribute to COVID-19 pathophysiology by increased platelet-platelet and platelet-leukocyte interactions.
Abstract
There is an urgent need to understand the pathogenesis of coronavirus disease 2019 (COVID-19). In particular, thrombotic complications in patients with COVID-19 are common and contribute to organ failure and mortality. Patients with severe COVID-19 present with hemostatic abnormalities that mimic disseminated intravascular coagulopathy associated with sepsis, with the major difference being increased risk of thrombosis rather than bleeding. However, whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection alters platelet function to contribute to the pathophysiology of COVID-19 remains unknown. In this study, we report altered platelet gene expression and functional responses in patients infected with SARS-CoV-2. RNA sequencing demonstrated distinct changes in the gene-expression profile of circulating platelets of COVID-19 patients. Pathway analysis revealed differential gene-expression changes in pathways associated with protein ubiquitination, antigen presentation, and mitochondrial dysfunction. The receptor for SARS-CoV-2 binding, angiotensin-converting enzyme 2 (ACE2), was not detected by messenger RNA (mRNA) or protein in platelets. Surprisingly, mRNA from the SARS-CoV-2 N1 gene was detected in platelets from 2 of 25 COVID-19 patients, suggesting that platelets may take-up SARS-COV-2 mRNA independent of ACE2. Resting platelets from COVID-19 patients had increased P-selectin expression basally and upon activation. Circulating platelet-neutrophil, -monocyte, and -T-cell aggregates were all significantly elevated in COVID-19 patients compared with healthy donors. Furthermore, platelets from COVID-19 patients aggregated faster and showed increased spreading on both fibrinogen and collagen. The increase in platelet activation and aggregation could partially be attributed to increased MAPK pathway activation and thromboxane generation. These findings demonstrate that SARS-CoV-2 infection is associated with platelet hyperreactivity, which may contribute to COVID-19 pathophysiology.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel positive-sense single-stranded RNA betacoronavirus and the causative pathogen for the ongoing coronavirus disease 2019 (COVID-19) pandemic. Although SARS-CoV-2 infection is associated with the development of acute respiratory distress syndrome (ARDS), recent reports also describe patients who develop multiorgan failure and thrombosis, including myocardial infarction and ischemic stroke.1 Patients with cardiovascular risk factors such as obesity, diabetes, and hypertension are at increased risk for the development of thrombotic complications with 20% to 30% of critically ill COVID-19 patients having thrombosis during SARS-CoV-2 infection.2-4 Although thrombotic complications are common during SARS-CoV-2 infection, the pathological drivers of thrombosis in COVID-19 remain unclear.
In addition to their traditional role in thrombosis and hemostasis, platelets (and their precursor cells megakaryocytes) mediate key aspects of inflammatory and immune processes.5-10 Similar to innate immune cells, platelets express a broad array of receptors, including Toll-like receptors (TLRs), C-type lectin receptors, and nucleotide-binding and oligomerization domain–like receptors.8 These, and other receptors, are known to help recognize viral pathogens such as dengue, HIV-1, and influenza.11-15 Once platelets sense the invading pathogens, they mediate immune responses indirectly through the release of cytokines and antimicrobial peptides and directly through interaction with neutrophils, monocytes, and lymphocytes to amplify the immune response.16-19 Because platelet activation often occurs when platelets respond to invading pathogens, inflammatory and infectious illnesses are frequently associated with a prothrombotic response, termed immunothrombosis. Immunothrombosis may drive adverse immunological and hemostatic processes, thereby contributing to adverse clinical outcomes such as vascular thrombosis, organ failure, and death.20 However, whether platelets contribute to the pathophysiology of SARS-CoV-2 infection, including thrombosis, is unknown.
In this prospective clinical study, we examined platelet gene expression and functional responses in acutely ill patients with COVID-19, compared with matched healthy donors. RNA-sequencing (RNA-seq) analysis from platelets isolated from SARS-CoV-2–infected patients revealed significant changes in platelet gene expression, including changes in immune pathways. Although platelet ultrastructure was intact, platelets were hyperreactive during SARS-CoV-2 infection, as evidenced by increased surface P-selectin expression basally and upon stimulation, and greater formation of circulating platelet-leukocyte aggregates (PLAs). Platelets from COVID-19 patients also exhibited increased aggregation, adhesion, and spreading. These hyperreactive responses were driven partly through increased generation and release of thromboxane A2. Taken together, our data provide new evidence that platelet gene expression is altered and functional responses are significantly increased during SARS-CoV-2 infection. We postulate that these changes may contribute to thrombotic events in COVID-19 patients.
Methods
For full details on methods, please see supplemental Methods (available on the Blood Web site).
Study design
Patients acutely ill with SARS-CoV-2 infection (n = 41) were recruited from the University of Utah Health Sciences Center in Salt Lake City. SARS-CoV-2 infection was confirmed by reverse transcription polymerase chain reaction (PCR; RT-PCR), in accordance with current standards. We collected acid citrate dextrose (ACD)-anticoagulated whole blood from patients hospitalized with COVID-19 from 17 March 17 to 5 June 2020. All COVID-19 patients were recruited under study protocols approved by the institutional review board (IRB) of the University of Utah (IRB# 00102638, 00093575). All patients enrolled in our study were hospitalized and were enrolled within 72 hours of hospital or intensive care unit (ICU) admission. Healthy, age-, and sex-matched donors were enrolled under a separate IRB protocol (IRB# 0051506). Each study participant or their legal authorized representative gave written informed consent for study enrollment in accordance with the Declaration of Helsinki. Enrollment criteria included age >18 years, respiratory symptoms (cough, shortness of breath) or fever, hospital admission, positive SARS-CoV-2 testing, and informed consent. We summarize demographic and illness severity data or Sequential Organ Failure Assessment (SOFA) scores in Table 1. All COVID-19 patients were hospitalized and were further stratified into non-ICU and critically ill ICU patients.
Table 1.
Clinical characteristics of healthy donors and hospitalized patients with COVID-19
| Healthy donors, n = 17 | COVID-19 patients | P | ||
|---|---|---|---|---|
| Non-ICU, N = 24 | ICU, N = 17 | |||
| Mean age (±SD), y | 49.9 (±26.8) | 48.1 (±15.9) | 62.5 (±14.4) | .023 |
| Male, % | 53 | 45.8 | 58.8 | .63 |
| Hispanic/Latino, % | 12 | 29.2 | 47.1 | .075 |
| Mean BMI (±SD) | 33.6 (±9.0) | 30.6 (±8.8) | .31 | |
| Diabetes, % | 0 | 25.0 | 64.7 | <.001 |
| Hypertension, % | 0 | 33.3 | 47.1 | .279 |
| Mean SOFA (±SD) | 1.8 (±1.1) | 4.7 (±1.2) | <.001 | |
| ARDS, % | 8.3 | 94.1 | <.001 | |
| MV, % | 0 | 53 | <.001 | |
| Survival to date, % | 100 | 64.7 | <.001 | |
| Aspirin, % | 12.5 | 11.8 | .95 | |
| Hydroxychloroquine, % | 16.7 | 29.4 | .34 | |
| Remdesivir, % | 20.8 | 23.5 | .84 | |
| Convalescent plasma, % | 0 | 5.9 | .24 | |
| Mean WBC (±SD) | 6.1 (±2.3) | 8.2 (±2.1) | .005 | |
BMI, body mass index; MV, mechanical ventilation; SD, standard deviation; SOFA, Sequential Organ Failure Assessment score; WBC, white blood count.
Cell isolation, differentiation, and nomenclature
Leukocyte-depleted platelets were isolated as previously described from whole blood drawn from SARS-CoV-2–infected patients and healthy donors.12,21-24
RNA preparation and sequencing
RNA-seq and analysis were performed on total RNA extracted from platelets isolated from SARS-CoV-2–infected patients and age- and sex-matched healthy donors, as before.12,21-24 RNA-seq files were deposited in NCBI short-read archives under PRJNA634489.
Real-time PCR
For messenger RNA (mRNA) real-time PCR, isolated RNA was reverse transcribed using Oligo dT and random hexamers as previously described.12,23,24
Flow cytometry for platelet activation and PLAs
Whole blood flow cytometry for platelet activation and PLAs was done as previously described.21,25,26
Aggregation
Aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37°C under stirred conditions as previously described.27,28
Measurement of thromboxane A2 generation
Washed platelets at a concentration of 2 × 108 platelets per milliliter were stimulated with 2-methylthio-adenosine-5′-diphosphate (2MeSADP) (5 nM, final) for 3.5 minutes. Levels of the stable analog thromboxane B2 were determined in duplicate using a Correlate-EIA thromboxane B2 enzyme immunoassay kit (Cayman Chemical) according to the manufacturer’s instructions.
Western blotting
Protein samples for platelets were prepared, and western blotting was performed, as previously described12,23,27,28
Confocal and transmission electron microscopy
High-resolution, confocal reflection microscopy was performed using an Olympus IX81, FV300 (Melville, NY) confocal-scanning microscope equipped with a 60×/1.42 NA oil objective. For ultrastructural analyses, platelets were adhered to Acylar coated with poly-l-lysine and fixed in 2.5% glutaraldehyde in phosphate-buffered saline and processed as previously described.24
Statistical analyses
Continuous variables from all experiments were assessed for normality with skewness and kurtosis tests. Data that were normally distributed were expressed as a mean plus or minus the standard error of the mean. For analyses involving 2 groups, a parametric 2-tailed Student t test was used. When 3 or more groups were analyzed, an analysis of variance with a Tukey post hoc test was performed. When data were not normally distributed, a Mann-Whitney was used when 2 groups were analyzed; a Kruskal-Wallis with a Dunn multiple comparison post hoc test was used for analyses of 3 or more groups. For correlations, we performed a Spearman correlation. Summary statistics were used to describe the study cohort and clinical variables were expressed as the mean plus or minus standard deviation or as a number and percentage. Statistical analyses were performed by using GraphPad Prism (version 7; San Diego, CA). A 2-tailed P value <.05 was considered statistically significant.
Results
COVID-19 patient cohort
COVID-19 patients were well matched by age, sex, and race to healthy donors (Table 1). SARS-CoV-2 virus was uniformly detected by PCR in patients diagnosed with SARS-CoV-2 infection. Approximately 41% of COVID-19 patients were in the ICU, of whom 53% required mechanical ventilation due to respiratory failure. Hospitalized non-ICU and ICU COVID-19 patients had comorbidities consistent with previously published studies, including hypertension, diabetes, and obesity.3,29 We observed an ~17% mortality rate among all COVID-19 patients, consistent with hospitalized mortality rates (13.4%) in COVID-19 patients in Utah per the Utah Department of Utah as of 8 June 2020.
The human platelet transcriptome is altered during SARS-CoV-2 infection
We and others have used transcriptomics to provide insights into how platelets are altered during acute inflammatory and infectious stressors, including viral pandemic pathogens.12,21,23 To determine whether the platelet transcriptome is altered in patients during SARS-CoV-2 infection, we performed RNA-seq on RNA isolated from highly purified platelets from 6 non-ICU and 4 ICU COVID-19 patients and 5 matched healthy donors recruited from the greater Salt Lake City area. As shown in Figure 1A, non-ICU and ICU COVID-19 patients clustered together, but separately from healthy controls in hierarchical clustering of transcriptome-wide gene expression, suggesting systemic alterations in platelet RNA expression. Differential gene-expression analysis revealed 3090 differentially expressed genes between non-ICU patients compared with healthy donors whereas 2256 were differentially expressed in ICU patients compared with healthy donors (false discovery rate < 0.05) (Figure 1B-D; supplemental Tables 1 and 2). Only 16 genes were differentially expressed in a direct comparison between non-ICU and ICU COVID-19 patients, suggesting minimal impact of ICU status (supplemental Table 3) on COVID-19–induced gene changes. Analysis of combined non-ICU and ICU patients (all COVID-19) compared with healthy donors identified 3325 differentially expressed genes (supplemental Figure 1; supplemental Table 4).
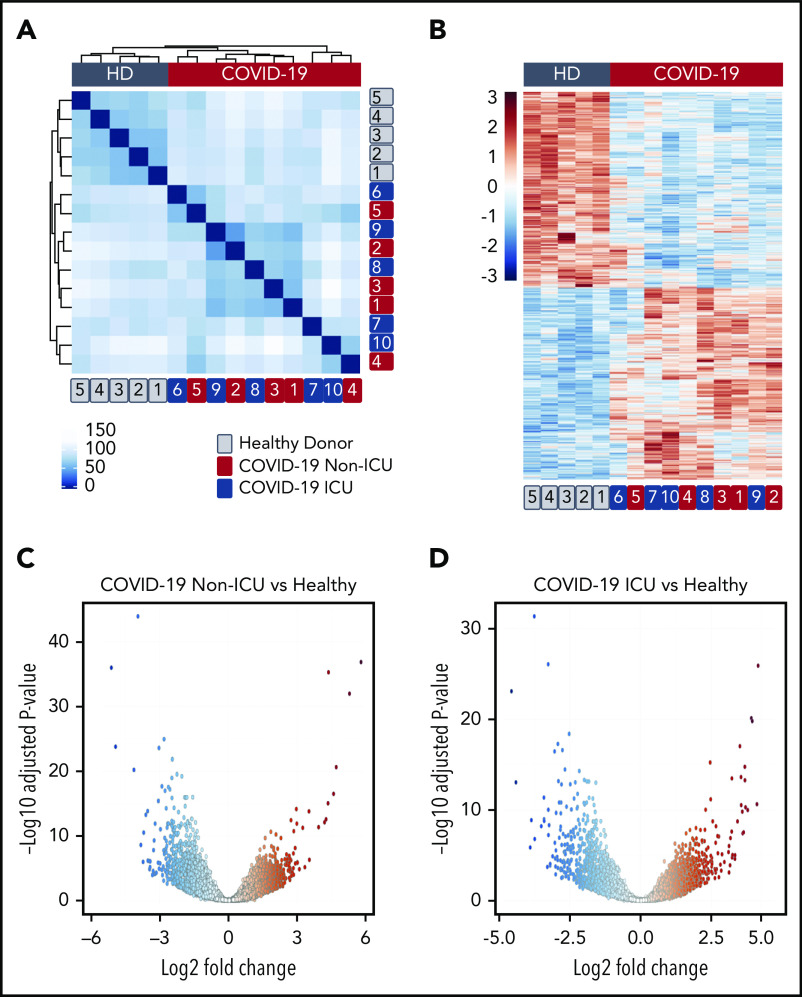
SARS-CoV-2 infection alters the platelet transcriptome. RNA-seq was performed on RNA isolated from highly purified platelets from 6 non-ICU and 4 ICU SARS-CoV-2 patients and 5 matched healthy donors (HD) as described in “Methods”. (A) Hierarchical clustering of samples according to global gene expression demonstrates non-ICU SARS-CoV-2–infected patients (red numbers) and ICU (blue numbers) cluster together whereas healthy donors (gray numbers) segregate together. (B) Heat map of significantly differentially expressed platelet transcripts from SARS-CoV-2–infected ICU and non-ICU patients and healthy control donors. Red indicates increased relative expression, and blue indicates decreased relative expression. Only coding mRNAs were examined in this analysis. (C-D) Volcano plot with significantly increased (red) and decreased (blue) transcripts from non-ICU and ICU COVID-19 patients.
Ingenuity pathway analysis using differentially expressed genes in all COVID-19 vs healthy donors identified enriched pathways associated with protein ubiquitination, antigen presentation, and mitochondrial dysfunction in patients infected with COVID-19 (supplemental Table 5). To further assess the relevance of mitochondrial pathway–related gene changes, we measured mitochondrial reactive oxygen species (ROS) and basal phosphatidylserine (PS) expression in patient platelets because platelet PS exposure is a marker of mitochondrial-dependent platelet apoptosis. COVID-19 patients (both ICU and non-ICU) had increased basal ROS compared with healthy donors (supplemental Figure 2A-B). However, mitochondrial dysfunction did not result in increased platelet apoptosis, as PS expression was not different between COVID-19 patients and healthy donors (supplemental Figure 2C-D).
We next compared previously published,12,21 platelet RNA-seq data sets from septic patients and influenza A/H1N1 patients studied during the 2009 pandemic. Of note, numerous gene changes were unique for each disease condition (supplemental Figure 3; supplemental Tables 6-8). However, of the differentially expressed genes that were shared between COVID-19 and sepsis or H1N1, nearly all (>96%) changed in the same direction, suggesting convergent mechanisms driving these changes. Accordingly, enrichment analysis indicated distinct pathway changes in sepsis and H1N1 compared with COVID-19, but with some pathway changes shared including those related to antigen presentation and immune regulation (supplemental Tables 5, 9, and 10).
Regarding immune regulation, we previously identified that interferon-induced transmembrane protein 3 (IFITM3), an antiviral immune protein, was highly expressed in platelets during influenza A/H1N1 and dengue infection, but absent in platelets from healthy donors.12 In human megakaryocytes, IFITM3 functions to limit dengue viral infectivity. Interestingly, a variant in IFITM3 causing an N-terminally truncated protein that is associated with loss of function and increased viral replication in vitro, correlates clinically with severe COVID-19.30 RNA-seq analysis from both non-ICU and ICU COVID-19 patients revealed IFITM3 as 1 of the top significantly differentially expressed genes in platelets (Figure 2A; ICU patient shown). Western blot analysis confirmed that IFITM3 was significantly upregulated in both non-ICU and ICU patients (Figure 2B). Because IFITM3 regulates viral infectivity, we further examined our RNA-seq data set for the presence of angiotensin-converting enzyme 2 (ACE2), the putative receptor for SARS-CoV-2 binding, in platelets. RNA-seq demonstrated no expression of ACE2 mRNA independent of ICU status (supplemental Tables 1, 2, and 4). Furthermore, we did not detect ACE2 in platelets either by quantitative real-time PCR in 25 COVID-19 patients (ICU and non-ICU) and 5 healthy donors or by western blot (Figure 2C-D). We also did not detect ACE2 mRNA in megakaryocytes cultured from healthy human donors (not shown). Consistent with this, in the majority of patients with SARS-CoV-2 infection (n = 23 of 25; 92%), the mRNA from the SARS-CoV-2 N1 gene was undetectable in isolated platelets. Interestingly, in isolated platelets from 2 ICU COVID-19 patients, for whom the ACE2 receptor was not expressed based on real-time PCR (n = 2 of 25; 8%), we did detect mRNA expression of the SARS-CoV-2 N1 gene (Figure 2E and data not shown). Taken together, our data suggest that the platelet transcriptome is altered during SARS-CoV-2 infection. These data also demonstrate that human platelets do not express ACE2 mRNA or protein.
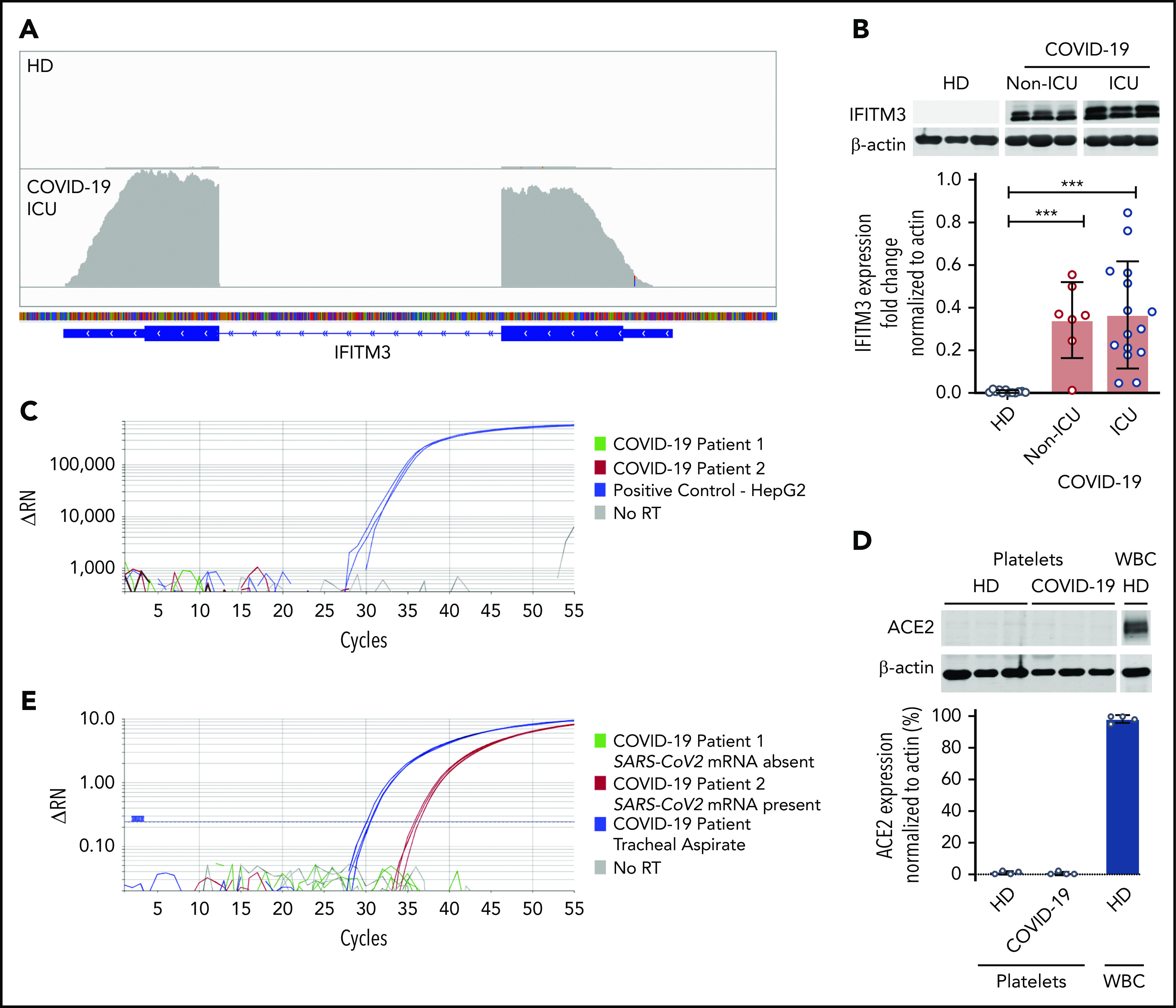
Platelets from COVID-19 patients express the antiviral protein IFITM3 but not the ACE2 receptor. (A) Integrated Genome Viewer plots demonstrating expression of IFITM3 from the RNA-seq data set. A representative healthy donor and COVID-19 patient are shown. The height of the bars indicates expression level. (B) Immunoblot and densitometric quantification of IFITM3 and β-actin expression in platelets isolated from healthy donors (n = 12) and SARS-CoV-2–infected non-ICU patients (n = 7) and COVID-19 ICU patients (n = 14). (C) RT-PCR analysis of ACE2 was performed on platelets from COVID-19 patients (5 healthy donors and 25 COVID-19 patients). Representative tracing from 2 COVID-19 patients (green and red). HepG2 cells served as a positive control (blue). No reverse transcriptase (RT) served as a negative control (gray). Reactions were performed in triplicate. (D) Immunoblot and densitometric quantification of ACE2 and β-actin expression in platelets isolated from healthy donors and ICU COVID-19 patients (n = 4). Leukocytes (white blood cells [WBC]) served as a positive control. (E) RT-PCR analysis of the SARS-CoV-2 N1 gene in platelets from non-ICU and ICU SARS-CoV-2–infected patients (n = 25). Representative tracing from 1 COVID-19 patient positive for SARS-CoV-2 mRNA presence (red) and 1 patient with SARS-CoV-2 mRNA absence (green). mRNA isolated from tracheal aspirates served as a positive control (blue). No reverse transcriptase served as a negative control (gray). Reactions were performed in triplicate. One PCR band at the correct size was observed in the tracheal aspirates and in the positive platelet samples. The PCR band in the platelet sample was confirmed by Sanger sequencing to be the N1 gene. ***P < .001. ΔRN, fluorescence intensity normalized to baseline.
Platelet number, size, and morphology in COVID-19 patients
As COVID-19 alters the platelet transcriptome, we next sought to determine whether SARS-CoV-2 infection induced changes in platelet function consistent with the thrombotic phenotype observed in COVID-19 patients.31-33 Viral and bacterial infections are commonly associated with thrombocytopenia and alterations in platelet morphology. Therefore, we tested whether patients with SARS-CoV-2 had changes in platelet count, mean platelet volume (MPV; a surrogate of platelet size and age), or their ultrastructure. Non-ICU and ICU COVID-19 patients generally had a platelet count and MPV in the normal reference range (Figure 3A-B). Although platelet counts and MPV were within normal ranges, plasma thrombopoietin (TPO) levels were significantly elevated in all COVID-19 patients (Figure 3C; supplemental Figure 4). TPO levels significantly correlated with platelet count in COVID-19 patients (supplemental Figure 4). Although TPO plasma levels were elevated in all COVID-19 patients, RNA-seq revealed that c-MPL, the gene encoding the TPO receptor, was significantly decreased (threefold in COVID-19 patients).
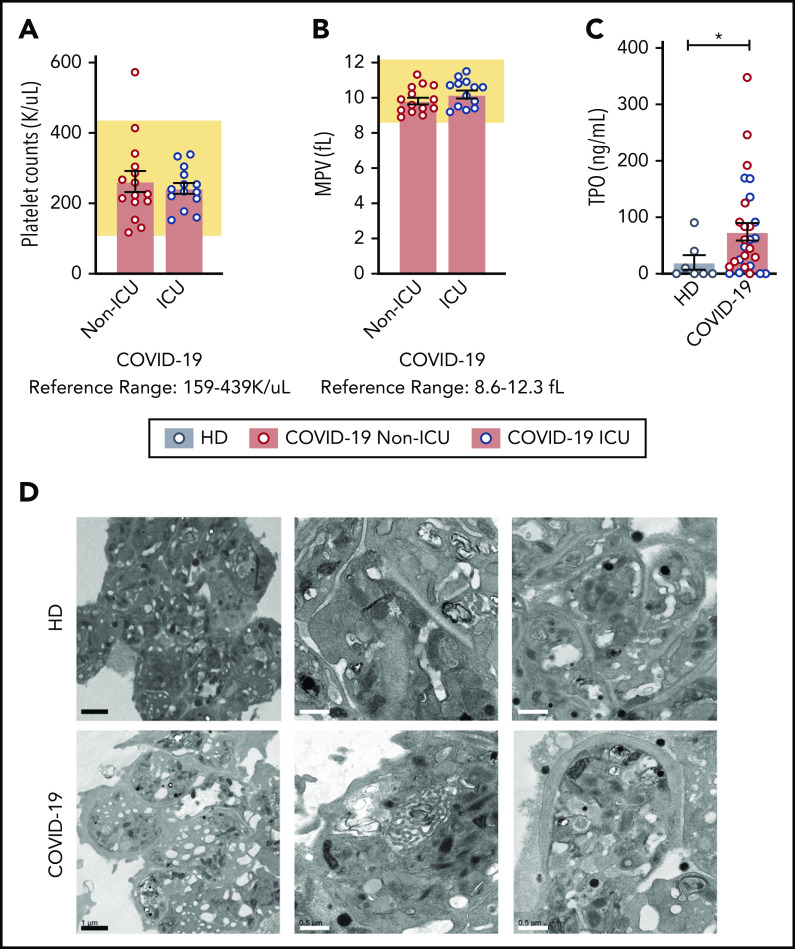
Platelet counts, MPV, and platelet morphology in COVID-19 patients are normal. (A) Platelet counts and (B) MPV are represented from COVID-19 non-ICU (red, n = 17) and ICU (blue, n = 12) patients. The reference range provided by ARUP is below the figure and represented by the shaded region. (C) TPO levels were measured by enzyme-linked immunosorbent assay (ELISA) in healthy donors (n = 7) and COVID-19 patients (non-ICU, n = 15; ICU, n = 14,). Blue dots indicate ICU patients whereas red dots indicate non-ICU patients. (D) Platelets were isolated from healthy donors (n = 4; HD; top panel) and COVID-19 patients (n = 4; COVID-19; bottom panel) and adhered to Acylar coated with poly-lysine and imaged with a JEOL JEM-1011 electron microscope. Digital images were captured with a side-mounted Advantage HR CCD camera. Lower power magnifications are provided on the left with representative images from 2 separate donors or patients on the right at a higher magnification. Scale bars: black bars = 1 μm; white bars = 0.5 μm. *P < .05. K/uL indicates ×103/μL.
We next examined platelet morphology and ultrastructure during SARS-CoV-2 infection using a transmission electron microscope. We analyzed >50 platelets from 4 COVID-19 patients (3 ICU and 1 non-ICU) and, for comparison, 4 matched healthy donors. Representative transmission electron microscope images are shown in Figure 3D. Platelets from both healthy donors and COVID-19 patients had similar appearances of the open canicular system, α and dense granules, mitochondria, and microtubular coils around the periphery of the platelet. In addition to examining morphology, we also examined platelets for the presence of SARS-CoV-2 virus as previous studies have demonstrated that platelets are capable of internalizing influenza virus.14 Consistent with our findings that the SARS-CoV-2 N1 gene was undetectable in the vast majority of isolated platelets (Figure 2E), we did not visually identify SARS-CoV-2 virus particles in these 4 platelets samples.
COVID-19 increases platelet surface P-selectin expression and circulating PLAs
Previous reports demonstrated that α-granule release and PLA formation are often increased during acute infections and may correlate with adverse clinical outcomes.34,35 We therefore next asked whether platelet P-selectin surface expression, a marker of platelet activation, was altered during COVID-19. Basal P-selectin surface expression was modestly, but significantly, increased in all COVID-19 patients independent of ICU status (Figure 4A; supplemental Figure 5B), compared with healthy donors. We also examined plasma levels another α-granule marker, platelet-derived growth factor (PDGF) AA/BB.36,37 Similar to basal levels of P-selectin expression, PDGF was significantly elevated in non-ICU and ICU patients compared with healthy donors (726.8 ± 106.8 ng/mL vs 2540.3 ± 347.9 ng/mL and 1988.3 ± 178.1 ng/mL; P < .05). Basal P-selectin expression correlated with soluble PDGF (r = 0.63; P = .076).
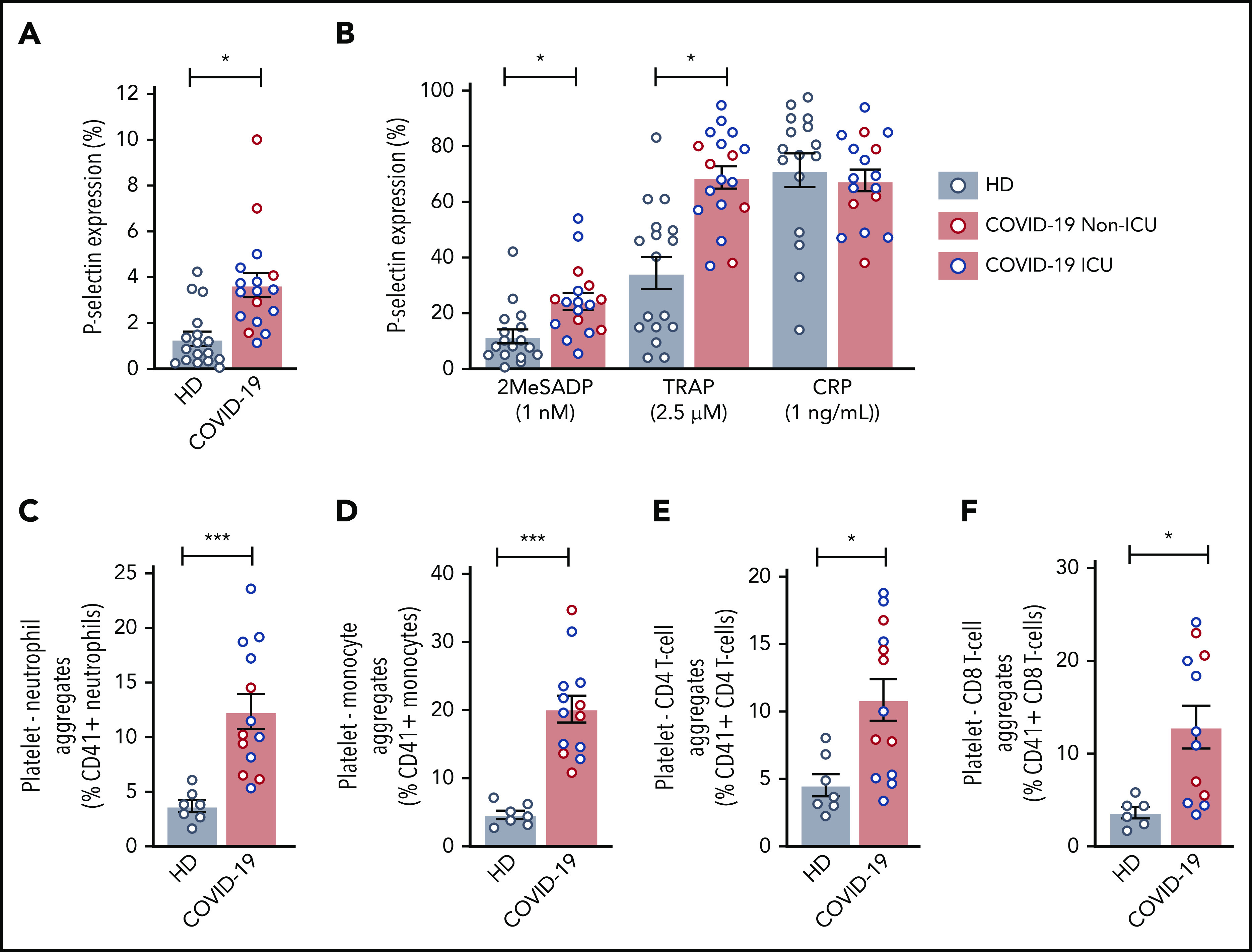
COVID-19 alters platelet activation and increases PLAs. (A) Platelet P-selectin expression was measured in whole blood by flow cytometry at baseline in 17 healthy donors and 5 non-ICU and 12 ICU COVID-19 patients. Blue dots indicate ICU patients; red dots indicate non-ICU patients. (B) P-selectin expression was measured after activation by platelet agonists. P2Y12 activation was induced by 1 ng/mL 2MeSADP, PAR1 was activated through 2.5 μM SFLLRN (TRAP), and GPVI was activated with 1 ng/mL CRP (N = 17-18 per group). Blue dots indicate ICU patients; red dots indicate non-ICU patients. (C-F) PLAs were measured in whole blood by flow cytometry. N = 7 for the healthy donors and N = 12-13 for COVID-19 patients. Blue dots indicate ICU patients; red dots indicate non-ICU patients. (C) PNAs were identified as CD66b+CD41+ leukocytes. (D) Platelet-monocyte aggregates were identified as CD14+CD41+ leukocytes. (E) Platelet-CD4 T-cell aggregates were identified as CD3+CD4+CD41+ leukocytes. (F) Platelet-CD8 T-cell aggregates were identified as CD3+CD8+CD41+ leukocytes. *P < .05; ***P < .001
Activating platelets with low-dose 2MeSADP and PAR1 peptide (SFLLRN or TRAP) induced significantly greater surface P-selectin expression in all COVID-19 patients (Figure 4B; supplemental Figure 5C). Similar results were observed in all COVID-19 patient platelets treated with higher concentrations of 2MeSADP to trigger maximal platelet activation (supplemental Figure 5D-E). No differences between ICU and non-ICU COVID-19 patients were observed in regards to P-selectin expression. However, platelet P-selectin expression in response to low-dose 2MeSADP significantly correlated with illness severity based on SOFA scores (r = 0.5296; P = .037). As P-selectin is an important receptor for forming PLAs, we next examined PLAs during SARS-CoV-2 infection. Platelet-neutrophil, platelet-monocyte, and platelet–T-cell (CD4+ and CD8+) aggregates were all significantly elevated in all COVID-19 compared with healthy donors (Figure 4C-F; supplemental Figure 6). Platelet-neutrophil aggregates (PNAs) were higher in ICU patients whereas platelet–T cell (CD4+) aggregates were generally higher in non-ICU COVID-19 patients. No differences were observed between ICU and non-ICU COVID-19 patients with platelet-monocyte and platelet-T cell (CD8+) aggregates (supplemental Figure 6).
Interestingly, platelet activation through 2MeSADP, SFLLRN (TRAP), and collagen-related peptide (CRP) resulted in decreased PAC-1 binding in all COVID-19 patients independent of ICU status compared with healthy donors (supplemental Figure 7). A similar decrease in PAC-1 binding was observed with high-dose agonists (supplemental Figure 7). The decrease in PAC-1 binding was not due to changes in αIIb expression as αIIb expression was similar among healthy donors and all COVID-19 patients (supplemental Figure 8).
Platelet aggregation is increased in COVID-19 patients
As procoagulant responses and clinical thrombosis are increased in patients with SARS-CoV-2 infection29,38-40 and, because we observed evidence of increase platelet activation in all COVID-19 patients (Figure 4; supplemental Figures 5 and 6), we next assessed platelet aggregation. Platelet aggregation in response to low-dose agonists (2MeSADP, thrombin, and collagen) was significantly increased in COVID-19 patients compared with healthy donors (Figure 5A-B; supplemental Figure 9). The potentiation in aggregation was more pronounced in ICU patients, especially at lower doses of thrombin and 2MeSADP (supplemental Figure 9). At higher doses, this effect was less marked with 2MeSADP and collagen, inducing significantly greater platelet aggregation (Figure 5A-B) with similar increases observed in all COVID-19 patients (supplemental Figure 9). Platelets from non-ICU and ICU COVID-19 patients also exhibited greater adhesion and spreading on fibrinogen and collagen (Figure 5C-F). Taken together, these findings indicate that during clinical infection with SARS-CoV-2, platelets may circulate, primed to be hyperreactive.
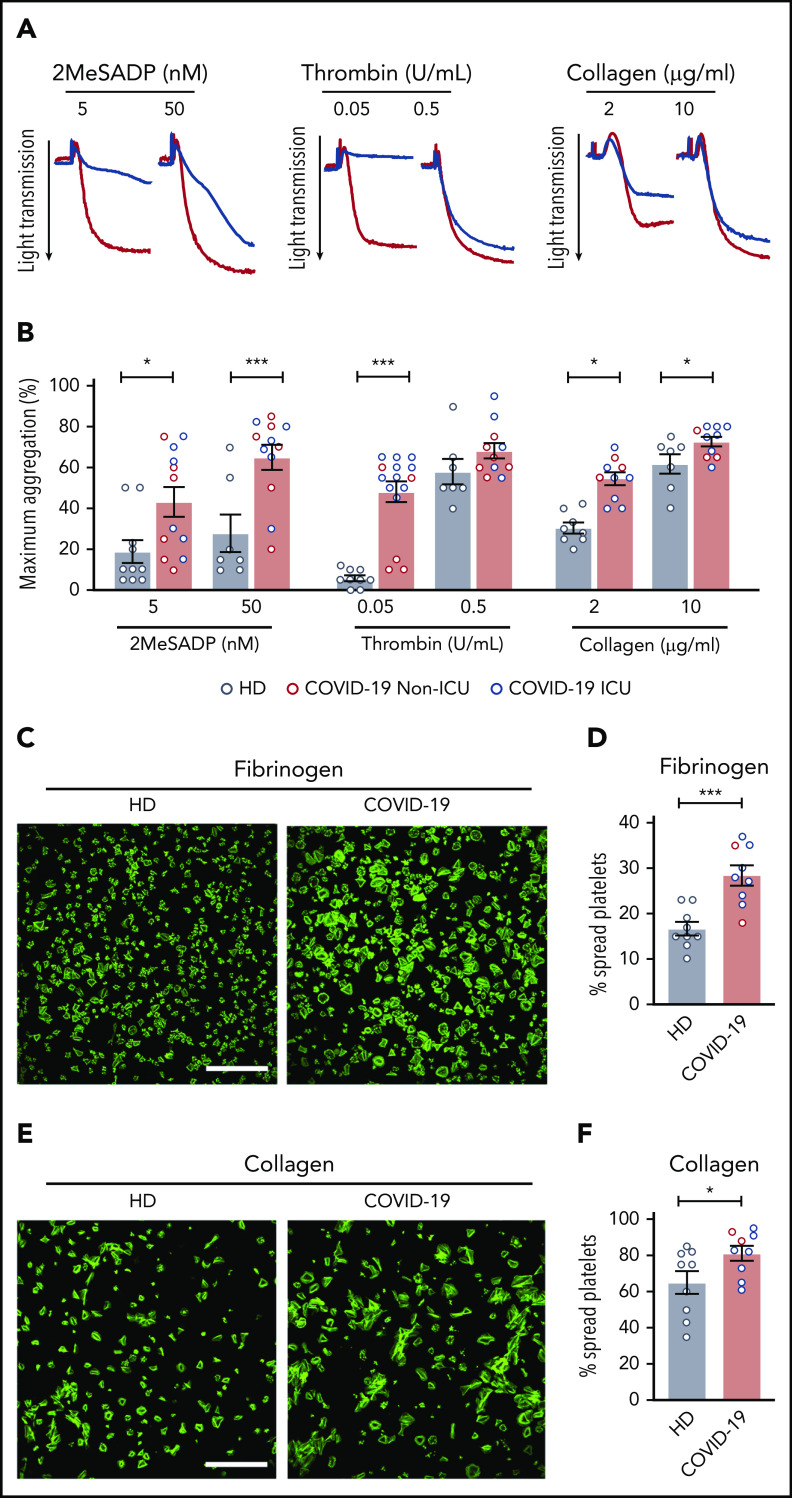
Platelets from COVID-19 patients are hyperreactive. Platelets were isolated from healthy donors and COVID-19 patients. (A) Representative aggregation traces of washed platelets from ICU COVID-19 patients or healthy donors treated with 2MeSADP (5 nM or 50 nM), thrombin (0.05 U/mL or 0.5 U/mL), or collagen (2 μg/mL or 10 μg/mL) under stirring conditions at 37°C. (B) Quantification of maximal aggregation (N = 7-10 for healthy donors; N = 10-15 for SARS-CoV-2–infected patients). Blue dots indicate ICU patients; red dots indicate non-ICU patients. (C) Washed platelets were allowed to spread on fibrinogen (scale bar, 50 μm; phalloidin stain). (D) After 30 minutes, platelets were fixed and platelet spreading was quantified. (E) Washed platelets were allowed to spread on collagen (scale bar, 50 μm; phalloidin stain). (F) After 30 minutes, platelets were fixed and platelet spreading was quantified. Images are representative for 9 independent experiments. Blue dots indicate ICU patients; red dots indicate non-ICU patients. *P < .05; **P < .01; ***P < .001.
Activation of MAPK-signaling pathway is upregulated in COVID-19 patient platelets
As platelet aggregation in COVID-19 patients was increased, especially in critically ill ICU patients, we next examined signaling events downstream of MAPK, a pathway mediating platelet aggregation. Phosphorylation of ERK1/2, p38, and eIF4E was significantly upregulated in platelets from critically ill ICU COVID-19 patients, indicating increased activation of the MAPK pathway (Figure 6A-B). Previous studies have shown that increased MAPK signaling promotes thromboxane generation by regulating cytosolic phospholipase A2 activation.28 Therefore, we next evaluated platelet cytosolic phospholipase A2 phosphorylation in patients infected with SARS-CoV-2. As shown in Figure 6C-D, cytosolic phospholipase A2 phosphorylation was significantly increased in SARS-CoV-2–infected patients both at baseline and upon activation. Our laboratory and others have shown that increases in cytosolic phospholipase A2 phosphorylation activate cytosolic phospholipase A2 activity, thereby upregulating thromboxane production.28 Consistent with these previous findings, stimulation with 2MeSADP resulted in increased thromboxane generation by platelets from COVID-19 ICU patients (Figure 6E). Platelet hyperreactivity in COVID-19 ICU patients could be reduced by pretreatment with high-dose aspirin (supplemental Figure 10). These results demonstrate that increased platelet activation (granule release and P-selectin expression) and aggregation during COVID-19 is due, at least in part, to increased MAPK pathway activation and thromboxane generation.
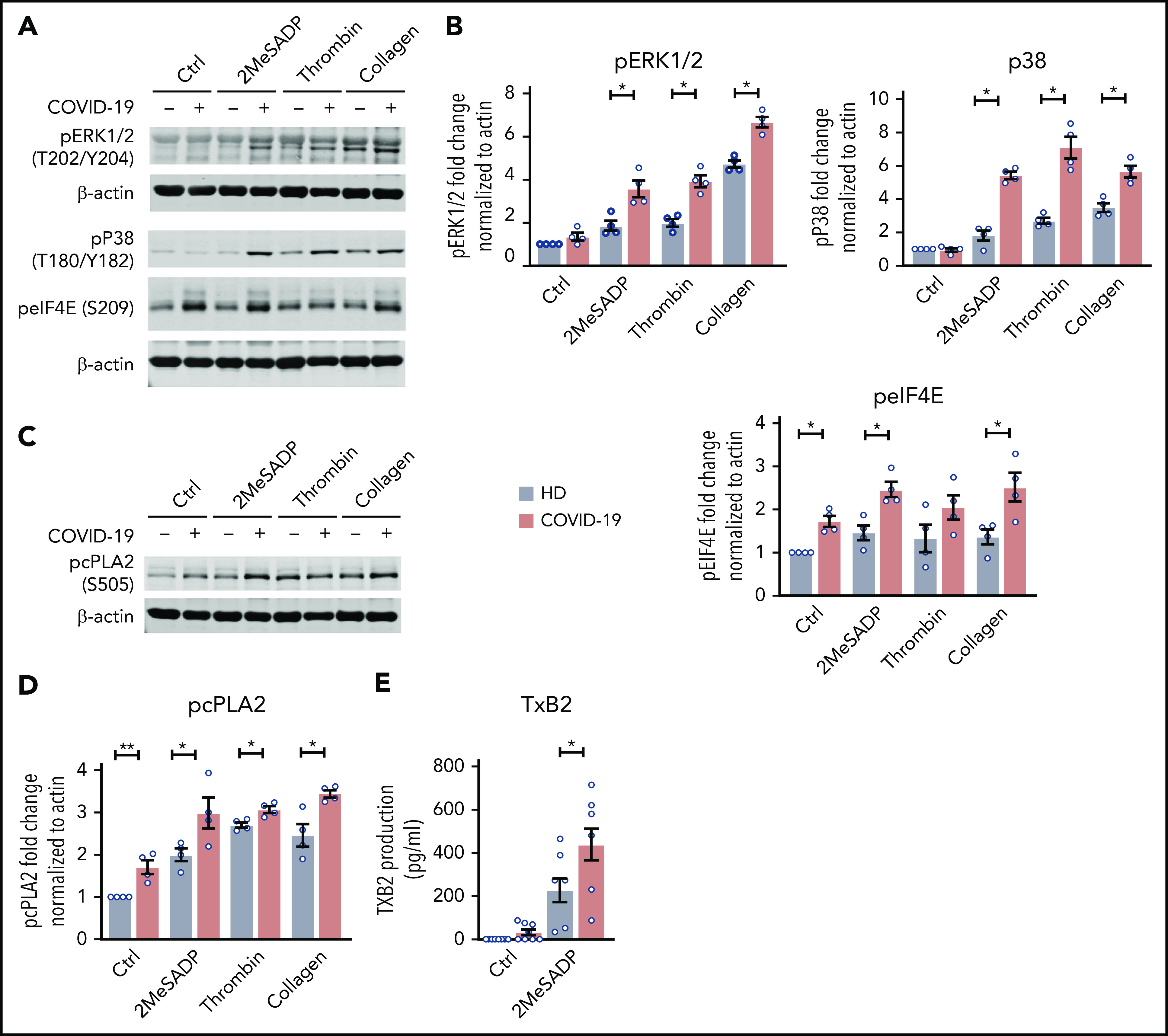
The MAPK-signaling pathway is upregulated in COVID-19 ICU patient platelets. (A) Washed platelets were stimulated with 2MeSADP (1 ng/mL), thrombin (0.05 U/mL), collagen (2 μg/mL), or vehicle under stirring conditions for 5 minutes. Platelet proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), subsequent western blots were probed for phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2) (T202/Y204), phosphorylated p38 (T180/Y182), and phosphorylated eIF4E (Ser209). β-actin was used as loading control. (B) Quantification of phosphorylation normalized to β-actin (n = 4). (C) Washed platelets were stimulated with 2MeSADP (1 ng/mL), thrombin (0.05 U), collagen (2 μg/mL), or vehicle under stirring conditions for 5 minutes. Platelet proteins were separated by SDS-PAGE, subsequent western blots were probed for phosphorylated cytosolic phospholipase A2 (cPLA2) (Ser505). (D) Quantification of phosphorylation normalized to β-actin (n = 4). (E) Thromboxane B2 (TXB2) generation was measured as described in “Methods” (n = 8). *P < .05; **P < .01.
Discussion
COVID-19 is most commonly associated with ARDS and hypoxemic respiratory failure. Significant derangements in the coagulation cascade have been observed in critically ill COVID-19 patients, including elevated D-dimers, fibrinogen, and von Willebrand factor levels.29,31,38 Furthermore, thrombosis, including pulmonary embolism, venous thrombosis, and ischemic stroke, are common among severely ill patients.39,41-43 Platelets are known to play a significant role in the development of these thrombotic complications. In addition, platelets are an important bridge between the hemostatic system and immune defense, including viral illness. Whether COVID-19 alters platelet gene expression and function is unknown. Here, we demonstrate for the first time that COVID-19 significantly alters platelet gene expression and triggers robust platelet hyperreactivity.
Previous studies from our group have demonstrated that infectious diseases including dengue, influenza, and sepsis are associated with dynamic changes in platelet gene expression.12,21 Similar to other infectious diseases, the platelet transcriptome was significantly altered in COVID-19 patients, as compared with healthy donors. Interestingly, non-ICU and ICU COVID-19 patients had similar changes in gene expression with only 16 genes differentially expressed between the 2 cohorts.
Although global transcriptomic changes were similar between both non-ICU and ICU COVID-19 patients, hundreds of platelet transcripts significantly differentially expressed during COVID-19 were distinctly different than those transcripts altered during pandemic influenza A/H1N1 or sepsis. This suggests there may be unique aspects of COVID-19 on platelets that are distinct from those changes triggered during sepsis and pandemic influenza A/H1N1. We highlight that there were also a number of transcripts that were similarly altered in platelets from patients with COVID-19 and pandemic influenza A/H1N1. This included the interferon-sensitive gene IFITM3, which prevents viral entry and replication.44 Interestingly, IFITM3 expression was similar among ICU and non-ICU COVID-19 patients. We recently reported that IFITM3 mRNA and protein are upregulated in platelets and megakaryocytes during viral infections, and functionally serve to limit viral infection of host cells, including megakaryocytes and stem cells.12 Whether upregulation of IFITM3 in platelets from COVID-19 patients serves to similarly restrict SARS-CoV-2 viral replication remains unknown at this time, but may be pursued in future studies.
Recent studies have suggested that viruses such as influenza can be found in platelets.14 Whether SARS-CoV-2 virus can bind to and enter platelets has not previously been established. Using complementary transcriptomic and proteomic assays, we were uniformly unable to detect ACE2, the putative receptor for SARS-CoV-2 viral entry, in platelets or megakaryocytes. Transmission electron microscopy also revealed the absence of SARS-CoV-2 viral particles in platelets. We did detect, in a very small number of COVID-19 patients (n = 2 of 25; 8% both COVID-19 ICU patients), transcriptional expression of the N1 SARS-CoV-2 gene in isolated platelets. Both patients had no detectable expression of the ACE2 receptor based on real-time PCR analysis. We were unable to determine whether SARS-CoV-2 virion particles were also present in platelets from these 2 COVID-19 patients as we did not perform electron microscopy on these patients. Because our findings indicate that the ACE2 receptor is absent in platelets and megakaryocytes, if SARS-CoV-2 viral uptake occurs, it would seemingly need to happen independently of the ACE2 receptor. As platelets express a host of TLRs, including TLR7, it is possible that SARS-CoV-2 could enter megakaryocytes and platelets through an alternative mechanism.45-48
Although thrombocytopenia is observed in some COVID-19 patients and correlates with mortality,32,49 patients we recruited with COVID-19 (ICU and non-ICU) generally had normal platelet number and size, consistent with at least 1 prior report.40 This is distinct from other viral infectious diseases including more severely ill influenza patients, as well as dengue and SARS-CoV-1.33,50-52 Interestingly, over one-half of SARS-CoV-1 patients present with thrombocytopenia with no change in TPO levels until the convalescence stage of the disease when TPO levels increase as well as platelet counts.53 Viral infections are known to modulate liver production of TPO due to alterations in host cytokine profiles such as increased tumor growth factor β.54,55 In our study, increased TPO levels in COVID-19 patients without alterations in platelet counts may reflect the systemic effects present in patients during infection altering liver production of TPO. Alternatively, we observed significantly decreased c-MPL RNA levels in COVID-19 patients. Decreased TPO receptor on platelets and megakaryocytes has been shown to affect circulating TPO levels and may also explain our observation in COVID-19 patients.56-58
Platelet activation is common during infectious diseases, including PS exposure.59,60 Although we validated enrichment of 1 ingenuity pathway analysis pathway (mitochondrial dysfunction) with functional changes demonstrating increased mitochondrial ROS generation in COVID-19 patients, we did not observe any increase in resting PS levels. These findings illustrate 1 difference in platelets during SARS-CoV-2 infection and other infectious setting, including HIV infection and sepsis.59,60 Platelets also contain many antiviral and antibacterial factors inside their α- and dense granules, which are secreted by activated platelets.51 Previous studies have observed increased P-selectin expression during viral infection including influenza.47,61 Increased expression of P-selectin promotes the formation of PLAs through P-selectin glycoprotein ligand-1 (PSGL-1). The formation of PLAs is a very sensitive marker of in vivo platelet activation during infectious disease, and we observed increased PLAs in COVID-19 patients.5 PLA formation, specifically with monocytes, also induces fibrin clot formation via interactions of PSGL-1 on leukocyte-derived microparticles with tissue factor on the platelet surface.62 Similar to other critically ill patient populations, circulating platelet-monocyte aggregates in blood were significantly increased independent of ICU status in COVID-19 patients.26 We also identified that circulating PNAs were increased in COVID-19 patients and were higher in ICU patients. The formation of PNAs recruits neutrophils to damaged lung capillaries.63-65 P-selectin neutralization reduces neutrophil recruitment and inhibits the development of acute lung injury in animal models.65 P-selectin blockade is being investigated during SARS-CoV-2 infection and we speculate that increased PNAs may contribute to the acute lung damage and ARDS observed in COVID-19.
Although not as often studied during infectious diseases, increased platelet T-cell interactions may also influence outcomes during SARS-CoV-2 infections. Previous studies have demonstrated increased neuroinflammation, commonly reported in COVID-19, and altered IL-17 and IFNγ production when platelets adhere to CD4+ T cells.66,67 In addition to regulating T-cell inflammatory responses, platelets express major histocompatibility class I (MHC-I) and are capable of cross-presenting antigens to CD8+ T cells.68 Viral infections such as dengue are known to effect platelet MHC expression and proteasome activity.69 Increased platelet T-cell interactions may modulate T-cell reactivity, inflammation, and immune responses during COVID-19 and warrant further investigation.
We observed increased platelet aggregation in response to 2MesADP, thrombin, and collagen as well as increased adhesion and spreading of platelets on fibrinogen and collagen during infection with SARS-CoV-2. Critically ill ICU COVID-19 patients had increased aggregation in response to low-dose agonist compared with non-ICU patients, suggesting more severe disease may induce greater platelet hyperreactivity. Increased platelet aggregation has been observed in other viral illness such as HIV,70 where subthreshold concentrations of agonists induce greater platelet aggregation similar to our observations during SARS-CoV-2 activation. Increased MAPK signaling may be partly responsible for the increased aggregation responses as we observed increase activation of cytosolic phospholipase A2 and subsequently increased thromboxane generation, which enhances platelet aggregation.28 Aspirin pretreatment of platelets from SARS-CoV-2–infected patients abolishes this hyperactivity (supplemental Figure 10), further supporting increased thromboxane generation as 1 mechanism triggering platelet hyperreactivity during COVID-19. Of note, platelet aggregation was assessed only on COVID-19 patients who were not on aspirin or other antiplatelet agents. Interestingly, platelet P-selectin expression in response to low-dose 2MeSADP significantly correlated with illness severity based on SOFA scores. A recent report indicated that ADP is a key player in the development of microvascular thrombosis in COVID-19 patients.71 As aspirin reduced platelet hyperactivity in COVID-19 patients, our findings of platelet activation correlating with illness severity further suggest that antiplatelet therapies may be warranted in treating COVID-19 patients. Enhanced MAPK signaling could also be responsible for increased α-granule release and P-selectin expression observed in COVID-19 patients.72
Increased activation of the MAPK pathway in platelets may be partly due to increased JAK3, which is known to be upstream of the MAPK pathway.73,74 RNA-seq analysis demonstrated increased JAK3 mRNA expression, which we subsequently validated at the protein level (supplemental Figure 11). In addition to JAK3, we also observed that other upstream regulators of the MAPK pathway were altered in platelets during COVID-19, including signaling by Rho family GTPases (supplemental Table 5).
However, not all aspects of platelet functional responses were enhanced in COVID-19. Integrin αIIbβ3 activation was reduced during COVID-19, compared with healthy donors. A similar finding was previously observed in critically ill influenza A/H1N1 (which caused the 2009 pandemic) patients, suggesting that reduced integrin activation may be a common feature of acute viral illness.26 The mechanism behind reduced integrin αIIbβ3 activation (and its significance) remains unknown. Our flow cytometry analyses indicated that αIIb expression was similar between COVID-19 patients and controls, however, suggesting that reduced αIIb receptor density was not responsible for this finding. We did observe that calpain mRNA expression was significantly upregulated in platelets during SARS-CoV-2 infection (supplemental Tables 2 and 4). As mitochondrial-dependent calpain activation regulates integrin αIIbβ3,75 it is possible that altered mitochondria function and increased calpain levels may contribute to our observation of reduced integrin αIIbβ3 activation in COVID-19 patients.
Based on the results of our study, we postulate that increased platelet reactivity during SARS-CoV-2 infection may contribute to immunothrombosis during COVID-19 as reported by others.4,31,43 As none of the COVID-19 patients enrolled in our study were clinically diagnosed with thrombotic complications, we are unable to examine clinical associations between platelet activation and thrombosis in our study. Interestingly, platelet counts, MPV, and markers of platelet activation, including platelet-leukocyte and plasma PDGF, did not correlate with markers of inflammation such as IL-6, IL-8, and TNF-α (data not shown). We also posit that platelets are likely not the sole contributors, as other blood cells and soluble coagulation factors also mediate injurious inflammatory, immune, and thrombotic responses.
In conclusion, our findings demonstrate that COVID-19 is associated with substantial alterations in the platelet transcriptome and proteome, and platelet hyperreactivity. Our data may serve as foundational to ongoing clinical studies investigating whether targeting platelets during COVID-19 may improve patient outcome.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the figure preparation expertise of Diana Lim and assistance with participant recruitment from Antoinette Blair, Macy Barrios, Amber Plante, Jordan Greer, Amy DeNardo, Amanda Bailey, and Lindsey Waddoups. The authors also acknowledge Nancy Chandler, Willisa Liou, and the University of Utah Electron Microscopy Core Laboratory for their assistance with the electron microscopy.
This work was supported by the following National Institutes of Health grants: Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01093826 (C.C.Y.); National Heart, Lung, and Blood Institute grant R01HL135265 (A.C.P.); National Institute on Aging grant K01AG059892 (R.A.C.); National Heart, Lung, and Blood Institute grants R35HL145237 (A.S.W.) and R01HL142804 (M.T.R. and A.S.W.); National Institute on Aging grants R01AG048022 and R56AG059877; and National Heart, Lung, and Blood Institute grant R01HL130541 (M.T.R.). This work was supported by grants from the University of Utah Triple I Program (E.A.M.), Fonds voor Wetenschappelijk Onderzoek Vlaanderen FWO 12U7818N (F.D.), and the American Heart Foundation (18Post340300200 [B.K.M.]). This work was also supported in part by Merit Review award number I01 CX001696 (M.T.R.) from the US Department of Veterans Affairs Clinical Sciences R&D (CSRD). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center (Salt Lake City, UT). Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1S10RR026802-01. Research reported in this publication used the High-Throughput Genomics and Bioinformatic Analysis Shared Resource at Huntsman Cancer Institute at the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA042014. The work was also supported by the Flow Cytometry Core at the University of Utah.
Footnotes
The RNA-seq data reported in this article are available at BioProject (accession #PRJNA634489).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.K.M., F.D., E.A.M., I.P., J.W.R., L.G., A.C.P., N.D.T., M.C., C.C.Y., M.T.R., and R.A.C. designed and performed experiments; B.K.M., F.D., E.A.M., I.P., J.W.R., C.S., N.D.T., M.T.R., and R.A.C. analyzed results and made the figures; B.K.M., F.D., E.A.M., I.P., J.W.R., A.S.W., M.T.R., and R.A.C. wrote the paper; and all authors reviewed and critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert A. Campbell, Eccles Institute of Human Genetics, University of Utah Health Sciences Center, 15 North 2030 East, Bldg 533, Suite 4225, Salt Lake City, UT 84112; e-mail: ude.hatu.2m2u@llebpmacr.
REFERENCES
Articles from Blood are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/blood.2020007214
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7483430
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Immunity and Coagulation in COVID-19.
Int J Mol Sci, 25(20):11267, 19 Oct 2024
Cited by: 0 articles | PMID: 39457048 | PMCID: PMC11508857
Review Free full text in Europe PMC
BALB/c mice challenged with SARS-CoV-2 B.1.351 β variant cause pathophysiological and neurological changes within the lungs and brains.
J Gen Virol, 105(10), 01 Oct 2024
Cited by: 0 articles | PMID: 39475775 | PMCID: PMC11524415
One immune cell to bind them all: platelet contribution to neurodegenerative disease.
Mol Neurodegener, 19(1):65, 27 Sep 2024
Cited by: 0 articles | PMID: 39334369 | PMCID: PMC11438031
Review Free full text in Europe PMC
Platelet Aggregation Alterations in Patients with Severe Viral Infection Treated at the Intensive Care Unit: Implications for Mortality Risk.
Pathogens, 13(9):778, 10 Sep 2024
Cited by: 0 articles | PMID: 39338970 | PMCID: PMC11435101
Adequate serum 25-hydroxy-vitamin D levels are correlated with low anti-PF4 levels in mild COVID-19 Patients: An observational study.
Medicine (Baltimore), 103(37):e39252, 01 Sep 2024
Cited by: 0 articles | PMID: 39287233 | PMCID: PMC11404891
Go to all (563) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
BioProject
- (2 citations) BioProject - PRJNA634489
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19.
Blood, 136(11):1330-1341, 01 Sep 2020
Cited by: 464 articles | PMID: 32678428 | PMCID: PMC7483437
SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19.
J Hematol Oncol, 13(1):120, 04 Sep 2020
Cited by: 397 articles | PMID: 32887634 | PMCID: PMC7471641
Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy.
Circulation, 142(12):1176-1189, 28 Jul 2020
Cited by: 337 articles | PMID: 32755393 | PMCID: PMC7497892
SARS-CoV-2 and coagulation disorders in different organs.
Life Sci, 260:118431, 15 Sep 2020
Cited by: 71 articles | PMID: 32946915 | PMCID: PMC7490584
Review Free full text in Europe PMC
Funding
Funders who supported this work.
CSRD VA (1)
Grant ID: I01 CX001696
NCATS NIH HHS (1)
Grant ID: UL1 TR002538
NCI NIH HHS (1)
Grant ID: P30 CA042014
NCRR NIH HHS (1)
Grant ID: S10 RR026802
NHLBI NIH HHS (6)
Grant ID: R00 HL135265
Grant ID: R35 HL145237
Grant ID: K99 HL135265
Grant ID: R01 HL130541
Grant ID: R01 HL142804
Grant ID: R01 HL144957
NIA NIH HHS (3)
Grant ID: R56 AG059877
Grant ID: R01 AG048022
Grant ID: K01 AG059892
NICHD NIH HHS (1)
Grant ID: R01 HD093826

 1,2
1,2