Abstract
Background
There is a paucity of data describing the effects of coronavirus disease 2019 on placental pathology, especially in asymptomatic patients. Although the pathophysiology of coronavirus disease 2019 is not completely understood, there is emerging evidence that it causes a severe systemic inflammatory response and results in a hypercoagulable state with widespread microthrombi. We hypothesized that it is plausible that a similar disease process may occur in the fetal-maternal unit.Objective
This study aimed to determine whether coronavirus disease 2019 in term patients admitted to labor and delivery, including women without coronavirus disease 2019 symptomatology, is associated with increased placental injury compared with a cohort of coronavirus disease 2019-negative controls.Study design
This was a retrospective cohort study performed at NYU Winthrop Hospital between March 31, 2020, and June 17, 2020. During the study period, all women admitted to labor and delivery were routinely tested for severe acute respiratory syndrome coronavirus 2 regardless of symptomatology. The placental histopathologic findings of patients with coronavirus disease 2019 (n=77) who delivered a singleton gestation at term were compared with a control group of term patients without coronavirus disease 2019 (n=56). Controls were excluded if they had obstetrical or medical complications including fetal growth restriction, oligohydramnios, hypertension, diabetes, coagulopathy, or thrombophilia. Multivariable logistic regression models were performed for variables that were significant (P<.05) in univariable analyses. A subgroup analysis was also performed comparing asymptomatic coronavirus disease 2019 cases with negative controls.Results
In univariable analyses, coronavirus disease 2019 cases were more likely to have evidence of fetal vascular malperfusion, that is, presence of avascular villi and mural fibrin deposition (32.5% [25/77] vs 3.6% [2/56], P<.0001) and villitis of unknown etiology (20.8% [16/77] vs 7.1% [4/56], P=.030). These findings persisted in a subgroup analysis of asymptomatic coronavirus disease 2019 cases compared with coronavirus disease 2019-negative controls. In a multivariable model adjusting for maternal age, race and ethnicity, mode of delivery, preeclampsia, fetal growth restriction, and oligohydramnios, the frequency of fetal vascular malperfusion abnormalities remained significantly higher in the coronavirus disease 2019 group (odds ratio, 12.63; 95% confidence interval, 2.40-66.40). Although the frequency of villitis of unknown etiology was more than double in coronavirus disease 2019 cases compared with controls, this did not reach statistical significance in a similar multivariable model (odds ratio, 2.11; 95% confidence interval, 0.50-8.97). All neonates of mothers with coronavirus disease 2019 tested negative for severe acute respiratory syndrome coronavirus 2 by polymerase chain reaction.Conclusion
Despite the fact that all neonates born to mothers with coronavirus disease 2019 were negative for severe acute respiratory syndrome coronavirus 2 by polymerase chain reaction, we found that coronavirus disease 2019 in term patients admitted to labor and delivery is associated with increased rates of placental histopathologic abnormalities, particularly fetal vascular malperfusion and villitis of unknown etiology. These findings seem to occur even among asymptomatic term patients.Free full text

Coronavirus disease 2019 infection and placental histopathology in women delivering at term
Abstract
Background
There is a paucity of data describing the effects of coronavirus disease 2019 on placental pathology, especially in asymptomatic patients. Although the pathophysiology of coronavirus disease 2019 is not completely understood, there is emerging evidence that it causes a severe systemic inflammatory response and results in a hypercoagulable state with widespread microthrombi. We hypothesized that it is plausible that a similar disease process may occur in the fetal-maternal unit.
Objective
This study aimed to determine whether coronavirus disease 2019 in term patients admitted to labor and delivery, including women without coronavirus disease 2019 symptomatology, is associated with increased placental injury compared with a cohort of coronavirus disease 2019–negative controls.
Study Design
This was a retrospective cohort study performed at NYU Winthrop Hospital between March 31, 2020, and June 17, 2020. During the study period, all women admitted to labor and delivery were routinely tested for severe acute respiratory syndrome coronavirus 2 regardless of symptomatology. The placental histopathologic findings of patients with coronavirus disease 2019 (n=77) who delivered a singleton gestation at term were compared with a control group of term patients without coronavirus disease 2019 (n=56). Controls were excluded if they had obstetrical or medical complications including fetal growth restriction, oligohydramnios, hypertension, diabetes, coagulopathy, or thrombophilia. Multivariable logistic regression models were performed for variables that were significant (P<.05) in univariable analyses. A subgroup analysis was also performed comparing asymptomatic coronavirus disease 2019 cases with negative controls.
Results
In univariable analyses, coronavirus disease 2019 cases were more likely to have evidence of fetal vascular malperfusion, that is, presence of avascular villi and mural fibrin deposition (32.5% [25/77] vs 3.6% [2/56], P<.0001) and villitis of unknown etiology (20.8% [16/77] vs 7.1% [4/56], P=.030). These findings persisted in a subgroup analysis of asymptomatic coronavirus disease 2019 cases compared with coronavirus disease 2019–negative controls. In a multivariable model adjusting for maternal age, race and ethnicity, mode of delivery, preeclampsia, fetal growth restriction, and oligohydramnios, the frequency of fetal vascular malperfusion abnormalities remained significantly higher in the coronavirus disease 2019 group (odds ratio, 12.63; 95% confidence interval, 2.40–66.40). Although the frequency of villitis of unknown etiology was more than double in coronavirus disease 2019 cases compared with controls, this did not reach statistical significance in a similar multivariable model (odds ratio, 2.11; 95% confidence interval, 0.50–8.97). All neonates of mothers with coronavirus disease 2019 tested negative for severe acute respiratory syndrome coronavirus 2 by polymerase chain reaction.
Conclusion
Despite the fact that all neonates born to mothers with coronavirus disease 2019 were negative for severe acute respiratory syndrome coronavirus 2 by polymerase chain reaction, we found that coronavirus disease 2019 in term patients admitted to labor and delivery is associated with increased rates of placental histopathologic abnormalities, particularly fetal vascular malperfusion and villitis of unknown etiology. These findings seem to occur even among asymptomatic term patients.
Introduction
The impact of coronavirus disease 2019 (COVID-19) infection on pregnancy outcomes and its effects on the fetal-placental unit are not clearly established. The literature on COVID-19 in pregnancy is mainly limited to case reports, case series, and systematic reviews1, 10, 11, 12, 13, 2, 3, 4, 5, 6, 7, 8, 9 involving primarily symptomatic patients. The majority of these reports have focused on severity of maternal disease, mode of delivery, and possible vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to the neonate.
A recent report identified SARS-CoV-2 virions in placenta villi by means of electron microscopy,5 and a more recent report documented that the virus can be transmitted to the fetus transplacentally with viral loads in the placenta being more than 2-fold higher than the maternal blood or nasopharynx.14 This high viral load in the placenta raises the possibility that the virus may elicit a placental inflammatory response, which could result in placental injury.
Although the pathophysiology of COVID-19 is not completely understood, there is emerging evidence that it can cause a severe systemic inflammatory response and may result in a hypercoagulable state with widespread microthrombi.15 Evidence of venous thromboembolism and microangiopathic disease in almost every organ system, including lungs, kidney, heart, and brain, has been identified both clinically and by autopsy examination.15 , 16 Thus, it is reasonable to investigate if a similar disease process occurs in the fetal-maternal unit.
We hypothesize that the placentas of pregnant women may have histologic evidence of injury because they could have harbored the virus for weeks before delivery, even if the women were asymptomatic. Therefore, we undertook this retrospective cohort study to compare placental pathology findings between women with COVID-19 at term vs a control group of COVID-19–negative term patients. A secondary objective was to determine the association between asymptomatic patients with COVID-19 and placental pathology.
Materials and Methods
This was a retrospective cohort study involving women who delivered at term at NYU Winthrop Hospital. Pregnant patients diagnosed with COVID-19 who delivered a singleton gestation between March 31, 2020, and June 17, 2020 were identified. At the study institution, all women admitted to Labor and Delivery during this time period were routinely tested via nasopharyngeal polymerase chain reaction (PCR) for SARS-CoV-2, regardless of symptomatology.17 The placentas of all patients with positive COVID-19 testing were sent for gross and microscopic histopathologic examination. COVID-19 cases were excluded from the study if they delivered at <37 weeks gestation. A control group was identified from COVID-19–negative deliveries during the same period of a singleton gestation at ≥37 weeks with placental histopathology available for review. Patients in the control group were excluded if they had obstetrical or medical complications including fetal growth restriction, oligohydramnios, chronic hypertension, pregnancy-induced hypertension (gestational hypertension or preeclampsia, including postpartum), preexisting or gestational diabetes, coagulopathy, or thrombophilia. Chart review was performed using electronic medical records (EMRs) to obtain relevant demographic and clinical information. This study was approved by the New York University Institutional Review Board.
Testing for COVID-19 infection was performed by means of SARS-CoV-2 PCR of a nasopharyngeal swab, using the Cepheid Xpert Xpress (Cepheid, Sunnyvale, CA) SARS-CoV-2 RT-PCR assay underemergency use authorization as per our institution’s policy. As per the manufacturer, the analytical sensitivity and specificity are reported as 100% (87/87 samples) and 100% (30/30 samples), respectively, with a limit of detection of 250 copies/mL or 0.0100 plaque-forming units/mL.
The placentas were submitted to the pathology laboratory without fixative. The placentas from COVID-19 cases were fixed in 10% buffered formalin for 72 hours, following which they were examined and measurements were taken. They were sectioned at 5 mm intervals and 3 mm–thick tissue sections were taken, including 2 from each quadrant, 2 cross sections of the umbilical cord and membranes, and any lesions. The control placentas were sectioned according to routine institutional protocol, which follows the guidelines put forth by the Amsterdam Placental Workshop Group Consensus Statement.18 Specifically, a minimum of 3 sections of placenta parenchyma were submitted, plus 1 block containing membranes and 2 cross sections of cord, plus additional sections when gross lesions are identified.
The tissue blocks were processed in a vasoactive intestinal polypeptide tissue processor (Skaura Tissue-Tek VIP, Torrance, CA). The tissue was then paraffin-embedded and sectioned, baked, and stained with hematoxylin and eosin in a Sakura Tissue-Tek Prisma stainer (Torrance, CA). For selected cases where villitis of unknown etiology or chronic intervillositis was identified, an immunohistochemistry stain for CD3 and CD68 was performed to confirm the presence of T-cell lymphocytic infiltrate in the villous or intervillous space. In addition, 4 μm–thick sections were obtained from paraffin-embedded tissue blocks. They were deparaffinized and then rehydrated with xylene and graded alcohols. The tissue slides and appropriate positive and negative controls were stained. Immunohistochemical staining was performed on a Ventana BenchMark Ultra (Roche, Basel, Switzerland) using Optiview DAB IHC detection kit (Ventana Medical Systems, Tucson, Arizona). Antigen retrieval was performed in 95°C for 64 minutes. CD68 primary antibody (clone number, KP-1) and CD3 primary antibody (clone number, 2GV6 rabbit monoclonal antibody) were obtained from Ventana Medical Systems. The slides were counterstained with hematoxylin.
Microscopic examination was performed by a single pathologist for all placentas from COVID-19 cases (P.K.), and by 1 of 3 pathologists for the control placentas (P.K., V.D., A.H.). The pathologists were not blinded to clinical information, including COVID-19 status. The findings recorded for the study were identical to that provided to the obstetrician-gynecologist for clinical purposes. Histopathologic lesions were categorized according to the 2016 Amsterdam criteria.18 A total of 71 neonates born to mothers with COVID-19 had test results available in the EMR for SARS-CoV-2 via PCR of nasal swabs using Cepheid Xpert Xpress.
Subsequently, we performed a second review on a subset of 26 COVID-19 placentas and 21 control placentas to assess interobserver reliability. Representative slides from each placenta were independently reviewed by the above 3 pathologists (P.K., V.D., A.H.) to assess for the presence or absence of the following: fetal vascular malperfusion with avascular villi, high grade; fetal vascular malperfusion with avascular villi, low grade; fetal vascular malperfusion with mural fibrin deposition; villitis of unknown etiology, high grade; and villitis of unknown etiology, low grade. The pathologists were blinded to COVID-19 status during the rereview and the previously collected data were not changed by the results of this interobserver reliability assessment.
Statistical analysis
Descriptive statistics (mean±standard deviation or median [25th–75th percentiles] for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group (cases vs controls). Groups were compared using the chi-square test or Fisher exact test, as deemed appropriate, for categorical variables and the 2 sample t-test or Mann-Whitney test for continuous data.
The interrater reliability for 5 classes of placental histopathologic lesions was calculated using Gwet’s first-order agreement coefficient (AC1).19 The AC1 coefficient was chosen because it overcomes some of the inherent weakness in the Kappa calculation and can adjust for both chance agreement and misclassification errors, which Kappa statistics do not allow.20 The AC1 can be interpreted using the following guidelines by Landis-Koch Benchmark21: 0.8 to 1.0=almost perfect; 0.6 to 0.8=substantial; 0.4 to 0.6=moderate; 0.2 to 0.4=slight; 0 to 0.2=poor.
A total of 2 separate multivariable logistic regression analyses were performed for the outcomes of fetal vascular malperfusion and villitis of unknown etiology. Model 1 adjusted for all variables that were significant (P<.05) in the univariate analyses and Model 2 excluded those factors that could be in the causative pathway, such as preeclampsia, fetal growth restriction, and oligohydramnios (as feasible based on sample size). Preexisting and gestational diabetes were not adjusted for in these models owing to inadequate numbers. A numeric measure of the accuracy of the model was obtained from the area under the curve (AUC), where an area of 1.0 signifies near perfect accuracy, whereas an area of <0.5 indicates that the model is worse than just flipping a coin. The following was used as a guide for AUC: 0.9 to 1.0, excellent; 0.8 to 0.9, very good; 0.7 to 0.8, good; 0.6 to 0.7, average; and 0.5 to 0.6, poor. The Hosmer-Lemeshow goodness of fit test was also used to test how well each model fits the data. Results from the multivariable logistic regression models are reported as odds ratios (ORs) with their corresponding 95% confidence intervals (CIs).
For the secondary objective, controls were compared with asymptomatic COVID-19 cases. Chi-square test or Fisher exact test, as deemed appropriate, was used to compare these 2 groups for pathologic outcomes. A result was considered statistically significant at the P<.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 77 COVID-19 cases and 56 COVID-19–negative controls were included in the study. A total of 71 neonates born to mothers with COVID-19 had available test results for SARS-CoV-2 via nasopharyngeal PCR and all were negative. Demographic and clinical variables for each group are presented in Table 1 . COVID-19 cases were significantly more likely to be younger (29.9 years vs 32.2 years, P=.02), Hispanic (55.8% [43/77] vs 17.6% [10/56], P=.001), and deliver vaginally (74% [57/77] vs 25% [14/56], P<.0001) than controls. The majority of study patients (67/77, 87%) were asymptomatic; the remaining 10 patients had mild COVID symptomatology including fever, cough, upper respiratory infection symptoms, and shortness of breath. None required supplemental oxygen administration or admission to the intensive care unit. Laboratory testing, such as inflammatory markers and coagulation studies, was not performed on these patients as they were not clinically indicated. Obstetrical and medical complications were noted in less than 10% of the COVID-19 cohort including: oligohydramnios (6/77, 7.8%); fetal growth restriction, defined as ultrasound estimated fetal weight less than the 10th percentile for gestational age (4/77, 5.2%); preeclampsia (5/77, 6.5%); and preexisting or gestational diabetes (7/77, 9.1%). Neonatal outcomes including birthweight; appearance, pulse, grimace, activity, and respiration (APGAR) scores; and neonatal intensive care unit (NICU) admissions were similar between groups.
Table 1
Demographic and clinical patient characteristics by COVID-19 status
| Variablea | COVID-19 cases (n=77) | COVID-19 negative (n=56) | P value |
|---|---|---|---|
| Age (y) | 29.9±6.2 | 32.3±5.0 | .02 |
| BMI (kg/m2) | 32.5±5.5 | 32.1±6.0 | .65 |
| Gestational age at delivery (wk) | 39.1±1.0 | 39.3± 1.1 | .17 |
| Birthweight (g) | 3280±402 | 3280±402 | .31 |
| APGAR (1-min) | 9 (9–9) | 9 (9–9) | .67 |
| APGAR (5-min) | 9 (9–9) | 9 (9–9) | .69 |
| NICU admission | 6 (7.8) | 6 (10.7) | .56 |
| Para | |||
| 0 | 24 (31.2) | 29 (51.8) | .13 |
| 1 | 29 (37.7) | 17 (30.4) | |
| 2 | 13 (16.9) | 7 (12.5) | |
| 3 | 9 (11.7) | 2 (3.6) | |
| ≥4 | 2 (3.5) | 1 (1.8) | |
| Race/ethnicity | |||
| White (non-Hispanic) | 18 (23.4) | 27 (48.2) | .001 |
| Hispanic | 43 (55.8) | 10 (17.9) | |
| Black (non-Hispanic) | 10 (13.0) | 7 (12.5) | |
| Asian (includes Indian) | 4 (5.2) | 9 (16.1) | |
| Other (non-Hispanic) | 2 (2.6) | 3 (5.4) | |
| Mode of delivery | |||
| Vaginal | 57 (74.0) | 14 (25.0) | <.0001 |
| Cesarean | 20 (26.0) | 42 (75.0) | |
| Labor before delivery | 7 (35.0) | 20 (47.6) | .350 |
| Oligohydramnios | 6 (7.8) | 0 | .04 |
| Fetal growth restriction | 4 (5.2) | 0 | .14 |
| Preeclampsia | 5 (6.5) | 0 | .07 |
| Diabetes | |||
| Gestational | 5 (6.5) | 0 | .02 |
| Pregestational | 2 (2.6) | 0 | |
APGAR, appearance, pulse, grimace, activity, and respiration; BMI, body mass index; COVID-19, coronavirus disease 2019; NICU, neonatal intensive care unit.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
There were 25 COVID-19 cases with placental histopathologic features of fetal vascular malperfusion. The majority (18/25) exhibited features of low grade vascular malperfusion with a global distribution; 1 of these had a recent small luminal fibrin thrombus in a chorionic plate vessel. Two cases were considered to have high grade fetal vascular malperfusion, 1 segmental and 1 global in distribution. One of these cases had 2 recent mural fibrin thrombi in chorionic plate vessels (arteries). The remaining 5 cases had intramural fibrin deposition in an isolated stem villous or chorionic plate vessel or single small basal aggregates of avascular villi and were classified as fetal vascular malperfusion of uncertain significance.
We had a total of 16 COVID-19 placentas with histopathologic findings of villitis of unknown etiology. Six cases were classified as high grade, 5 with associated avascular villi, and 1 with chronic deciduitis. Three cases were classified as patchy, low grade villitis of unknown etiology. Four cases had only 1 to 2 small foci of villitis; these were considered ungradable and favor low grade. Three cases had only basal villitis, 1 with mild chronic deciduitis. No chronic chorioamnionitis was identified in any of the cases.
A comparison of placental histopathologic findings between COVID-19 cases vs controls is shown in Table 2 . Placental weights were similar between groups. COVID-19 cases were more likely to have findings associated with fetal vascular malperfusion, including avascular villi (Figure 1 and and2 )2 ) and/or mural fibrin deposition (Figure 3 ) (32.5% [25/77] vs 3.6% [2/56], P<.0001; Table 2). In a multivariable model adjusting for maternal age, race and ethnicity, mode of delivery, fetal growth restriction, preeclampsia, and oligohydramnios, this difference remained statistically significant (OR=12.63; 95% CI, 2.40–66.40; Model 1, Table 3 ). When the adjustment did not include fetal growth restriction, preeclampsia, and oligohydramnios as possible colliders (Model 2), this difference remained statistically significant (OR=12.26; 95% CI, 2.44–61.77; Table 4 ).
Table 2
Comparison of placental histopathologic findings
| Variablea | COVID-19 cases (n=77) | COVID-19 negative (n=56) | P-value | |
|---|---|---|---|---|
| Placental weight | <10th percentile | 7 (9.1) | 7 (12.5) | .078 |
| 10th–25th percentile | 15 (19.5) | 14 (25.0) | ||
| 25th–50th percentile | 25 (32.5) | 9 (16.1) | ||
| 50th–90th percentile | 29 (37.7) | 21 (37.5) | ||
| >90th percentile | 1 (1.3) | 5 (8.9) | ||
| FVM (any feature) | Absent | 52 (67.5) | 54 (96.4) | <.0001 |
| Present | 25 (32.5) | 2 (3.6) | ||
| Avascular villi | Absent | 56 (72.7) | 55 (98.2) | <.0001 |
| Present | 21 (27.3) | 1 (1.8) | ||
| Mural fibrin deposition | Absent | 69 (89.6) | 55 (98.2) | .079 |
| Present | 8 (10.4) | 1 (1.8) | ||
| Chorioamnionitis | Absent | 58 (75.3) | 45 (80.4) | .15 |
| Present, stage 1 | 15 (19.5) | 5 (8.9) | ||
| Present, stage 2 | 4 (5.2) | 6 (10.7) | ||
| Chorioamnionitis fetal response | Absent | 67 (87.0) | 50 (89.3) | .069 |
| Present, stage 1 | 9 (11.7) | 2 (3.6) | ||
| Present, stage 2 | 1 (1.3) | 4 (7.1) | ||
| Villitis of unknown etiology | Absent | 61 (79.2) | 52 (92.9) | .030 |
| Present | 16 (20.8) | 4 (7.1) | ||
| Chronic intervillositis | Absent | 74 (96.1) | 56 (100) | .26 |
| Present | 3 (3.9) | 0 | ||
| Infarct | Absent | 57 (74.0) | 47 (83.9) | .17 |
| Present | 20 (26.0) | 9 (16.1) | ||
| Hematoma | Absent | 56 (72.7) | 45 (80.3) | .31 |
| Present | 21 (27.2) | 11 (19.6) | ||
| Meconium | Absent | 61 (79.2) | 52 (92.9) | .030 |
| Present | 16 (20.8) | 4 (7.1) | ||
| Decidual vasculopathy | Absent | 74 (96.1) | 56 (100) | .51 |
| Present—OC | 2 (2.6) | 0 | ||
| Present—PM | 1 (1.3) | 0 | ||
| No placental lesions | 11 (14.3) | 27 (48.2) | <.0001 | |
COVID-19, coronavirus disease 2019; FVM, fetal vascular malperfusion; OC, occlusive; PM, persistent muscularization.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
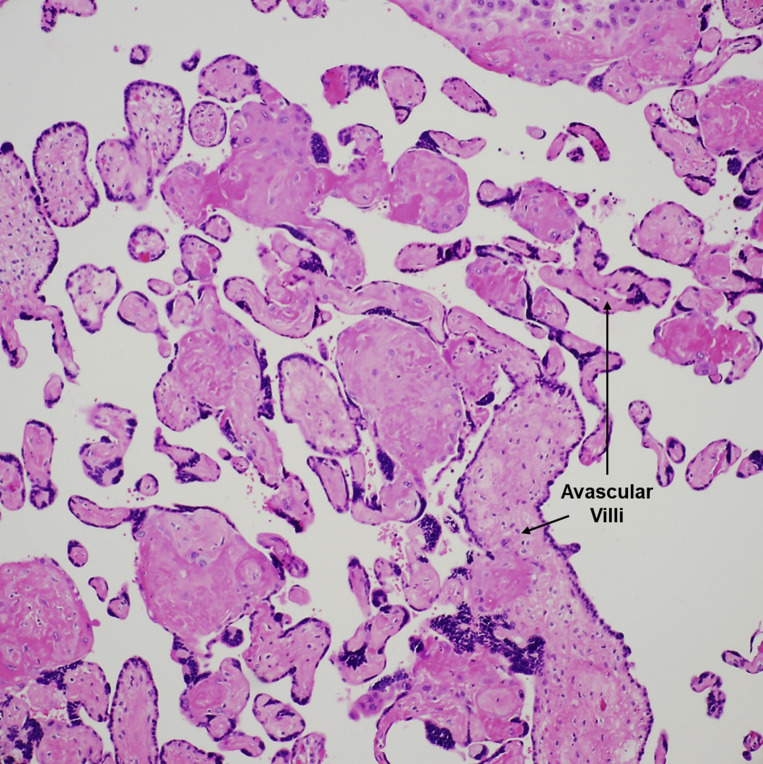
Fetal vascular malperfusion
Large aggregate (>10 villi) of avascular villi, rimmed by preserved syncytiotrophoblasts, with total loss of villous capillaries and hyaline fibrosis of the stroma. H&E stain ×200.
H&E, hematoxylin and eosin.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
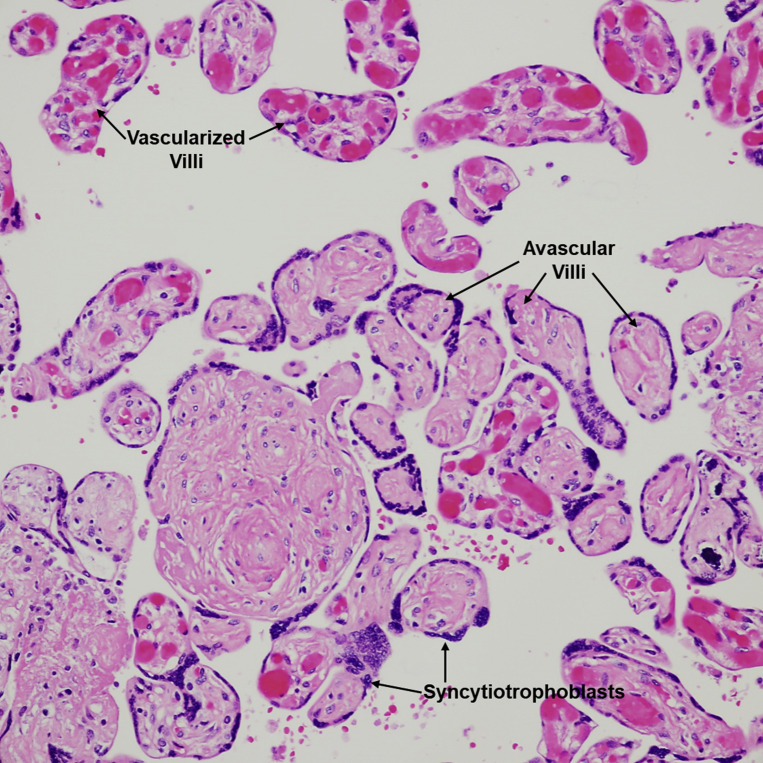
Fetal vascular malperfusion
Intermediate-sized aggregate of villi, rimmed by preserved syncytiotrophoblasts, with total loss of villous capillaries and hyaline fibrosis of the stroma. Well-vascularized villi are seen in the upper portion of the figure; a few are scattered in-between the avascular villi. H&E stain ×200.
H&E, hematoxylin and eosin.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
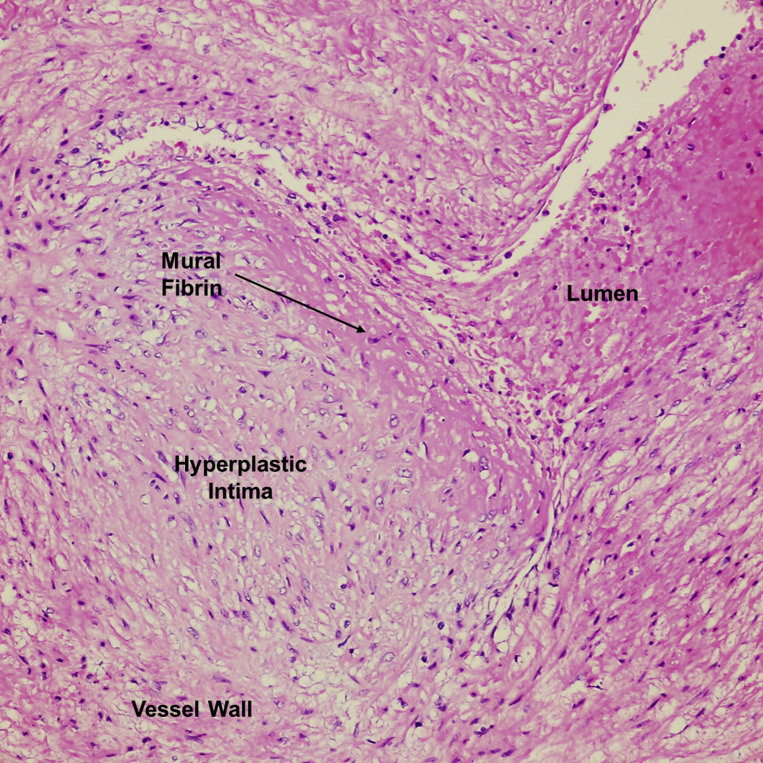
Intramural fibrin deposition in a stem villous vessel
Vessel with an area of eccentric intimal hyperplasia and subendothelial fibrin deposition. Blood is seen in the lumen. H&E stain ×400.
H&E, hematoxylin and eosin.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Table 3
Multivariable logistic regression of FVM, Model 1
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence limits | P value | |
|---|---|---|---|---|---|---|
| Intercept | −2.46 | 1.71 | .1496 | |||
| Group | ||||||
| COVID-19 cases vs negative controls | 1.27 | 0.42 | 12.63 | 2.40 | 66.40 | .0027 |
| Age | 0.01 | 0.04 | 1.01 | 0.93 | 1.10 | .8020 |
| Race/ethnicity | ||||||
| Asian/other, non-Hispanic vs White, non-Hispanic | 0.40 | 0.64 | 2.23 | 0.34 | 14.54 | .5335 |
| Black, non-Hispanic vs White, non-Hispanic | 0.33 | 0.52 | 2.08 | 0.45 | 9.71 | .5268 |
| Hispanic vs White, non-Hispanic | −0.33 | 0.38 | 1.08 | 0.33 | 3.52 | .3946 |
| Mode of delivery | ||||||
| Vaginal vs cesarean delivery | 0.21 | 0.30 | 1.51 | 0.46 | 4.93 | .4924 |
| Preeclampsia | 0.01 | 0.51 | 1.02 | 0.14 | 7.55 | .9817 |
| Yes vs no | ||||||
| Fetal growth restriction | −0.34 | 0.65 | 0.51 | 0.04 | 6.57 | .6026 |
| Yes vs no | ||||||
| Oligohydramnios | 0.08 | 0.47 | 1.17 | 0.19 | 7.38 | .8659 |
| Yes vs no | ||||||
AUC, area under the curve; COVID-19, coronavirus disease 2019; FVM, fetal vascular malperfusion.
Hosmer-Lemeshow goodness of fit test (chi-square=5.92, df=8, P<.6559); AUC=0.74.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Table 4
Multivariable logistic regression of FVM, Model 2
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence limits | P value | |
|---|---|---|---|---|---|---|
| Intercept | −2.17 | 1.36 | .1105 | |||
| Group | ||||||
| COVID-19 cases vs negative controls | 1.25 | 0.41 | 12.26 | 2.44 | 61.77 | .0024 |
| Age | 0.01 | 0.04 | 1.01 | 0.93 | 1.10 | .8325 |
| Race and ethnicity | ||||||
| Asian or other, non-Hispanic vs white, non-Hispanic | 0.26 | 0.59 | 1.84 | 0.33 | 10.20 | .4859 |
| Black, non-Hispanic vs white, non-Hispanic | 0.39 | 0.50 | 2.09 | 0.47 | 9.29 | .3352 |
| Hispanic vs white, non-Hispanic | −0.30 | 0.37 | 1.05 | 0.33 | 3.39 | .9326 |
| Mode of delivery | ||||||
| Vaginal vs cesarean delivery | 0.18 | 0.29 | 1.43 | 0.45 | 4.52 | .5407 |
AUC, area under the curve; COVID-19, coronavirus disease 2019; FVM, fetal vascular malperfusion.
Hosmer-Lemeshow goodness of fit test (chi-square=5.78, df=8, P<.6722); AUC=0.73.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Patients with COVID-19 were also more likely than controls to have villitis of unknown etiology (Figure 4, Figure 5, Figure 6 ) on placental histopathology (20.8% [16/77] vs 7.1% [4/56], P=.030). In a multivariable logistic regression model adjusting for maternal age, race and ethnicity, mode of delivery, oligohydramnios, and fetal growth restriction, this difference was no longer statistically significant (OR=2.11; 95% CI, 0.50–8.97; Model 1, Table 5 ). Similarly, there was no difference in the incidence of villitis of unknown etiology when the adjustment did not include fetal growth restriction and oligohydramnios as possible colliders (Model 2) (OR=2.71; 95% CI, 0.68–10.84; Table 6 ).
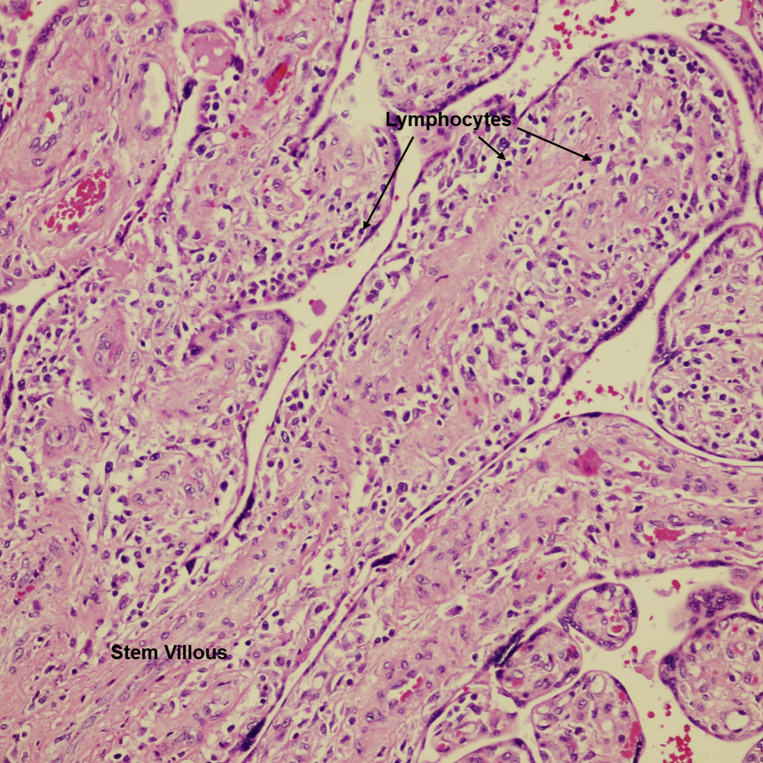
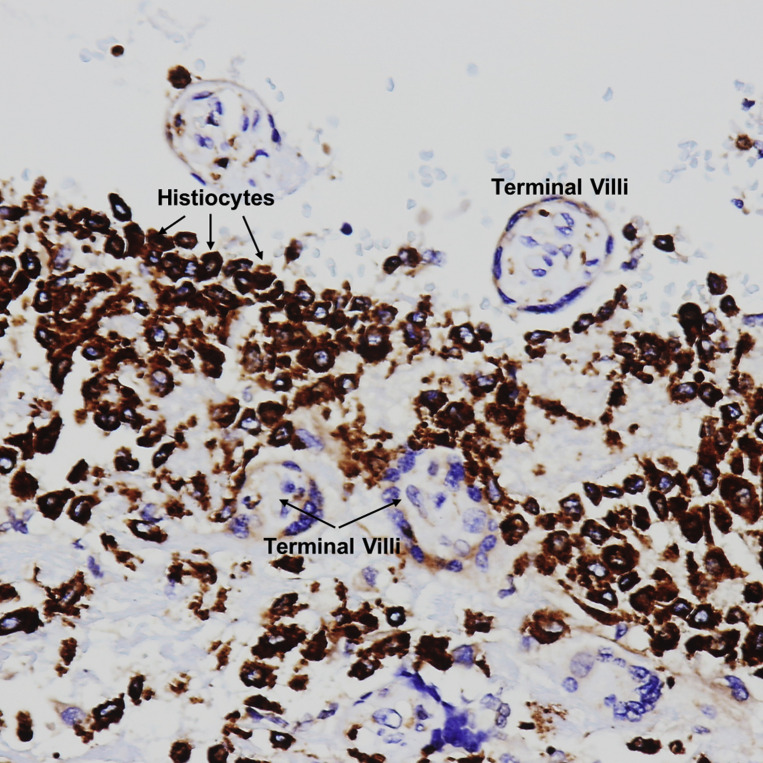
Villitis of unknown etiology
Stem villous and terminal villi infiltrated by a lymphohistiocytic inflammatory infiltrate in the stroma. VUE = villi infiltrated by lymphocytes and histiocytes. H&E stain ×400.
H&E, hematoxylin and eosin; VUE, villitis of unknown etiology.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
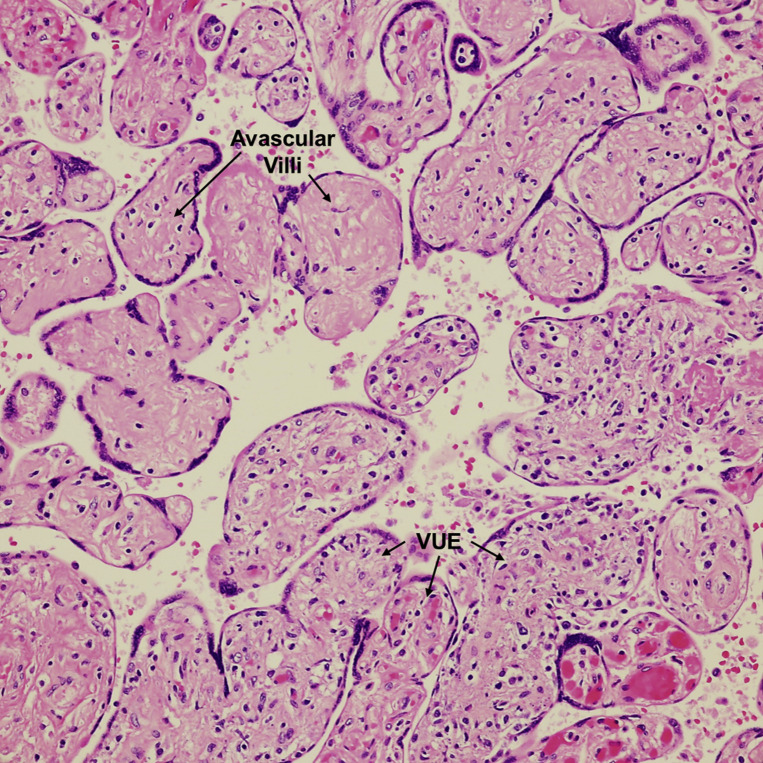
Villitis of unknown etiology with avascular villi
Terminal villi infiltrated by lymphocytes (bottom right). Aggregate of avascular villi, rimmed by preserved syncytiotrophoblasts, with complete loss of vessels and hyaline fibrosis of the stroma (upper left). Suggests vascular occlusion of proximal stem villi (not indicated in this figure). VUE=terminal villi with inflammatory cells. H&E stain ×200.
H&E, hematoxylin and eosin; VUE, villitis of unknown etiology.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
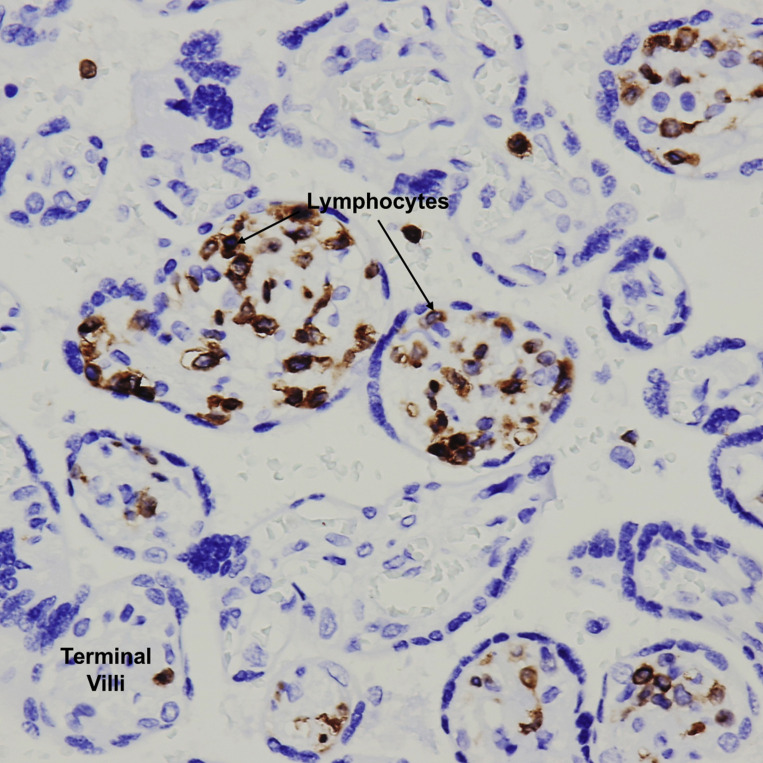
Villitis of unknown etiology
Terminal villi with a T-cell lymphocytic inflammatory infiltrate in the villous stroma. CD3 immunohistochemical stain ×400.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Table 5
Multivariable logistic regression of villitis of unknown etiology (Model 1)
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence limits | P value | |
|---|---|---|---|---|---|---|
| Intercept | −0.43 | 1.71 | .8024 | |||
| Group | ||||||
| COVID-19 cases vs negative controls | 0.37 | 0.37 | 2.11 | 0.50 | 8.97 | .3126 |
| Age | −0.02 | 0.05 | 0.98 | 0.89 | 1.09 | .7463 |
| Race and ethnicity | ||||||
| Black/Asian/other, non-Hispanic vs white, non-Hispanic | −0.81 | 0.57 | 0.61 | 0.09 | 4.34 | .6207 |
| Hispanic vs white, non-Hispanic | 1.13 | 0.41 | 4.22 | 1.01 | 17.74 | .0490 |
| Mode of delivery | ||||||
| Vaginal vs cesarean delivery | −0.35 | 0.33 | 0.49 | 0.13 | 1.83 | .2899 |
| Fetal growth restriction | ||||||
| Yes vs no | 1.01 | 0.64 | 7.54 | 0.62 | 91.51 | .1128 |
| Oligohydramnios | ||||||
| Yes vs no | 0.43 | 0.50 | 2.38 | 0.34 | 16.75 | .3824 |
AUC, area under the curve; COVID-19, coronavirus disease 2019.
Hosmer-Lemeshow goodness of fit test (chi-square=6.41, df=8, P<.602); AUC=0.77.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Table 6
Multivariable logistic regression of villitis of unknown etiology (Model 2)
| Parameter | Estimate | Standard error | Odds ratio | 95% confidence limits | P value | |
|---|---|---|---|---|---|---|
| Intercept | −1.72 | 1.50 | .2532 | |||
| Group | ||||||
| COVID-19 cases vs negative controls | 0.50 | 0.35 | 2.71 | 0.68 | 10.84 | .1589 |
| Age | −0.02 | 0.05 | 0.98 | 0.90 | 1.08 | .7259 |
| Race and ethnicity | ||||||
| Black/Asian/other, non-Hispanic vs white, non-Hispanic | −0.68 | 0.54 | 0.75 | 0.11 | 4.88 | .7595 |
| Hispanic vs white, non-Hispanic | 1.06 | 0.40 | 4.22 | 1.03 | 17.22 | .0447 |
| Mode of delivery | ||||||
| Vaginal vs cesarean delivery | −0.38 | 0.33 | 0.47 | 0.13 | 1.67 | .2407 |
AUC, area under the curve; COVID-19, coronavirus disease 2019.
Hosmer-Lemeshow goodness of fit test (chi-square=7.93, df=8, P<.4407); AUC=0.75.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
COVID-19 cases were more likely to have evidence of meconium on placental evaluation than controls (20.8% [16/77] vs 7.1% [4/56], P=.030; Table 2). Although 14.3% of COVID cases had no substantial histopathologic abnormalities, this was significantly less common than for the controls (48.2% [27/56] vs 14.3% [11/77], P<.0001). Other placental histopathologic findings were similar between groups (Table 2).
For the secondary objective, when comparing asymptomatic patients with COVID-19 (n=67) with the controls, a significant difference was again observed in the frequency of fetal vascular malperfusion and villitis of unknown etiology (31.3% [21/67] vs 3.6% [2/56], P<.0001 and 22.4% [15/67] vs 7.1% [4/56], P=.020, respectively; Table 7 ). In another subgroup analysis of patients with COVID-19 comparing those with symptoms (n=10) with those without (n=67), there was no difference in any of the placental histopathologic findings between groups (data not shown).
Table 7
Comparison of placental histopathologic findings in a subgroup analysis of asymptomatic COVID-19 cases vs negative controls
| Variablea | COVID-19 cases, asymptomatic (n=67) | COVID-19 negative (n=56) | P value | |
|---|---|---|---|---|
| Placental weight | <10th percentile | 7 (10.5) | 7 (12.5) | .098 |
| 10th–90th percentile | 59 (88.0) | 44 (78.6) | ||
| >90th percentile | 1 (1.5) | 5 (8.9) | ||
| Fetal vascular malperfusion (any feature) | Absent | 46 (68.7) | 54 (96.4) | <.0001 |
| Present | 21 (31.3) | 2 (3.6) | ||
| Avascular villi | Absent | 48 (71.6) | 55(98.2) | <.0001 |
| Present | 19 (28.4) | 1 (1.8) | ||
| Mural fibrin deposition | Absent | 61 (91.0) | 55 (98.2) | .125 |
| Present | 6 (9.0) | 1 (1.8) | ||
| Chorioamnionitis stage | Absent | 49 (73.1) | 45 (80.4) | .069 |
| Present, stage 1 | 13 (22.4) | 5 (8.9) | ||
| Present, stage 2 | 3 (4.5) | 6 (10.7) | ||
| Chorioamnionitis fetal response | Absent | 58 (86.6) | 50 (89.3) | .096 |
| Present, stage 1 | 8 (11.9) | 2 (3.6) | ||
| Present, stage 2 | 1 (1.5) | 4 (7.1) | ||
| Villitis of unknown etiology | Absent | 52 (77.6) | 52 (92.9) | .020 |
| Present | 15 (22.4) | 4 (7.1) | ||
| Chronic intervillositis | Absent | 64 (95.5) | 56 (100) | .250 |
| Present | 3 (4.5) | 0 | ||
| Infarct | Absent | 48 (71.6) | 47 (83.9) | .132 |
| Present | 19 (28.4) | 9 (16.0) | ||
| Hematoma | Absent | 49 (73.1) | 45 (80.4) | .400 |
| Present | 18 (26.9) | 11 (19.6) | ||
| Meconium | Absent | 51 (76.1) | 52 (92.9) | .014 |
| Present | 16 (23.9) | 4 (7.1) | ||
| Decidual vasculopathy | Absent | 64 (95.5) | 56 (100) | .500 |
| Present – OC | 2 (3.0) | 0 | ||
| Present – PM | 1 (1.5) | 0 | ||
COVID-19, coronavirus disease 2019; OC, occlusive; PM, persistent muscularization.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Comment
Principal findings
We found that placental histopathologic abnormalities were more frequent among COVID-19 cases than controls. Both fetal vascular malperfusion and villitis of unknown etiology were significantly more common in the COVID-19 cases than the controls (32.5% [25/77] vs 3.6% [2/56], P<.001 and 20.8% [16/77] vs 7.1% [4/56], P=.030, respectively). The difference in fetal vascular malperfusion lesions persisted in multivariable analysis after adjusting for potential confounders; however, this difference did not persist in a similar multivariable model for villitis of unknown etiology. The statistically significant increase in incidence of fetal vascular malperfusion and villitis of unknown etiology persisted even among asymptomatic COVID-19 cases compared with controls (31.3% [21/67] vs 3.6% [2/56], P<.0001 and 22.4% [15/67] vs 7.1% [4/56], P=.020, respectively). We did not observe an increase in the other placental histopathologic lesions, including those related to maternal vascular malperfusion, except for presence of meconium, which was likely attributable to higher rate of vaginal delivery in the COVID-19 group and of little clinical significance.
Results in the context of what is known
In a recent structured review including 20 studies with histopathology findings in third trimester placentas following maternal SARS-CoV-2 infection, evidence of fetal vascular malperfusion was reported in 35% of cases, which is similar to the rate observed in our cohort (32.5%).22 In this review, the rate of maternal vascular malperfusion was reported to be higher than that observed in our cohort, whereas villitis of unknown etiology was less frequent than in our study. The authors note that limited conclusions can be drawn regarding the effects of SARS-CoV-2 on the placenta because most of the studies included few patients and lacked control groups. This review also found no evidence of vertical transmission to the fetus in any of the identified articles, which we also observed. The exact mechanism by which SARS-CoV-2 may cross the placental barrier is not fully elucidated. However, it is hypothesized that the observed low rate of vertical transmission is due, in part, to the fact that 2 key receptors that must be present for viral entry into host cells (angiotensin-converting enzyme 2 and transmembrane protease serine 2) are rarely coexpressed in third trimester placentas.23
We identified 6 studies that reported third trimester placental histopathology findings in >5 patients with COVID-19 and included a control group24, 25, 26, 27, 28, 29 (Table 8 ). Prahbu et al24 found a higher rate of lesions of fetal vascular malperfusion in the COVID-19 cohort than the controls, similar to our study. Two studies25 , 28 reported a higher frequency of lesions associated with maternal vascular malperfusion than controls, which we did not observe, possibly because we included only term patients. Of note, both studies included preterm placentas, which are known to be associated with maternal vascular malperfusion lesions. Three studies26 , 27 , 29 reported no specific placental histopathologic features in patients with COVID-19 compared with their respective controls. The studies varied considerably in their design, choice of control group, inclusion and exclusion criteria, and classification of placental lesions, limiting our ability to compare findings with those of this study.
Table 8
Summary of literature reporting placental histopathology findings in third trimester placentas following maternal SARS-CoV-2 infection which contain >5 patients and a control group
| Author | #COVID cases | Control group | Histologic findings of SARS-CoV-2–positive placentas |
|---|---|---|---|
| Prahbu et al24 | 29 | Consecutive SARS-CoV-2–negative deliveries >20 wk GA, matched for delivery time period; all had another clinical indication for placental pathology (n=106) | Fetal vascular malperfusion: 14/29 (48%)a |
| Meconium: 18/29 (62%)a | |||
| Maternal vascular malperfusion: 8/29 (28%) | |||
| Chronic villitis: 5/29 (17%) | |||
| Acute chorioamnionitis: 3/29 (10%) | |||
| Shanes et al25 | 15 |
| Maternal vascular malperfusion: 12/15 (80%)a |
| Fetal vascular malperfusion – formal diagnosis: 1/15 (7%) | ||
| Findings suggestive of FVM (any feature): | |||
| Clustered avascular villi: 4/15 (27%) | |||
| Mural fibrin deposition: 1/15 (7%) | |||
| Delayed villous maturation: 4/15 (27%) | |||
| Chorioamnionitis with fetal response: 1/15 (7%) | |||
| Chronic inflammatory pathology | |||
| Low-grade chronic lymphocytic villitis: 2/15 (7%) | |||
| Chronic deciduitis: 2/15 (7%) | |||
| Hecht et al2 | 19 |
| Maternal vascular malperfusion: 5/19 (25%) |
| Low grade: 3/19 (16%) | ||
| High grade: 2/19 (11%) | |||
| Fetal vascular malperfusion: 3/19 (16%) | |||
| Acute chorioamnionitis: 6/19 )32%) | |||
| Fetal inflammatory response: 4/19 (21%) | |||
| Villitis of unknown etiology: 1/19 (5%) | |||
| Histiocytic intervillositis: 1/19 (5%) | |||
| Zhang et al27 | 74 | Consecutive term and preterm SARS-CoV-2–negative placentas submitted based on “institutional criteria of maternal and fetal conditions” and matched for delivery time period (n=290) | Maternal vascular malperfusion |
| “Vasculopathy”: 36/74 (49%) | |||
| Infarcts: 7/74 (9%) | |||
| Fetal vascular malperfusion | |||
| “Thrombosis”: 18/74 (24%) | |||
| Avascular villi: 5/74 (7%) | |||
| Chorioamnionitis: 48/74 (65%) | |||
| Meconium: 24/74 (32%) | |||
| Villitis: 17/74 (23%) | |||
| Maternal floor infarct: 2/74 (3%) | |||
| Smithgall et al28 | 51 | Consecutive term and preterm singleton placentas from SARS-CoV-2–negative deliveries matched for delivery time period (n=25); subgroup analysis of symptomatic vs asymptomatic infections performed | Maternal vascular malperfusion |
| Decidual vasculopathy: 3/51 (6%) | |||
| Accelerated villous maturity: 10/51 (20%) | |||
| Villous agglutination: 21/51 (41%)a | |||
| Infarcts: 7/51 (14%) | |||
| Intervillous thrombus: 8/51 (16%) | |||
| Subchorionic thrombus: 9/51 (18%)a | |||
| Fetal vascular malperfusion | |||
| Avascular villi (segmental): 5/51 (10%) | |||
| Fetal thrombotic vasculopathy: 4/51 (8%) | |||
| Chorangiosis: 8/51 (16%) | |||
| Chronic villitis, unknown etiology: 2/51 (4%) | |||
| Acute intrauterine infection | |||
| Maternal response: 17/51 (33%) | |||
| Fetal response: 9/51 (18%) | |||
| Gulersen et al29 | 50 | Term and preterm singleton placentas from historic controls submitted to pathology at “discretion of the delivery physician;” matched for GA at delivery (n=50); subgroup analysis of symptomatic vs asymptomatic infections performed | Maternal vascular malperfusion |
| Accelerated villous maturation: 0/50 | |||
| Decidual vasculopathy: 0/50 | |||
| Distal villous hypoplasia: 2/50 (4%) | |||
| Excessive infarction: 4/50 (8%) | |||
| Old hemorrhage in membranes: 1/50 (2%) | |||
| Fetal vascular malperfusion: 4/50 (8%) | |||
| Chorinitis: 11/50 (22%) | |||
| Amnionitis: 9/50 (18%) | |||
| Meconium staining: 9/50 (18%) |
COVID-19, coronavirus disease 2019; FVM, fetal vascular malperfusion; GA, gestational age; GBS, Group B Streptococcus; HIE, hypoxic-ischemic encephalopathy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Clinical implications
Pregnant women with COVID-19 can present with COVID-19–related symptoms; obstetrical complications such as pregnancy loss, fetal death, preeclampsia, and preterm birth (among others); or be asymptomatic at term.30, 31, 32, 33 Despite the fact that nearly all neonates born to COVID-19–infected mothers are SARS-CoV-2 PCR-negative,33 , 34 the virus could impact perinatal outcomes through placental injury. Indeed, placental injury has been reported in symptomatic cases and those with adverse pregnancy outcomes.14 , 30, 31, 32 However, neonatal outcomes in our study, including birthweight, NICU admissions, and APGAR scores, were similar between COVID-19 and control groups, despite the higher rate of placental abnormalities in the COVID-19 cohort. This suggests that the placenta may act as a defensive biologic filter, offering a degree of protection to the neonate; modify its immune status; or actively produce factors that may protect the fetus in utero. However, our findings suggest that there is injury to the placenta with possible adverse effects in the neonates, warranting follow-up for possible long-term side effects.
The etiology and significance of the increased rate of lesions related to fetal vascular malperfusion in our COVID-19 cohort is unclear. In our COVID-19 placentas, most fetal vascular malperfusion lesions had a global distribution, suggesting a partial or intermittent obstruction in umbilical blood flow, rather than segmental, as is seen in the case of localized thrombosis and occlusion of chorionic plate or stem villous vessels. In this global pattern, endothelial damage may reflect altered flow rather than thrombosis per se.35 Usually, but not always, this pattern is seen in cases with predisposing umbilical cord lesions, none of which were observed in our cohort. If or how SARS-CoV-2 or COVID-19 may lead to such alterations in fetal umbilical blood flow and ensuing endothelial damage should be a focus of future research. We suspect that indirect immune- and/or viral-mediated systemic damage, rather than local pathophysiologic changes (ie, hypoxemia) of COVID-19 may be responsible, because our findings were similar when we included only asymptomatic cases in the analysis. We found only 2 cases of fetal vascular thrombi (both in chorionic plate vessels), in comparison to others who found that many of the cases of fetal vascular malperfusion had evidence of fetal vascular thrombosis,24 , 36 , 37 which they hypothesized may be attributable to the increased hypercoagulable state induced by COVID-19.
We did not observe an increase in the incidence of placental lesions associated with maternal vascular malperfusion, including placenta weight <10th percentile, intraparenchymal or retroplacental hematomas, infarcts, or decidual vasculopathy, compared with controls. Maternal vascular malperfusion lesions in the placenta are associated with adverse pregnancy outcomes, including stillbirth, preterm delivery, and placental abruption, and have been observed with increased frequency compared with controls in 1 study on placental pathology in patients with COVID-19.25 Similarly, we did not find an increase in the incidence of pregnancy outcomes related to ischemic placental disease (fetal growth restriction, oligohydramnios, and preeclampsia).This may be related to the severity of the disease, with more severe COVID-19 infection resulting in greater damage on the maternal unit of the placenta. Alternatively, the timing and duration of infection may play an important role, with immature placentas being more vulnerable to certain types of viral-mediated damage than term placentas. Our study included only term placentas, whereas others have included a substantial proportion of preterm deliveries, in which lesions of maternal vascular malperfusion are known to be more common.
Unlike other reports to date, our study observed an increase in the incidence of villitis of unknown etiology in placentas of patients with COVID-19 (20.8% [16/77] vs 7.1% [4/56], P=.030). Although this statistical significance did not persist in multivariable modeling, this was likely secondary Type II error from a small sample size. Nevertheless, the rate of villitis of unknown etiology in our COVID-19 cohort is 3 times higher than in controls and is higher than expected in term placentas (5%–15%).35 This may be due to the study time period extending several months past the peak of COVID-19 infections in our region, with this lesion developing more often in women who have a longer interval from infection to delivery. In all cases of villitis of unknown etiology in our COVID-19–positive cohort, there was no clinical history that could explain this finding and placental weights were normal (between 10th and 90th percentile) in these patients. The presence of villitis of unknown etiology in otherwise healthy patients with normal placental weights suggests that exposure to SARS-CoV-2 may have occurred earlier in the third trimester, with villitis of unknown etiology developing as a sequelae, after weeks of harboring the virus. The etiology of the villitis of unknown etiology seen in these placentas is uncertain; it may be a direct or indirect consequence of viral infection resulting in an exaggerated systemic immune response, including increased levels of cytokines, as has been noted following other respiratory viral infections.38 It appears unlikely that pathophysiologic changes from severe COVID-19 disease, such as maternal hypoxia, influenced these findings because our results were similar in a subgroup analysis including only asymptomatic cases.
Chronic intervillositis was observed in placentas from 3 (4.5%) patients with COVID-19 (Figures 7 and and8 ),8 ), all of whom were asymptomatic, and none in the control group. This difference was not statistically significant (P=.26), although the comparison is limited by small sample size. Two previous case reports on placental histopathology in patients with COVID-19 have described chronic intervillositis as a more common finding14 , 30; however, both cases were preterm gestations with severe COVID-19 symptomatology. The fact that our study included only term gestations, the majority of whom were asymptomatic or with mild symptoms, may contribute to the observation that chronic intervillositis as an uncommon abnormality in our COVID-19–positive cohort.
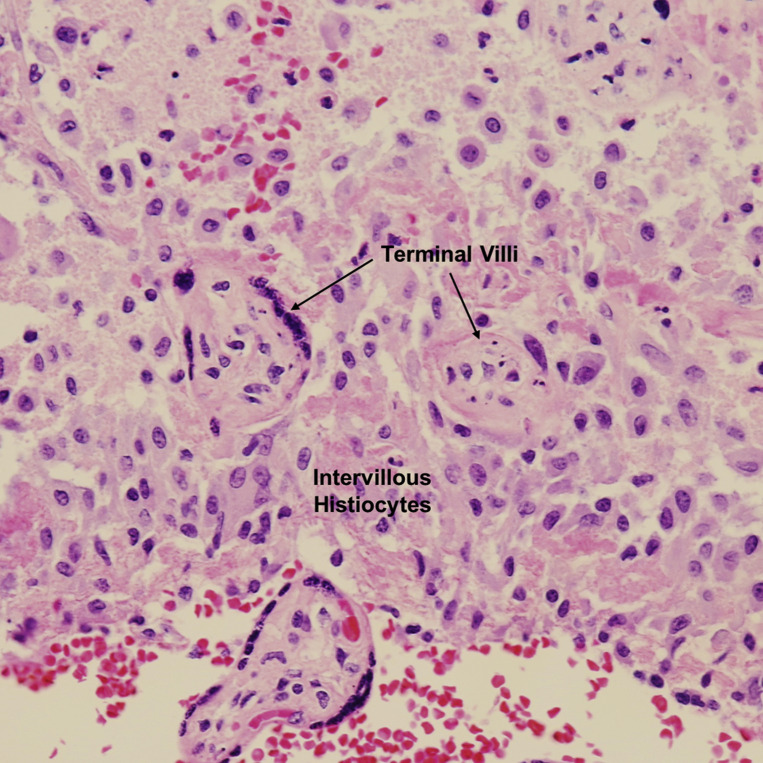
Chronic intervillositis
Aggregates of histiocytes between terminal villi. H&E stain ×200.
H&E, hematoxylin and eosin.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Research implications
Clarifying the relationship between adverse obstetrical outcomes and COVID-19–related placental histopathologic lesions should be the focus of further research. Future investigation may be directed at determining if the increased rate of placental abnormalities, particularly fetal vascular malperfusion, translates into differences in neonatal outcomes. Long-term follow-up of these infants may be warranted and may be the focus of future studies if indeed our findings are confirmed by other investigators. In addition, further studies are warranted to examine placental pathology of patients who were infected with COVID-19 earlier in gestation, as both duration of infection and placental maturity may impact type and frequency of various histopathologic lesions.
Strengths and limitations
To our knowledge, this is the largest study describing histopathological findings in the placentas of term women diagnosed with COVID-19 infection during their admission to Labor and Delivery. In addition, this is the only study to include a contemporaneous comparison group of term COVID-19–negative controls without other potentially confounding medical or obstetrical complications. Other studies that have used a control group have included preterm gestations, historic controls, and/or those with comorbid conditions.24, 25, 26, 27, 28, 29
Another strength is that our sample of women with COVID-19 consists of consecutive patients who were routinely tested on admission to Labor and Delivery, regardless of symptomatology, allowing us to compare findings among women with and without symptoms, which few others have done.28 , 29 This study design captured all eligible patients who met the inclusion criteria.
One of our study’s limitations is that we were not able to assess timing of COVID-19 infection in our study patients; however, in a limited (1-month) audit, we identified that for 75% of these patients, the finding of positive COVID-19 status was new and the remaining 25% patients were known to be COVID-19–positive by a previous PCR test before admission. Another limitation is to identify the ideal control group because placentas are sent to pathology predominantly in patients with high-risk maternal or fetal conditions in our institution. Therefore, we had to include many placentas from patients whose only indication for placental evaluation was delivery through cesarean section, which decreased our sample size and ability to match for certain clinical characteristics. This also accounts for the significantly higher rate of cesarean delivery in the control group (42/56, 75% vs 20/77, 26%; P<.0001). However, the majority of patients in the control group who delivered via cesarean delivery underwent labor before delivery, and there was no statistically significant difference between groups in predelivery labor (7/20, 35% vs 20/42, 47.6%; P=.350), nor would we expect mode of delivery to affect placental lesions related to fetal vascular malperfusion or villitis of unknown etiology.
Control placentas had fewer sections examined per placenta than COVID-19 cases, which may contribute to the higher frequency of certain lesions in the latter group. However, if this were the case, we would expect all lesions to be more frequent in the COVID-19 group, which was not our observation. It is unlikely that oversampling alone could account for the 3-fold increase in lesions of villitis of unknown etiology or the nearly 10-fold increase in lesions associated with fetal vascular malperfusion in cases compared with controls. Although differences in pathologists’ interpretation could influence differences in findings between groups, we found excellent interobserver reliability (AC1 >0.9; Table 9 ) on rereview of approximately one-third of all placentas in our study (26 cases and 21 controls). Finally, pathologists were not blinded to COVID-19 status, as this study was performed in a pragmatic fashion. However, during the reliability assessment, pathologists were blinded to COVID-19 status and we found excellent interclass correlation.
Table 9
Pathologist interobserver reliability for select placental histopathology findings in a subset of placentas from COVID-19 cases (n=26) and controls (n=21)
| Placental lesion | AC1 (SE) |
|---|---|
| FVM with avascular villi, low grade | 0.961 (0.028) |
| FVM with avascular villi, high grade | 0.929 (0.038) |
| FVM with mural fibrin deposition | 1.000 (0) |
| VUE, low grade | 0.954 (0.027) |
| VUE, high grade | 0.941 (0.036) |
AC1, Gwet’s first-order agreement coefficient; COVID-19, coronavirus disease 2019; FVM, fetal vascular malperfusion; SE, standard error; VUE, villitis of unknown etiology.
Patberg et al. Coronavirus disease 2019 placental pathology. Am J Obstet Gynecol 2021.
Conclusions
We found that COVID-19 infection during pregnancy results in increased rates of placental histopathologic abnormalities, particularly fetal vascular malperfusion and villitis of unknown etiology. These findings seem to occur regardless of whether SARS-CoV-2 positive testing leads to COVID-19 symptomatology in pregnant patients. This suggests that placental damage is mediated by virus-induced injury on the fetal-placental unit, rather than as a sequelae of COVID-19 pathophysiology in the mother. Long-term neonatal follow-up may be warranted if our findings are confirmed by future studies.
Acknowledgments
We would like to thank the pathology physician assistants and resident physicians who were integral to our study and responsible for the processing of placentas for this study. We would also like to thank Rosanne Vertichio for her assistance in data collection.
Footnotes
The authors have no conflict of interest to report.
Cite this article as: Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol 2021;224:382.e1-18.
Supplementary Data
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ajog.2020.10.020
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7571377
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ajog.2020.10.020
Article citations
Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women.
J Clin Med, 13(18):5546, 19 Sep 2024
Cited by: 0 articles | PMID: 39337033 | PMCID: PMC11432756
Comparing the immunogenicity of COVID-19 infection and vaccination in pregnant women as measured by anti-S IgG.
BMC Infect Dis, 24(1):935, 09 Sep 2024
Cited by: 0 articles | PMID: 39251937 | PMCID: PMC11386373
Vitamin D Receptor-Interplay in COVID-19-Negative, -Infected, and -Vaccinated Women during Pregnancy.
J Clin Med, 13(20):6140, 15 Oct 2024
Cited by: 0 articles | PMID: 39458089 | PMCID: PMC11508755
SARS-CoV-2 infection by trimester of pregnancy and adverse perinatal outcomes: a Mexican retrospective cohort study.
BMJ Open, 14(4):e075928, 11 Apr 2024
Cited by: 0 articles | PMID: 38604636 | PMCID: PMC11015228
Collateral Damage in the Placenta during Viral Infection in Pregnancy: A Possible Mechanism for Vertical Transmission and an Adverse Pregnancy Outcome.
Diseases, 12(3):59, 20 Mar 2024
Cited by: 0 articles | PMID: 38534983 | PMCID: PMC10969698
Review Free full text in Europe PMC
Go to all (80) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection.
Am J Obstet Gynecol MFM, 2(4):100211, 15 Aug 2020
Cited by: 48 articles | PMID: 32838277 | PMCID: PMC7428686
Diagnostic utility of serial circulating placental growth factor levels and uterine artery Doppler waveforms in diagnosing underlying placental diseases in pregnancies at high risk of placental dysfunction.
Am J Obstet Gynecol, 227(4):618.e1-618.e16, 27 May 2022
Cited by: 14 articles | PMID: 35644246
COVID-19 as an independent risk factor for subclinical placental dysfunction.
Eur J Obstet Gynecol Reprod Biol, 259:7-11, 29 Jan 2021
Cited by: 37 articles | PMID: 33556768 | PMCID: PMC7845516
A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection.
Placenta, 101:13-29, 23 Aug 2020
Cited by: 126 articles | PMID: 32911234 | PMCID: PMC7443324
Review Free full text in Europe PMC