Abstract
Free full text

Leukoencephalopathy Associated with Severe COVID-19 Infection: Sequela of Hypoxemia?
SUMMARY:
There is increasing evidence to suggest that complications of coronavirus disease 2019 (COVID-19) infection are not only limited to the pulmonary system but can also involve the central nervous system. Here, we report 6 critically ill patients with COVID-19 infection and neuroimaging findings of leukoencephalopathy. While these findings are nonspecific, we postulate that they may be a delayed response to the profound hypoxemia the patients experienced due to the infection. No abnormal enhancement, hemorrhage, or perfusion abnormalities were noted on MR imaging. In addition, Severe Acute Respiratory Syndrome coronavirus 2 was not detected in the CSF collected from the 2 patients who underwent lumbar puncture. Recognition of COVID-19-related leukoencephalopathy is important for appropriate clinical management, disposition, and prognosis.
There is mounting evidence that Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) can affect the central nervous system, with hematogenous spread or direct neural propagation via the olfactory pathway proposed as possible mechanisms of SARS-CoV-2 neurotropism.1 The angiotensin converting enzyme 2 receptor plays a role in the mechanism by which the SARS-CoV-2 gains cellular entry and is expressed in the brain.2,3 Furthermore, the SARS-CoV-2 virus has been detected in the CSF of a patient with coronavirus disease 2019 (COVID-19).4 Neurologic symptoms are commonly reported in patients with COVID-19 infection; a study from France reported that 91% of patients with COVID-19 infection demonstrated neurologic symptoms, while a study from China reported a lower prevalence of 36%.5,6 Moreover, there are case reports of encephalitis, including a case of hemorrhagic necrotizing encephalopathy, associated with COVID-19 infection.7-10 Recently, there is emerging evidence of white matter injury associated with COVID-19 infection in the form of demyelinating lesions and leukoencephalopathy without a clear etiology.11,12 Here, we report a case series of suspected white matter injury in patients with COVID-19 infection.
Case Series
Our study was performed retrospectively, at a large, urban, academic medical center and was approved by the institutional review board with a waiver of informed consent. A total of 42 patients who tested positive for COVID-19 via real-time reverse transcription polymerase chain reaction underwent brain MR imaging, and the imaging was reviewed. Six patients were found to have imaging findings of leukoencephalopathy and were included in this study.
All 6 patients presented to our hospital for increased difficulty in breathing, and all without neurologic impairment at the time of presentation. The average age of the patients was 64 years (range, 60–76 years; 4 men and 2 women). No patients had a history of neurologic disease or were immunocompromised. One patient had brain MR imaging 1 year prior, which demonstrated minimal nonspecific white matter changes without other abnormalities. All 6 patients required intubation in the emergency department for hypoxic respiratory failure. The lowest recorded arterial oxygen level (partial pressure of oxygen [PaO2]) was between 46 and 66 mm Hg during their hospital course, with an average of 55 mm Hg (On-line Table). All 6 patients were weaned off sedation between admission days 9 and 24 when their respiratory status improved, with 2 patients successfully extubated. All patients exhibited altered mental status and neurologic symptoms between admission days 15 and 30.
Five of the 6 patients underwent noncontrast-enhanced CT imaging first, with a mean time to CT of 23 days (range, 14–30 days) from date of admission. Four of the 5 CT studies were unremarkable, with only patient 2 exhibiting multiple bilateral hypodense lesions in the deep cerebral white matter (Fig 1).
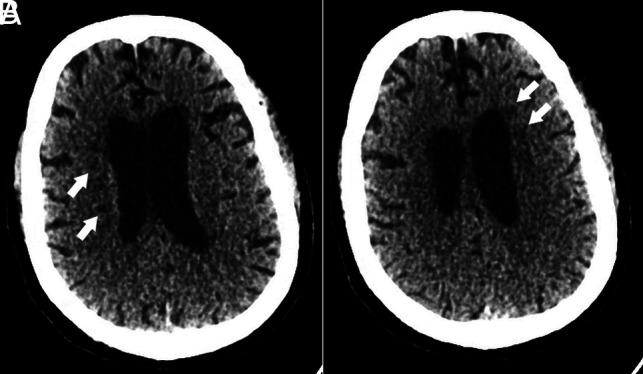
Head CT of a critically ill patient (patient 2) with COVID-19 infection. Axial CT images demonstrate multiple hypodense foci within the bilateral periventricular white matter (white arrows) without evidence of hemorrhage.
All 6 patients eventually underwent MR imaging of the brain with a mean time to MR imaging of 26 days (range, 14–34 days) from date of admission. The mean duration between the PaO2 nadir and MR imaging was 22 days (range, 13–29 days). All MR imaging examinations performed at our institution included axial diffusion-weighted imaging (b = 1000), susceptibility-weighted imaging, axial T2 FLAIR, and axial T2-weighted imaging. Three patients had contrast-enhanced T1-weighted imaging, 2 patients had MRA, and 3 patients had arterial spin-labeling.
On MR images, all 6 patients exhibited symmetric T2 FLAIR hyperintense signal and restricted diffusion involving the deep white matter of both cerebral hemispheres, with relative sparing of the subcortical U-fibers (Figs 2 and and3).3). Additional sites of white matter involvement were also observed in the 6 patients: corpus callosum in patient 2 (Fig 2); middle cerebellar peduncles in patients 1, 2, 4, 5, and 6 (Fig 4); and corticospinal tracts in patients 2, 3 and 4. On the fractional anisotropy map, focal areas of white matter tract disruption were observed only in patient 2 (Fig 2). No evidence of brain stem or basal ganglia involvement, mass effect, hemorrhage, or other acute findings was observed. Of the patients who also underwent contrast-enhanced MR imaging, MRA, or arterial spin-labeling, no abnormal gadolinium enhancement, vascular pathology, or perfusion abnormality was observed (Figs 4 and and5).5). The imaging findings of restricted diffusion with corresponding T2 FLAIR hyperintensity in the white matter are consistent with leukoencephalopathy.
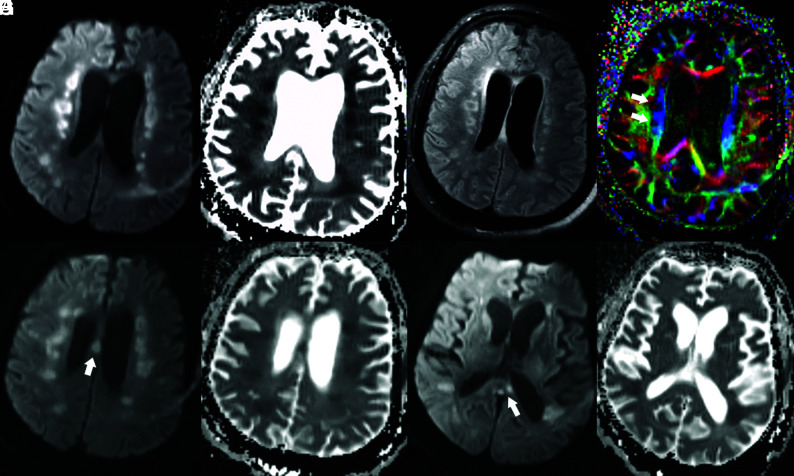
Brain MR images of a critically ill patient (Patient 2) with COVID-19 infection exhibiting impaired arousal, aphasia, and lethargy. A and B, Paired axial DWI and ADC map show symmetric foci of restricted diffusion involving the deep white matter of both cerebral hemispheres. C, Axial T2/FLAIR image through the same level shows associated increased T2/FLAIR hyperintensity corresponding to the regions of restricted diffusion. D, Axial fractional anisotropy map shows focal disruption of white matter tracts in the regions of diffusion restriction (white arrows). E and F, Paired axial DWI and ADC map show restricted diffusion of the body of the corpus callosum (arrow). G and H, Paired axial DWI and ADC map show restricted diffusion of the splenium of the corpus callosum (arrow).
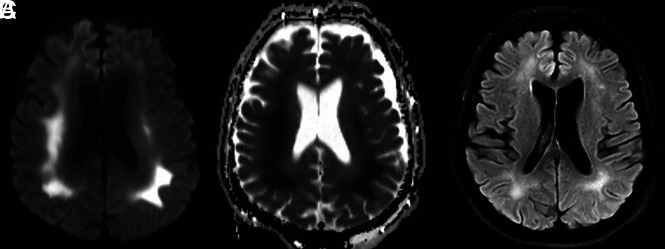
Brain MR images of a critically ill patient (patient 1) with COVID-19 infection exhibiting altered mental status. A and B, Paired axial DWI and ADC map show symmetric restricted diffusion of the deep white matter of both cerebral hemispheres. C, Axial T2 FLAIR image through the same level shows associated increased T2 FLAIR hyperintensity corresponding to the regions of restricted diffusion.
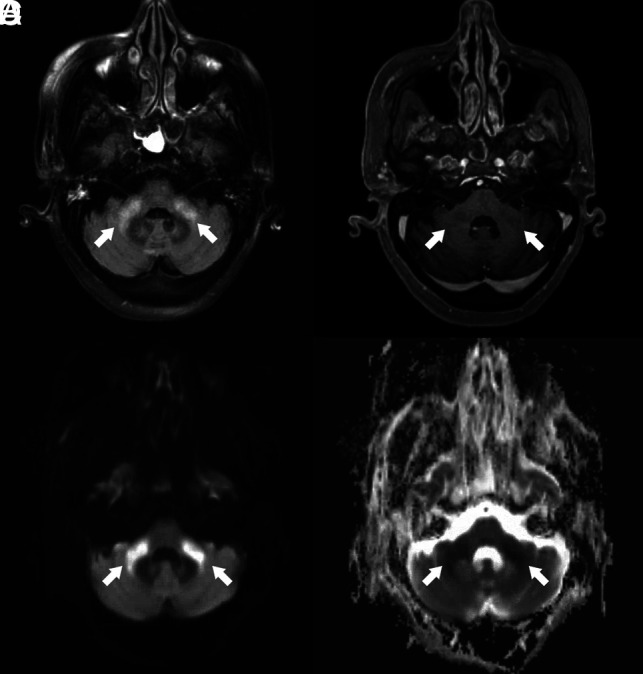
Brain MR images of a critically ill patient (patient 5) with COVID-19 infection exhibiting impaired arousal and diffuse hypertonicity. A, Axial FLAIR image shows increased T2 FLAIR signal in the bilateral middle cerebellar peduncles (arrows). B, Contrast-enhanced T1-weighted image shows absence of abnormal enhancement of the bilateral middle cerebellar peduncles (arrows). C and D, Paired axial DWI and ADC map show corresponding restricted diffusion of bilateral middle cerebellar peduncles (arrows).
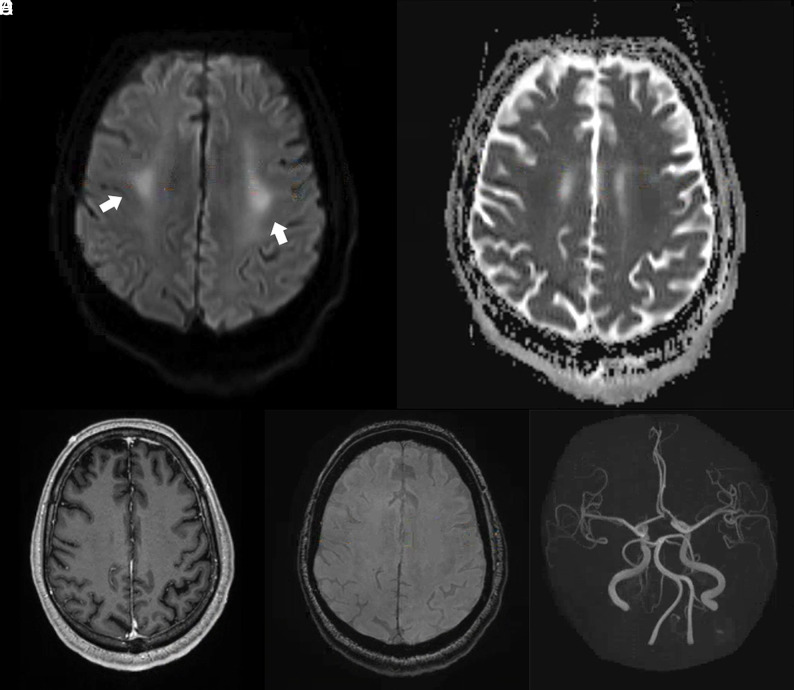
Brain MR images of a critically ill patient (patient 4) with COVID-19 infection exhibiting impaired arousal and left distal lower extremity paresis. A and B, Paired axial DWI and ADC map show symmetric restricted diffusion of the bilateral perirolandic white matter (arrows). C, Axial contrast-enhanced T1-weighted image at the same level demonstrates no corresponding abnormal enhancement. D, Susceptibility-weighted image at the same level demonstrates no susceptibility effect to suggest hemorrhage. E, 3D reconstruction of the circle of Willis from time-of-flight imaging demonstrates no vascular abnormality.
DISCUSSION
Neurologic complications related to COVID-19 infection are not uncommon, but associated neuroimaging manifestations have not been extensively investigated.5,7,10,13 Initial reported neuroimaging findings related to COVID-19 infection included infarction, hemorrhage, and encephalopathy.5,7,13-15 Recently, leukoencephalopathy with intracranial microhemorrhages was reported.11 Specifically, the authors observed posterior predominant white matter FLAIR hyperintensity, posterior circulation hyperperfusion, and susceptibility signal in the corpus callosum. The mechanism underlying leukoencephalopathy in the setting of COVID-19 infection, however, remains unclear. While neurotropism has been proposed as an underlying cause for neurologic symptoms related to COVID-19 infection, there may be additional mechanisms at play.1 Specifically, the brain is sensitive to oxygen deprivation, and severe COVID-19 infection is known to be associated with hypoxemia.16 In our case series, we present evidence of white matter–specific injury, which may be the sequela of COVID-19-related hypoxemia.
On MR imaging, all patients in our case series exhibited sparing of the subcortical U-fibers and brain stem, with relatively symmetric involvement of the deep cerebral white matter, which is reminiscent of delayed posthypoxic leukoencephalopathy.17 Severe cases of COVID-19 infection are associated with profound hypoxemia, and all 6 patients in our series required intubation and experienced marked hypoxemia. It has been postulated that prolonged or severe hypoxemia can lead to myelin sheath damage through dysfunction of the adenosine triphosphate–dependent enzymes responsible for myelin secretion and maintenance—a cycle of approximately every 23 days.18 Neurologic symptoms in all 6 patients were first detected between 14 and 23 days after the date of the lowest recorded PaO2. Furthermore, the mean duration between MR imaging and the PaO2 nadir was 22 days, which could be consistent with a posthypoxemic etiology.
Infection-related leukoencephalopathy due to neurotropism is also possible, and similar features have been reported with other infectious agents, including human immunodeficiency virus, human polyomavirus JC, and Borrelia burgdorferi (Lyme disease).17 SARS-CoV-2, however, was not detected in the CSF samples of 2 patients in our series who underwent lumbar puncture, and no other abnormalities were noted in the CSF samples (On-line Table).17 There is the possibility of false-negative CSF findings as the sensitivity and specificity of SARS-CoV-2 detection in the CSF has not been established. Confirmation of tropism to the cerebral white matter by SARS-CoV-2 requires further pathology and postmortem investigation. Vascular and toxic/metabolic causes of leukoencephalopathy are also possible; however, the absence of perfusion abnormalities, infarction, hemorrhage, and the lack of clear toxin or medication-related culprits in these patients make these etiologies less likely.17 Finally, neural injury related to the cytokine storm syndrome has been proposed, with Poyiadji et al7 reporting neuroimaging findings of acute necrotizing encephalopathy in a case of COVID-19 infection.19 These imaging features, however, were absent in our case series but this may be related to differences in disease severity, imaging at different stages of the disease, or different underlying processes. Further investigation into the inflammatory response associated with neurologic injury in patients with COVID-19 infection is warranted.
There are several limitations of our case series, the first of which is the small number of patients. Second, only 1 patient had brain MR imaging 1 year prior, which showed minimal nonspecific white matter changes. The remaining 5 patients had no baseline imaging studies. However, given the normal neurologic status of the 6 patients before admission, the white matter abnormalities seen in our case series were unlikely to be chronic in nature. Third, given the critical condition of these patients, serial imaging was not performed to assess the ongoing evolution of these neuroimaging findings. Larger cohort studies with standardized imaging sequences are required to better evaluate the spectrum of neuroimaging findings related to COVID-19 infection.
Recognition of a clinical syndrome is an initial step in deciphering disease mechanisms, introducing and testing therapeutic modalities, and ultimately modifying the course for improved patient outcomes. Health care providers should be aware of the potential for a COVID-19-related delayed leukoencephalopathy syndrome in patients infected with SARS-CoV-2 and displaying subacute neurologic deficits, despite improvement in respiratory status. The consequences of delayed posthypoxic leukoencephalopathy can be severe, including both morbidity from chronic neurologic deficits and mortality. Further research is necessary to better understand this entity.
ABBREVIATIONS:
| COVID-19 | coronavirus disease 2019 |
| PaO2 | partial pressure of oxygen |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome coronavirus 2 |
| MR | magnetic resonance |
| CT | computed tomography |
| CSF | cerebrospinal fluid |
Footnotes
Disclosures: Karen Buch—UNRELATED: Employment: Massachusetts General Hospital. William A. Mehan, Jr—UNRELATED: Consultancy: Independent Review Committee member Kura Oncology, Comments: radiology reviewer for head and neck cancer clinical trial; Expert Testimony: medicolegal expert consulting work. Thabele M. Leslie-Mazwi—UNRELATED: Employment: Massachusetts General Hospital.
References
Articles from AJNR: American Journal of Neuroradiology are provided here courtesy of American Society of Neuroradiology
Full text links
Read article at publisher's site: https://doi.org/10.3174/ajnr.a6671
Read article for free, from open access legal sources, via Unpaywall:
https://www.ajnr.org/content/ajnr/41/9/1641.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3174/ajnr.a6671
Article citations
COVID-19-Associated Neurological Complications in Children.
Iran J Child Neurol, 18(4):47-60, 29 Sep 2024
Cited by: 0 articles | PMID: 39478943
Significance of Persistent Systemic Support in the Clinical Course of Delayed Post-hypoxic Leukoencephalopathy Following Severe Coronavirus Disease 2019.
Intern Med, 63(8):1167-1172, 01 Feb 2024
Cited by: 1 article | PMID: 38296478 | PMCID: PMC11081903
Can Patients with Asymptomatic/Mild Illness and Moderate Illness COVID-19 Have White Matter Damage?
Int J Gen Med, 16:4585-4593, 10 Oct 2023
Cited by: 2 articles | PMID: 37840824 | PMCID: PMC10576465
Case report: Bilateral globus pallidus lesions and delayed progressive leukoencephalopathy in COVID-19: Effects of hypoxia alone or combination of hypoxia and inflammation?
Front Neurol, 13:1084831, 09 Jan 2023
Cited by: 0 articles | PMID: 36698885 | PMCID: PMC9868960
Brain fog as a Long-term Sequela of COVID-19.
SN Compr Clin Med, 5(1):9, 24 Nov 2022
Cited by: 24 articles | PMID: 36466122 | PMCID: PMC9685075
Review Free full text in Europe PMC
Go to all (45) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Brain abnormalities in COVID-19 acute/subacute phase: A rapid systematic review.
Brain Behav Immun, 89:543-554, 17 Jul 2020
Cited by: 61 articles | PMID: 32682993 | PMCID: PMC7366124
Review Free full text in Europe PMC
COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy.
J Neurol Sci, 415:116945, 27 May 2020
Cited by: 25 articles | PMID: 32480073 | PMCID: PMC7251359
COVID-19-associated Leukoencephalopathy.
Radiology, 296(3):E184-E185, 14 May 2020
Cited by: 44 articles | PMID: 32407258 | PMCID: PMC7437492
The pathophysiology of 'happy' hypoxemia in COVID-19.
Respir Res, 21(1):198, 28 Jul 2020
Cited by: 260 articles | PMID: 32723327 | PMCID: PMC7385717
Review Free full text in Europe PMC
 a
a 




