Abstract
Free full text

Risk factors for poor health outcomes for male dairy calves undergoing transportation in western Canada
Abstract
The condition of 640 male dairy calves was recorded and their health deterioration, morbidity, and mortality evaluated after long-distance transport. Assessments included a health examination, weight estimation, and measure of failed transfer of passive immunity (FTPI). A McNemar Test and logistic regression analysis were used to evaluate the effects of pre-transport condition on subsequent health. Before transport, calf health and age at shipping varied between farms; overall, 17%, 8%, and 12% of calves had diarrhea, navel disease, and FTPI, respectively, and calves were transported at a median age of 5 days. In their first 2 weeks after transportation, 23% and 44% of calves were treated for diarrhea and bovine respiratory disease (BRD), respectively, and 4% died. Calves with navel disease, low body weight, and a depressed attitude at the farm of origin were more likely to experience negative health outcomes. Better health before transportation is needed to protect the subsequent health and welfare of young calves.
Résumé
Facteurs de risque associés à un résultat de mauvaise santé pour des veaux laitiers mâles soumis à un transport dans l’ouest canadien. La condition de 640 veaux laitiers mâles fut enregistrée et la détérioration de leur santé, la morbidité et la mortalité évaluées après un transport de longue distance. Les évaluations incluaient un examen de santé, une estimation du poids et une mesure de l’échec du transfert d’immunité passive (FTPI). Un test de McNemar et une analyse de régression logistique furent utilisés pour évaluer les effets des conditions pré-transport sur la santé subséquente. Avant le transport, la santé des veaux et l’âge au moment de l’expédition variaient entre les fermes; globalement, 17 %, 8 % et 12 % des veaux avaient de la diarrhée, une pathologie de l’ombilic et FTPI, respectivement, et les veaux furent transportés à l’âge médian de 5 jours. Durant les deux premières semaines après le transport, 23 % et 44 % des veaux furent traités pour de la diarrhée et des problèmes respiratoires (BRD), respectivement, et 4 % sont décédés. Les veaux avec une pathologie à l’ombilic, un faible poids corporel et une attitude déprimée à la ferme d’origine étaient plus susceptibles d’avoir des conséquences négatives sur leur santé. Une meilleure santé avant le transport est nécessaire pour protéger la santé ultérieure et le bien-être des jeunes veaux.
(Traduit par Dr Serge Messier)
Introduction
Disease and mortality in male calves remain important topics for research in animal welfare and calf production. Recent studies in Belgium and the Netherlands reported that 25% of white veal calves developed at least 1 disease during their lifetime (1) and that 15% and 61% developed diarrhea and bovine respiratory disease (BRD), respectively (2). Other studies have reported 4% to 10% total mortality throughout the feeding period for veal calves in eastern Canada and Switzerland (3,4). Such health challenges have resulted in high levels of antimicrobial use in the male calf industry contributing to the emergence of antimicrobial resistance (4,5).
The care of calves at the dairy farm of origin may influence subsequent health. Relevant factors include the quality and quantity of colostrum provided, the type and cleanliness of housing, and exposure to adverse events such as dystocia (4,6–8). Additionally, calves that are marketed at a young age may be vulnerable to health challenges as low body weight has been associated with higher mortality in calves arriving at veal facilities (8). Better management at the dairy farm and improved marketing practices could increase male calf health and welfare (9).
Long-distance transportation can also affect calf health (10). Transportation of young calves has been associated with dehydration, weight loss, and mobilization of body reserves as identified by increased serum glucose and cortisol levels (11,12). Additionally, a North American survey found 4% of male dairy calves became compromised (dead or non-ambulatory) after transportation, compared to 0.045% in other classes of cattle (13). Due to Canada’s large geography, many young dairy calves are transported long distances between dairy farms and calf-rearing facilities, but the effects of long-distance transportation have not been systemically evaluated (14).
Because calf age and health are considered important for good transportation outcomes, many countries have specific requirements for calf condition before transportation. For example, New Zealand (15) and the European Union (16) require calves to have healthy navels, and be a minimum age of 4 and 14 d before transportation, respectively. Recently, Canada introduced regulations to limit transportation time for unweaned calves to 12 h, prohibit transport of calves with unhealed or infected navels, and require that transport of calves less than 9 d of age be limited to movements directly to the final destination (14). However, there has been little research to inform these policies, and no studies have followed calves from dairy farms to relate pre-transport health to subsequent morbidity and mortality at calf rearing facilities.
This prospective single-cohort study was designed to i) describe the condition and management of male calves at the dairy farm of origin before transportation; ii) evaluate risk factors associated with immediate deterioration of calf condition after long-distance transportation; and iii) determine how calf condition before transport related to subsequent morbidity and mortality.
Materials and methods
Study participants
Dairy farms and calf growers were recruited in September 2017 through veterinary clinics in western Canada. Inclusion criteria for dairy farms included proximity to the Fraser Valley region of British Columbia and selling male dairy calves at least weekly. Of 19 dairy farms approached, 17 agreed to participate. Basic dairy farm management data (herd size, calf housing type, and calf feeding practices) were collected using a short survey. Farms were visited regularly (weekly or biweekly depending on the farm management) and calves were evaluated within 24 h before transportation to either a local auction market or to 1 of 2 calf growers. Inclusion criteria for the calf growers (CG) in Alberta included willingness to participate, and purchase of male dairy calves from British Columbia. Two calf growers (CG1, CG2) agreed to participate, both located approximately 1000 km from the dairy farms. No calf growers who purchased calves from the auction were willing to participate. A sample size calculation comparing 2 independent proportions showed that a total of 282 calves would be required to detect a change between 5% diarrhea (17) in calves before transport to 15% after transportation (18,19) using a 95% confidence level and 80% power. This target sample size was achieved at the dairy farms of origin, but the number of calves available for follow-up was lower mostly because of logistical challenges with confirming transport and arrival dates.
Calf assessments
From October 1, 2017 to March 31, 2018, 885 calves were assessed by a single veterinarian (DJW) using a standardized health examination. Calves were marked with highly visible livestock paint (Tell Tail Aerosol; FIL Industries, New Zealand) to allow them to be identified after transportation. The health examination was conducted using the Calf Health Scorer App (University of Wisconsin-Madison, Madison, Wisconsin, USA), as described previously (3). Briefly, the App allows scoring of symptoms on a 4-point scale for each of respiratory disease (20), diarrhea (21), and navel and joint inflammation (22). The sum of ear, eye, nose, cough, and temperature scores comprise the respiratory disease score (20). Each health score was collapsed from 4 levels into a binary variable denoting “normal” and “abnormal” based on biologically relevant values using the criteria in Table 1. This was done because of the low frequency of abnormal scores in the sample, to improve intra-observer agreement, and for ease of interpretation.
Table 1
Criteria for converting raw calf health scores from the Calf Health Scorer App into dichotomized scores (0 for normal, 1 for abnormal).
| Health score | Normal | Abnormal | Description of abnormal clinical signs |
|---|---|---|---|
| Attitude | ≤ 1 | > 1 | Dull, unwilling or unable to rise |
| Pyrexia | ≤ 2 | > 2 | Body temperature greater than 39.3°C |
| BRD | ≤ 4 | > 4 | Elevated body temperature, and/or ocular or nasal discharge, and/or coughing |
| Fecal | ≤ 1 | > 1 | Feces watery in consistency |
| Navel | ≤ 1 | > 1 | Navel swollen and painful with or without malodourous discharge |
BRD — Bovine respiratory disease.
Along with health assessments, a jugular blood sample was collected from all calves; 567 of these animals were confirmed (based on birthday or communication with farmers during weekly visits) to be aged 1 to 9 d (23) and were therefore included in the analysis. At the farm level, 2 of the 17 farms had fewer than 5 calves with eligible blood tests and were not included in the calculation of median and interquartile range (IQR). The pre-transport health assessment was conducted on the same day as blood sampling for calves transported at ≤ 9 d; otherwise the health assessment was conducted at a subsequent visit. This occurred, for example, if farms kept calves for an extended period due to illness, transporter availability, or as a standard management practice. Serum total protein (STP) was measured with an optical refractometer (Reichert Vet 360; Reichert, Depew, New York, USA) and failure of transfer of passive immunity (FTPI) was defined as < 5.2 g of STP per dL (24). Calf age was calculated using the birthday recorded in each farm’s record system for farms that kept accurate records for male calves. As an estimation of calf weight, a circumferential measurement (heart girth; HG) was taken of each calf ’s chest just caudal to the elbow joint using a soft measuring tape (The Coburn Company, Whitewater, Wisconsin, USA) (25).
The same veterinarian evaluated 161 of the calves 1 to 5 h after they were off-loaded at CG1 or CG2 on a total of 8 separate occasions between November 1, 2017, and March 31, 2018. The remaining 221 calves were evaluated by research assistants, but due to assistant turnover and resulting poor intra-observer agreement, the data were not included. Calf transport times varied because trucks collected calves at multiple farms, but trip duration generally ranged from 12 to 24 h. Calves destined for CG1 arrived at the farm in the evening and remained in the transport trailer overnight before being off-loaded and evaluated the following morning. At CG2, calves arrived and were immediately off-loaded and evaluated within 3 h. Both facilities housed approximately 1500 calves and used individual housing for the first 6 to 8 wk. The evaluation at the calf growers included the standardized calf health examination and a jugular blood sample for a second measurement of STP. The transportation protocol practiced by CG1 included provision of oral meloxicam suspension (Solvet, Calgary, Alberta) at the trip mid-point, but administration of the medication could not be verified for calves individually. Protocols for animal assessments and sampling were approved by the University of British Columbia Animal Care Committee (A16–0336-001).
Morbidity and mortality
During their first 2 wk at the calf growers, calf health was assessed daily, and any disease treatments or deaths were recorded by farm staff. Diarrhea was defined as watery fecal consistency and BRD was recorded when an increased rate, sound, or effort of respiration was identified in combination with pyrexia plus 1 or more of coughing, nasal discharge, depression, decreased appetite, or rough hair coat. Producers were trained on the case definitions with the veterinarian, but farm treatment protocols were not otherwise altered for the study. Calves were treated individually, primarily for diarrhea and BRD; other diseases uncommonly identified by the producers included bloat, otitis, and navel disease. Treatments for diarrhea included supportive therapy (orally administered activated charcoal) and antimicrobials for calves with severe symptoms. Bovine respiratory disease treatment consisted of antimicrobial therapy with or without non-steroidal anti-inflammatory drugs. At CG1, calves were treated by either 1 experienced employee or the farm owner. At CG2, all disease diagnoses and treatments were completed by the farm owner.
Statistical analyses
Due to logistical constraints in following calves throughout the study, each analysis used a different subset of calves (Figure 1).
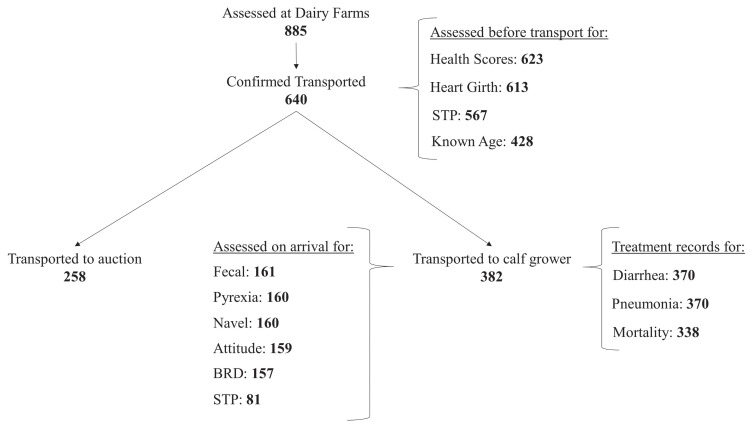
Flowchart showing the number of calves for the various statistical analyses.
BRD – Bovine respiratory disease; STP – Serum total protein.
Pre-transport descriptive data
Only calves with complete health scores that were confirmed to be transported to the calf grower or auction (n = 623) were included to ensure that evaluations reflected calf condition within 24 h of transport. Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to tabulate descriptive data. Median and mean measures of central tendency are reported depending on the data distribution. Health score deterioration was evaluated using RStudio version 1.2.1335 as an indication of decline in condition; all other data analysis was performed using SAS University Edition (SAS Institute, Cary, North Carolina, USA).
Health deterioration after transport
Calves (n = 161) were evaluated for changes in health score. The correlation between STP measured before versus after transportation was based on 81 calves because 80 calves were missing an STP measurement due to a malfunctioning refractometer or were > 9 d at the time of the second sample. A McNemar test with Durkalski’s adjustment for the effect of farm was used to evaluate changes (26), using the “clust.bin.pair” package of R package version 0.1.2 (27). A paired t-test and Pearson correlation (r) were used to compare STP levels before and after transportation (Proc Univariate). The assumption of normality was confirmed with data visualization and a Shapiro-Wilk test for normality.
For modelling purposes, for each health category (attitude, pyrexia, BRD, fecal, navel), calves were scored 1 if they had deteriorated after transport and 0 if condition was unchanged or improved. Logistic mixed-effect models were then used to test the effect of age, HG, and FTPI on heath deterioration, with dairy farm of origin included as a random effect to account for variation in management, including the differences in transportation. Model building was completed using Proc glimmix with a binary distribution and logit link function. Pearson correlations between explanatory variables were calculated to assess collinearity, with none showing a coefficient > 0.6. Univariable regression models were used to determine if age, HG or FTPI was associated with health deterioration. Only FTPI was associated with health score deterioration, so multivariable modelling was not pursued.
Morbidity and mortality
Similar model-building was used for morbidity and mortality at the calf grower using Proc glimmix. Three models were created with calves scoring 1 if they were treated for diarrhea in the first 2 wk (model 1), treated for BRD in the first 2 wk (model 2), or died during these 2 wk (model 3). In each model, calves were scored 0 if they remained untreated in the 2 wk (models 1 and 2) or survived the 2 wk (model 3). Initially, univariable logistic mixed-effect models were used to test for associations between these outcomes and age, HG, FTPI, and the dichotomized health scores taken on the dairy farm of origin (Table 2). Dairy farm within calf grower was considered a random effect. Factors liberally associated in univariable modelling (P < 0.2) were offered to the multivariable model using backwards elimination. Six calves were confirmed arrived but were lost to follow-up and did not have treatment or mortality records. The 6 calves that died before the end of week 2 without being treated for diarrhea or BRD were not included in morbidity regression analysis. A further 38 calves that were lost to follow-up and could not be confirmed alive after 2 wk were not included in mortality analysis.
Table 2
Univariable model for morbidity (diarrhea, bovine respiratory disease) and mortality outcomes for male dairy calves in the first 2 weeks after arrival at a calf grower, including variables associated at P < 0.2.
| Health outcome | N | Variablea | n | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Diarrhea | 370 | FTPI | ||||
 Fail Fail | 50 | 2.0 | 1.0 to 4.2 | 0.067 | ||
 Pass Pass | 300 | Referent | ||||
| HG | ||||||
 Every 1 cm increase Every 1 cm increase | 364 | 0.9 | 0.8 to 1.0 | 0.009 | ||
| Navel | ||||||
 Abnormal Abnormal | 39 | 2.2 | 1.0 to 4.9 | 0.046 | ||
 Normal Normal | 329 | |||||
| Pyrexia | ||||||
 Abnormal Abnormal | 17 | 3.5 | 1.2 to 10.9 | 0.024 | ||
 Normal Normal | 350 | Referent | ||||
| BRD | 370 | BRD | ||||
 Abnormal Abnormal | 7 | 6.5 | 0.8 to 52.3 | 0.080 | ||
 Normal Normal | 355 | Referent | ||||
| HG | ||||||
 Every 1 cm increase Every 1 cm increase | 364 | 1.1 | 1.0 to 1.2 | 0.068 | ||
| Mortality | 338 | Attitude | ||||
 Abnormal Abnormal | 11 | 11.7 | 2.7 to 51.4 | 0.001 | ||
 Normal Normal | 324 | Referent | ||||
| BRD | ||||||
 Abnormal Abnormal | 7 | 4.3 | 0.5 to 39.0 | 0.190 | ||
 Normal Normal | 323 | Referent | ||||
| Fecal | ||||||
 Abnormal Abnormal | 64 | 3.9 | 1.3 to 12.1 | 0.020 | ||
 Normal Normal | 272 | Referent | ||||
| HG | ||||||
 Every 1 cm increase Every 1 cm increase | 329 | 0.9 | 0.8 to 1.0 | 0.050 | ||
FTPI — Failure of transfer of passive immunity, defined as serum total protein < 5.2 g/dL; HG — Heart girth circumference (cm); BRD — Bovine respiratory disease; OR — Odds ratio; CI — Confidence interval; N — Total number.
Intra-observer agreement
To verify intra-observer agreement for health measures in the standardized examination, 25 calves at the University of British Columbia’s Dairy Education and Research Centre were assessed twice on 1 d, approximately 3 h apart. Weighted (for measures with multiple levels) and unweighted (for binary measures) kappa coefficients were used to determine agreement (Proc Freq). Cicchetti-Allison (linear) weight type was used for the weighted kappa calculation. Intraobserver agreement for HG was calculated by a concordance correlation for 106 calves that were assessed within 1 h before transportation (as part of this study) and again approximately 3 h later at an auction market.
Results
For the 25 calves assessed for intra-observer agreement, the percent agreement ranged from 36% to 100% using the 4-point health scale. With health scores converted to dichotomized scores (0 for normal and 1 for abnormal), percent agreement ranged from 88% to 100% (Table 3). For HG measured on 106 calves, the correlation coefficient for intra-observer agreement was 0.95.
Table 3
Intra-observer agreement between health scores of 25 dairy calves assessed 3 h apart using raw health scores (0 to 4) and dichotomized scores (0 or 1).
| Health score | Raw scores | Dichotomized scores | ||
|---|---|---|---|---|
|
|
| |||
| Kappaa | Percent agreement | Kappa | Percent agreement | |
| Attitude | 0.78* | 96% | 1.00 | 100% |
| Pyrexia | 0.48 | 60% | 0.46 | 92% |
| BRD | 0.48 | 36% | 0.59 | 88% |
| Fecal | 0.54 | 68% | 0.86 | 96% |
| Navel | 1.00* | 100% | 1.00 | 100% |
BRD — Bovine respiratory disease, calculated as the sum of individual ear, eye, nose, cough, and temperature scores and ranged from 0 to 6.
Health management at the farm of origin
The median dairy herd size was 290 milking cows (IQR: 205 to 401), and a median of 30 (IQR: 21 to 49) calves were transported per farm. Calves received a median of 6 L (range: 4 to 8 L) of milk per day provided as 2 daily meals. Three farms did not feed calves on the day they were transported. Farms fed calves unpasteurized waste milk (9 farms), milk replacer (6 farms), pasteurized waste milk (1 farm), or both unpasteurized waste milk and milk replacer (1 farm). Male calves were raised in a different area or type of housing than female calves on 12 farms, while 5 farms used similar housing for both sexes. Individual hutches or pens were used for male calves on 10 farms, while 7 used a group pen or a mix of individual and group or pair housing.
The prevalence of disease and disease symptoms in male calves varied widely among the different dairy farms (Figure 2; Table A1). The median (IQR) level of FTPI was 12% (range: 4% to 19%), with 2 farms showing no cases of FTPI. At least 1 health abnormality was identified in 233/623 calves (37%) in their pre-transportation assessment, but this ranged from 3/20 calves (15%) on 1 farm to 14/21 calves (67%) on another. From graphical evaluation, the variation did not appear related to the farm-level variables such as type of milk fed, herd size, or housing type.
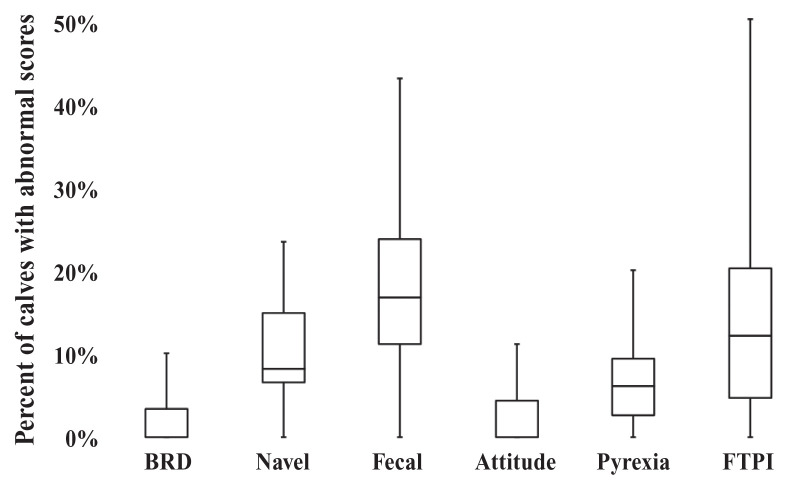
Box and whisker plot of the prevalence of disease and disease symptoms (n = 623) and failure of transfer of passive immunity (n = 567) in male dairy calves assessed ≤ 24 h before transportation from 17 BC dairy farms. Boxes show medians with upper and lower quartiles; whiskers indicate range. BRD — Bovine respiratory disease; FTPI — Failure of transfer of passive immunity, defined as serum total protein < 5.2 g/dL.
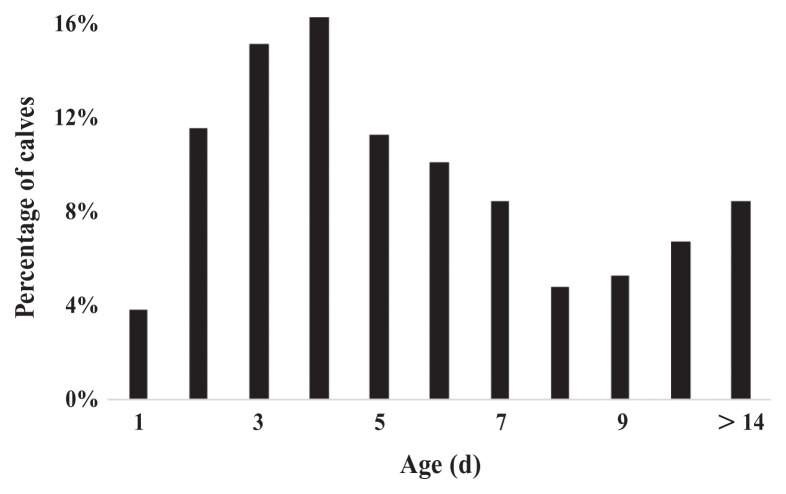
The median age of calves at transportation was 5 d (IQR: 3 to 7 d), ranging from 1 to 54 d for the 428 calves of known age (Figure 3). Mean ± SD heart girth of calves was 82 ± 4 cm, which corresponds to 44 kg body weight (BW) (25), with calves ranging from 67 cm (~27 kg) to 98 cm (~77 kg).
Health change after transport
Of the 640 transported calves, 258 went to an auction market and 382 to 1 of 2 calf growers. For the 161 calves assessed at the calf growers just after long-distance transportation, a larger proportion had an elevated body temperature after transport and a smaller proportion had evidence of diarrhea (Table 4). Calves with an STP < 5.2 g/dL measured at the dairy farm had greater odds of developing a depressed attitude after transport [odds ratio (OR): 11.6; 95% confidence interval (CI): 2.7 to 50.9; P = 0.001]. Mean ± standard error (SE) STP was similar before and after transport at 5.9 ± 0.07 g/dL and 6.0 ± 0.07 g/dL (P = 0.16), and levels before and after transport were highly correlated (r = 0.80).
Table 4
The percentage of male dairy calves with health abnormalities at the dairy farm and after arriving at the calf grower, with statistical analysis by McNemar test with Durkalski’s adjustment.
| Abnormal health score | Location | n | P-value | |
|---|---|---|---|---|
|
| ||||
| Dairy farm (%) | Calf grower (%) | |||
| Attitude | 15.7 | 17.4 | 159 | 0.721 |
| Pyrexia | 3.8 | 8.7 | 160 | 0.037 |
| BRD | 1.9 | 2.5 | 157 | 0.436 |
| Fecal | 18.0 | 7.5 | 161 | 0.028 |
| Navel | 9.4 | 14.3 | 160 | 0.095 |
BRD — Bovine respiratory disease.
Morbidity and mortality
During the first 2 wk after transportation, complete morbidity and mortality records were available for 370 and 338 of the calves, respectively. During these 2 wk, 86/370 calves (23%) were treated for diarrhea, 164/370 calves (44%) were treated for BRD, and 13/338 (4%) died. A larger proportion of calves was treated for disease at CG2 compared to CG1 [75/189 (40%) versus 11/181 (6%) for diarrhea, 147/189 (78%) versus 17/181 (9%) for BRD], but similar mortality was observed at both locations [7/185 (3.8%) versus 6/153 (3.9%)]. In the final logistic regression model, smaller HG and abnormal navel score were associated with increased diarrhea treatment in the first 2 wk (Table 5). However, an interaction was found between navel score and HG, such that calves with a small heart girth that had navel disease had increased odds of being treated for diarrhea. In addition, calves with a depressed attitude at the dairy farm had increased odds of dying in the first 2 wk at the calf grower (Table 5). No factors measured at the dairy farm were associated with BRD treatment at the calf grower.
Table 5
Logistic mixed regression models of associated risk factors for male dairy calves being treated for diarrhea or dying in the first 2 weeks at a calf grower.
| Outcome | Variable | Estimate | SE | t | P-value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Diarrhea | Intercept | 5.5 | 3.5 | 1.57 | 0.14 | — | — |
| Abnormal navel | 23.2 | 10.6 | 2.18 | 0.03 | 2.8 | 1.2–6.7 | |
| Normal navel | Reference | — | — | — | — | ||
| HGa (cm) | −0.09 | 0.04 | −2.05 | 0.04 | 0.9 | 0.8–1.0 | |
| HGa navel interaction | |||||||
 HG with abnormal navelb HG with abnormal navelb | −0.3 | 0.13 | −2.09 | 0.04 | 0.7 | 0.5–0.9 | |
 HG with normal navelb HG with normal navelb | Reference | — | — | — | — | ||
| Mortality | Abnormal attitude | 2.5 | 0.75 | 3.29 | 0.001 | 11.8 | 2.7–51.4 |
| Normal attitude | Reference | ||||||
SE — Standard error; t — calculated difference; OR — Odds ratio; CI — Confidence interval.
Discussion
Of the 640 calves that were assessed before transportation, many had an identifiable health abnormality and the prevalence of these abnormalities varied widely, depending on the dairy farm of origin. Calf disease on dairy and veal farms is a considerable challenge, and has been linked to provision of colostrum, cleanliness of housing, and body weight upon arrival (4,8). The importance of reducing calf disease has been emphasized because of the negative welfare implications and to minimize the use of antimicrobials in food animals (4). However, the use of good calf management practices is inconsistent among farms (28), so the variability in calf condition seen in our study is not surprising.
Some calves in this study were transported at a very young age and light weight. However, age itself was not a predictor of immediate health deterioration, morbidity or mortality, and age was not highly correlated with HG in this data set. While weight of calves entering veal facilities is an important risk factor for mortality (8), the optimal age and weight for transport of dairy calves has not been well-established. Regulations in New Zealand, the EU, and Canada have considered age an important criterion for calves to be considered fit for transportation, but it remains unclear whether transporting older calves will improve their welfare. The effect of age may also interact with the time and conditions of transport, for example, with younger calves being more susceptible to extreme weather or lengthy transport. Further research using a wider range of ages and transportation conditions is warranted to determine if there is an optimal age to transport young calves.
Interpretation of herd-level factors is limited because of the small number of farms used in the current study. Similar to our findings, other work has shown that male calves were more likely than female calves to be housed individually (3), perhaps because farmers wanted to keep male calves healthy before transport. Regarding feeding practices, calves were provided a smaller quantity of milk than is currently recommended, and some were provided unpasteurized waste milk, which has been associated with reduced immunity and productivity (29).
Levels of FTPI in this study were comparable to levels seen in some recent studies (3), but lower than those reported in earlier survey results (30). This could be due to sampling bias as well-managed farms may have been more willing to participate, or it could reflect improved colostrum management in recent years. In a 2017 Canadian survey, 91% of farmers reported always feeding colostrum to male calves (28), as is consistent with our observations of FTPI. The variation between farms was notable, however, with 2 farms having no calves with FPTI and others having a very high rate of failure. This finding, together with the observed differences in the environmental and nutritional management, underlines the inconsistency observed in care of male calves on dairy farms (9).
Of the 161 calves that were assessed before and after transportation, the percentage with diarrhea was lower at the calf grower than at the farm of origin. This may reflect dehydration as calves did not receive milk or water for an estimated 24 to 36 h before and during transport, and/or the use of oral meloxicam. Indeed, better gastrointestinal health has been observed when meloxicam was given before long distance (8.5 h) transportation of Jersey calves (31). More calves had pyrexia after transportation which could reflect an inability of young calves to closely regulate their body temperature during transportation as seen in previous studies (11). As this study took place during the winter months, it is unlikely that high environmental temperature or humidity played a role in pyrexia.
Calf STP concentrations were similar and highly correlated before and after transport. This supports the results of previous work which compared calves transported up to 12 h to control calves that remained on their home farm (32). Serum total protein taken from male dairy calves upon arrival at rearing facilities has been used as an indication of FTPI and has been predictive of respiratory disease and mortality (2). Our results support that STP measured upon arrival at calf-rearing facilities reflects FTPI accurately.
Calves with FTPI were more likely to develop a depressed attitude after transportation. While “depressed attitude” is not a clinical diagnosis, it can be a symptom of septicemia, fatigue, and dehydration (33), and may indicate an inability of the calf to cope with external conditions. Calf “attitude,” although not specific to any one disease condition, may be predictive of poor health outcomes and be useful for assessing fitness for transport.
In this study, calves with swollen navels and small HG were especially likely to be treated for diarrhea. Previous work has identified weight and navel disease (6,8) as important risk factors for disease and mortality in veal calves. Navel disease did not increase the probability of being treated for diarrhea for calves with larger HG, which may have been more resistant to developing illness due to age (34) or a higher plane of nutrition (35). Other unmeasured factors may also have been important in this finding including poor environmental cleanliness and lack of preventative measures such as navel dipping that may have resulted in both poor growth and navel infection.
In the first 2 wk at the calf grower, 44% of calves were treated for BRD; this is comparable to previous work which found a 60% cumulative incidence of BRD over the first 18 d after arrival at a veal facility (2). Interestingly, none of the calf conditions measured at the dairy farm of origin predicted BRD outcomes at the calf grower. Perhaps environmental and management factors (air quality, nutrition, mingling of animals) had a greater influence on the development of BRD.
This study builds on previous research which suggests the quality of care of male dairy calves can predispose them to poor health outcomes at calf-rearing facilities. However, the study was limited by an inability to follow all the calves to the calf grower, resulting in smaller sample size which might have affected the findings related to morbidity and mortality. In future studies, improved data collection at the calf grower could increase the sample size and might reveal further calf-level risk factors.
In summary, some calves in this study were transported at a young age and light weight, and some displayed disease symptoms that should preclude transport. These factors varied widely depending on the farm of origin. Calves transported at a light weight with navel disease, and those with a depressed attitude, were more likely to experience poor health outcomes during the first 2 wk at calf-grower facilities. These findings indicate a need to improve the health of male dairy calves before they are transported. Further clarification on the optimum age and weight, and other criteria for transport of calves are necessary to inform policy and protect male dairy calf health and welfare.
Acknowledgments
We thank the dairy farmers and calf growers who allowed us to conduct this study on their farms, and the veterinary clinics that helped recruit participants. We are thankful for the support of our colleagues at the BC Ministry of Agriculture (Abbotsford, British Columbia), particularly Dr. Jane Pritchard, Terri Giacomazzi, Tom Droppo, Dr. Brian Radke, and Erin Cuthbert, who helped in data collection. This research was supported by the BC Ministry of Agriculture through Growing-Forward 2, a federal-provincial-territorial initiative. The UBC Animal Welfare Program is generously supported by the NSERC Industrial Research Chair Program with industry contributions from the Dairy Farmers of Canada (Ottawa, Ontario) and many others listed at http://awp.landfood.ubc.ca/
Appendix
Table A1
The farm-level prevalence of disease and disease symptoms (n = 623) and failure of transfer of passive immunity (n = 567) in male dairy calves assessed ≤ 24 h before transportation from 17 BC dairy farms.
| Farm-level prevalence | BRD | Navel | Fecal | Attitude | Pyrexia | FTPI |
|---|---|---|---|---|---|---|
| Minimum | 0% | 0% | 0% | 0% | 0% | 0% |
| Quartile 1 | 0% | 7% | 11% | 0% | 3% | 5% |
| Median | 0% | 8% | 17% | 0% | 6% | 12% |
| Quartile 2 | 3% | 15% | 24% | 4% | 9% | 20% |
| Maximum | 10% | 23% | 43% | 11% | 20% | 50% |
BRD — Bovine respiratory disease; FTPI — Failure of transfer of passive immunity.
Footnotes
Support for this research was provided by the Canadian Federal government and BC Ministry of Agriculture through the policy framework Growing Forward 2.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (gro.vmca-amvc@nothguorbh) for additional copies or permission to use this material elsewhere.
References
Articles from The Canadian Veterinary Journal are provided here courtesy of Canadian Veterinary Medical Association
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/146897668
Article citations
Surveillance of Antimicrobial Resistance of Escherichia coli Isolates from Intestinal Contents of Dairy and Veal Calves in the Veneto Region, Northeaster Italy.
Animals (Basel), 14(10):1429, 10 May 2024
Cited by: 0 articles | PMID: 38791647 | PMCID: PMC11117218
Dairy calf transportation in the United States: Challenges and strategies to improve animal welfare.
JDS Commun, 5(3):259-263, 17 Nov 2023
Cited by: 0 articles | PMID: 38646578 | PMCID: PMC11026933
Growth Performance and Feed Intake Assessment of Italian Holstein Calves Fed a Hay-Based Total Mixed Ration: Preliminary Steps towards a Prediction Model.
Vet Sci, 10(9):554, 03 Sep 2023
Cited by: 18 articles | PMID: 37756076 | PMCID: PMC10536390
Transmission of antimicrobial resistance (AMR) during animal transport.
EFSA J, 20(10):e07586, 25 Oct 2022
Cited by: 10 articles | PMID: 36304831 | PMCID: PMC9593722
Antimicrobial Use and Resistance in Surplus Dairy Calf Production Systems.
Microorganisms, 10(8):1652, 16 Aug 2022
Cited by: 1 article | PMID: 36014070 | PMCID: PMC9413162
Review Free full text in Europe PMC
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age.
Prev Vet Med, 113(2):231-240, 01 Nov 2013
Cited by: 175 articles | PMID: 24269039
Factors associated with body weight of young surplus dairy calves on arrival to a calf rearing facility.
Prev Vet Med, 203:105630, 24 Mar 2022
Cited by: 4 articles | PMID: 35367936
A randomized controlled trial investigating the effect of transport duration and age at transport on surplus dairy calves: Part I. Impact on health and growth.
J Dairy Sci, 106(4):2784-2799, 14 Feb 2023
Cited by: 4 articles | PMID: 36797186
Perspectives on the Management of Surplus Dairy Calves in the United States and Canada.
Front Vet Sci, 8:661453, 13 Apr 2021
Cited by: 16 articles | PMID: 33928141 | PMCID: PMC8076512
Review Free full text in Europe PMC