Abstract
Background
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors + endocrine therapy (ET) prolonged progression-free survival as first- or second-line therapy for hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer prognosis. Given the recent publication of overall survival (OS) data for the 3 CDK4/6-inhibitors, we performed a meta-analysis to identify a more precise and reliable benefit from such treatments in specific clinical subgroups.Methods
We conducted a systematic literature search to select all available phase II or III randomized clinical trials of CDK4/6-inhibitors + ET reporting OS data in first- or second-line therapy of HR+/HER2-negative pre- or postmenopausal metastatic breast cancer. A random effect model was applied for the analyses. Heterogeneity was assessed with I2statistic. Subgroup analysis was performed to explore the effect of study-level factors. The project was registered in the Open Science Framework database (doi: 10.17605/OSF.IO/TNZQP).Results
Six studies were included in our analyses (3421 patients). A clear OS benefit was observed in patients without (hazard ratio [HR] = 0.68, 95% confidence interval [CI] = 0.54 to 0.85, I2 = 0.0%) and with visceral involvement (HR = 0.76, 95% CI = 0.65 to 0.89, I2 = 0.0%), with at least 3 metastatic sites (HR = 0.75, 95% CI = 0.60 to 0.94, I2 = 11.6%), in an endocrine-resistant (HR = 0.79, 95% CI = 0.67 to 0.93, I2 = 0.0%) and sensitive subset (HR = 0.73, 95% CI = 0.61 to 0.88, I2 = 0.0%), for younger than 65 years (HR = 0.80, 95% CI = 0.67 to 0.95, I2 = 0.0%) and 65 years or older (HR = 0.71, 95% CI = 0.53 to 0.95, I2 = 44.4%), in postmenopausal (HR = 0.76, 95% CI = 0.67 to 0.86, I2 = 0.0%) and pre- or perimenopausal setting (HR = 0.76, 95% CI = 0.60 to 0.96, I2 = 0.0%) as well as in chemotherapy-naïve patients (HR = 0.72, 95% CI = 0.55 to 0.93, I2 = 0.0%).Conclusions
CDK4/6-inhibitors + ET combinations compared with ET alone improve OS independent of age, menopausal status, endocrine sensitiveness, and visceral involvement and should be preferred as upfront therapy instead of endocrine monotherapy.Free full text

Overall Survival of CDK4/6-Inhibitor–Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis
Abstract
Background
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors + endocrine therapy (ET) prolonged progression-free survival as first- or second-line therapy for hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer prognosis. Given the recent publication of overall survival (OS) data for the 3 CDK4/6-inhibitors, we performed a meta-analysis to identify a more precise and reliable benefit from such treatments in specific clinical subgroups.
Methods
We conducted a systematic literature search to select all available phase II or III randomized clinical trials of CDK4/6-inhibitors + ET reporting OS data in first- or second-line therapy of HR+/HER2-negative pre- or postmenopausal metastatic breast cancer. A random effect model was applied for the analyses. Heterogeneity was assessed with I2statistic. Subgroup analysis was performed to explore the effect of study-level factors. The project was registered in the Open Science Framework database ( 10.17605/OSF.IO/TNZQP).
Results
Six studies were included in our analyses (3421 patients). A clear OS benefit was observed in patients without (hazard ratio [HR] = 0.68, 95% confidence interval [CI] = 0.54 to 0.85, I2 = 0.0%) and with visceral involvement (HR = 0.76, 95% CI = 0.65 to 0.89, I2 = 0.0%), with at least 3 metastatic sites (HR = 0.75, 95% CI = 0.60 to 0.94, I2 = 11.6%), in an endocrine-resistant (HR = 0.79, 95% CI = 0.67 to 0.93, I2 = 0.0%) and sensitive subset (HR = 0.73, 95% CI = 0.61 to 0.88, I2 = 0.0%), for younger than 65 years (HR = 0.80, 95% CI = 0.67 to 0.95, I2 = 0.0%) and 65 years or older (HR = 0.71, 95% CI = 0.53 to 0.95, I2 = 44.4%), in postmenopausal (HR = 0.76, 95% CI = 0.67 to 0.86, I2 = 0.0%) and pre- or perimenopausal setting (HR = 0.76, 95% CI = 0.60 to 0.96, I2 = 0.0%) as well as in chemotherapy-naïve patients (HR = 0.72, 95% CI = 0.55 to 0.93, I2 = 0.0%).
years or older (HR = 0.71, 95% CI = 0.53 to 0.95, I2 = 44.4%), in postmenopausal (HR = 0.76, 95% CI = 0.67 to 0.86, I2 = 0.0%) and pre- or perimenopausal setting (HR = 0.76, 95% CI = 0.60 to 0.96, I2 = 0.0%) as well as in chemotherapy-naïve patients (HR = 0.72, 95% CI = 0.55 to 0.93, I2 = 0.0%).
Conclusions
CDK4/6-inhibitors + ET combinations compared with ET alone improve OS independent of age, menopausal status, endocrine sensitiveness, and visceral involvement and should be preferred as upfront therapy instead of endocrine monotherapy.
Hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer (MBC) represents the most frequent subgroup of advanced breast tumors (1). The most relevant therapeutic improvement of the last few years in this subset has been represented by the introduction of cyclin-dependent kinases (CDK) 4 and 6 (CDK4/6) inhibitors (palbociclib, ribociclib, and abemaciclib) combined with endocrine therapy (ET). These drugs bind to the CDK4 and 6, preventing their correct functioning and leading to cell-cycle arrest and apoptosis. They also seem to induce a broad spectrum of immunological events, which, however, need further investigation to be fully understood (2).
Pivotal trials led to the approval of CDK4/6-inhibitors plus ET combinations after showing very similar statistically significant and clinically meaningful improvements in progression-free survival (PFS) in a first- or second-line setting of both premenopausal (3–5) and postmenopausal (3,4,6–9) patients with HR+/HER2-negative MBC. The median PFS of all the comparison arms roughly doubled, as well as overall response rates, compared with standard ET (3–9). Notably, a recent network meta-analysis confirmed the superiority of CDK4/6-inhibitor regimens over single agent ET, showed a substantial equivalence among the 3 inhibitors and no difference with chemotherapy (CT) (10). However, all these studies were based on PFS as their primary endpoint and, until recently, overall survival (OS) data were available only for palbociclib-containing phase II PALOMA 1 and phase III PALOMA 3 trials (11,12) and the ribociclib-containing MONALEESA 2 trial (7). Previous studies had observed a statistically significant association between PFS and OS in MBC (13), in general and specifically in HR+/HER2-negative disease, overall suggesting that the first might be a good surrogate endpoint for the latter (14). Nevertheless, the prediction of OS based on PFS is still matter of debate, because the number of subsequent treatment lines, cross-over from the control arm to active treatment, and nonrandomized use of second-line agents might interfere with this association (13,15). Finally, OS results for the pivotal phase III trials MONARCH 2, MONALEESA 3, and MONALEESA 7 were recently published, providing additional data regarding abemaciclib- and ribociclib-based regimens (16–18). Considering all available results, a 4- to 10-month improvement in median survival with a 19%-29% relative reduction in the risk of death has been observed so far (7,11,12,16–18). However, results were not statistically significant for each trial or for each subgroup of patients, probably due to the study being substantially under-powered in demonstrating possible OS differences (19). For these reasons and also given the current lack of effective biomarkers capable of identifying patients that might benefit most from these novel therapeutic agents, we decided to perform this meta-analysis in different clinically relevant subgroups of HR+/HER2-negative MBC.
Materials and Methods
Search Strategy and Selection Criteria
We conducted a systematic literature search on PubMed at the end of October 2019 to select all available phase II or III randomized controlled trials(RCT) of CDK4/6 inhibitors plus ET showing OS data in the first- or second-line treatment setting of HR+/HER2-negative pre- or postmenopausal MBC. European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology meetings’ and San Antonio Breast Cancer Symposium’ online databases were also consulted. The query included the terms “palbociclib,” “ribociclib,” “abemaciclib,” “breast,” “metastatic,” and “advanced.” Duplicate reports were excluded. No language restriction was adopted. The research and data extraction were conducted by 2 investigators (F Schettini and F Giudici) and a third one (D Generali) was consulted in case of controversy. Details about study design, patient characteristics, interventions, and previous treatments were extracted from each article. The primary outcome was OS measured in various subgroups of interest. Hazard ratios (HR) and associated 95% confidence intervals (CI) were extracted for OS from published articles. Subgroups of interest were the following: visceral disease (yes vs no), bone-only disease (yes vs no), number of metastatic sites (<3 sites vs ≥3), endocrine sensitivity and resistance (yes vs no), previous CT for the metastatic setting (yes vs no), age (<65 vs ≥65 years), and menopausal status (pre-perimenopausal vs postmenopausal). Endocrine resistance and sensitivity were defined according to ESO-ESMO International Consensus Guidelines (20).
years), and menopausal status (pre-perimenopausal vs postmenopausal). Endocrine resistance and sensitivity were defined according to ESO-ESMO International Consensus Guidelines (20).
Data Analysis
Analyses were performed applying a priori the random-effect model from DerSimonian and Laird (21). Pooled data were presented in forest plots. All study-specific estimates were combined using inverse variance-weighted averages of logarithmic hazard ratios in random-effects models. Statistical significance was set at P less than
less than .05. All tests were 2-sided. The degree of heterogeneity between studies was assessed by visual inspection of the forest plots and I2 statistic estimate (22). Using subgroup analysis, we planned to explore the effect of the following study-level factors: visceral involvement status (no involvement vs involvement), bone-only disease condition (yes vs no), number of metastatic sites (<3 vs ≥3), endocrine sensitive status (resistant vs sensitive), previous CT for the metastatic setting (untreated vs treated), age (<65
.05. All tests were 2-sided. The degree of heterogeneity between studies was assessed by visual inspection of the forest plots and I2 statistic estimate (22). Using subgroup analysis, we planned to explore the effect of the following study-level factors: visceral involvement status (no involvement vs involvement), bone-only disease condition (yes vs no), number of metastatic sites (<3 vs ≥3), endocrine sensitive status (resistant vs sensitive), previous CT for the metastatic setting (untreated vs treated), age (<65 years vs ≥65
years vs ≥65 years), and menopausal status (postmenopausal vs pre/perimenopausal). Subgroup analyses were performed if at least 2 studies for each of the previously mentioned subgroups of interest were available. Q-test of homogeneity (Q Statistic: Q within and Q between) was performed to compare the pooled effect in 2 or more groups.
years), and menopausal status (postmenopausal vs pre/perimenopausal). Subgroup analyses were performed if at least 2 studies for each of the previously mentioned subgroups of interest were available. Q-test of homogeneity (Q Statistic: Q within and Q between) was performed to compare the pooled effect in 2 or more groups.
Publication bias was not assessed due to inadequate numbers of included trials to properly assess a funnel plot or more advanced regression-based assessments. Statistical analyses were performed using R software version 3.5.0 (package meta) (23). The risk of bias for each trial was assessed by using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (24). Internal validity of eligible studies was assessed according to the Cochrane Collaboration’s “Risk of Bias” tool in Review Manager (25). Each domain related to a risk of bias was assessed in each included trial, because there is evidence that these issues are associated with biased estimates of treatment effect. The domains were the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias. Review authors’ judgments were categorized as “low risk,” “high risk,” or “unclear risk” of bias.
The project was registered in the Open Science Framework online public database (http://osf.io with 10.17605/OSF.IO/TNZQP).
Results
Included Studies’ Characteristics
Six out of 8 (75.0%) studies reported OS results and were therefore included in the analyses for a total of 3421 patients (7,11,12,16–18). The study selection process is summarized in Supplementary Figure 1 (available online). Three of the 6 (50.0%) included studies enrolled only postmenopausal patients, 1 (17.0%) study exclusively enrolled premenopausal patients, to whom an analogue of gonadotropin-releasing hormone agonist (GnRH) was administered to induce ovarian function suppression, and the 2 (33.0%) remaining studies enrolled both post- and premenopausal patients. For the latter group, a GnRH analogue was administered along with study treatments. Three out of 6 (50.0%) studies were set in first line, while the remaining (50.0%) were set in first or second line. Five (83.0%) studies were phase III RCT and 1 (17.0%) was a phase II trial. The experimental arms included in these trials were fulvestrant plus ribociclib, palbociclib or abemaciclib, letrozole plus palbociclib or ribociclib, and a nonsteroidal aromatase inhibitor or tamoxifen plus ribociclib. Trial characteristics and main outcomes are reported in Table 1 and full results are shown in Table 2. An overall pooled OS benefit was observed for CDK4/6-inhibitor combinations compared with standard ET (HR = 0.76, 95% CI = 0.68 to 0.85, I2 = 0.0%; Supplementary Figure 2 available online).
Table 1.
Characteristics and results of published randomized phase II or III trials of CDK4/6-inhibitors combined with ET in HR+/HER2-negative MBC
| Features | Published randomized Phase II or III trials | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PALOMA 1 | PALOMA 2 | PALOMA 3 | MONALEESA 2 | MONALEESA 7 | MONALEESA 3 | MONARCH 3 | MONARCH 2 | MONARCH plus | |
| Phase | II | III | III | III | III | III | III | III | III |
| No. of patients | 165 | 666 | 521 | 668 | 672 | 726 | 493 | 669 | 463 |
| Treatment | Palbociclib + letrozole vs letrozole | Palbociclib + letrozole vs letrozole - | Palbociclib + fulvestrant vs fulvestrant (+ GnRHa in pre/peri pts) | Ribociclib + letrozole vs letrozole | Ribociclib + tamoxifen or AI + GnRHa vs tamoxifen or AI + GnRHa | Ribociclib + fulvestrant vs fulvestrant | Abemaciclib + NSAI vs NSAI | Abemaciclib + fulvestrant vs fulvestrant (+ GnRHa in pre/peri pts) | Abemaciclib + NSAI or fulvestrant vs NSAI or fulvestrant |
| Menopausal status at moment of trial enrollment | Post | Post | Pre/post | Post | Pre | Post | Post | Pre/post | Post |
| Setting | 1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC |
| Median PFS, mo | 20.2 vs 10.2 | 24.8 vs 14.5 | 9.5 vs 4.6 | 25.3 vs 16.0 | 23.8 vs 13.0 | 20.5 vs 12.8 | NR vs 14.7 | 16.4 vs 9.3 | NR and 11.5 vs 14.7 and 5.6 |
| PFS HR (95% CI) | 0.49 (0.32 to 0.75) | 0.58 (0.46 to 0.72) | 0.46 (0.36 to 0.59) | 0.57 (0.46 to 0.70) | 0.55 (0.44 to 0.69) | 0.59 (0.48 to 0.73) | 0.54 (0.41 to 0.72) | 0.55 (0.45 to 0.68) | 0.50 (0.35 to 0.72) and 0.38 (0.24 to 0.59) |
| ORRa | 43% vs 33% | 42% vs 35% | 25% vs 11% | 43% vs 29% | 51% vs 36% | 41% vs 9% | 59% vs 44% | 48% vs 21% | 56% and 39% vs 30% and 8% |
| Median OS, mo | 37.5 vs 33.3 | NM | 35.0 vs 28.0 | NR | NR vs 40.9 | NR vs 40.0 | NM | 46.7 vs 37.3 | NM |
| OS HR (95% CI) | 0.81 (0.49 to 1.35) | NM | 0.81 (0.64 to 1.03) | 0.75 (0.52 to 1.08) | 0.71 (0.54 to 0.95) | 0.72 (0.57 to 0.92) | NM | 0.76 (0.61 to 0.95) | NM |
| Journal/Congressb | Lancet Oncol/J Clin Oncol | N Engl J Med | New Engl J Med | Ann Oncol | New Engl J Med | N Engl J Med | J Clin Oncol | JAMA Oncol | Ann Oncol |
| First authorb | Finn RS | Finn RS | Turner NC | Hortobagyi G | Im S-A | Slamon DJ | Goetz MP | Sledge GW | Jiang Z |
| Yearb | 2014/2017 | 2016 | 2018 | 2018 | 2019 | 2019 | 2017 | 2019 | 2019 |
Table 2.
Full subgroup analyses resultsa
| Variables | No. of Pts | No. of studies | Pooled HR (95% CI) | I2, % | P pooled | P H | P sub. diff. |
|---|---|---|---|---|---|---|---|
| Age, y | 1916 | 3 | 0.77 (0.66 to 0.88) | 12.0 | <.001 | .34 | .49 |
 <65 <65 | 1203 | 3 | 0.80 (0.67 to 0.95) | 0.0 | .01 | .45 | |
 ≥65 ≥65 | 713 | 3 | 0.71 (0.53 to 0.95) | 44.4 | .003 | .17 | |
| Menopausal status | 3417 | 6 | 0.75 (0.67 to 0.84) | 0.0 | <.001 | .95 | .99 |
 Pre- or perimenopausal Pre- or perimenopausal | 894 | 3 | 0.76 (0.60 to 0.96) | 0.0 | .02 | .41 | |
 Postmenopausal Postmenopausal | 2523 | 5 | 0.76 (0.67 to 0.86) | 0.0 | <.001 | .89 | |
| Bone-only disease | 1577 | 3 | 0.74 (0.62 to 0.89) | 0.0 | <.001 | .61 | .47 |
 Yes Yes | 492 | 3 | 0.82 (0.60 to 1.13) | 0.0 | .23 | .45 | |
 No No | 1085 | 2 | 0.71 (0.58 to 0.88) | 0.0 | .002 | .47 | |
| Metastatic sites | 1600 | 3 | 0.77 (0.65 to 0.91) | 0.0 | .002 | .63 | .74 |
 <3 <3 | 891 | 2 | 0.79 (0.62 to 1.01) | 0.0 | .06 | .63 | |
 ≥3 ≥3 | 709 | 3 | 0.75 (0.60 to 0.94) | 11.6 | .02 | .32 | |
| Previous CT in metastatic setting | 979 | 2 | 0.77 (0.62 to 0.94) | 0.0 | .01 | .74 | .42 |
 Yes Yes | 271 | 2 | 0.85 (0.61 to 1.18) | 0.0 | .34 | .45 | |
 No No | 708 | 2 | 0.72 (0.55 to 0.93) | 0.0 | .01 | .82 | |
| Visceral involvement | 2291 | 4 | 0.73 (0.65 to 0.83) | 0.0 | <.001 | .89 | .91 |
 No No | 901 | 3 | 0.68 (0.54 to 0.85) | 0.0 | <.001 | .96 | |
 Yes Yes | 1390 | 4 | 0.76 (0.65 to 0.89) | 0.0 | <.001 | .69 | |
| Endocrine sensitivity status | 2834 | 5 | 0.77 (0.68 to 0.86) | 0.0 | <.001 | .73 | .55 |
 Resistance Resistance | 1331 | 4 | 0.79 (0.67 to 0.93) | 0.0 | .004 | .45 | |
 Sensitive Sensitive | 1503 | 4 | 0.73 (0.61 to 0.88) | 0.0 | .001 | .70 |
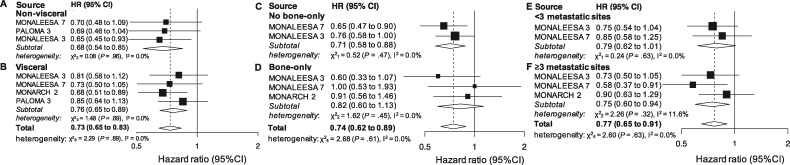
Visceral Involvement Status
A subgroup analysis in patients without visceral involvement was provided in 3 studies (901 patients). The cumulative effect was statistically significant (HR = 0.68, 95% CI = 0.54 to 0.85, P <
< .001, I2 = 0.0%; Figure 1A). Four studies (1390 patients) reported OS results for patients with visceral involvement. The cumulative effect was statistically significant (HR = 0.76, 95% CI = 0.65 to 0.89, P
.001, I2 = 0.0%; Figure 1A). Four studies (1390 patients) reported OS results for patients with visceral involvement. The cumulative effect was statistically significant (HR = 0.76, 95% CI = 0.65 to 0.89, P <
< .001, I2 = 0.0%; Figure 1B). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.73, 95% CI = 0.65 to 0.83, P
.001, I2 = 0.0%; Figure 1B). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.73, 95% CI = 0.65 to 0.83, P <
< .001, I2 = 0.0%; Figure 1) and the 2 groups did not statistically significantly differ (P
.001, I2 = 0.0%; Figure 1) and the 2 groups did not statistically significantly differ (P =
= .91).
.91).
Bone-Only Status
Two studies (1085 patients) reported OS results for patients without bone-only disease. The cumulative effect was statistically significant (HR = 0.71, 95% CI = 0.58 to 0.88, P =
= .002, I2 = 0.0%; Figure 1C). Three studies reported results for bone-only disease (492 patients). A non-statistically significant cumulative benefit was observed (HR = 0.82, 95% CI = 0.60 to 1.13, P
.002, I2 = 0.0%; Figure 1C). Three studies reported results for bone-only disease (492 patients). A non-statistically significant cumulative benefit was observed (HR = 0.82, 95% CI = 0.60 to 1.13, P =
= .23, I2 = 0.0%; Figure 1D). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.74, 95% CI = 0.62 to 0.89, P
.23, I2 = 0.0%; Figure 1D). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.74, 95% CI = 0.62 to 0.89, P <
< .001, I2 = 0.0%; Figure 1) and there was no statistically significant difference between the 2 groups (P
.001, I2 = 0.0%; Figure 1) and there was no statistically significant difference between the 2 groups (P =
= .47).
.47).
Number of Metastatic Sites
Two studies reported results for patients with less than 3 metastatic sites (891 patients). An almost statistically significant cumulative effect was observed (HR = 0.79, 95% CI = 0.62 to 1.01, P =
= .06, I2 = 0.0%; Figure 1E). Three studies reported results for patients with at least 3 metastatic sites (709 patients). The cumulative effect was statistically significant (HR = 0.75, 95% CI = 0.60 to 0.94, P
.06, I2 = 0.0%; Figure 1E). Three studies reported results for patients with at least 3 metastatic sites (709 patients). The cumulative effect was statistically significant (HR = 0.75, 95% CI = 0.60 to 0.94, P =
= .02, I2 = 11.6%; Figure 1F) as well as the result obtained when joining the 2 subpopulations (HR = 0.77, 95% CI = 0.65 to 0.91, P
.02, I2 = 11.6%; Figure 1F) as well as the result obtained when joining the 2 subpopulations (HR = 0.77, 95% CI = 0.65 to 0.91, P =
= .002, I2 = 0.0%; Figure 1), with no statistically significant between-group difference (P
.002, I2 = 0.0%; Figure 1), with no statistically significant between-group difference (P =
= .74).
.74).
Endocrine Sensitivity Status
Four studies provided results for the endocrine resistant subset (1331 patients). The effect in the subgroup was statistically significant (HR = 0.79, 95% CI = 0.67 to 0.93, P =
= .004, I2 = 0.0%; Figure 2A).
.004, I2 = 0.0%; Figure 2A).
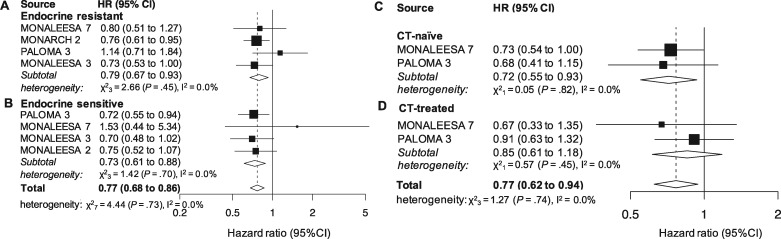
Pooled overall survival (OS) according to endocrine resistance status and previous chemotherapy (CT). Pooled OS in patients younger than 65 years (A), 65 years or older (B), postmenopause (C) and pre- or perimenopause (D). CI = confidence interval; HR = hazard ratio.
Four studies (1503 patients) reported results for the endocrine sensitive subset, which was statistically significant as well (HR = 0.73, 95% CI = 0.61 to 0.88, P =
= .001, I2 = 0.0%; Figure 2B). The overall effect in the joint analysis of the 2 subgroups was also statistically significant (HR = 0.77, 95% CI = 0.68 to 0.86, P
.001, I2 = 0.0%; Figure 2B). The overall effect in the joint analysis of the 2 subgroups was also statistically significant (HR = 0.77, 95% CI = 0.68 to 0.86, P <
< .001, I2 = 0.0%; Figure 2), whereas the between-group difference for the endocrine resistance and sensitive setting was not (P
.001, I2 = 0.0%; Figure 2), whereas the between-group difference for the endocrine resistance and sensitive setting was not (P =
= .55).
.55).
Previous CT for Metastatic Disease
Two studies reported results for CT-naïve (708) and CT-pretreated (271) patients in a metastatic setting. A statistically significant cumulative effect was demonstrated for the first (HR = 0.72, 95% CI = 0.55 to 0.93, P =
= .01, I2 = 0.0%; Figure 2C) but not for the latter group (HR = 0.85, 95% CI = 0.61 to 1.18, P
.01, I2 = 0.0%; Figure 2C) but not for the latter group (HR = 0.85, 95% CI = 0.61 to 1.18, P =
= .34, I2 = 0.0%; Figure 2D). The joint analysis of the 2 subpopulations was statistically significant (HR = 0.77, 95% CI = 0.62 to 0.94, P
.34, I2 = 0.0%; Figure 2D). The joint analysis of the 2 subpopulations was statistically significant (HR = 0.77, 95% CI = 0.62 to 0.94, P =
= .01, I2 = 0.0%; Figure 2), and there was no statistically significant difference between the 2 groups (P
.01, I2 = 0.0%; Figure 2), and there was no statistically significant difference between the 2 groups (P =
= .42).
.42).
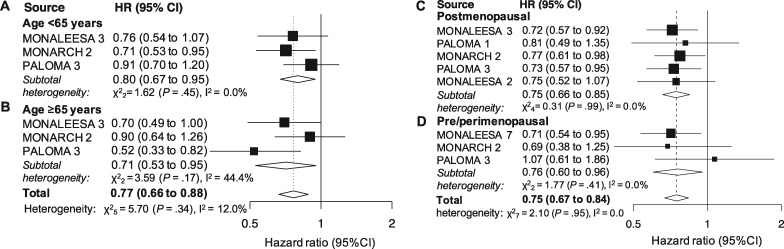
Age
Three studies reported results for patients younger than 65 years and those 65 years and older (1203 and 713 patients, respectively). A statistically significant effect was demonstrated in both subgroups (HR = 0.80, 95% CI = 0.67 to 0.95, P
years and older (1203 and 713 patients, respectively). A statistically significant effect was demonstrated in both subgroups (HR = 0.80, 95% CI = 0.67 to 0.95, P =
= .01, I2 = 0.0%; and HR = 0.71, 95% CI = 0.53 to 0.95, P
.01, I2 = 0.0%; and HR = 0.71, 95% CI = 0.53 to 0.95, P =
= .003, I2 = 44.4%; Figure 3, A and B, respectively). The cumulative effect observed in the joint analysis was statistically significant (HR = 0.77, 95% CI = 0.66 to 0.88, P
.003, I2 = 44.4%; Figure 3, A and B, respectively). The cumulative effect observed in the joint analysis was statistically significant (HR = 0.77, 95% CI = 0.66 to 0.88, P <
< .001, I2 = 12.0%; Figure 3), whereas the between group difference was not (P
.001, I2 = 12.0%; Figure 3), whereas the between group difference was not (P =
= .49).
.49).
Menopausal Status
Five studies (2523 patients) provided results for postmenopausal patients. The cumulative effect was statistically significant (HR = 0.76, 95% CI = 0.67 to 0.86, P <
< .001, I2 = 0.0%; Figure 3C). Three studies (894 patients) provided results for the pre- or perimenopausal setting. The pooled result was statistically significant as well (HR = 0.76, 95% CI = 0.60 to 0.96, P
.001, I2 = 0.0%; Figure 3C). Three studies (894 patients) provided results for the pre- or perimenopausal setting. The pooled result was statistically significant as well (HR = 0.76, 95% CI = 0.60 to 0.96, P =
= .02, I2 = 0.0%; Figure 3D). The overall effect in the joint analysis was statistically significant (HR = 0.75, 95% CI = 0.67 to 0.84, P
.02, I2 = 0.0%; Figure 3D). The overall effect in the joint analysis was statistically significant (HR = 0.75, 95% CI = 0.67 to 0.84, P <
< .001, I2 = 0.0%; Figure 3) with no difference observed between subgroups (P
.001, I2 = 0.0%; Figure 3) with no difference observed between subgroups (P =
= .99).
.99).
Risk of Bias Analysis
The studies included in our analyses did not show any relevant risk of bias within the 7 domains considered. Risk of bias pooled results are reported in Supplementary Figure 3 (available online). A detailed assessment for each single study is reported in Supplementary Figure 4 (available online).
Discussion
We focused our meta-analysis on specific subgroups of clinical relevance. Results show for the first time, to our knowledge, that CDK4/6-inhibitors plus ET combinations, compared with ET monotherapy, improve OS in HR+/HER2-negative MBC as first- or second-line treatment independent of age (<65 vs ≥65 years), menopausal status (pre- or peri- vs postmenopausal), endocrine sensitivity (sensitive vs resistant), and visceral involvement. More specifically, we observed a 24% and 32% relative reduction in the risk of death for patients with or without visceral metastasis, respectively, which was also accompanied by a statistically significant 29% risk reduction in patients without bone-only disease, irrespective of other metastatic sites. The OS benefit was comparable in both pre- or peri- and postmenopausal settings, with statistically significant 24% relative decreases in the risk of death in both cases. Notably, CDK4/6-inhibitor–based therapies produced a statistically significant relative reduction in the risk of death of 20% and 29% for patients younger than 65 years and those 65
years), menopausal status (pre- or peri- vs postmenopausal), endocrine sensitivity (sensitive vs resistant), and visceral involvement. More specifically, we observed a 24% and 32% relative reduction in the risk of death for patients with or without visceral metastasis, respectively, which was also accompanied by a statistically significant 29% risk reduction in patients without bone-only disease, irrespective of other metastatic sites. The OS benefit was comparable in both pre- or peri- and postmenopausal settings, with statistically significant 24% relative decreases in the risk of death in both cases. Notably, CDK4/6-inhibitor–based therapies produced a statistically significant relative reduction in the risk of death of 20% and 29% for patients younger than 65 years and those 65 years and older, respectively. Given the acceptable and manageable toxicity profile, it is reassuring that CDK4/6-inhibitor combinations also proved to be statistically significantly effective in older patients, confirming and strengthening results from a previous meta-analysis of targeted agents combined with standard ET in elderly patients based on PFS as survival endpoint (26). Importantly, CDK4/6-inhibitor–based treatments were also able to statistically significantly reduce the risk of death by 21% in an endocrine resistance setting and 27% in endocrine sensitive setting. Notably, in the endocrine-resistant subgroup, the only statistically significant individual result was the one obtained within the MONARCH 3 study, which specifically enrolled endocrine-resistant patients to be treated with an abemaciclib-based combination. In this trial, endocrine resistance was defined according to the previously mentioned ESO-ESMO definition (20). Differently, in the endocrine-sensitive setting, only the palbociclib-containing PALOMA 3 trial was associated with a statistically significant result, and no abemaciclib-containing study was available for this analysis. Additionally, palbociclib and ribociclib proved, overall, to produce a statistically significant relative decrease in the risk of death for CT-naïve patients in the metastatic setting (28% death risk reduction) and also in patients with at least 3 metastatic sites (25% death risk reduction).
years and older, respectively. Given the acceptable and manageable toxicity profile, it is reassuring that CDK4/6-inhibitor combinations also proved to be statistically significantly effective in older patients, confirming and strengthening results from a previous meta-analysis of targeted agents combined with standard ET in elderly patients based on PFS as survival endpoint (26). Importantly, CDK4/6-inhibitor–based treatments were also able to statistically significantly reduce the risk of death by 21% in an endocrine resistance setting and 27% in endocrine sensitive setting. Notably, in the endocrine-resistant subgroup, the only statistically significant individual result was the one obtained within the MONARCH 3 study, which specifically enrolled endocrine-resistant patients to be treated with an abemaciclib-based combination. In this trial, endocrine resistance was defined according to the previously mentioned ESO-ESMO definition (20). Differently, in the endocrine-sensitive setting, only the palbociclib-containing PALOMA 3 trial was associated with a statistically significant result, and no abemaciclib-containing study was available for this analysis. Additionally, palbociclib and ribociclib proved, overall, to produce a statistically significant relative decrease in the risk of death for CT-naïve patients in the metastatic setting (28% death risk reduction) and also in patients with at least 3 metastatic sites (25% death risk reduction).
On the other hand, the observed OS benefits in patients with less than 3 metastatic sites (21% death risk reduction) or bone-only disease (18% death risk reduction) and in CT-pretreated patients in an advanced setting (15% death risk reduction) were not statistically significant. However, these data must be put into context. Firstly, it is important to point out that each of the clinical subsets examined had a different sample size and biological plausibility for a different effect size (as reflected by different HR). This also translates in a different power to identify a statistically significant treatment effect. In fact, it is highly likely that the analysis regarding the CT-pretreated subgroup was negatively affected by the low number of patients (94 of 572 and 177 of 521 from MONALEESA 7 and PALOMA 3 trials, respectively), probably insufficient to demonstrate a clear benefit in terms of OS. Additionally, when putting together CT-naïve and CT-pretreated patients, the cumulative effect observed was a statistically significant 23% relative reduction in the risk of death, with no statistically significant difference between the 2 groups, suggesting that CDK4/6-inhibitor–based treatments are effective in both subsets, albeit a more pronounced effect could be obtained in CT-naïve patients. Furthermore, a posthoc subgroup analysis of the recently published Young-PEARL phase II trial comparing palbociclib + exemestane or fulvestrant vs capecitabine in premenopausal patients similarly showed a statistically significantly improved PFS for the CDK4/6-inhibitor arm in patients pretreated with CT for metastatic disease (HR = 0.62, 95% CI = 0.38 to 0.99) with a more uncertain benefit for CT-pretreated patients (HR = 0.82, 95% CI = 0.36 to 1.92) (27). Taken together, these results clearly support the recommendation of international guidelines concerning the need to delay CT in HR+/HER2-negative MBC, except in case of visceral crisis (20,28), and support the use of CDK4/6-inhibitor–based treatments as upfront therapy.
When considering the subgroup of patients with less than 3 metastatic sites, the result was only marginally non-statistically significant (P =
= .06, 95% CI = 0.62 to 1.01), reflecting a clear trend for improved survival. Additionally, when joining together patients with less than 3 and at least 3 metastatic sites, the pooled effect was statistically significant, with a meaningful 31% relative reduction in the risk of death and a statistically non-significant test for subgroup differences (P
.06, 95% CI = 0.62 to 1.01), reflecting a clear trend for improved survival. Additionally, when joining together patients with less than 3 and at least 3 metastatic sites, the pooled effect was statistically significant, with a meaningful 31% relative reduction in the risk of death and a statistically non-significant test for subgroup differences (P =
= .74), suggesting a potentially more pronounced effect in patients with higher tumor burden compared with patients with a low tumor burden metastatic disease. Similarly, the OS benefit obtained with CDK4/6-inhibitor–based combinations in bone-only disease was not statistically significant. In fact, patients with bone-only metastatic tumors usually show a more indolent and less rapidly evolving disease, with an improved survival over patients with other metastatic sites (29). Therefore, it is highly likely that more patients, longer follow-up, and more events might be needed to obtain more conclusive results. Moreover, within pivotal trials the interaction tests between treatment effect and metastatic sites were not statistically significant. Additionally, another meta-analysis demonstrated a statistically significantly improved PFS for CDK4/6-inhibitor–based therapies as first line in bone-only metastatic disease (29), further confirmed by a patient-level pooled analysis from the US Food and Drug Administration (30). Furthermore, when taken together with the result obtained in the subset of patients with no bone-only tumors, the benefit was clinically meaningful (26% risk reduction) and statistically significant (P
.74), suggesting a potentially more pronounced effect in patients with higher tumor burden compared with patients with a low tumor burden metastatic disease. Similarly, the OS benefit obtained with CDK4/6-inhibitor–based combinations in bone-only disease was not statistically significant. In fact, patients with bone-only metastatic tumors usually show a more indolent and less rapidly evolving disease, with an improved survival over patients with other metastatic sites (29). Therefore, it is highly likely that more patients, longer follow-up, and more events might be needed to obtain more conclusive results. Moreover, within pivotal trials the interaction tests between treatment effect and metastatic sites were not statistically significant. Additionally, another meta-analysis demonstrated a statistically significantly improved PFS for CDK4/6-inhibitor–based therapies as first line in bone-only metastatic disease (29), further confirmed by a patient-level pooled analysis from the US Food and Drug Administration (30). Furthermore, when taken together with the result obtained in the subset of patients with no bone-only tumors, the benefit was clinically meaningful (26% risk reduction) and statistically significant (P <
< .001), with no statistically significant subgroup difference (P
.001), with no statistically significant subgroup difference (P =
= .55). Overall, this result should thus be interpreted carefully and be updated in the near future with still unpublished OS subgroup data from other CDK4/6-inhibitors trials in order to draw more definitive conclusions.
.55). Overall, this result should thus be interpreted carefully and be updated in the near future with still unpublished OS subgroup data from other CDK4/6-inhibitors trials in order to draw more definitive conclusions.
This meta-analysis has some limitations that need to be addressed. Firstly, some of the subgroups from published trials were not totally identical. More specifically, it was not possible to extract a clean result concerning all the patients untreated with CT in metastatic setting due to different subgroup characterization, which led to a potential underestimation of the number of patients untreated with CT specifically in the metastatic setting (this happened for the PALOMA 3 study). Similarly, for visceral and nonvisceral disease, both MONALEESA 3 and 7 studies used the categorization “liver or lung involvement” instead of visceral and nonvisceral. Secondly, data about crossover after progression in the mono-ET arms have not been reported, except for MONALEESA 2 trial, where crossover was explicitly not admitted. Therefore, its impact on survival outcomes could not be clearly elucidated. Furthermore, subgroup analyses differed among trials; thus, for each of the subgroups considered, not all of the RCT could be included. In fact, for some of the trials included (7,16,17), OS data were published before final analysis, possibly because more mature results for worse subgroups (patients with visceral metastases, primary endocrine resistance) provided more OS events that drove the early stopping rules data. This might have produced a theoretical risk of “enriched” meta-analysis in positive trials because a negative trial is only published at the time of the final analysis. Therefore, intention-to-treat and subgroup OS data from the remaining PALOMA 2 and MONARCH 3 trials and final OS results from the MONALEESA 2, and MONARCH 2 trials are awaited. Nevertheless, based on PFS data and current results, we expect a substantial confirmation of the effects already observed, specifically in the subgroups with non-statistically significant results (e.g. tumors with very limited tumor burden and bone-only disease) for which current data might not be sufficiently mature. In fact, more patients and longer follow-up might be needed to observe a statistically significant effect. At the same time, due to the potentially more indolent disease course, it cannot be excluded that patients with bone-only metastases or very small tumor burden may experience prolonged PFS and OS even when receiving ET alone initially.
To our knowledge, this is the first meta-analysis exploring the impact and benefit of CDK4/6-inhibitor–based regimens on OS in specific clinically relevant subgroups. Results from our study address clinically relevant questions that might help the clinicians in better tailoring patients’ treatments. In this perspective, we also would like to point out that a study-level meta-analysis like ours, compared with patient-level meta-analysis, provides more rapid results and does not need large, time-consuming, and potentially more expensive collaborations between major competitors to obtain individual patients’ data from each trial, making it more suitable for addressing clinically relevant questions in a reasonable timeline. What most, results were not affected by significant heterogeneity and, overall, there was no truly relevant risk of bias concerning the included trials. It is also noteworthy that when the meta-analysis is based on only a few studies (2 or 3), the heterogeneity is difficult to estimate and standard random-effects meta-analysis methods are usually performed even if the obtained results may be influenced by the small number of studies (wide pooled confidence intervals). Nevertheless, it is unclear whether or to what extent small-sample-size behavior could be improved by more sophisticated modeling systems.
In conclusion, CDK4/6-inhibitors plus ET combinations are substantially effective in improving OS in HR+/HER2-negative MBC as first- or second-line treatment in young or adult (<65 years) as well as in older patients independently from visceral involvement, endocrine sensitivity, and menopausal status. Ribociclib-based combinations might be preferred for the premenopausal setting, because the major contribution on the overall positive subgroup analysis result came from the ribociclib-based MONALEESA 7 trial, which specifically enrolled pre- and perimenopausal patients (a total of 672), whereas the other studies included only contributed with relatively small subgroups of the overall patients enrolled (108 and 114 for PALOMA 3 and MONARCH 3, respectively). On the other hand, abemaciclib-based combinations might be preferred for endocrine-resistant tumors, being the only CDK4/6-inhibitor clearly providing a statistically significant effect in this subset. However, it must be considered that this is only speculative, because no currently published data support the superiority of 1 of the 3 molecules, or the same CDK4/6-inhibitor with a different ET companion (AI, fulvestrant or tamoxifen), over the others (10,31). Furthermore, the degree of benefit shown across pivotal trials for the intention-to-treat populations is quite similar (3–9). Standard ET without CDK4/6-inhibitors might still be an option for bone-only and very limited disease given a more uncertain OS benefit. However, a clear PFS benefit demonstrated elsewhere (29,30) and a the current OS pooled analysis being substantially under-powered suggest that more data are needed to draw definitive conclusions. CDK4/6-based regimens should thus be considered in these subsets as upfront therapy, although they could still be used as a second-line option in case of different first-line treatment choice. Finally, it could be preferable avoiding CT as the upfront therapy in the metastatic setting. Apart from toxicity, activity, and efficacy concerns reported elsewhere (10,20,28,29,31), our analysis shows that upfront CT might also reduce the beneficial impact on OS for CDK4/6-inhibitor–based treatments.
years) as well as in older patients independently from visceral involvement, endocrine sensitivity, and menopausal status. Ribociclib-based combinations might be preferred for the premenopausal setting, because the major contribution on the overall positive subgroup analysis result came from the ribociclib-based MONALEESA 7 trial, which specifically enrolled pre- and perimenopausal patients (a total of 672), whereas the other studies included only contributed with relatively small subgroups of the overall patients enrolled (108 and 114 for PALOMA 3 and MONARCH 3, respectively). On the other hand, abemaciclib-based combinations might be preferred for endocrine-resistant tumors, being the only CDK4/6-inhibitor clearly providing a statistically significant effect in this subset. However, it must be considered that this is only speculative, because no currently published data support the superiority of 1 of the 3 molecules, or the same CDK4/6-inhibitor with a different ET companion (AI, fulvestrant or tamoxifen), over the others (10,31). Furthermore, the degree of benefit shown across pivotal trials for the intention-to-treat populations is quite similar (3–9). Standard ET without CDK4/6-inhibitors might still be an option for bone-only and very limited disease given a more uncertain OS benefit. However, a clear PFS benefit demonstrated elsewhere (29,30) and a the current OS pooled analysis being substantially under-powered suggest that more data are needed to draw definitive conclusions. CDK4/6-based regimens should thus be considered in these subsets as upfront therapy, although they could still be used as a second-line option in case of different first-line treatment choice. Finally, it could be preferable avoiding CT as the upfront therapy in the metastatic setting. Apart from toxicity, activity, and efficacy concerns reported elsewhere (10,20,28,29,31), our analysis shows that upfront CT might also reduce the beneficial impact on OS for CDK4/6-inhibitor–based treatments.
Overall, our results strongly support the recommendations from major international treatment guidelines (20,28) and recent pooled analyses (10,30–32).
Funding
There was no funding source for this study.
Notes
Disclosures: FS has declared travel and accommodation expenses paid by Pfizer and Celgene. MG, GA and SDP have declared honoraria from Roche, Pfizer, Astra-Zeneca, Novartis, Celgene, Eli Lilly, Amgen and Eisai. AP has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. GJ has reported grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Lilly, personal fees from Celgene, personal fees and non-financial support from Amgen, personal fees and non-financial support from BMS, personal fees from Puma Technology, personal fees and non-financial support from Astra-Zeneca, personal fees from Daiichi Sankyo, personal fees from Abbvie, outside the submitted work. LDM has declared honoraria from Roche, Pfizer, Ipsen, Eli Lilly, Eisai, Novartis, Takeda and MSD, consulting/advisory role for Roche and Eli Lilly, travels, accommodations and expenses from Roche, Pfizer and Celgene. MDL has declared consulting fees from Pfizer, Novartis, Eli Lilly, Roche, Eisai, Celgene. MC has declared consulting fees from Novartis and Pfizer. FP has declared honoraria for advisory boards, activities as a speaker, travel grants, research grants from Amgen, Astra-Zeneca, Celgene, Eisai, Eli Lilly, Ipsen, MSD, Novartis, Pierre-Fabre, Pfizer, Roche and Takeda, and research funding from Astra-Zeneca, Eisai, Roche and Italian Ministry of Health. PFC has declared consultant role for Novartis, Eli Lilly, Astra Zeneca and Tesaro, honoraria from BMS, Roche, Eli Lilly, Novartis and AstraZeneca, research funding from Novartis, Roche, BMS, Merck-KGa, Italian Ministry of Health, Veneto Secretary of Health and University of Padova. IP has declared honoraria for advisory boards, activities as a speaker, and travel grants from Eisai, Eli Lilly, Ipsen, Italfarmaco, Novartis, Pierre-Fabre, Pfizer, Roche, Gentili and Astra-Zeneca. DJ has served as a consultant/advisory board at Novartis, Genentech, Eisai, Ipsen, and EMD Serono. AL-C reports grants, personal fees and non- financial support from Novartis, Roche, AstraZeneca, Eli Lilly, and Pfizer; grants and non-financial support from EISAI, grants and personal fees from Genomic Health, GlaxoSmithKline Tesaro, personal fees from MSD, personal fees and non-financial support from Bristol-Myers Squibb. ADL has reported advisory board, consultant and honoraria from AZ, Bayer, Celgene, Daiichi-Sankyo, Eisai, Genomic Health, Genentech, Ipsen, Lilly, Novartis, Puma Biotechnology, Pfizer and Roche. DG has declared consulting fees from Novartis, Lilly and Pfizer, research funding from LILT, Novartis Astra-Zeneca and University of Trieste. The other authors have nothing to declare.
References
Articles from JNCI Journal of the National Cancer Institute are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/jnci/djaa071
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7669227?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/jnci/djaa071
Article citations
The different sequences of CDK4/6 inhibitor and mTOR inhibitor in HR+/HER2-advanced breast cancer: A multicenter real-world study.
Heliyon, 10(19):e38147, 19 Sep 2024
Cited by: 0 articles | PMID: 39386840 | PMCID: PMC11462034
Confirming the efficacy and safety of CDK4/6 inhibitors in the first-line treatment of HR+ advanced breast cancer: a systematic review and meta-analysis.
Front Pharmacol, 15:1369420, 05 Aug 2024
Cited by: 0 articles | PMID: 39161906 | PMCID: PMC11330780
Review Free full text in Europe PMC
Identifying drivers of first-line HR+/HER2- metastatic breast cancer treatment choices.
Future Oncol, 20(29):2165-2177, 04 Jun 2024
Cited by: 0 articles | PMID: 38861295 | PMCID: PMC11508941
Fibroblast growth factor receptor signaling in estrogen receptor-positive breast cancer: mechanisms and role in endocrine resistance.
Front Oncol, 14:1406951, 08 Jul 2024
Cited by: 0 articles | PMID: 39040443 | PMCID: PMC11260626
Review Free full text in Europe PMC
The Use of Cyclin-Dependent Kinase 4/6 Inhibitors in Elderly Breast Cancer Patients: What Do We Know?
Cancers (Basel), 16(10):1838, 11 May 2024
Cited by: 1 article | PMID: 38791919 | PMCID: PMC11119337
Review Free full text in Europe PMC
Go to all (54) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.
Breast Cancer Res Treat, 180(1):21-32, 22 Jan 2020
Cited by: 18 articles | PMID: 31970560
Review
Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis.
JAMA Netw Open, 3(10):e2020312, 01 Oct 2020
Cited by: 36 articles | PMID: 33048129 | PMCID: PMC8094425
Review Free full text in Europe PMC
CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis.
Lancet Oncol, 21(2):250-260, 16 Dec 2019
Cited by: 136 articles | PMID: 31859246
Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis.
Lancet Oncol, 20(10):1360-1369, 04 Sep 2019
Cited by: 95 articles | PMID: 31494037
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001863