Abstract
Free full text

Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy
Abstract
Myocarditis is an inflammatory disease of the heart that may occur because of infections, immune system activation, or exposure to drugs. The diagnosis of myocarditis has changed due to the introduction of cardiac magnetic resonance imaging. We present an expert consensus document aimed to summarize the common terminology related to myocarditis meanwhile highlighting some areas of controversies and uncertainties and the unmet clinical needs. In fact, controversies persist regarding mechanisms that determine the transition from the initial trigger to myocardial inflammation and from acute myocardial damage to chronic ventricular dysfunction. It is still uncertain which viruses (besides enteroviruses) cause direct tissue damage, act as triggers for immune-mediated damage, or both. Regarding terminology, myocarditis can be characterized according to etiology, phase, and severity of the disease, predominant symptoms, and pathological findings. Clinically, acute myocarditis (AM) implies a short time elapsed from the onset of symptoms and diagnosis (generally <1 month). In contrast, chronic inflammatory cardiomyopathy indicates myocardial inflammation with established dilated cardiomyopathy or hypokinetic nondilated phenotype, which in the advanced stages evolves into fibrosis without detectable inflammation. Suggested diagnostic and treatment recommendations for AM and chronic inflammatory cardiomyopathy are mainly based on expert opinion given the lack of well-designed contemporary clinical studies in the field. We will provide a shared and practical approach to patient diagnosis and management, underlying differences between the European and US scientific statements on this topic. We explain the role of histology that defines subtypes of myocarditis and its prognostic and therapeutic implications.
Definitions, Epidemiology, and Pathophysiology
Myocarditis is an inflammatory disease of the heart that may occur as a consequence of infections, exposure to toxic substances, and immune system activation1,2 and is included among secondary cardiomyopathies in the 1996 World Health Organization classification.3 Myocarditis has a wide spectrum of clinical presentations and trajectories, with most cases resolving spontaneously. It is also a relatively common cause of sudden cardiac death (SCD) in young people (from 6% to 10% in autopsy-based series; Table I in the Data Supplement).4,5,119–121 Furthermore, in some patients, inflammation may cause extensive scarring that triggers left ventricular (LV) remodeling, leading eventually to dilated cardiomyopathy (DCM)6 or alternatively to a predominant hypokinetic nondilated phenotype of cardiomyopathy. Myocarditis can be characterized according to etiology, phase, and severity of the disease, predominant symptoms, and pathological findings. Clinically, acute myocarditis (AM) implies a short time elapsed from the onset of symptoms and diagnosis (generally <1 month), while chronic inflammatory cardiomyopathy (infl-CMP) indicates myocardial inflammation with established DCM or hypokinetic nondilated phenotype generally with a longer duration of symptoms (>1 month; Figure Figure1).1). Based on the cell types infiltrating, myocarditis can be classified as eosinophilic, lymphocytic, giant cells, or granulomatous (Figure (Figure2).2). Chronic myocarditis could represent an intermediate stage between AM and chronic infl-CMP in patients with persisting myocardial inflammation (Figure I in the Data Supplement). Due to evolving diagnostic criteria and differences in the conceptual view and interpretation of myocarditis within the medical community, definitions associated with myocarditis have changed over the last decades. A list of definitions used in this document is presented in Table Table11.
Table 1.
Glossary of the Terms Used in this Document Regarding Myocarditis

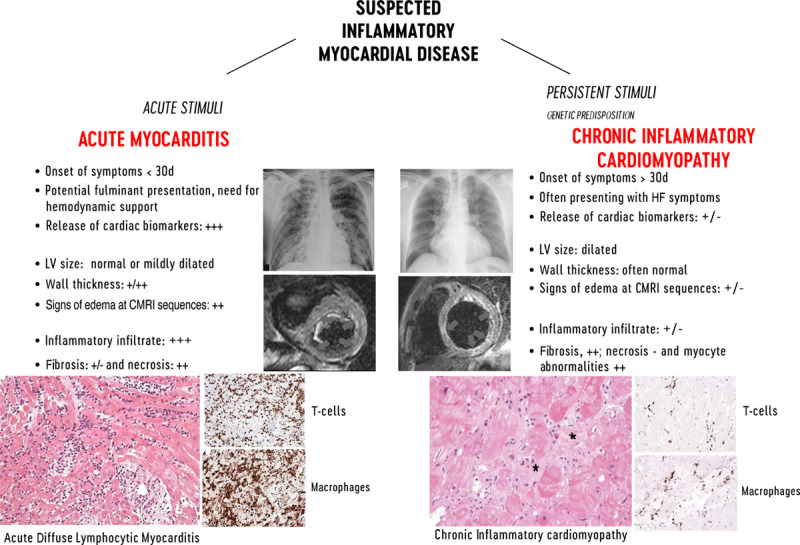
Characteristic features of lymphocytic acute myocarditis and chronic inflammatory cardiomyopathy. Left, Imaging features of acute myocarditis: chest radiograph of a patient admitted for chest pain and suspected acute myocarditis with no enlargement of the cardiac silhouette and cardiac magnetic resonance imaging (CMRI) showing normal left ventricular (LV) volume and significantly increased cardiac mass with diffuse high signal in T2-weighted images (arrows) suggesting diffuse edema. Histology shows acute lymphocytic myocarditis with myocyte necrosis and diffuse mononuclear cell infiltrates by hematoxylin-eosin and immunohistological stain on CD3+ T cells and CD68+ macrophages, compatible with an active myocarditis based on Dallas criteria (magnitude ×200). Right, Imaging features of chronic lymphocytic cardiomyopathy: chest radiograph of a patient admitted with heart failure (HF) symptoms, showing enlargement of cardiac silhouette; at CMRI, the LV is dilated, with normal thickness and focal areas of high signal intensity at T2-weighted images suggesting localized edema (arrows). At histology, chronic inflammatory cardiomyopathy typically presents fibrosis (*) within areas with inflammatory cellular infiltrates and myocyte abnormalities (magnitude ×200). CD indicates cluster of differentiation.
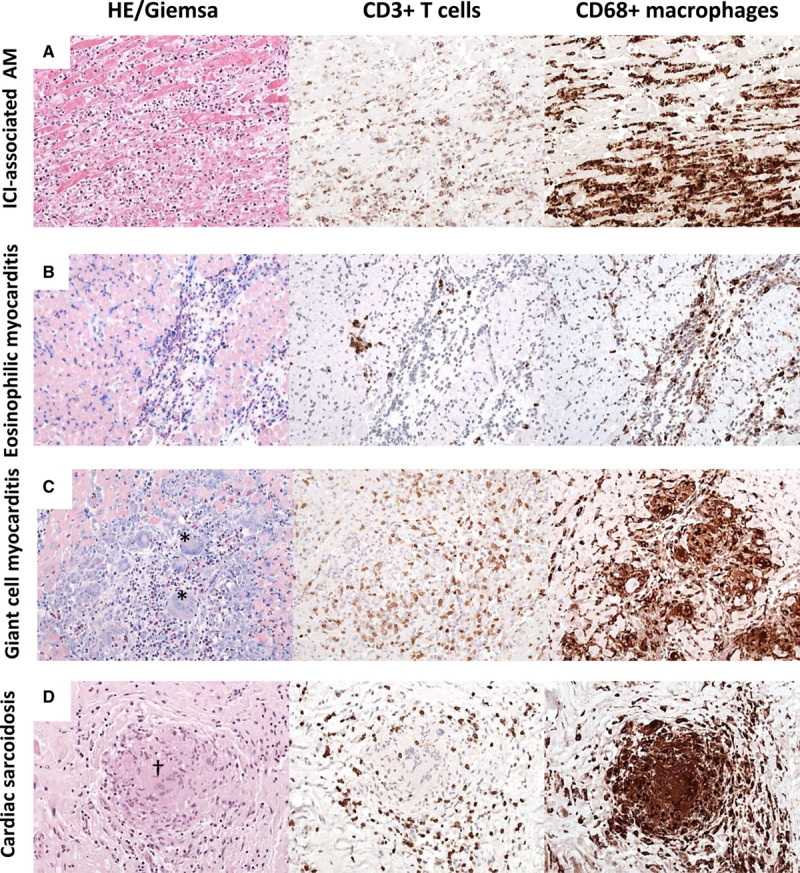
Different patterns of myocardial inflammation demonstrated by histological and immunohistological stainings on endomyocardial biopsy. A, Immune checkpoint inhibitor (ICI)–associated acute myocarditis (AM) frequently reveals diffuse mononuclear infiltrates composed of CD3+ T cells and CD68+ macrophages. Based on hematoxylin-eosin (HE) and immunohistological stainings, ICI-associated myocarditis resembles a diffuse lymphocytic myocarditis. B, In eosinophilic myocarditis, prominent inflammatory cells are eosinophilic granulocytes (Giemsa) and macrophages. C, Giant cell myocarditis is characterized by large mononucleated infiltrates with the presence of giant cells (*) and eosinophils (Giemsa). D, Cardiac sarcoidosis can be differentiated from giant cell myocarditis by the presence of granuloma (†) and absence of necrotic myocytes (magnitude, ×200 in all pictures). CD indicates cluster of differentiation.
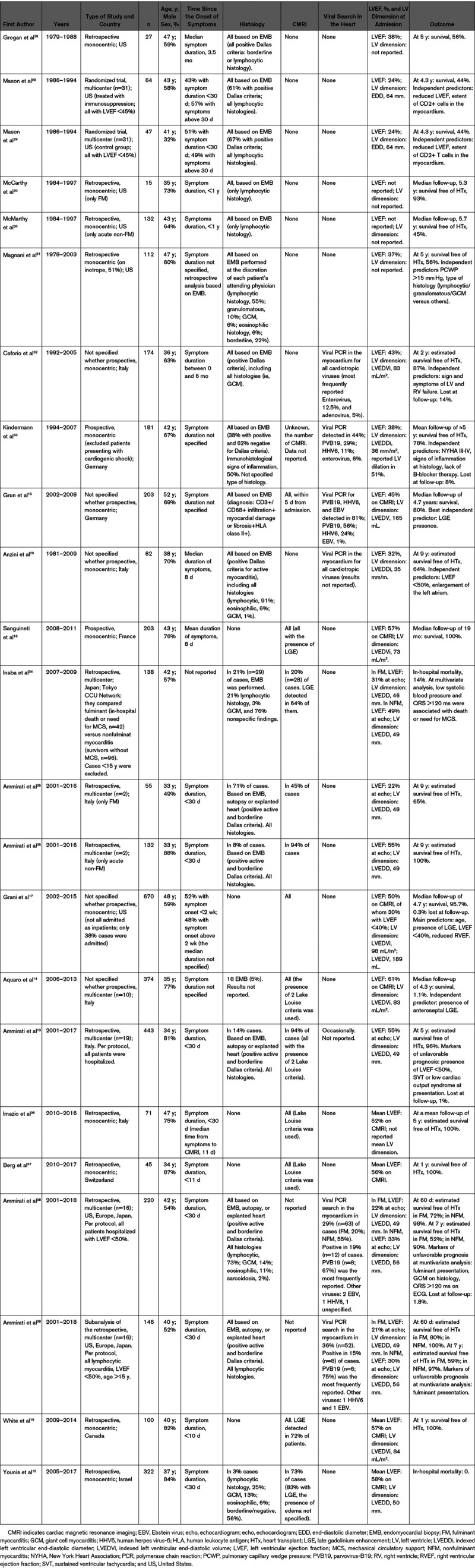
The disease burden of myocarditis is difficult to define. Based on hospital discharge forms between 1990 and 2013, an incidence of 22 cases of 100 000 patients annually was estimated by the Global Burden of Disease Study.10 However, this report did not distinguish between AM or chronic infl-CMP and other cardiomyopathies, with possible overestimation of myocarditis. Among patients presenting to the emergency department, AM was the second most common cardiac cause of chest pain (3%) in a French registry.11 Furthermore, ≈33% of the patients initially labeled as myocardial infarction with nonobstructed coronary arteries are later diagnosed as AM.12 According to contemporary registries, AM is a cardiac condition affecting relatively young patients (median age of onset ranges between 30 and 45 years in most of the series) and men more than women (male prevalence ranges between 60% and 80%; Table Table22).13–20 The absolute prevalence and relative proportion of different etiologies may vary over time and according to endemic diseases. For example, immune checkpoint inhibitor (ICI)–associated myocarditis is a recently recognized entity, whose rate of diagnosis has increased due to larger awareness and to the larger population of patients with cancer eligible for treatment with ICI.21 On the other hand, AM and chronic infl-CMP may have a different incidence in specific geographic areas according to local epidemiology (such as Chagas disease in South America). Controversies still exist regarding the mechanisms that determine the transition from the initial trigger to myocardial inflammation and from acute myocardial injury to chronic dysfunction. To date, it is not known which viruses other than enteroviruses may cause direct tissue damage in humans or act mainly as triggers for autoimmunity-mediated damage or both.22,23 It must be considered that the experimental evidences on murine models of viral myocarditis are based on infections with Coxsackie B viruses, whereas for the most common agent in virus-positive myocarditis patients, parvovirus-B19 (PVB19),24,25 no animal models are available. A possible association between genetic abnormalities and susceptibility to inflammation has been suggested. In particular, patients with mutations responsible for arrhythmogenic cardiomyopathy may be at risk for AM and share clinical and pathological aspects with chronic infl-CMP,26,27 although further studies are required to elucidate this association and understand its mechanistic underpinnings.
000 patients annually was estimated by the Global Burden of Disease Study.10 However, this report did not distinguish between AM or chronic infl-CMP and other cardiomyopathies, with possible overestimation of myocarditis. Among patients presenting to the emergency department, AM was the second most common cardiac cause of chest pain (3%) in a French registry.11 Furthermore, ≈33% of the patients initially labeled as myocardial infarction with nonobstructed coronary arteries are later diagnosed as AM.12 According to contemporary registries, AM is a cardiac condition affecting relatively young patients (median age of onset ranges between 30 and 45 years in most of the series) and men more than women (male prevalence ranges between 60% and 80%; Table Table22).13–20 The absolute prevalence and relative proportion of different etiologies may vary over time and according to endemic diseases. For example, immune checkpoint inhibitor (ICI)–associated myocarditis is a recently recognized entity, whose rate of diagnosis has increased due to larger awareness and to the larger population of patients with cancer eligible for treatment with ICI.21 On the other hand, AM and chronic infl-CMP may have a different incidence in specific geographic areas according to local epidemiology (such as Chagas disease in South America). Controversies still exist regarding the mechanisms that determine the transition from the initial trigger to myocardial inflammation and from acute myocardial injury to chronic dysfunction. To date, it is not known which viruses other than enteroviruses may cause direct tissue damage in humans or act mainly as triggers for autoimmunity-mediated damage or both.22,23 It must be considered that the experimental evidences on murine models of viral myocarditis are based on infections with Coxsackie B viruses, whereas for the most common agent in virus-positive myocarditis patients, parvovirus-B19 (PVB19),24,25 no animal models are available. A possible association between genetic abnormalities and susceptibility to inflammation has been suggested. In particular, patients with mutations responsible for arrhythmogenic cardiomyopathy may be at risk for AM and share clinical and pathological aspects with chronic infl-CMP,26,27 although further studies are required to elucidate this association and understand its mechanistic underpinnings.
Table 2.
Principal Studies That Evaluated the Long-Term Outcome of Adult Patients With Myocarditis Based on Histology or Cardiac Magnetic Resonance Imaging Findings or the Combination of Both Published Since 1995
This review will try to summarize a shared and practical approach to patients presenting with AM or chronic infl-CMP, meanwhile pointing out the areas of controversies and uncertainties and the unmet clinical needs. Specific conditions such as pediatric myocarditis, including rheumatic carditis, Chagas disease, and HIV cardiomyopathy deserve separate discussion and are not addressed in this document.
Diagnostic Approach to AM and infl-CMP
AM: Symptoms and Signs
Patients with suspected AM are generally evaluated in the emergency room due to chest pain, dyspnea, fatigue, palpitations, or syncope.1 Based on large registries, chest pain is the most frequent symptom (85%–95% of cases),13–16,18 followed by dyspnea (19%–49% of cases),13,16,17 whereas syncope occurs in about 6%.13 Fever is common (about 65%),13,15 while other prodromal manifestations, such as flu-like symptoms, gastrointestinal disorders, sore throat, or respiratory tract infections, may have preceded the acute phase by a few days or weeks, with a prevalence ranging from 18% to 80%.13,14,18
In a recent retrospective registry of 443 AMs, 26.6% had a presentation complicated by LV systolic dysfunction, ventricular arrhythmias, or cardiogenic shock (ie, fulminant myocarditis [FM] that accounted for 8.6% of total cases). On the other hand, the majority of AMs (73.4%) had no such complications (uncomplicated AM) and presented chest pain in 97% of cases and ST-segment elevation on ECG in 62.3% of cases, and they had no deaths or heart transplantation (HTx) at 5 years.13 When collecting patient history, attention should focus on specific causes including recent exposure to drugs (eg, antibiotics, clozapine, ICI) or toxic substances (eg, cocaine or amphetamine)2 or to infectious agents (eg, ingestion of raw meat suggesting helminthic infections,39 travels to areas where viruses associated with AM, such as Dengue, are endemic). A proposed approach to AM is summarized in Figure Figure33.
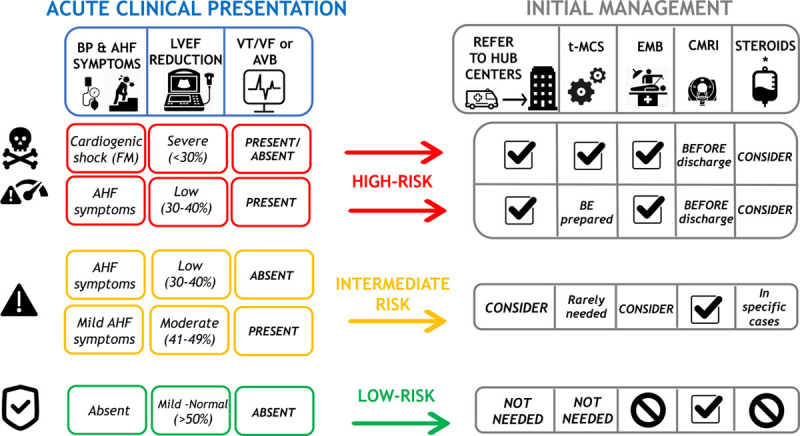
Proposed risk-based approach to acute myocarditis. Left, Clinical features that characterize high (red boxes), intermediate (orange boxes), or low (green boxes) risk are summarized, according to the presence of low blood pressure (BP) and severity of acute heart failure (AHF), initial left ventricular ejection fraction (LVEF) on first echocardiogram, and ECG (presence of ventricular tachycardia [VT] or ventricular fibrillation [VF] or advanced atrioventricular block [AVB]). Right, How these risk features may influence patient management in terms of referral to expert centers, temporary mechanical circulatory support (t-MCS), need for endomyocardial biopsy (EMB) or cardiac magnetic resonance imaging (CMRI), and consideration for steroid treatment. Tag sign indicates recommended actions. No symbol indicates not recommended. *Immunosuppression with intravenous steroids may be considered and often used in patients with fulminant myocarditis; however, clinical studies that demonstrate their efficacy are lacking.
AM: ECG
The ECG is abnormal in about 85% of cases,13,15 ST-segment elevation mimicking acute myocardial infarctions is the most frequent abnormality13,15; inferior and lateral leads are commonly involved. QRS width >120 ms, atrioventricular block, symptomatic bradycardia, or tachycardia and ventricular arrhythmias should increase the suspicion of AM and suggest high-risk forms.38 Second- or third-degree atrioventricular block is rarely observed in patients with normal LV ejection fraction (LVEF) >50%, except in cardiac sarcoidosis (CS), Lyme carditis, as well as ICI-associated myocarditis.40
AM: Laboratory Tests
Recommended laboratory tests for identification of patients with suspected AM are myocardial necrosis biomarkers (high-sensitivity troponins, creatinine kinase-MB). Only a weak correlation exists between troponin release and the severity of cardiac dysfunction.41 Other laboratory tests routinely requested include markers of inflammation such as C-reactive protein that is positive in 80% to 95% in recent series.13,14 Erythrocyte sedimentation rate is also commonly increased, but it is generally not available in the emergency department. A persistently increased erythrocyte sedimentation rate can suggest an associated autoimmune disorder. Furthermore, differential white blood count can show eosinophilia, suggesting the presence of eosinophilic myocarditis (EM).39 Finally, peripheral blood serological and virological tests are rarely informative,2 with some exceptions (eg, HIV and Borrelia burgdorferi antibodies). A search for viral genomes with polymerase chain reaction in aerial tract fluids and pharyngeal swabs can identify viruses of the respiratory tract, such as influenza, and severe acute respiratory syndrome coronavirus-2, which can trigger an AM.42,43 Autoantibodies (eg, antinuclear antibody test) and other tests may be indicated in patients with known or possible history of autoimmune disorders.2
AM: Echocardiography
Echocardiography is part of the standard evaluation of patients with a suspected acute cardiac condition and may show a broad spectrum of findings. Even when LVEF is normal, the presence of increased wall thickness, mild segmental hypokinesia, in particular, in the inferior and inferolateral walls, diastolic dysfunction, abnormal tissue Doppler imaging, mild right ventricular dysfunction, pericardial effusion, and abnormal myocardial echogenicity may suggest AM. In the early phase, LV dimensions are generally normal even when LVEF is low or very low38—a condition that may result in severe stroke volume reduction and tachycardia. LVEF on admission is a powerful prognostic marker.13,15,38 Furthermore, cardiac function may evolve rapidly during AM, either spontaneously or after treatment.13,15
Suggested Indications for Cardiac Magnetic Resonance Imaging in AM and Chronic infl-CMP
Cardiac magnetic resonance imaging (CMRI) has emerged as a powerful noninvasive diagnostic tool for tissue characterization, including recognition and quantification of inflammation and replacement fibrosis in the setting of AM, and infl-CMP.44,45 Furthermore, CMRI is the gold standard for the quantification of biventricular volumes, ejection fraction, and cardiac mass (Table II in the Data Supplement).44 CMRI is recommended in patients with clinically suspected AM or in patients with chest pain, normal coronaries, and raised troponin, for the differential diagnosis of ischemic versus nonischemic origin,46 with the exception of those in critical condition or with usual contraindication for this diagnostic tool.2,44 CMRI should be performed in patients who initially presented with fulminant forms to assess the presence, extent, and localization of residual inflammation and replacement fibrosis when they are hemodynamically stable (Table (Table3).3). Unless recurrent flares occur, edema tends to decline 4 weeks after disease onset.47 Therefore, to rule in or rule out myocardial inflammation reliably, CMRI should be performed within 2 to 3 weeks from the onset of symptoms, although accuracy may be lower during the first days. The availability of high-sensitivity troponins and CMRI have improved the accuracy of noninvasive diagnosis of AM,44 which resulted in the identification of more low-risk patients than before, when diagnosis was mainly based on endomyocardial biopsy (EMB), which was performed more often in sicker patients. Thus, observational studies reported a more favorable prognosis of AM over the last decades (Table (Table22).13–20,28–38 In fact, in 5 of 6 studies with EMB-based diagnosis, the mean echocardiographic LVEF was <40%.20,28,29,31–33 In 2009, a consensus group published the original Lake Louise Criteria, which identified 3 hallmarks of myocardial inflammation with corresponding CMRI markers45: (1) hyperemia, that is, intense signal in early gadolinium enhancement images; (2) tissue edema, that is, increased myocardial T2 relaxation time or an increased signal intensity in T2-weighted images; and (3) necrosis/fibrosis based on late gadolinium enhancement (LGE) images. If 2 of these 3 criteria are positive, AM can be diagnosed with 74% sensitivity and 86% specificity.48 With mounting evidence that CMRI mapping increases the overall diagnostic accuracy, the Lake Louise Criteria have been recently updated (Figure II in the Data Supplement).44 The updated criteria include T2 mapping for edema and native T1, as well as extracellular volume for inflammatory injury.44 A study has confirmed an increased sensitivity of the updated criteria (87.5%) while keeping a high specificity in AM (96.2%).49 A single positive criterion can support diagnosis of myocardial inflammation if clinical suspicion is strong.44 CMRI cannot identify specific cause of myocardial inflammation, and the histological subtypes, although regional distribution of inflammatory changes in the tissue provide diagnostic clues (eg, basal septal involvement in CS). In patients with de novo DCM or unexplained ventricular arrhythmias, CMRI can suggest previous myocardial inflammation based on the regional distribution of LGE; however, its sensitivity is not high in chronic forms.50 The presence and location in the mid layer of the septum (mid-wall strip) of LGE and low LVEF at baseline appear to be the strongest negative predictors of outcome.17,51 CMRI is useful also in the follow-up of AM and is generally performed 6 to 12 months after the index event (Table (Table3).3). Disappearance of edema is frequent at follow-up (up to 84% of cases), whereas LGE generally persists (in up to 89%), although its extent is reduced from 6.2% to 4.1% of LV mass after 6 months in the Italian Multicenter Study on Acute Myocarditis registry including 187 cases.52 This finding is in keeping with other studies that analyzed the changes in LGE and edema at follow-up.16,53 The extent of LGE is a dynamic process in AM, mainly related to tissue edema in the acute phase that progressively vanishes over time, whereas in the late phase, LGE mainly reflects postinflammatory replacement fibrosis.53 Persistence of LGE and disappearance of edema are markers of unfavorable prognosis compared with complete resolution or persistence of both LGE and edema.52 A potential explanation is that persistent edema can suggest a still active process with some residual chance of recovery,52 further stressing the role of CMRI also in monitoring patients with AM and infl-CMP over time.
Table 3.
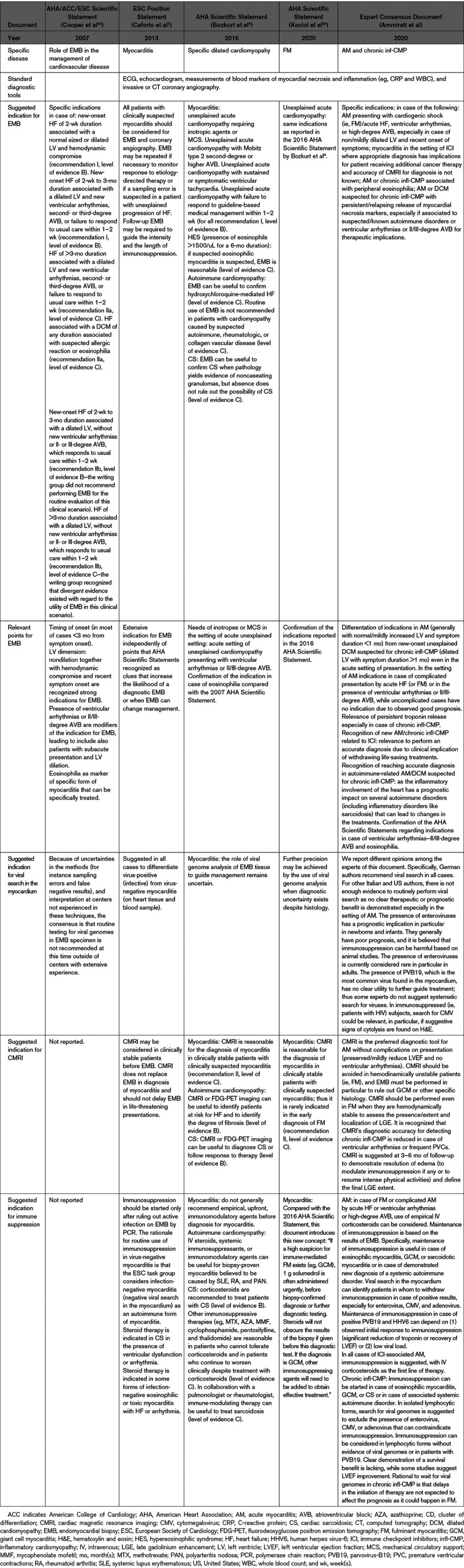
Comparison of Suggested Indications for Endomyocardial Biopsy, Viral Search, and Cardiac Magnetic Resonance Imaging Among Previous US and European Scientific Statements and Current Document
Suggested Indications for Positron Emission Tomography in AM and Chronic infl-CMP
Although positron emission tomography (PET) is not usually used in the setting of AM or chronic infl-CMP, it can be considered as an alternative noninvasive diagnostic tool in stable patients with contraindication to CMRI or in patients with suspected systemic autoimmune disease where other organs could be involved by the inflammatory process.56 PET is especially useful for the diagnosis and monitoring of CS.57,58 T cells, macrophages, or granulocytes that infiltrate the myocardium, either as a nonspecific response to cell injury or as primary lesion in CS, are characterized by an enhanced glucose metabolism that can be detected by the focal uptake of the glucose analogue 18F-fluorodeoxyglucose. PET can reveal hypermetabolic mediastinal and hilar lymph nodes differentiating CS from other autoimmune disease with cardiac involvement (eg, vasculitis). This technique provides a tool to monitor the progression of damage and its regression in response to immunosuppressive therapy.57 Recent development of additional immuno-PET tracers in oncology has dramatically expanded the usefulness of PET imaging to detect endogenous immune cells and may provide novel diagnostic and prognostication strategies in the myocarditis patients.
Suggested Indication for EMB in AM and Chronic infl-CMP
EMB is considered the reference standard for the diagnosis of myocarditis2,7; however, it is an invasive procedure that portends some risks. Cardiac complications have been reported in 1% to 2% of the patients at expert centers but in up to 8.9% at low-volume centers.59,60 The sensitivity of EMB is relatively low when evaluated with standard hematoxylin-eosin staining,61 since sampling sites do not always correspond to the distribution of inflammation. Sensitivity may be increased by increasing the number of collected specimens over the minimum recommended number (from 4 to 6 specimens).2 Immunohistochemistry-specific antibodies for leukocytes (CD45), macrophages (CD68), T cells (CD3) and their main subtypes, helper (CD4) and cytotoxic (CD8) cells, and B cells (CD19/CD20) can also increase the sensitivity of EMB.2 Quantitative criteria to improve the diagnostic yield of EMB in myocarditis include the Marburg criteria, based on the presence of >14 mononuclear leukocytes/mm2 on bioptic samples,62 with the presence of >7 T lymphocytes per mm2.63 These criteria were adopted in a position statement by the European Society of Cardiology experts.2 Despite relatively low sensitivity, the information derived from EMB is fundamental for identifying the mechanisms and deciding therapy in specific clinical scenarios both in AM and chronic infl-CMP (Table (Table33):
AM presenting with severe heart failure (HF) or cardiogenic shock (ie, FM)55
AM complicated by severe myocardial dysfunction, acute HF, ventricular arrhythmias, or high-degree atrioventricular block;
AM or suspected chronic infl-CMP associated with peripheral eosinophilia;
AM or chronic infl-CMP with persistent or relapsing release of biomarkers of myocardial necrosis, particularly if associated to a suspected/known autoimmune disorder or ventricular arrhythmias or high-degree atrioventricular block; and
Myocarditis in the setting of ICI, where appropriate diagnosis has implications for patients receiving additional cancer therapy.64
Most of these recommendations were first released in the 2007 American Heart Association/American College of Cardiology (ACC)/European Society of Cardiology Scientific Statement on the role of EMB in the management of cardiovascular disease,54 which were validated by a retrospective analysis on 851 patients with unexplained HF who underwent EMB.59 The diagnostic yield of EMB in the setting of AM is considered to be higher if performed within 2 weeks since symptom onset and in case of normal sized or mildly dilated LV or in presence of markers (ventricular arrhythmias or high-degree atrioventricular blocks) of specific subsets such as giant cell myocarditis (GCM) or CS. AM is often a self-limiting disease and can be managed noninvasively in low-risk patients.60 However, at present, EMB is largely underutilized also in the recommended settings, as shown by some reports on the use of temporary mechanical circulatory supports (MCS) in FM.65 Thus far, a relationship between the extent of inflammatory infiltrates with prognosis and its therapeutic implications have not been consistently found across different settings of inflammatory cardiac disorders. In a retrospective study, patients with acute lymphocytic myocarditis who received an MCS or died during hospitalization had more inflammatory infiltrates compared with patients who survived without MCS.35
Differential Diagnosis
Invasive coronary arteriography or computed tomography angiography are often necessary to rule out an acute coronary syndrome.2 Furthermore, patients with AM and acute pericarditis can complain of similar symptoms. Elevation of high-sensitivity troponin can steer the diagnosis toward AM. AM can be also associated with signs of pericarditis (ie, pericardial effusion on echocardiogram or CMRI; evidence of inflammation of pericardial layers on CMRI). FM should be differentiated from other conditions that may cause hypotension,55 acute myocardial dysfunction, and cardiac shock (such as septic shock, Shoshin beriberi syndrome, systemic capillary leak syndrome, and pheochromocytoma).
Chronic infl-CMP
Chronic infl-CMP can be found in patients at their first evaluation for new-onset HF symptoms or in patients with subacute/chronic HF and DCM or hypokinetic nondilated phenotype. Chronic infl-CMP may represent the evolution of ≥1 AM episodes that, either diagnosed or missed in the acute phase, caused myocardial damage and systolic dysfunction. A mild elevation of troponin out of proportion compared with LVEF impairment, associated with a dilated LV with normal or mildly increased wall thickness, can suggest a chronic infl-CMP over AM.8 Accordingly, CMRI and EMB may show less florid inflammation, and replacement fibrosis may prevail. Furthermore, cardiomyocytes with morphological abnormalities may be found at histology (Figure (Figure1;1; Figure I in the Data Supplement). Due to progressive LV remodeling, patients presenting with a chronic infl-CMP have chronic HF symptoms (generally >1 month) but may be hemodynamically stable and are treated as patients with DCM.9 Anamnestic clues, subtle electrocardiographic alterations (such as low voltage or fragmentation of QRS in peripheral leads, minor conduction disturbances, and nonspecific ST-T abnormalities), low-grade, persistent elevation of troponin, and failure to respond to standard HF treatment should promote the search for an inflammatory cause.9 In chronic infl-CMP, the presence of high number of T lymphocytes or macrophages on EMB has a unique value in predicting an increased risk of mortality or transplantation over the next decade.66 The extent of myocardial fibrosis should be reported as it could be related to the likelihood of recovery. Finally, the additional analysis to search for cardiotropic viruses (eg, RNA enteroviruses and DNA adenoviruses), bacteria, and parasites in biopsy specimens using quantitative real-time polymerase chain reaction is recommended by the European Society of Cardiology experts to guide immunosuppressive therapy in the setting of chronic infl-CMP.2,67 Conversely, American Heart Association experts do not recommend routine viral genome analysis outside of centers with experience,9 even if they consider it as an additional option when diagnostic uncertainty exists.55
Virus-Induced and Immune-Mediated Lymphocytic AM and infl-CMP
Lymphocytic AM and chronic infl-CMP have been attributed to a variety of pathogens, mainly viruses (by direct virus-mediated or indirect immune-mediated myocardial injury), toxic effect of drugs or radiation, and autoimmune injury in the setting of systemic inflammatory disorders.
Virus-induced AM can refer to both virus-mediated myocarditis and virus-triggered myocarditis. Enteroviral (coxsackievirus) myocarditis are examples of virus-mediated AM, as viral replication can cause direct cardiomyocyte injury.68 The cases of enterovirus-mediated AM have been mainly reported in newborns and infants in recent years.69 Respiratory viruses, such as influenza and coronaviruses, are examples of common viruses that can trigger an immune-mediated lymphocytic myocarditis in the absence of viral genome in the myocardium.42,70 In virus-triggered AM, molecular mimicry between viral and cardiac antigens, which can result in autoreactive T-cell infiltration in the myocardium in predisposed individuals, is suspected to be the underlying mechanism of myocardial injury.22 The resolution of the viral syndrome with the extinction of viral antigens could explain the frequent self-resolving natural history of most AMs. Of note, PVB19 appears to cause both virus-mediated and virus-triggered myocarditis. In children in particular, PVB19 may cause a systemic infection associated with AM where PVB19 can be detected both in plasma and myocardium. In adults, PVB19 has been associated with both AM and chronic infl-CMP,71 and the viral genome has been detected with different titers in the myocardium of these subjects, while it is not generally detected in the bloodstream. PVB19 has been the only virus found in patients with lymphocytic FM in an international registry.25,38 A preliminary report showing a benefit from immunosuppression in chronic infl-CMP with PVB19 presence in the myocardium seems to support the hypothesis that the immune response plays a role in the development of myocardial inflammation after viral infection.72 Alternatively, low copy number of PVB19 DNA may reflect latent infection and should be interpreted as a bystander, since they can be found also in normal myocardium.73 These findings may suggest that immunosuppression is not contraindicated in all virus-positive myocarditis, but the involved virus (eg, it may be considered with PVB19 but not with coxsackievirus), the host (eg, infants versus adults or immunodeficient versus immunocompetent individuals), and the setting (noncomplicated versus FM) should be considered in the decision to start immunosuppressive drugs. According to several researchers, high viral loads and replicating (versus nonreplicating) viruses could stand against the use of immunosuppression and possibly in favor of treatment with antiviral drugs or with agents that reinforce native immune response, for example, interferon-β,74 even if clinical data from trials are substantially lacking in the setting of AM. Similarly to PVB19, human herpes virus-6 has been occasionally found in the myocardium of patients with AM and chronic infl-CMP, but its pathogenetic role is unclear.19
Immune-Mediated AM and Chronic infl-CMP
AM or chronic infl-CMP can be associated with systemic autoimmune disorders (eg, systemic lupus erythematosus or dermatomyositis) or organ/system-specific autoimmune/inflammatory diseases (eg, inflammatory bowel disorders). The immune system activation stimulated by an intercurrent infection could favor a flare of the underlying immune disorder involving the heart. The identification of the myocarditis-associated condition is relevant for the specific treatments, given that not infrequently AM is the first manifestation of a systemic inflammatory/autoimmune disease.39 The Lombardy registry of AM reported that 7.2% of patients had associated autoimmune or systemic disorders, and this condition was more frequent in patients with complicated presentation (15.4%).13
ICI-Associated Myocarditis
ICIs have transformed cancer treatment, with regulatory approval in ≈20 different cancer types. The percentage of patients with cancer who were eligible for ICI increased from 1.5% in 2011 to ≈50% in 2020.75 These agents include monoclonal antibodies, which block immune brakes or regulators, termed CTLA-4 (cytotoxic T-lymphocyte antigen-4), PD-1 (programmed death receptor-1), and its ligand (PD-L1 [programmed death-ligand 1]) that, when stimulated, can dampen the immune response to an immunologic stimulus. By blocking these checkpoints from binding with their partner proteins, ICIs inhibit the off signal, activating T cells and promoting killing of cancer cells. By activating the immune system, ICI can also lead to immune-mediated adverse events (such as colitis, dermatitis, and pneumonitis).76
Etiology and Pathogenesis
In 2016, Johnson et al77 described 2 cases of fatal FM after treatment with ICI. These patients presented with refractory electrophysiological disturbances and concomitant myositis, with pathology indicating T-cell and macrophage-dependent myocardial infiltration. Other case series of ICI-associated AM reported an incidence between 1% and 2% when ICIs are used in combination.21,78 Preclinical data suggest a critical role for CTLA-4 and PD-1 in the cross talk between the cardiovascular and immune systems. Inhibition of CTLA-4 and PD-1, either genetically or pharmacologically, was found to contrast ICI-associated myocarditis and other cardiovascular toxicities in mice.79
Diagnosis
The largest case series of 122 patients with ICI-associated myocarditis had an early onset of symptoms (median of 30 days after initial exposure to ICI), and up to 50% died.40 The increasing number of reports in the past few years is consistent with the growing awareness of this new clinical syndrome, as well as with the more widespread use of ICI. Patients on combination ICI treatment (eg, ipilimumab and nivolumab) should have an ECG and troponin assay at baseline. Once started on therapy, troponin should be checked weekly during the first 6 weeks. In addition, given concomitant myositis in a substantial number of cases of ICI-associated myocarditis, a defined workup for myositis (including checking for creatine kinase [CK] and possibly skeletal muscle biopsy) is recommended in suspected cases.
Treatment
High-dose intravenous corticosteroids associated with withdrawal of ICI are considered the first-line therapy, although mortality remains high. Alemtuzumab (anti-CD52 antibody), antithymocyte globulin (anti-CD3 antibody), and abatacept (a CTLA-4 agonist) have been proposed as second-line therapy (Table (Table33).100–102 A better mechanistic understanding of ICI-associated cardiovascular toxicity by using preclinical models could help for defining preventive and treatment strategies in patients.
Eosinophilic Myocarditis
EM is relatively uncommon, but it is often unrecognized; thus its incidence may be underestimated. The rate of death or HTx in patients with EM and a fulminant presentation was over 26% at 60 days after admission.38
Etiology and Pathogenesis
EM is generally associated with hypersensitivity reactions to chemicals (in particular, clozapine, carbamazepine, minocycline, and β-lactam antibiotics and occasionally vaccination) or with systemic conditions such as eosinophilic granulomatosis with polyangiitis (EGPA; former Churg-Strauss syndrome) or hypereosinophilic syndrome (HES; idiopathic or clonal) or with a parasitic infection, mainly due to Toxocara canis transmitted by raw meat.39 In rare circumstances, EM can be associated with solid-organ malignancy as a paraneoplastic event (ie, lung cancer). A 3-phase process of the eosinophilic injury has been proposed: an initial inflammatory/necrotic phase (observed during AM), followed by thrombotic and fibrotic remodeling of the endomyocardium (typical of Loeffler cardiomyopathy; Figure Figure44).

Eosinophilic myocardial injury: associated conditions and transition from acute myocarditis to inflammatory cardiomyopathy. A, Eosinophilic myocarditis can be idiopathic or associated with a systemic disorder. The associated conditions can be (1) eosinophilic granulomatosis with polyangiitis (EGPA), which is often associated with asthma, pulmonary nonfixed infiltrates (arrows on a chest computed tomographic scan image in the yellow inset), and paranasal sinus abnormalities; (2) hypereosinophilic syndromes (HES) characterized by persistent peripheral eosinophilia (≥1.5×109/L for over 6 mo), which can be a complex idiopathic form or a myeloproliferative variant like the clonal form associated with FIP1L1/PDGFRA fusion gene; (3) parasitic infections; (4) hypersensitivity reactions to drugs and drug reaction with eosinophilia and systemic symptoms (DRESS) that are generally characterized by fever and diffuse skin rush (like in the patient with DRESS showed in the rose inlet), with frequent delay onset after drug initiation (up to 2–6 wk); and rarely, (5) solid tumors. While in the acute phase, the eosinophilic myocarditis is the main determinant of prognosis, the associated conditions can be the major determinants of prognosis in the mid and long term. B, An acute intense exposure to eosinophilia can cause an acute eosinophilic myocarditis (left), which in some case can be described as necrotizing due to extensive areas of the cardiomyocyte necrosis (*) caused by diffuse eosinophilic infiltrates on endomyocardial biopsy histology. The acute inflammatory phase can cause subendocardial and transmural injuries, identified by late gadolinium enhancement on cardiac magnetic resonance imaging (CMRI). If the eosinophilic exposure persists, eosinophilic injury evolves to a thrombotic and fibrotic stage, with diffuse subendocardial fibrosis with apical thrombi (green arrows), as identified by CMRI; the latter are characteristic features of Loeffler cardiomyopathy (right).
Diagnosis
EM may affect middle-aged individuals, with similar prevalence in both sexes, presenting mainly with chest pain and dyspnea, with evidence of LV systolic dysfunction. The diversity of possible underlying causes may be responsible of the variety of clinical scenarios; specifically, fever and skin rash are more common in hypersensitivity-related EM and asthma in EGPA-related EM.39 Eosinophilia can be evident in the course of the disease,80 but it is absent in about 25% of the patients at admission.39 Echocardiography and CMRI provide information on cardiac function and may detect intracardiac thrombosis (particularly at the LV apex), mostly described in HES-related EM (up to 29% of cases) and EGPA-related EM (up to 19% of cases).39 In contrast with the typical subepicardial LGE pattern observed in other forms of myocarditis, EM is generally associated with subendocardial LGE.39
Treatment
A meta-analysis of 179 cases has shown a lower incidence of in-hospital mortality with the use of corticosteroids, although randomized trials are lacking.39 Identification and treatment of the underlying causes should be promptly considered. In particular, immediate withdrawal of the offending substance in combination with corticosteroid administration is recommended in hypersensitivity-related EM. Albendazole and corticosteroids should be given in EM associated with Toxocara canis infection,39 and imatinib is utilized in myeloproliferative variants of HES. Combined immunosuppressive therapy, including corticosteroids and cyclophosphamide, azathioprine, or methotrexate, may be considered in EM associated with EGPA and HES.39 The rate of recurrence is not known, but fatal recurrences have been reported.39 Patients with HES and EGPA are at increased risk of late recurrence, in particular if immunosuppressive agents are withdrawn.
Giant Cell Myocarditis
GCM is a form of rapidly progressing necrotizing myocarditis with a poor prognosis including an ≈85% rate of death or HTx at 3 years.38,81 GCM is responsible for ≈1 in 200 cases of myocarditis and ≈10% of all FM.
Etiology and Pathogenesis
GCM is characterized by myocardial destruction mediated by a large number of cytotoxic T cells, macrophages, giant cells, and eosinophils. This leads to LV dysfunction and ventricular arrhythmias. Associated autoimmune disorders, in particular, inflammatory bowel diseases and thyroid disorders, have been reported in ≈20% of cases.81
Diagnosis
GCM affects equally men and women. Median age at onset is between 43 and 53 years, higher than observed in lymphocytic myocarditis.38,81 GCM frequently presents as acute HF or cardiogenic shock and with ventricular tachycardia or complete atrioventricular block.82 EMB is generally the first diagnostic tool. GCM shares some histological features with CS; therefore, the differential diagnosis can be challenging.82
Treatment
Immunosuppressive therapy should be initiated promptly. Treatment with anti–T-lymphocyte–based (ie, antithymocyte globulin) and calcineurin inhibitor therapy can lead to clinical remission in up to two-thirds of patients, in particular, in those not requiring MCS.83 The initial approach may vary based on the clinical presentation. In case of FM, antithymocyte globulin associated with pulse high dose of corticosteroids is preferred, and cyclosporine is titrated to trough levels of 150 to 250 ng/L a few days after the administration of antithymocyte globulin (Table (Table44).84,94–96 There is a variable rate of LVEF recovery without transplant, among published series.38,81,83,85 Dosage of oral corticosteroids after the acute phase is 1 mg/kg in the first months with subsequent slow tapering over 1 year, while cyclosporine is generally maintained >2 years, with a target plasma through level of 80 to 100 ng/L. Azathioprine at 1 to 2 mg/kg per die divided into 2 daily doses or mycophenolate mofetil (500–1000 mg BID) can be added. There are anecdotal but consistent data suggesting that discontinuation of immunosuppression after 1 year of treatment may be followed by relapse and death.84 GCM patients have a high risk of ventricular tachycardia, and placement of an implantable cardiac defibrillator (ICD) is generally recommended in all patients including those with full recovery of LVEF.86 Compared with historical results, current combined immunosuppressive treatment suggests an improvement in transplant-free survival from 11% to 55% at 1 year.83,85 HTx is an effective therapy, with similar posttransplant survival in patients with GCM as in those with other causes. Nevertheless, recurrence of GCM on the transplanted hearts and a higher rate of early cellular rejection have been reported.87
Table 4.
Immunosuppressive Treatment Used for Fulminant myocarditis or Acute Myocarditis Complicated by Severe Heart Failure Not Supported by Evidences From Clinical Trials

Sarcoidotic Myocarditis
Sarcoidosis is a worldwide disease with a prevalence of about 4.7 to 64 in 100 000; the highest rates are reported in Northern European and African American individuals, particularly in women.57
000; the highest rates are reported in Northern European and African American individuals, particularly in women.57
Etiology and Pathogenesis
Sarcoidosis is a multisystem, granulomatous disease of unknown etiology. Accumulating evidence suggests an immunologic response to an unidentified antigenic trigger in genetically susceptible individuals. Organ involvement is variable, but most patients have pulmonary and lymph node involvement.57 Clinically manifest cardiac involvement occurs in about 5% of patients with pulmonary/systemic sarcoidosis.57 Sarcoidotic myocarditis is characterized by infiltration by activated macrophages, which in some cases can lead to chronic inflammation and fibrotic replacement with non-necrotizing granulomas. Eosinophils and necrosis are rare or absent.82 The macrophages within sarcoid granulomas tend to become epithelioid and form multinucleated giant cells.
Diagnosis
Most cases occur in patients 25 to 60 years of age. The 3 principal manifestations of CS are conduction abnormalities, ventricular arrhythmias, and HF.57 There is a growing awareness that CS can be the first manifestation of sarcoidosis in any organ.108 For example, between 16% and 35% of patients presenting with complete atrioventricular block (<60 years of age) or ventricular tachycardia of unknown etiology had previously undiagnosed CS as the underlying etiology.108 The ventricular septum and LV basal free wall are most commonly affected. EMB has only 20% to 30% sensitivity, 57 if not imaging guided.109 Experts’ position statements propose criteria to reach diagnosis of CS that are mainly based on positive histology in the heart or extracardiac histological evidence of sarcoidosis plus demonstration of cardiac involvement based on imaging (Table III in the Data Supplement).57
Treatment
Corticosteroids therapy is advocated for the treatment of CS by most experts. It is unknown whether all patients with CS should be treated or only those with clinical manifestations of the disease.57 Optimal doses of corticosteroids and how best to assess response to therapy is unknown. Methotrexate is often used as a second-line agent in refractory cases or if there are significant steroid side effects. Other therapies that have been used in CS include azathioprine, cyclophosphamide, infliximab,57 and rarely rituximab (Table (Table44).104–107
Patients with CS are at risk of SCD, and there are limited data to help with risk stratification (Table III in the Data Supplement). In a recent Finnish nationwide study, 10-year survival was 92.5% in 102 patients.110 Notably, CS can recur in transplanted hearts.105 Key unresolved questions related to treatment are whether we should treat clinically silent CS and which drugs should be first- and second-line therapies for CS.
Specific Treatments
Patients with AM or chronic infl-CMP associated with autoimmune disorders are treated according to indications regarding the systemic condition. Corticosteroids are generally the cornerstone of therapy, frequently in combination with another agent. During the acute phase, drugs with a rapid onset of action such as intravenous immunoglobulin, cyclophosphamide, and rituximab may be preferred, while for maintenance therapy, mycophenolate mofetil, methotrexate, and azathioprine may be used to allow tapering of corticosteroids over time. Plasmapheresis is occasionally used in the acute setting, for instance in AM associated with antiphospholipid syndromes. Excluding AM associated with systemic inflammatory conditions, no specific evidence-based treatments are available for lymphocytic AM. Only one trial is currently recruiting patients with AM (https://www.clinicaltrials.gov; unique identifier: NCT03018834), testing the efficacy of anakinra. AM is often a self-limiting disease, and spontaneous recovery of myocardial dysfunction may occur. There is a rationale for using immunosuppressive treatments in high-risk AM, but no trial has tested this hypothesis in the acute phase. Thus, there are no specific recommendations for therapy in the acute phase beyond standard therapy for LV dysfunction and acute HF. The only study that assessed the efficacy of immunosuppression in AM, the MTT (Myocarditis Treatment Trial), reported no benefit from immunosuppression.29 However, the initiation of treatment was delayed, since patients were enrolled between 2 weeks and 1 year from symptom onset. Almost all studies with corticosteroids focused on chronic infl-CMP with 6-month history of HF symptoms. An improvement of cardiac function has been observed, but most studies were inadequately powered,67 and there was no improvement in survival.111 The single-center Tailored IMmunosuppression in Inflammatory Cardiomyopathy trial that randomized 85 patients with virus-negative chronic infl-CMP at a 6-month course of prednisone plus azathioprine or standard HF medications only showed a significant improvement of symptoms and echocardiographic parameters (median LVEF from 27% to 46% after 6 months) in the prednisone and azathioprine group. Given these findings, a large randomized trial is needed to assess the benefit and risk of long-term immunosuppression. Furthermore, few data exist supporting treatments for patients with virus-positive chronic infl-CMP. Usual HF treatments are recommended in those patients with chronic inf-CMP or AM with reduced LVEF and stable hemodynamics. β-Blockers are often used after an AM also in patients with uncomplicated presentation (53.8% based on the Lombardy registry of AM),13 probably due to the perceived protection against arrhythmic events. Finally concerning the prevention of SCD at discharge, patients with infl-CMP follow the general indication for ICD, with the abovementioned exceptions concerning GCM and CS. In patients with AM, a multiparametric stratification of risk is reasonable for decision on ICD implantation. It may include family history of SCD or arrhythmogenic cardiomyopathy, ventricular tachycardia on presentation,112 presence and septal localization of LGE on CMRI,14,17 and histology compatible with CS or GCM. Currently, ICD is rarely implanted after an AM with preserved LVEF (2% in the Lombardy registry13 and 1.6% in the ITAMY registry). The cumulative percentage of SCD, resuscitated cardiac arrest, and appropriate ICD shock was 2.1% at 4.3 years of follow-up in the ITAMY registry.14
MCS and HTx
Patients with AM complicated by refractory HF or cardiogenic shock require inotropic agents or MCS.55 Myocarditis is often a reversible condition; thus temporary devices such as intra-aortic balloon pumps, venoarterial extracorporeal membrane oxygenator, rotary pumps, or intra-aortic axial pumps should be considered first. Observational studies and multicenter registries report a short-term transplant-free survival of 55% to 80% in patients with FM who received temporary MCS.113,114 An analysis of trends in myocarditis incidence and management in the United States between 2005 and 2014115 has reported a growing rate of use of any temporary MCS, from 4.5% to 8.6%, with a significant trend for all devices except intra-aortic balloon pump, which anyway was the most frequently used support (3.8% overall). In theory, the use of devices that reduce LV afterload, such as centrifugal pumps or intra-aortic axial pumps, alone or in combination with venoarterial extracorporeal membrane, could favor myocardial recovery more than venoarterial extracorporeal membrane alone, through both hemodynamic and anti-inflammatory mechanisms.116 Nonetheless, a multicenter registry on intra-aortic axial pump use for FM (34 patients from 2009 to 2016) showed a survival to discharge of 62%,117 not different from the 61% discharge rate reported among 185 patients supported with venoarterial extracorporeal membrane in Taiwan from 2001 to 2011.118 If there is no weaning from MCS after 2 to 3 weeks, long-term LV assist device or urgent HTx may be considered.
Knowledge Gaps and Perspective
Critical knowledge gaps exist regarding diagnosis, prognostication, and treatment of AM and chronic infl-CMP, which need to be addressed. Though EMB is the gold reference for diagnosis, it is not available or is underperformed in most hospitals60 and has a relatively low sensitivity using conventional histology. Hence, novel sensitive and specific biomarkers and imaging modalities are needed. The advent of novel technologies developed in the immuno-oncology space (eg, single-cell RNA sequencing, mass cytometry, high-frequency and deeper T-cell receptor sequencing, multiplex immunofluorescence, and other technologies) should become novel research strategies and further advance the usefulness of tissue analysis. Prospective large interventional trials or registries in the field of AM and chronic infl-CMP could help standardize the diagnostic and therapeutic approaches, which currently vary widely. Prospective registries aimed at identifying low- versus high-risk patients at the time of hospitalization and to refine and characterize the risk for specific events beyond death or HTx (eg, recurrence, evolution to DCM, and arrhythmias) at discharge and during follow-up are needed. Finally, a common terminology to describe cases of AM and infl-CMP, and shared clinical pathways for patient management, could increase our knowledge on this condition, potentially improving patient outcome.
Acknowledgments
Drs Ammirati and Frigerio acknowledge the Fondazione Centro Cardiologia e Cardiochirurgia A. De Gasperis, Niguarda Hospital, Milano, Italy, for its research support. Dr Basso is supported by the Registry of Cardio-Cerebral-Vascular Pathology, Veneto Region, Italy. Dr Tschöpe acknowledges the Federal Ministry of Education and Research, Germany, for the Cortisone in Parvovirus Inflammatory Cardiomyopathy program and the German Centre for Cardiovascular Research for the voltage mapping–guided and magnetic resonance imaging–guided endomyocardial biopsy in myocarditis and inflammatory dilated cardiomyopathy study.
Disclosures
Dr Adler is a consultant for Abbott, Abiomed, AstraZeneca, Endotronix, Ionis, Medtronic, and Novartis, is on the board of directors of Genstem Therapeutics, and is a shareholder of Rocket Pharmaceuticals. Dr Brambatti is an employee at Ionis Pharmaceuticals. Dr Moslehi has served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron and has been supported by National Institutes of Health grants R56 HL141466 and R01 HL141466. Dr Tschöpe is a consultant at Cardiotropic Labs, Miami, FL. Dr Camici is a consultant at Servier. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- ACC
- American College of Cardiology
- AM
- acute myocarditis
- CMRI
- cardiac magnetic resonance imaging
- CS
- cardiac sarcoidosis
- CTLA-4
- cytotoxic T-lymphocyte antigen-4
- DCM
- dilated cardiomyopathy
- EGPA
- eosinophilic granulomatosis with polyangiitis
- EM
- eosinophilic myocarditis
- EMB
- endomyocardial biopsy
- FM
- fulminant myocarditis
- GCM
- giant cell myocarditis
- HES
- hypereosinophilic syndrome
- HF
- heart failure
- HTx
- heart transplantation
- ICD
- implantable cardiac defibrillator
- ICI
- immune checkpoint inhibitor
- infl-CMP
- inflammatory cardiomyopathy
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- LVEF
- left ventricular ejection fraction
- MCS
- mechanical circulatory support
- MTT
- Myocarditis Treatment Trial
- PD-1
- programmed death receptor-1
- PD-L1
- programmed death-ligand 1
- PET
- positron emission tomography
- PVB19
- parvovirus-B19
- SCD
- sudden cardiac death
*Drs Ammirati and Frigerio contributed equally to this work.
†Drs Cooper and Camici contributed equally to this work as senior authors.
This manuscript was sent to Kenneth B. Margulies, MD, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.120.007405.
For Sources of Funding and Disclosures, see page 683.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circheartfailure.120.007405
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCHEARTFAILURE.120.007405
Citations & impact
Impact metrics
Article citations
The Role of MicroRNA in the Pathophysiology and Diagnosis of Viral Myocarditis.
Int J Mol Sci, 25(20):10933, 11 Oct 2024
Cited by: 0 articles | PMID: 39456716 | PMCID: PMC11507602
Review Free full text in Europe PMC
Cardiac MRI in infarct-like myocarditis: transmural extension of late gadolinium enhancement is associated with worse outcomes.
Insights Imaging, 15(1):246, 11 Oct 2024
Cited by: 0 articles | PMID: 39392565 | PMCID: PMC11469985
Return-to-Play Post-Myocarditis for Athletes: To Play or Not to Play?
Diagnostics (Basel), 14(19):2236, 07 Oct 2024
Cited by: 0 articles | PMID: 39410640 | PMCID: PMC11475062
Review Free full text in Europe PMC
Emerging Biomarkers in Cardiac Sarcoidosis and Other Inflammatory Cardiomyopathies.
Curr Heart Fail Rep, 21(6):570-579, 04 Oct 2024
Cited by: 0 articles | PMID: 39365404 | PMCID: PMC11511729
Review Free full text in Europe PMC
Myocarditis: Differences in Clinical Expression between Patients with ST-Segment Elevation in Electrocardiogram vs. Patients without ST-Segment Elevation.
J Pers Med, 14(10):1057, 13 Oct 2024
Cited by: 0 articles | PMID: 39452564 | PMCID: PMC11508978
Go to all (251) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT03018834
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[ANMCO/SIC Consensus document on the management of myocarditis].
G Ital Cardiol (Rome), 21(12):969-989, 01 Dec 2020
Cited by: 2 articles | PMID: 33231216
Diagnosis and treatment of myocarditis and inflammatory cardiomyopathy. Consensus document of the SEC-Working Group on Myocarditis.
Rev Esp Cardiol (Engl Ed), 77(8):667-679, 17 May 2024
Cited by: 0 articles | PMID: 38763214
Review
[Indication for myocardial biopsy in myocarditis and dilated cardiomyopathy].
Med Klin (Munich), 100(9):553-561, 01 Sep 2005
Cited by: 3 articles | PMID: 16170644
Review
[Endomyocardial biopsy should be performed in every patient with suspected myocarditis].
G Ital Cardiol (Rome), 16(10):533-538, 01 Oct 2015
Cited by: 1 article | PMID: 26444210
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL141466
Grant ID: R01 HL155990

 1,*
1,* 



