Abstract
Free full text

Regulatory T cell-derived TGF-β1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity
Summary
The mechanisms by which regulatory T (Treg) cells differentially control allergic and autoimmune responses remain unclear. We show that Treg cells in food allergy (FA) had decreased expression of transforming growth factor beta 1 (TGF-β1) due to interleukin-4 (IL-4) and signal transducer and activator of transciription-6 (STAT6)-dependent inhibition of Tgfb1 transcription. These changes were modelled by Treg cell-specific Tgfb1 monoallelic inactivation, which induced allergic dysregulation by impairing microbiota-dependent Retinoic acid receptor-related orphan receptor gamma t (ROR-γt)+ Treg cell differentiation. This dysregulation was rescued by treatment with Clostridiales species, which upregulated Tgfb1 expression in Treg cells. Biallelic deficiency precipitated fatal autoimmunity, with intense autoantibody production and dysregulated T follicular helper and B cell responses. These results identify a privileged role for Treg cell-derived TGF-β1 in regulating allergy and autoimmunity at distinct checkpoints in a Tgfb1 gene dose and microbiota-dependent manner.
Introduction
The incidence of immune dysregulatory diseases, including allergic and autoimmune diseases, has increased in recent decades, reflecting altered environmental influences (Bach, 2002, 2018; Platts-Mills, 2015). A key mechanism by which these diseases develop involves perturbation of immune tolerance mediated by regulatory T (Treg) cells (Grant et al., 2015; Noval Rivas and Chatila, 2016). Of the several mechanisms implicated in Treg cell-mediated enforcement of peripheral immune tolerance, the role of Treg cell-derived transforming growth factor-β (TGF-β) remains enigmatic given the broad expression of TGF-β species among different immune and non-immune cells (Travis and Sheppard, 2014). In particular, whereas TGF-β has been implicated in immunosuppression by Treg cells (Cuende et al., 2015; Li et al., 2007; Marie et al., 2005), other studies have indicated that Treg cell-derived TGF-β1, the major TGF-β species expressed by T cells, is largely redundant in immune regulation (Edwards et al., 2016; Gutcher et al., 2011). In this study, we demonstrate that despite being abundantly available from other cell sources, TGF-β1 derived from Treg cells fulfils non-redundant functions in controlling allergic and autoimmune responses by distinct mechanisms segregated by Treg cell Tgfb1 gene dose and microbiota dependency.
Results
T helper-2 (Th2) cell-like reprogramming of Treg cells promotes allergic dysregulation by suppressing Tgfb1 transcription.
Oral tolerance is dependent on the differentiation of Foxp3+Helios− induced Treg (iTreg) cells from naïve CD4+Foxp3− T cells (CD4+ Teff) upon their activation by antigen-presenting CD103+ dendritic cells (DCs) in the presence of TGF-β1 (Apostolou and von Boehmer, 2004; Esterhazy et al., 2016; Haribhai et al., 2009; Mucida et al., 2005). In FA, allergen-specific Treg cells undergo Th2 cell-like reprogramming, which disables their regulatory function and promotes disease (Noval Rivas et al., 2015). Interruption of this process by deletion of Stat6 or Treg cell-specific Il4 and Il13 restores Treg cell regulation and suppresses FA (Abdel-Gadir et al., 2018; Noval Rivas et al., 2015). We employed FA-prone mice that carry a gain of function mutation in the IL-4Rα chain gene (Il4raF709) to investigate the mechanisms by which FA develops in these mice (Noval Rivas et al., 2015; Tachdjian et al., 2010). Epigenetic analysis of mesenteric lymph node Treg cells isolated from Il4raF709 mice using Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) revealed decreased access at the Tgfb1 locus, which was reversed by deletion of Stat6 (Fig. 1A). Chromatin immunoprecipitation (ChIP) studies demonstrated increased binding of STAT6 to the Tgfb1 promoter of Il4raF709 Treg cells in association with increased H3K4me1 but not H3K27me3, a histone marker configuration indicative of an active enhancer state (Fig. 1B) (Heintzman et al., 2007).
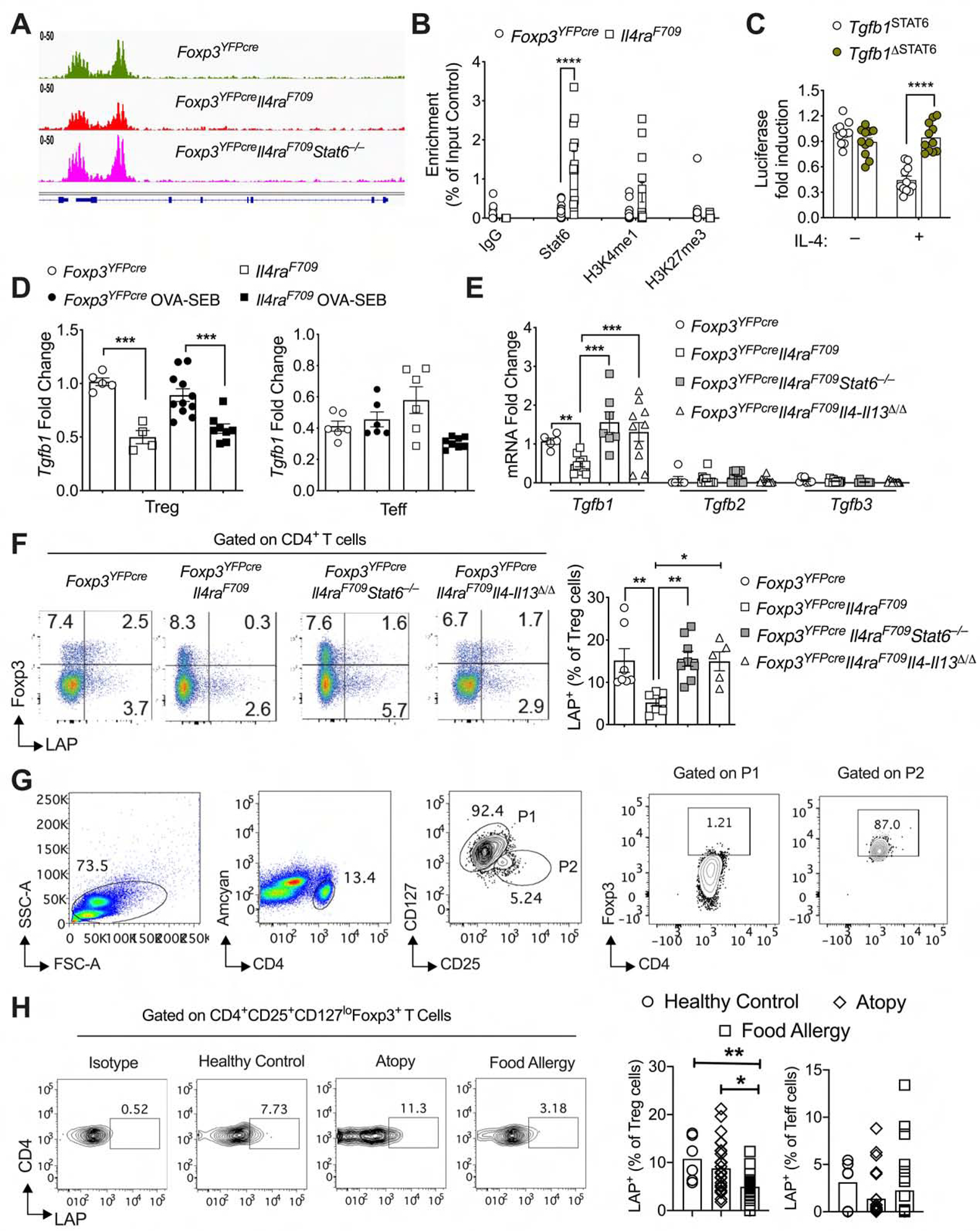
(A) ATAC-seq analysis of the Tgfb1 locus in Treg cells of the respective genotypes. (B) ChIP assays for the binding of STAT6, H3K4me1, H3K27me3 and control (IgG) antibodies to the Tgfb1 promoter in Treg cells of Foxp3YFPcre mice. (C) Luciferase reporter assays for Tgfb1 promoters with or without STAT6 binding site treated as indicated (D) RT-PCR of Tgfb1 transcripts in splenic Treg and Teff cells sorted from Foxp3YFPcre and Foxp3YFPcreIl4raF709 sham and OVA-SEB sensitized mice. Transcript expression in CD4+ Teff cells is also shown. (E) Tgfb1–3 transcripts in Foxp3YFPcre, Foxp3YFPcreIl4raF709, Foxp3YFPcreIl4raF709 Il4-l13Δ/Δ and Foxp3YFPcreIl4raF709Stat6−/− splenic Treg cells, the latter either sufficient of deficient in Il4-l13 or Stat6. (F) Flow cytometric analysis and frequencies of LAP+ staining in Treg cells sorted from the respective mouse strains. (G) Fow cytometry (FACS) plots showing the gating strategy for human Treg and Teff cells. (H) LAP staining on Treg cells isolated from PBMCs of FA, atopics without FA and healthy subjects. Each symbol represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: Student’s t-test (B), two-way ANOVA (C); One-way ANOVA with Dunnett’s post hoc analysis (D, E, F, G). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of two or three independent experiments. n=5–13 mice per group (B), 11 replicates per group (C), 4–14 mice per group for panels (D, E, F), and 6 to 26 subjects for panel G. Please also see Figure S1.
Consistent with a transcriptional suppression mechanism of the Tgfb1 promoter by STAT6, luciferase reporter assays revealed that treatment with IL-4 decreased Tgfb1 promoter activity by about 50%, and that inactivation of a candidate STAT6 binding element within the promoter reversed that inhibition (Fig. 1C). In agreement with these findings, expression of Tgfb1 transcripts was decreased by 50% in the Treg but not the Teff cells of Il4raF709 mice as compared to Il4ra wild-type mice. This decrease was normalized upon deletion of Stat6 or Treg cell-specific deletion of Il4 and Il13 using a yellow fluorescent protein (YFP)-Cre recombinase fusion protein encoded by the endogenous Foxp3 locus (Foxp3YFPcre) (Fig. 1, ,DD and andE)E) (Rubtsov et al., 2008). In contrast, there was minimal expression of Tgfb2 and Tgfb3 in the Treg cells, which was not affected by Stat6 or Il4 and Il13 deletion (Fig. 1E). Expression of the TGF-β1 precursor latency associated protein (LAP) was also decreased on Treg cells of Il4raF709 mice as compared to control mice but normalized upon deletion of Il4-Il13 or Stat6 (Fig. 1F). Importantly, subjects with FA, whose circulating Treg cells (CD4+CD25+CD127loFOXP3+; see gating strategy in Fig. 1G) exhibit Th2 cell-like reprograming (Noval Rivas et al., 2015), had decreased LAP staining of their Treg cells as compared to non-FA atopic or non-atopic controls (Fig. 1G, ,H;H; See Table S1 for subject demographics). Overall, these results established that FA-promoting Th2 cell-like reprogramming of Treg cells is associated with decreased LAP expression in mice and human subjects with FA.
A direct role for decreased Tgfb1 expression in Treg cells in promoting FA was corroborated by over-expressing a Foxp3YFPcre-regulated Tgfb1 transgene (Tgfb1Tg) in Treg cells of Il4raF709 mice (Hall et al., 2010). Analysis of Il4raF709Foxp3YFPcreTgfb1Tg mice revealed that transgene expression upregulated Tgfb1 transcripts and LAP surface expression in Il4raF709 Treg cells but not in CD4+ Teff cells (Fig. S1). Whereas oral sensitization of Il4raF709 mice with chicken egg ovalbumin (OVA) mixed with the mucosal adjuvant staphylococcal enterotoxin B (OVA/SEB) followed by oral challenge with OVA resulted in profound anaphylaxis, Il4raF709Foxp3YFPcreTgfb1Tg mice were completely protected. Tgfb1 transgene expression in Treg cells of Il4raF709 mice completely suppressed the elevation in total and OVA-specific IgE antibodies induced by oral allergic sensitization. It also suppressed the mast cell degranulation following oral challenge with OVA and the CD4+ Teff Th2 cell response (Fig. S1). These results indicated that the Th2 cell-like reprogramming in Treg cells suppresses Tgfb1 transcription to promote FA.
Treg cell-specific Tgfb1 haploinsufficiency promotes allergic dysregulation.
To further determine the role of decreased Treg cell-derived TGF-β1 in mediating FA, we employed mice heterozygous for a single floxed Tgfb1 allele crossed with Foxp3YFPcre (Fig. 2A). Oral sensitization of Foxp3YFPcreTgfb1Δ/+ mice but not Foxp3YFPcre mice with OVA/SEB followed by oral challenge with OVA resulted in anaphylaxis, as monitored by the drop in core body temperature. Total serum IgE concentrations were elevated in Foxp3YFPcreTgfb1Δ/+ mice at baseline, and OVA-specific IgE responses rose sharply post-sensitization, indicative of allergic dysregulation (Fig. 2B). Mast cell degranulation, as evidenced by increased serum mouse mast cell protease-1 (MMCP-1), was increased in sensitized Foxp3YFPcreTgfb1Δ/+ mice following oral challenge with OVA, in association with exaggerated gut tissue mastocytosis, impaired differentiation of iTreg cells and increased Th2 cell responses (Fig. 2, ,BB to toGG).

(A) Core body temperature drop following OVA challenge of OVA/SEB sensitized WT, Il4raF709 and Foxp3YFPcreTgfb1Δ/+ mice. (B) Serum total and OVA-specific IgE and MMCP-1, mast cell counts from the histology of the small intestinal (C) Microscopic pictures (original magnification; x600) of toluidine blue stained histological sections from the jejunum of mice sensitized and challenged in (A). Arrows indicate mast cells. (D) Flow cytometric analysis of mast cells in the SI-LPL of mice challenged in (A). (E, G) Flow cytometric analysis and frequencies of Helios+ and Helios− Treg cells (E) IL-4+ (F) and GATA-3+ (G) Treg and Teff cells in SI-LPL of mice from panel (A). Each symbol represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: repeat measure two-way ANOVA (A); One-way ANOVA with Dunnett’s post hoc analysis (B, E, F). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of at least three independent experiments with 9–31 mice per group for (A) and 5–17 mice per group for panels (B, E, F, G). *p<0.05, **p<0.01 and p****<0.0001.
ROR-γt+ iTreg cells differentiate under the influence of microbial signals and play a critical role in suppressing Th2 cell responses, including FA (Abdel-Gadir et al., 2019; Ohnmacht et al., 2015; Sefik et al., 2015). In particular, a bloom in Clostridiales and Bacteroidales species at the time of weaning sets a critically timed window for the differentiation of a durable ROR-γt+ iTreg cells population that suppresses aberrant gut pathologies (Al Nabhani et al., 2019; Stephen-Victor et al., 2020). Analysis of small intestinal lamina propria lymphocytes (SI-LPL) following weaning (day 28) revealed decreased frequencies of ROR-γt+ Treg cells in Foxp3YFPcreTgfb1Δ/+ mice to become similar to those seen in Il4raF709 mice (Abdel-Gadir et al., 2019). In contrast, biallelic Tgfb1 inactivation in Treg cells of Foxp3YFPcreTgfb1Δ/Δ mice abrogated ROR-γt+ Treg cell differentiation (Fig. 3A). In contrast, GATA-3+ Treg cells were reciprocally increased. Consistent with these results, ROR-γt+ Treg cells were enriched in the LAP+ Treg cell population in the small intestinal lamina propria (SI-LP) of microbiota-sufficient specific pathogen free (SPF) mice (Fig. 3B). Furthermore, gut Treg cells of germ-free (GF) mice, which are lacking in the microbiota-dependent ROR-γt+ subpopulation, also exhibited decreased LAP staining and Tgfb1 mRNA expression as compared to SPF mice (Fig. 3, ,CC and andD).D). Reconstitution of GF mice with a consortium of immunomodulating Clostridiales species, but not one composed of Proteobacteria species (Abdel-Gadir et al., 2019), upregulated ROR-γt, LAP and Tgfb1 expression in gut Treg cells to become similar to those seen in Treg cells of SPF mice (Fig. 3, ,CC and andD).D). These results indicated that immunomodulatory commensal bacteria upregulate Tgfb1 expression in gut Treg cells, a necessary step in promoting their differentiation into ROR-γt+ Treg cells.

(A) Flow cytometric analysis and cell frequencies of ROR-γt and GATA3 staining in SI-LPL Treg cells isolated from 4-week-old Foxp3YFPcre, Foxp3YFPcreTgfb1Δ/+, Foxp3YFPcreTgfb1Δ/Δ mice strains. (B) Flow cytometric analysis and cell frequencies of ROR-γt expression in LAP+ and LAP− SI-LPL Treg cells. (C) Flow cytometric analysis and cell frequencies of LAP and ROR-γt expression in SI-LPL Treg cells of GF mice, GF mice reconstituted with Clostridiales or Proteobacteria consortia and SPF mice. (D) RT-PCR analysis of Tgfb1 mRNA expression in the mouse groups in (C). (E) Temperature changes in OVA/SEB-sensitized Foxp3YFPcreTgfb1Δ/+ and Il4raF709 mice that were either left untreated or treated with the Clostridiales during sensitization then challenged with OVA. (F) Serum total and OVA-specific IgE and serum MMCP-1. (G) Frequencies of ROR-γt+ Treg cells in the MLN and SI-LPL and of IL-4+ Treg cells in the MLN of the mouse groups in (E). (H) Flow cytometric analysis of SI c-Kit+FcεRI+ mast cells and their frequencies in the mouse groups in (E). Each symbol represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: One-way ANOVA with Dunnett’s post hoc analysis (A-D, F-H); repeat measure two-way ANOVA (E); *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of at least three independent experiments with 5–8 mice per group for (A and B) and 4–19 mice for (C, E to H).
Given that immunomodulating Clostridiales species suppress FA by inducing ROR-γt+ Treg cells (Abdel-Gadir et al., 2019), we examined the capacity of treatment with the Clostridiales consortium to rescue allergic dysregulation in Foxp3YFPcreTgfb1Δ/+ mice.
Treatment with the Clostridiales consortium suppressed anaphylaxis in OVA/SEB-sensitized and OVA-challenged Foxp3YFPcreTgfb1Δ/+ mice in a manner similar to that seen in similarly sensitized and challenged Clostridiales treated Il4raF709 mice, in association with profoundly decreased OVA-specific IgE and MMCP-1 release (Fig. 3, ,EE and andF)F) (Abdel-Gadir et al., 2019). Microbial therapy with the Clostridiales consortium upregulated the induction of ROR-γt+ Treg cells and suppressed IL-4 production by T cells and mast cell expansion in the mesenteric lymph nodes (MLN) and SI-LPL of both mouse strains (Fig. 3, ,GG and andH).H). These results are consistent with a critical role for Treg cell-derived TGF-β1 in mediating microbiota-dependent oral tolerance by promoting ROR-γt+ Treg cell differentiation.
The contribution of Treg cell-intrinsic TGF-β1 signaling to the allergic response was addressed by means of Treg cell-specific deletion of Tgfbr2, encoding TGF-β receptor subunit II (TGF-βR2). As previously documented, these mice are generally healthy (Konkel et al., 2017; Wu et al., 2017). Oral sensitization of Foxp3YFPcreTgfbr2Δ/Δ mice with OVA/SEB rendered the mice susceptible to robust anaphylaxis upon their oral challenge with OVA (Fig. 4, ,AA and andB).B). Notably, Foxp3YFPcreTgfbr2Δ/Δ mice exhibited increased iTreg cells (Foxp3+Helios−) in the MLN whereas the differentiation of iTreg cells in the SI-LPL was impaired in allergen sensitized mice (Fig. 4, ,DD and andE).E). Critically, the differentiation of ROR-γt+ Treg cells was profoundly suppressed in the MLN and SI-LP of allergen sensitized Foxp3YFPcreTgfbr2Δ/Δ mice. Reciprocally, the expansion of GATA3+ Treg cells was increased in Foxp3YFPcreTgfbr2Δ/Δ mice and were associated with heightened T helper cell responses (Fig. 4, ,DD to toF).F). Overall, these findings indicated a requisite role for TGF-β1 signaling in nascent gut Treg cells for the differentiation of ROR-γt+ Treg cells and in controlling FA.

(A) Core body temperature drop following OVA challenge of OVA-SEB sensitized WT and Foxp3YFPcreTgfbr2Δ/Δ mice. (B) Serum concentrations of IgE, OVA-specific IgE, and MMCP-1. (C) Flow cytometric analysis of mean fluorescence intensity (MFI) of Foxp3 in Treg cells isolated from MLN. (D and E) Flow cytometric analysis and frequencies of Helios+, Helios− and ROR-γt+ cells among CD4+Foxp3+ Treg cells from MLN (D) and SI-LPL (E) of mice from panel (A). (F) Flow cytometric analysis and frequencies of GATA3+ cells among CD4+Foxp3+ Treg cells from MLN and SI-LPL of mice from panel (A). (G) Flow cytometric analysis and frequencies of Teff cytokines. Each symbol represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: Two-way ANOVA (A, D, E); Student’s t-test (B to G). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of two independent experiments. n=4–16 mice per group.
Treg cell-derived TGF-β1 restrains mast cell expansion and activation.
The mechanisms by which Treg cell-derived TGF-β1 impacted different components of the allergic response was analyzed. Previous studies have invoked a role for TGF-β1 in regulating mast cell activation, leading us to investigate the role of Treg cell-derived TGF-β1 in regulating mast cell functions (Gomez et al., 2005). Treatment of IgE anti-DNP-sensitized mast cells with DNP-BSA resulted in mast cell activation and degranulation, marked by the increased surface expression of the granule marker LAMP-1 (CD107a) (Burton et al., 2013; Grutzkau et al., 2004; Noval Rivas et al., 2015). Addition of Treg cells derived from MLN of Foxp3YFPcre mice or recombinant TGFβ1 attenuated LAMP-1 induction upon mast cell exposure to antigen, while Treg cells derived from the MLN of Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcreTgfb1Δ/Δ mice failed to do so in a Tgfb1 gene dose-dependent manner (Fig. 5, ,AA and andB).B). Reciprocally, deletion of a floxed Tgfbr2 allele in mast cells using a mast cell protease 5 gene promoter-driven Cre recombinase (Mcpt5cre) precipitated susceptibility to FA and anaphylaxis (Fig. 5, ,CC and andD).D). Collectively, these results indicate a critical function for Treg cell-derived TGF-β1 signaling in regulating mast cell responses.

(A, B) Flow cytometric analysis of LAMP1 staining (A), and quantification of percent LAMP1 MFI inhibition (B), from in vitro bone-marrow mast cell suppression assay with sorted MLN Treg cells from 8 weeks old Foxp3YFPcre (+/+), Foxp3YFPcreTgfb1Δ/+ (Δ/+) and Foxp3EGFPcreTgfb1Δ/Δ (Δ/Δ) mice, or mast cells were treated with recombinant TGFβ1 (5ng/ml). (C) Core body temperature drop following OVA challenge of OVA-SEB sensitized WT and Mcpt5creTgfbr2Δ/Δ mice. (D) Serum concentrations of total IgE, OVA-specific IgE, and MMCP-1. Each symbol represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: One-way ANOVA with Dunnett’s post hoc analysis (B), Two-way ANOVA (C); Student’s t-test (D); *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of two independent experiments. n=6–12 replicates per group (A, B), and 5–8 mice per group (C, D).
Treg cell-specific biallelic Tgfb1 deletion precipitates intense autoimmunity.
Whereas partial depletion of Tgfb1 transcripts effected by Treg cell-specific inactivation of a single Tgfb1 allele rendered the mice highly allergic, their near complete depletion in Treg cells by means of cre-mediated deletion of both alleles (Foxp3YFPcreTgfb1Δ/Δ) in the face of sustained Tgfb1 expression in Teff cells precipitated a rapidly fatal autoimmune lymphoproliferative disease that was phenotypically similar to that associated with Foxp3 deficiency (Scurfy phenotype) (Fig. 6, ,AA to toDD and Fig. S2A) (Charbonnier et al., 2019; Lin et al., 2005). Flow cytometric analysis revealed decreased LAP expression on Treg cells of Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcreTgfb1Δ/Δ as a function of Tgfb1 allele deficiency (Fig. S2B). In contrast, the low expression of LAP on Teff cells in the respective strains was marginally increased (Fig. S2C). Notably, Treg cell expression of glycoprotein-A repetitions predominant protein (GARP), which binds to LAP and enables its activation and is itself implicated in regulating allergy and autoimmunity (Lienart et al., 2018; Nasrallah et al., 2020; Tran et al., 2009), was also decreased in a step wise manner as a function of Tgfb1 allele deficiency (Fig. S2D). Consistent with these results, TGFβ1 production by activated Treg cells was progressively decreased in Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcreTgfb1Δ/Δ as a function Treg cell-specific allele deficiency. In contrast, TGF β1 production in Teff cells, B cells and monocytes was increased in Foxp3YFPcreTgfb1Δ/Δ in comparison to Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcreTgfb1Δ/Δ mice (Fig. S2E to G). Further analysis revealed that expression of LAP in MLN CD11c+MHCII+ DCs stimulated with LPS was similar in magnitude between the Foxp3YFPcre and Foxp3YFPcreTgfb1Δ/+ mice, whereas LAP expression was enhanced in the equivalent DCs from Foxp3YFPcreTgfb1Δ/Δ mice, likely due to their activation (Fig. S3D). Foxp3YFPcreTgfb1Δ/Δ mice exhibited hypergammaglobulinemia, and skewing of their T cells towards an effector memory phenotype as compared to Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcre littermates (Fig. 6, ,EE and andG).G). There were increased frequencies of IL-4+ and IFN-γ+ Teff cells (Fig. 6F), while those of IL-17+ Teff cells were unchanged (data not shown). The frequencies of peripheral Treg cells was decreased in Foxp3YFPcreTgfb1Δ/Δ mice in association with decreased expression of certain markers, including Foxp3, PD-1 and NRP-1 in the face of normal or increased expression of others including CD25, CTLA4, OX40 and ICOS, and normal expression of the proliferation marker Ki-67 (Fig. 6, ,HH and andI;I; Fig. S3, A and B). IL-10 expression was similar in the MLN Treg cells of Foxp3YFPcre and Foxp3YFPcreTgfb1Δ/+ mice, while it was decreased in Foxp3YFPcreTgfb1Δ/Δ (Fig. S3C). In vitro, the suppressive capacity of Foxp3YFPcreTgfb1Δ/Δ Treg cells was decreased as compared to Foxp3YFPcre Treg cells, whereas that of Foxp3YFPcreTgfb1Δ/+ Treg cells was unchanged (Fig. S3E). Development of TGF-β1-deficient Treg cells in vivo was not altered in healthy Foxp3YFPcre/+ females homozygous for the Tgfb1 floxed allele (Foxp3YFPcre/+Tgfb1Δ/Δ) which, due to the phenomenon of X-linked inactivation, harbor both TGF-β1-sufficient and -deficient cells (Fig. S3F).

(A) Appearance of Foxp3YFPcre, Foxp3YFPcreTgfb1Δ/+, and Foxp3YFPcreTgfb1Δ/Δ littermate mice. (B) Body weights at 19 d of age. (C) Survival curves of the respective mouse strains. (D) Histological pictures of skin, lung and liver tissues stained with hematoxylin and eosin (original magnification; ×200). (E) Serum immunoglobulin concentrations. (F) Flow cytometric analysis and cell frequencies of IL-4 and IFNγ expression in splenic Treg and Teff cells. (G) Flow cytometric analysis of CD62 and CD44 staining on splenic CD4+ T cells (left), and cell frequencies of CD62lowCD44high Teff cells. (H) Flow cytometric analysis and cell frequencies of CD4+Foxp3+ Treg cells. (I) Flow cytometric analysis and MFI of Foxp3 and PD-1 expression in splenic Treg cells. Each symbol represents an independent sample. The age of the mice in A and C to H were between 3 to 4 weeks age. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: log-rank test (A), Student’s t-test (C, F-J), and One-way ANOVA with Dunnett’s post hoc analysis (D). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Data representative of two to three independent experiments with 4–16 mice per group. Please also see Figure S2–S6.
The role of Treg cell Tgfb1 in regulating allergic and autoimmune responses in a gene dose dependent manner was further highlighted in experiments using a bacterial artificial chromosome transgene that enabled the expression of a Cre recombinase and the enhanced green fluorescent protein (EGFP) in Foxp3+ T cells (Foxp3EGFPcre). Unlike the Foxp3YFPcreTgfb1Δ/Δ mice, the BAC Foxp3EGFPcreTgfb1Δ/Δ mice did not suffer lymphoproliferation and their survival was similar to control mice. Lineage tracing analysis using a cre-regulated Rosa26-YFP reporter (Rosa26YFP) revealed that the majority of Treg cells in Foxp3EGFPcreTgfb1Δ/ΔRosa26YFP mice did not express the Foxp3 BAC-driven EGFP-Cre transgene (Foxp3+EGFP−YFP−). Accumulation of EGFP− Treg cells reached up to 60% of the peripheral Treg cell pool (Fig. S4, A and B). We further analyzed the expression of LAP and GARP in Cre-expressing Treg cells (EGFP+Foxp3+), escaped Treg cells (EGFP−Foxp3+) and Teff cells (EGFP−Foxp3−) of Foxp3EGFPcreTgfb1Δ/Δ mice. Results revealed that whereas the escaped Treg cells exhibited increased expression of LAP and GARP, the expression of those proteins was substantially decreased in BAC-expressing Treg cells, reflecting the effective deletion of Tgfb1 in those cells. In contrast, LAP and GARP expression was negligible in Teff cells (Fig. S4, B and C). Of note, oral sensitization of Foxp3EGFPcreTgfb1Δ/Δ, but not Foxp3EGFPcre, mice rendered them susceptible to robust anaphylaxis upon oral challenge with OVA (Fig. S5A). The OVA food allergic response of Foxp3EGFPcreTgfb1Δ/Δ mice mirrored that observed in similarly treated Il4raF709 mice, showing increased total and OVA-specific IgE, elevated serum concentrations of MMCP-1 following anaphylaxis, with small intestinal tissue mast cell expansion (Fig. S5, B to D). The mesenteric lymph node Treg cells were increased in OVA food allergic Foxp3EGFPcreTgfb1Δ/Δ mice, indicating that the propensity of these mice to develop FA was not due to a relative deficiency of Treg cells (Fig. S5, E and F). Rather, transfer experiments with TGF-β1-sufficient and deficient Foxp3EGFPcre Treg cells bearing the OVA323–339 peptide-specific DO11.10 transgenic T cell receptor revealed the capacity of the former but not the latter cells to prevent the induction of FA in Il4raF709 mice and to suppress existing disease, indicating that it was the relative deficiency of Tgfb1-expressing Treg cells that rendered Foxp3EGFPcreTgfb1Δ/Δ mice susceptible to FA (Fig. S6). Overall, the development of an allergic response and the transition to a fulminant autoimmune phenotype are regulated by the availability of Treg cell-derived TGF-β1 in a cell frequency and gene dose-dependent manner.
Treg cell-specific biallelic Tgfb1 deletion dysregulates T follicular helper cell (Tfh) and B cell responses.
Analysis of Foxp3YFPcreTgfb1Δ/Δ mice revealed intense dysregulation of the Tfh cell response, characterized by increased frequencies of CD4+CXCR5+PD1+ Tfh and B220+GL-7+ germinal center B cells, as well as increased activation markers (CD80, CD86 and MHC class II) on classical dendritic cells in the secondary lymphoid organs. T follicular regulatory T (Tfr) cell frequencies were similar to Foxp3YFPcre mice, suggestive of defective Tfh suppression by TGF-β1-deficient Tfr cells (Fig. 7, ,AA to toD).D). In contrast, these abnormalities were largely absent in Foxp3YFPcreTgfb1Δ/+ mice. The dysregulated Tfh response in Foxp3YFPcreTgfb1Δ/Δ mice was associated with increased auto-antibody production involving all immunoglobulin isotypes including IgE, while the auto-antibody production in Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcre mice were similar (Fig. 7E and Fig. S7, A to C). Depletion of B cells by treatment with anti-CD20 mAb prolonged the survival of Foxp3YFPcreTgfb1Δ/Δ mice (Fig. 7F). These findings highlight the differential requirement for Treg cell-derived TGF-β1 in regulating the allergic versus the autoimmune antibody responses, with the latter being effectively controlled in mice with Treg cell specific Tgfb1 haploinsufficiency.
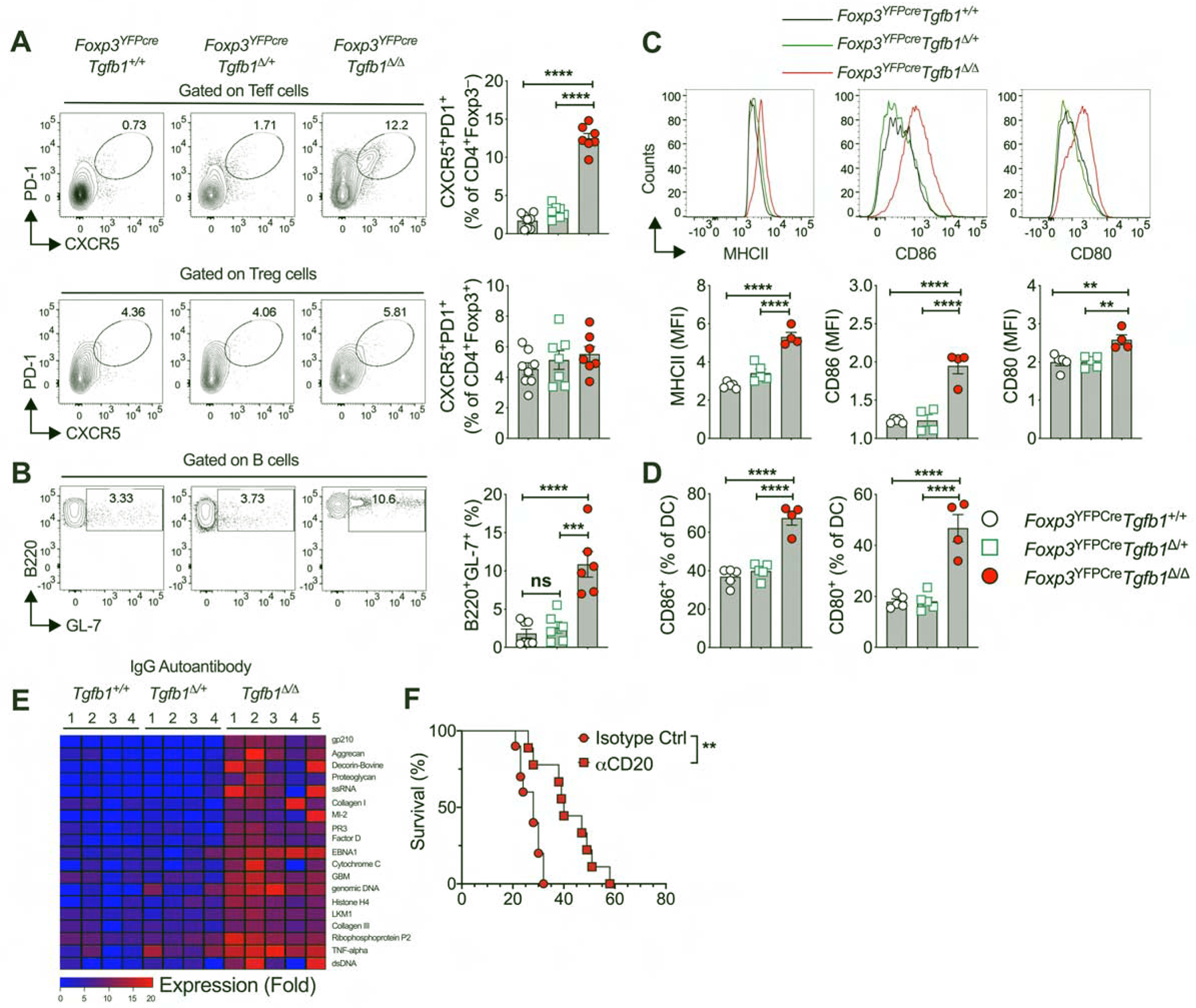
(A) Flow cytometric analysis and frequencies of CXCR5 and PD-1 expression in splenic Teff and Treg cells. (B) Flow cytometric analysis and frequencies of germinal center B cells (B220+GL-7+) within the splenic B cell population. (C) Frequencies of CD80 and CD86 expressing cells within CD11c+MHCII+ splenic dendritic cell population. (D) Flow cytometric analysis and MFI of MHCII, CD80 and CD86 expression within the CD11c+MHCII+ splenic dendritic cell population. (E) Heat map representation of serum IgG autoantibodies in littermate mice of the respective genotypes. (F) Survival curves of Foxp3YFPcreTgfb1Δ/Δ mice treated with isotype control or anti-CD20 mAb. The age of the mice in panels A- F were between 3 to 4 weeks of age. Each symbol or column number represents an independent sample. Numbers in flow plots indicate percentages. Error bars indicate SEM. Statistical tests: One-way ANOVA with Dunnett’s post hoc analysis (A-D), R package ‘limma’ and multiple comparisons corrections adjusted to p<0.05 (E), and log-rank test (F). *P<0.05, **P<0.01, ****P<0.0001. Data representative of two to three independent experiments with 4–8 mice per group. Please also see Figure S7.
Discussion
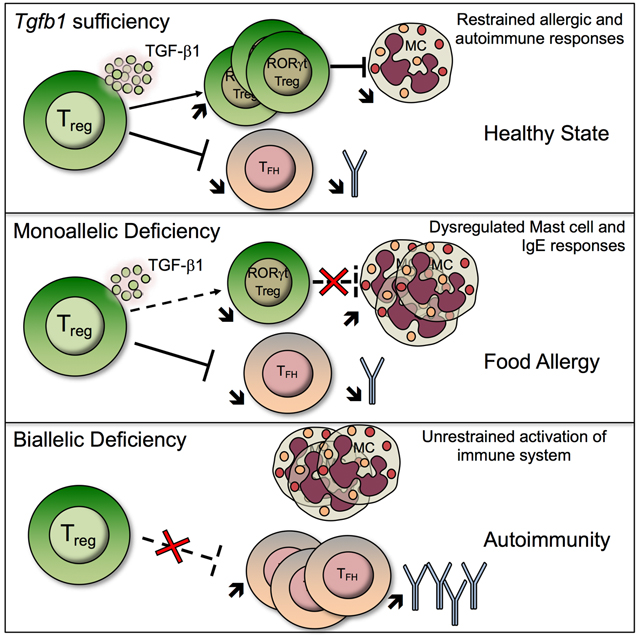
Our studies have demonstrated a pivotal role for Treg cell-derived TGF-β1 in regulating allergic and autoimmune responses, with stepwise decrease in Tgfb1 transcription in Treg cells precipitating contrasting immune dysregulatory outcomes. Thus, an incremental decrease in Tgfb1 transcription due to Th2 cell like-reprogramming of Treg cells, a pathologic process triggered by dysbiosis in FA subjects or by enhanced IL-4R signaling in Il4raF709 mice (Abdel-Gadir et al., 2019; Noval Rivas et al., 2015), promotes allergic dysregulation and susceptibility to FA. This outcome was phenocopied by Treg cell-specific Tgfb1 haploinsufficiency or by Tgfb1 deletion by a partially penetrant, BAC-based Foxp3-driven Cre recombinase, and was abrogated by Tgfb1 overexpression in Treg cells of FA-prone Il4raF709 mice. Importantly, the allergic dysregulation was linked to decreased expression of Tgfb1 in gut Treg cells, whose upregulation by the commensal microbiota was essential for their differentiation into oral tolerance promoting ROR-γt+ Treg cells. In contrast, homozygous Tgfb1 deficiency in Treg cells precipitated fatal autoimmunity, related to Tfh and dendritic cell dysregulation, an outcome echoed in rare patients with biallelic loss of function mutations in Tgfb1 (Kotlarz et al., 2018). Overall, these results establish that Treg cell-derived TGF-β1 differentially regulates allergic and autoimmune responses in a Treg cell Tgfb1 gene dose and microbiota-dependent manner.
A key finding in our studies was the demonstration of a privileged role for Treg cell-derived TGF-β1 in the maintenance of oral and peripheral tolerance. The original paradigm for the differentiation of antigen-specific induced Treg cells in the gut has stipulated the dependence of this process on TGF-β derived from specialized CD103+ antigen-presenting dendritic cells (Coombes et al., 2007; Sun et al., 2007). Our results indicate an additional non-redundant role for Treg cell-derived TGF-β1 in the further differentiation of these cells into oral tolerance-promoting RORγt+ Treg cells, whose deficiency results in profound allergic dysregulation (Abdel-Gadir et al., 2019; Ohnmacht et al., 2015). The action of Treg cell-derived TGF-β1 in promoting RORγt+ Treg cell differentiation appeared to be cell-intrinsic, as evidenced by the failure of such differentiation upon Treg cell-specific deletion of Tgfbr2. The demonstration that commensals upregulated Tgfb1 expression in gut Treg cells points to a commensal-regulated Treg cell TGF-β1-RORγt axis operative in mucosal tolerance and relevant to the weaning reaction, whose disruption by dysbiosis gives rise to allergic dysregulation and FA (Abdel-Gadir et al., 2019; Al Nabhani et al., 2019).
Treg cell Tgfb1 haploinsufficiency gave rise to profound gut tissue mast cell expansion and a steep increase in serum IgE concentrations, conducive to intense anaphylactic responses upon oral allergen challenges. In vitro assays revealed that TGF-β1 deficiency impaired the capacity of Treg cells to suppress mast cell activation. Of particular interest was the demonstration that deletion of Tgfbr2 in mast cells precipitated susceptibility to FA, thus emphasizing the role of TGFβ signaling in restraining the mast cell responses (Gomez et al., 2005). The essential role of RORγt+ Treg cells in this process may relate to the enrichment of LAP expression in this subpopulation, pursuant to its induction by the microbiota, and also to the biased response by this population to gut-derived microbial and food antigens (Abdel-Gadir et al., 2019; Ohnmacht et al., 2015).
Whereas Treg cell Tgfb1 haploinsufficieny precipitated allergic dysregulation, biallelic Tgfb1 deletion resulted in systemic lymphoproliferation and autoimmunity. Previous studies have identified a critical role for TGF-β1 in suppressing autoimmunity by employing mouse models of global and T cell-specific Tgfb1 deletion, and have also implicated TGF-β signaling in T cells and DC in such regulation (Gorelik and Flavell, 2000; Gutcher et al., 2011; Li et al., 2007; Marie et al., 2005; Ramalingam et al., 2012; Shull et al., 1992). Our studies now specifically identify Treg cells as the critical source of TGF-β1 in enforcing peripheral tolerance and restraining autoimmunity. Biallelic Tgfb1 deletion in Treg cells unleashed intense DC dysregulation, Teff cell activation and auto-antibody production. The latter phenotype was associated with heightened Tfh and germinal center B cell responses and dysregulated DC activation. These abnormalities were largely absent in Foxp3YFPcreTgfb1Δ/+ mice, consistent with the capacity of Tgfb1 haploinsufficient Treg cells to restrain these pathogenic autoimmune responses. This dichotomy in the regulation of allergic versus autoimmune responses points to distinct check points governing the regulation by Treg cells of the respective immune responses in a Tgfb1 gene dose-dependent manner. Previous studies have linked deletion of Tgfbr2 in CD4+ T cells with Tfh cell dysregulation by a mechanism involving the loss of Treg cell TGF-β1 mediated suppression of Tfh cells (McCarron and Marie, 2014). Our results would indicate that Treg cells are a critical source of TGF-β1 mediating regulation of the Tfh cell responses, the loss of which engenders dysregulated auto-antibody production.
While the essential role of CD4+ T cell-derived TGF-β1 in peripheral tolerance has been previously established, the contribution of Treg cell-derived TGF-β1 to peripheral tolerance remained unclear. A previous study employed a complex targeting approach to deplete TGF-β1 expression in Treg cells. This approach involved deletion of a different floxed Tgfb1 allele with Foxp3YFPcre, and inactivation of the other allele by insertion of an in-frame EGFP cDNA into exon 1 of Tgfb1 (Gutcher et al., 2011). The resulting mice were otherwise healthy, while their Treg cell frequency was increased, suggesting that Treg cell-derived TGF-β1 plays a role in controlling Treg cell homeostasis. These results echoed those we obtained with the BAC-Foxp3EGFPcreTgfb1Δ/Δ mice, suggesting either incomplete deletion of the floxed allele, a hypomorphic function of the Tgfb1EGFP allele and/or permissiveness of the exon 1 targeting strategy for expression of a TGFβ1 peptide from a downstream open reading frame. Overall, the fatal autoimmunity observed in Foxp3YFPcreTgfb1Δ/Δ mice, which unlike the BAC-Foxp3EGFPcreTgfb1Δ/Δ mice do not show evidence of Treg cell escape from Tgfb1 deletion, argues for an essential, non-redundant role of Treg cell Tgfb1 in peripheral tolerance. Notwithstanding the abundance of TGF-β production in different tissues and by different cell types, our results further emphasize the importance of the context of TGF-β action in orchestrating unique immune responses, as recently demonstrated for migratory DC-activated TGF-β in preconditioning naïve CD8 T cells to become epithelial tissue resident cells (Mani et al., 2019).
In conclusion, our studies reveal the primacy of Treg cells as a source for TGF-β1 in immunoregulation and outline genetic and environmental mechanisms by which Treg cell-derived TGF-β1 can separately exercise control over both allergic and autoimmune responses.
STAR+METHODS
RESOURCE AVAILABILITY
Lead Contact:
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Talal A. Chatila ([email protected])
Materials Availability Statement:
All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability:
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice.
BALB/cByJ (WT) and all the following strains, except where indicated, were obtained from or rederived at the JAX lab. C.129X1-Il4ratm3.1Tch (Il4raF709) have been previously described (Noval Rivas et al., 2013; Tachdjian et al., 2010). Tgfb1tm2.1Doe/J, NOD/ShiLt-Tg(Foxp3-EGFPcre)1cJbs/J (Foxp3EGFPcre), B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (R26YFP/YFP) and B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr/J (Foxp3YFPcre) were backcrossed 12 generations on BALB/cBYJ (Azhar et al., 2009; Rubtsov et al., 2008; Srinivas et al., 2001; Zhou et al., 2008). C.129P2(Cg)-Il4-Il13tm1.1Lky (Il4-Il13fl/fl) mice were crossed with Il4raF709Foxp3YFPcre mice as indicated (Noval Rivas et al., 2015; Voehringer et al., 2009). C.129S2-Stat6tm1Gru (Stat6−/−) mice were crossed with Il4raF709Foxp3YFPcre mice (Kaplan et al., 1996). FVB/N-Tg(CAG-EGFP,TGFB1*)C8Kul/J (Tgfb1Tg) mice were backcrossed on C57BL/6 for 6 generations (Hall et al., 2010). DO11.10Foxp3EGFPcre were generated from the respective transgenic mice (Murphy et al., 1990). B6.Tg(Mcpt5cre) and B6.Foxp3YFPcre mice were interbred with B6;129-Tgfbr2tm1Karl/J mice to create Mcpt5creTgfbr2Δ/Δ and Foxp3YFPcreTgfbr2Δ/Δ mice, respectively (Burton et al., 2014; Scholten et al., 2008). Mice were maintained under specific pathogen-free conditions and used according to the guidelines of the institutional Animal Research Committee at the Boston Children’s Hospital.
Human study population.
Three groups of subjects, aged from 6 months to 20 years were recruited under a protocol approved by the Institutional Review Board at the Boston Children’s Hospital: 1) healthy subjects without a history of FA or atopy (n=6), 2) non-food allergic subjects with other atopic diseases (including asthma, atopic dermatitis and allergic rhinitis) (n=27) and 3) subjects who have FA (n=22), as determined by the World Allergy Organization diagnostic criteria (2010). Subject demographics and allergen reactivity are detailed in Table S1, and inclusion and exclusion criteria are detailed as previously described (Abdel-Gadir et al., 2019).
METHOD DETAILS
Sensitization and challenge protocol.
Mice were treated intragastrically with either sterile PBS or OVA (Sigma-Aldrich) (250μg) together with 10μg SEB (Toxin Technology) in PBS (Treg) once weekly for 8 weeks (Abdel-Gadir et al., 2019). On week 9, mice were challenged intragastrically with 150 mg of OVA. Anaphylaxis was assessed by measuring changes in total body core temperature with transponders placed subcutaneously 2 days before challenge (IPTT-300; Bio Medic Data Systems) and a DAS-6001 console (Bio Medic Data Systems).
ATAC-Seq.
20,000 Treg cells/sample were used for preparation of ATAC-seq libraries according to the protocol described previously; however, with few modifications (Buenrostro et al., 2015). Briefly, cells were suspended in 100μl of cold hypotonic lysis buffer [10mM Tris-HCl (pH 7.5), 10mM NaCl, 3mM MgCl2 and 0.5% NP40], followed by immediate centrifugation at 550g for 30 min and re-suspension of the pellet in 5μl of transposition reaction mix [1μl of Tagment DNA Enzyme and 2.5μL of Tagment DNA Buffer from Nextera DNA Sample Prep Kit (Illumina), 1.5μl H2O]. The transposition reaction was incubated for 60 min at 37°C which led to fragmentation and tagging of DNA. For library preparation, 7-cycles of PCR were performed followed by selection of small fragments (less than 600bp) using SPRI beads. A second round of PCR (7-cycles) with similar conditions was performed in order to obtain the final library. Libraries were sequenced on the NextSeq 500 instrument to generate paired-end short reads (50bp, forward; 33bp, reverse). Paired-end sequencing reads were aligned to the mouse reference genome (mm10) using bowtie aligner version 2.2.9 (Langmead and Salzberg, 2012), followed by removal of reads with multiple alignments as well as reads mapping to mitochondrial DNA. De-duplication of reads was performed with picard (v2.8.0). makeTagDirectory command of Homer package (v4.9) was used to create the tag directory followed by identification of peaks using the ‘factor’ parameter in the find Peaks command (Heinz et al., 2010). For visualization of ATAC-seq data, replicate specific bam output files from picard were merged and converted into normalized bigwig format using the bamCoverage function in deepTools with options - fragmentLength 200 -normalizeUsingRPKM (Ramirez et al., 2014), and bigwig file was visualized on Integrative Genomics Viewer (IGV) (Robinson et al., 2011).
Chromatin Immunoprecipitation.
Treg cells were sorted from either Foxp3YFPcre or Foxp3YFPcreIl4raF709 mice. The cells were then kept for 8 min in 1 ml of 1% paraformaldehyde (PFA; Sigma-Aldrich) at room temperature (RT). Next, the sample incubated with lysis buffer I (5mM PIPES pH 8, 85mM KCl, 0,5% NP40 (Igepal-CA630), Protease Inhibitor) for 20 min at RT, and then centrifuged for 5 min at 7,870 g at RT. The pellet was lysed with lysis buffer II (10mM Tris-HCl pH 7,5, 150mM NaCl, 1% NP40 (Igepal-CA630), 1% DOC (Natrium Deoxycholate), 0,1% SDS, 1mM EDTA, Protease Inhibitor). The chromatin was further sonicated to a length of 25–500 bp using bioruptor (Diagenode, USA) for 35 cycles (30 sec on, 30 sec off). The chromatin was then precipitated using either IgG mock control, STAT6 (Cell Signaling Technology), H3K4me3 (Active Motif, USA) or H3k27me3 (Active Motif USA) histone methylation overnight. The chromatin was washed twice with each of the different washing buffers [wash buffer I (20mM Tris-HCl pH 8, 150mM NaCl, 2mM EDTA, 0,1% SDS, 1% Triton X100), wash buffer II (20mM Tris-HCl pH 8, 500mM NaCl, 2mM EDTA, 0,1% SDS, 1% Triton X100) and wash buffer III (10mM Tris-HCl pH 8, 1% NP40 (Igepal-CA630) 1% DOC, 1mM EDTA, 0,25M LiCl)]. The chromatin was then cleaned using 1X TE buffer (10mM Tris-HCl pH 8, 1mM EDTA) and eluted using an elution buffer (1% SDS, 0,1M NaHCO3). The DNA was then cleaned using QIAquick PCR purification kit (Qiagen, USA). The DNA was then used in polymerase chain reaction (PCR) were conducted with the relevant primers (Harb et al., 2015). PCR primers for putative STAT6 binding in the Tgfb1 promoter are: Forward - TCCTTGACACTCTCATCCGC and Reverse - GGCACTGTCTTCATCTTAGCG. Percent enrichment to the input control was calculated for each target locus and separately for mock (IgG), STAT6, H3K4me1 or H3k27me3 antibodies. Intra- and inter-assay coefficients of variation calculated for percent enrichment did not exceed 10%. All samples were processed according to the same standardized protocol and analyzed blinded and in a randomized order.
Plasmids and reagents:
pGL3-Tgfb1 was obtained from Addgene (Yeh et al., 2018). The mouse Tgfb1 promoter plasmid was generated using C57BL/6 cDNA using the following primers: mWTTgfb1-F: attaggtaccacatgagcagggcccactgt, mWTTgfb1-R: taataagcttgcgaagggcggcggcggcgg. The Tgfb1 promoter harboring a deletion of the STAT6 binding site was generated using the Q5 Site-Directed Mutagenesis Kit (NEB) and the following primer set: mMUTTgfb1-f: ACATGAGCAGGGCCCACTGTTAAAGCGTGT, and mMUTTgfb1-r: ACCCATGAGAAATACACGCTTTAACAGTGG. The sequences of both the native (Tgfb1STAT6) and mutant (Tgfb1ΔSTAT6) Tgfb1 promoter plasmids were confirmed by Sanger sequencing.
Luciferase assay:
EL4 cells were seeded in 6-well plates with non-antibiotic X-VIVO 15 medium and transfected with reporter gene plasmids as indicated. The total amount of DNA was kept constant. pTK-Renilla was co-transfected to normalize transfection efficiency. 24 hours (hr) post transfection, EL4 cells bearing the Tgfb1STAT6 or Tgfb1ΔSTAT6 plasmid constructs were stimulated with 20ng/ml of mouse IL-4 for 24 hr before luciferase reporter assays were performed. Luciferase activity was analyzed with the Dual Luciferase Reporter Assay System (Promega).
Isolation of Spleen, MLN and LP lymphocytes.
Spleen and MLNs were isolated and homogenized in PBS containing 2% FCS buffer. Red blood cells from splenic suspensions were lysed with ACK buffer. Cells were washed once with PBS containing 2% FCS and used for experiments. Small intestines were dissected from mice and the fecal contents were flushed out using PBS containing 2% FCS. Peyer’s patch was excised and the intestines were cut into 1cm pieces and treated with PBS containing 2% FCS, 1.5 mM DTT, and 10mM EDTA at 37 °C for 30 min with constant stirring to remove mucous and epithelial cells. The tissues were then minced and the cells were dissociated in RPMI containing collagenase (2 mg/ml collagenase II; Worthington), DNase I (100 μg/ml; Sigma-Aldrich), 5mM MgCl2, 5mM CaCl2, 5mM HEPES, and 10% FBS with constant stirring at 37 °C for 45 min. Leukocytes were collected at the interface of a 40%/70% Percoll gradient (GE Healthcare). The cells were washed with PBS containing 2% FCS and used for experiments.
Flow cytometry.
The following anti-mouse monoclonal antibodies (mAb) were used: CD3 (17A2), CD4 (RM4–5), IgE (RME-1), LAMP-1 (1D4B), CD86 (GL-1), CTLA-4 (UC10–4B9), ICOS (C398.4A), LAP (TW7–16B4), B220 (A3–6B2), cKit (2B8), IL-4 (11B11), IL-17a (TC11–18H10.1), IFN-γ (XMG1.2), Gr-1 (RB6–8C5), CD11b (M1/70), CD19 (6D5), I-Ad (39-10-8), CD45 (30-F11), and Ly-6C (HK1.4) were from Biolegend. Foxp3 (FJK-16S), GATA-3 (TWAJ), ROR-γt (BD2), Helios (22F6), IRF4 (3E4), Ki67 (SolA15), FcεR1a (MAR-1), CD11c (N418), CD4 (RM4–5), CXCR5 (SPRCl5), CD44 (IM7), CD62L (MEL-14), OX40 (OX86), GL-7 (GL-7), LAP (TW7–16B4), GARP (YGIC86), CD80 (16–10A1). CD25 (PC61.5), CD127 (A7R34), CD279 (J43) and cKit (2B8) were from eBiosciences. Anti-human mAb used in this study included CD3 (SK7), CD127 (A019D5), (Biolegend); CD4 (RPA-T4), LAP (TW4–6H10), FOXP3 (PCH101); CD25 (2A3), and ROR-γt (Q31–378) were from BD Biosciences; Cell viability dye (eFluor506) and (eF780) was from eBioscience. For cytokines cells were stimulated during 4 hours with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) in the presence of Golgi Plug (BD Biosciences), then stained with the BD Cytofix/Cytoperm buffers (BD Biosciences) and the indicated anti-cytokine antibody. For intracellular staining of nuclear factors, the Foxp3 Transcription Factor buffer set (eBioscience) was used. Dead cells were routinely excluded from the analysis based on the staining of eFluor 506 fixable viability dye (eBioscience), and analyses were restricted to single cells using FSC-H and FSC-A signals. Stained cells were analyzed on an LSR Fortessa (BD Biosciences) and data were processed using Flowjo (Tree Star Inc.).
Quantitative real-time PCR for host immunological Targets.
RNA was extracted from cells using RNeasy Mini kit (Qiagen) according to the manufacturer protocol. Reverse transcription was performed with the SuperScript III RT-PCR system and random hexamer primers (Invitrogen) and quantitative real-time reverse transcription (RT)-PCR with Taqman® Fast Universal PCR master mix, internal housekeeping gene mouse (Gapdh FAM dye) and specific target gene primers for murine Tgfb1, Tgfb2, and Tgfb3, as indicated (FAM Dye) (Applied Biosystems) on Step-One-Plus machine. Relative expression was normalized to Gapdh and calculated as fold change compared to Foxp3YFPcre Treg cells or Teff cells.
Bacterial consortia.
The composition and preparation of the Clostridiales and Proteobacteria consortia has been previously detailed (Abdel-Gadir et al., 2019).
ELISA.
Total, OVA-specific IgE and Murine mast cell protease 1 (MMCP-1) concentrations were measured in the sera of treated mice by ELISAs, as previously described (Abdel-Gadir et al., 2019).
TGF-β1 ELISA assays:
CD4+ T cells were enriched from mouse spleen by positive selection with anti-CD4 microbeads (Miltenyi Biotec). Enriched CD4+ T cells were further purified with a cell sorter by gating on YFP+CD4+ T cells for Treg cells and YFP− CD4+ T cells for Teff cells. For monocyte purification, CD4 T cell-depleted splenocytes were stained with PE conjugated CD11b mAb, and the CD11b+ cells were enriched by positive selection of anti-PE microbeads (Miltenyi Biotec). Monocytes were further purified from the enriched CD11b+ cells with a cell sorter by gating on CD11b+Ly6C+ cells. B cells were sorted from CD4 and CD11b depleted splenocytes by gating on CD19+ cells. Sorted cells were cultured at 0.5 × 106 cells/well (Treg and Teff cells) or at 1 × 106 cells/well (B cells and monocytes) in X-VIVO 15 media. CD4+ T cells were activated in the presence of anti-CD3/CD28 dynabeads (Thermofisher) and 1μg/ml of IL-2 (eBioscience) for 48h. B cells were activated with 10μg/ml F(ab′)2 goat anti-mouse IgM antibodies (αIgM ; Jackson ImmunoResearch Laboratories) and 1μg/ml recombinant CD40 ligand (CD40L; eBioscience) for 48h. Monocytes were activated with 1μg/ml lipopolysaccharides (LPS; Sigma-Aldrich) for 48h. TGF-β1 cytokine amounts in the tissue-culture supernatant was detected using the TGF-β1 ELISA kit from R&D Systems (DY1679–05) according to the manufacturer’s protocol.
Histology.
Intestinal mast cells were counted by microscopic examination of jejunal sections fixed in 10% formaldehyde and stored in ethanol 70% before staining with toluidine blue by the Harvard Rodent Histopathology Facility.
Autoantibody array analysis.
Autoantibody-by-autoantibody antibody analyses between group for differential expression were conducted using the R package ‘limma’ and multiple comparisons correction was performed in R (Ritchie et al., 2015). An antibody was considered statistically differentially antibody only if the Benjamini Hochberg-adjusted p value < 0.05 between the tested groups and the mean of expression of mutant group is 2-fold increase over wild group. The statistically significant autoantibodies were used to generated heatmap by heatmap.2 in gplots R package
B cell depletion:
B cell depletion in 15 days old Foxp3YFPcreTgfb1Δ/Δ mice was performed by intraperitoneal injection of 200μg anti-CD20 (clone AISB12, BioXCell) or IgG2a isotype control (clone 2A3, BioXCell) mAb per mouse weekly until the end of the survival analysis.
Mast cell suppression assay:
Treg cells were purified from either Foxp3YFPcre, Foxp3YFPcreTgfb1Δ/+ and Foxp3YFPcreTgfb1Δ/Δ. Mast cells were differentiated from bone marrow as previously described (Burton et al., 2013). Mast cells and Treg cells were dispensed 5X104 cells per well, respectively in conic 96 well plates, with 5ng/ml of IgE SPE7 (Sigma-Aldrich), 3ng/ml of mouse IL-3 (eBioscience), and CD3/CD28 activation beads were added accordingly to the manufacturer instructions (eBioscience), for a final volume of 100μl of RPMI. In some conditions mast cells were plated without Treg cells in the same final volume of 100μl of RPMI. After an overnight incubation at 37°, cells were stimulated for 10 min at 37°with DNP-BSA to assess the degranulation status of mast cells. 10μl of the 10x stimulus mix (500ng/ml DNP/BSA (sigma-Aldrich), 1/100 anti-LAMP-1 APC (Biolegend), 1/100 anti-ckit PE (eBioscience), 1/100 anti-CD4 FITC (Biolegend) and 1/1000 fixable viability dye eFluor 780 (eBioscience) were added per well. The reaction was stopped by adding 100μl/well of cold PBS/BSA containing 2mM EDTA. the plate was centrifuged for 3min at 1400rpm, the supernatants were discarded and the cells were suspended in 200μl/well of cold PBS/BSA and 2mM EDTA. The plate was centrifuged again for 3min at 1400rpm. The cells were suspended in 200μl of cold PBS/BSA and 2mM EDTA and the flow cytometry was performed immediately before losing the degranulation effect.
Treg cells adoptive transfer.
EGFP+CD4+DO11.10+ Treg cells were cell sorted from Foxp3EGFPcre and Foxp3EGFPcreTgfb1Δ/Δ mice, respectively. For treatment of established FA, Il4raF709 recipients were sensitized with OVA-SEB during 8 weeks. At week 9, the Treg-sensitized mice were given retro-orbitally at 5×105 Treg cells of the respective genotype, sensitized with OVA-SEB for 4 more weeks and challenged at week 5 (150mg OVA). For FA prevention, CD4+ DO11.10+Foxp3EGFP+ Treg cells were given retro-orbitally at 5×105 cells/mouse on day 0 of the sensitization protocol. The mice were then sensitized with OVA-SEB for 8 weeks then challenged with OVA.
Quantification and Statistical Analysis.
All experiments were performed using randomly assigned mice without investigator blinding. Results of Anaphylaxis temperature curves were analyzed by using 2-way ANOVA. Student’s unpaired two tailed t test were used for 2 groups comparisons. For more than 2 groups, 1-way ANOVA with Tukey or Bonferroni post-test analysis using Prism 8 (GraphPad). Results are presented as means (horizontal lines or rectangular bars) and SEM where each point represents one sample. Differences in mean values were considered significant at a p < 0.05.
The cytokine TGFβ−1 is implicated in controlling allergic and autoimmune responses, However the specific role of regulatory T (Treg) cell-derived TGFβ−1 in governing these responses has been unclear. Turner et al demonstrate that Treg cell-derived TGF-β1 is critical in restraining allergic and autoimmune diseases in a gene dose-dependent manner.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| eBioscience Fixable Viability Dye eFluor 506 and eFluor 780 | Thermo Fischer | Cat# 65-0866-18 |
| APC-Cy7 and FITC anti-mouse CD3 Antibody | Biolegend | Cat# 100222 Cat# 100204 |
| Brilliant Violet 605™ anti-mouse CD4 Antibody | Biolegend | Cat# 100451 |
| PE anti-mouse IgE Antibody | Biolegend | Cat# 406908 |
| Brilliant Violet 605™ anti-mouse CD86 Antibody | Biolegend | Cat# 105036 |
| Brilliant Violet 421™ anti-human/mouse/rat CD278 (ICOS) Antibody | Biolegend | Cat# 313524 |
| PE and APC anti-mouse LAP (TGF-β1) Antibody | Biolegend | Cat# 141404 Cat# 141406 |
| Alexa Fluor® 488 and APC anti-mouse/human CD45R/B220 Antibody | Biolegend | Cat# 103225 Cat# 103212 |
| PE/Cyanine7 anti-mouse CD117 (c-Kit) Antibody | Biolegend | Cat# 105814 |
| PE and PE/Dazzle™ 594 anti-mouse IL-4 Antibody | Biolegend | Cat# 504104 Cat# 504132 |
| PE/Cyanine7 anti-mouse IL-17A Antibody | Biolegend | Cat# 506922 |
| APC anti-mouse IFN-γ Antibody | Biolegend | Cat# 505810 |
| FITC anti-mouse Ly-6G/Ly-6C (Gr-1) Antibody | Biolegend | Cat# 108406 |
| Alexa Fluor® 647 anti-mouse Ly-6C Antibody | Biolegend | Cat# 128010 |
| FITC anti-mouse CD19 Antibody | Biolegend | Cat# 115506 |
| FITC anti-mouse I-Ad Antibody | Biolegend | Cat# 115006 |
| Brilliant Violet 605™ anti-mouse CD45 Antibody | Biolegend | Cat# 103140 |
| PE anti-mouse CD152 (CTLA-4) Antibody | Biolegend | Cat# 106306 |
| Alexa Fluor® 647 anti-mouse CD279 (PD-1) Antibody | Biolegend | Cat# 109118 |
| APC anti-mouse CD107a (LAMP-1) Antibody | Biolegend | Cat# MA5–28671 |
| FITC and PE anti mouse CD11b Antibody | Thermo Fischer | Cat# 11-0112-82 Cat# 12-0112-82 |
| APC anti-mouse FceR1 alpha Antibody | Thermo Fischer | Cat# 121614 |
| FITC and PE anti mouse CD11c Antibody | Thermo Fischer | Cat# 11-0114-82 Cat# 25-0114-82 |
| eFluor 610 anti-mouse CD185 (CXCR5) Antibody | Thermo Fischer | Cat# 61-7185-82 |
| eFluor 450 anti-mouse CD44 Antibody | Thermo Fischer | Cat# 48-0441-82 |
| APC anti-mouse CD62L Antibody | Thermo Fischer | Cat# 17-0621-82 |
| APC anti-mouse CD134 (OX40) Antibody | Thermo Fischer | Cat# 17-5905-82 |
| PE anti-mouse GL7 Antibody | Thermo Fischer | Cat# 12-5902-82 |
| PE/Cyanine7 anti-mouse GARP Antibody | Thermo Fischer | Cat# 25-9891-82 |
| PE anti-mouse CD80 Antibody | Thermo Fischer | Cat# 12-0801-82 |
| PE/Cyanine7 anti-mouse CD25 Antibody | Thermo Fischer | Cat# 25-0251-82 |
| APC-eFluor 780 anti-mouse HELIOS Antibody | Thermo Fischer | Cat# 47-9883-42 |
| eFluor 660 and PerCP-eFluor 710 anti-mouse GATA3 Antibody | Thermo Fischer | Cat# 50-9966-42 Cat# 46-9966-42 |
| PE anti-mouse Ki-67 Antibody | Thermo Fischer | Cat# 12-5698-82 |
| PE anti-mouse Neuropilin-1 (CD304) Antibody | Thermo Fischer | Cat# 12-3041-82 |
| PE anti-mouse RORγt Antibody | Thermo Fischer | Cat# 12-6981-82 |
| BV421 Anti-Mouse RORγt Antibody | BD Biosciences | Cat# 562894 |
| PE/Cyanine7 anti-human CD127 (IL-7Rα) Antibody | Biolegend | Cat# 351320 |
| APC/Cyanine7 anti-human CD3 Antibody | Biolegend | Cat# 344818 |
| PE anti-human LAP (TGF-β1) Antibody | Biolegend | Cat# 349704 |
| PerCP-Cyanine5.5 anti-human CD4 Antibody | Thermo Fischer | Cat# 45-0049-42 |
| APC anti-human FOXP3 Antibody | Thermo Fischer | Cat# 17-4776-42 |
| FITC anti-human CD25 Antibody | BD Biosciences | Cat# 347643 |
| Critical Commercial Assays | ||
| MCPT-1 (mMCP-1) Mouse Uncoated ELISA Kit | Thermo fisher | Cat# 7400488-7503-86 |
| Mouse TGF-beta 1 DuoSet ELISA | R&D Systems | Cat# DY1679-05 |
| RNeasy Mini Kit | QIAGEN | Cat# 74106 |
| Q5® Site-Directed Mutagenesis Kit | NEB | Cat# E0554S |
| Plasmid | Addgene | Cat# 101762 |
| Experimental Models: Organisms/Strains | ||
| BALB/cByJ | Jax | Stock No: 000651 |
| Il4raF709Foxp3YFPcre | Talal Chatila, Boston Children’s Hospital | N/A |
| Foxp3EGFPcreR26YFP/YFP | In this paper | N/A |
| Foxp3EGFPcreTgfb1fl/flR26YFP/YFP | In this paper | N/A |
| Foxp3YFPcre | Jax | Stock No: 016959 |
| Il4raF709Foxp3YFPcreIl4-Il13fl/fl | PMID: 25769611 | N/A |
| Il4raF709Foxp3YFPcreTgfb1Tg | In this paper | N/A |
| Il4raF709Foxp3YFPcre Stat6–/– | PMID: 25769611 | N/A |
| Foxp3YFPcreTgfb1fl/fl | In this paper | N/A |
| DO11.10 | Jax | N/A |
| DO11.10Foxp3EGFPcreTgfb1fl/fl | In this paper | N/A |
| Mcpt5creTgfbr2Δ/Δ | In this paper | N/A |
| Foxp3YFPcreTgfbr2Δ/Δ | PMID: 28423340 | N/A |
| Foxp3EGFPcre | Jax | Stock No: 023161 |
| Tgfb1fl/fl | Jax | Stock No: 010721 |
| Oligonucleotides | ||
| Tgfb1 TaqMan Assays | Thermo Fisher | Mm00441729_g1 |
| Tgfb2 TaqMan Assays | Thermo Fisher | Mm00436955_m1 |
| Tgfb3 TaqMan Assays | Thermo Fisher | Mm00436960_m1 |
| Software and Algorithms | ||
| GraphPad Prism 7 | GraphPad Software | N/A |
| FlowJo 10.4.2 | Tree Star | https://www.flowjo.com/solutions/flowjo/downloads |
Acknowledgements.
This work was supported by NIH NIAID grants 5R01AI1269151 and 5R01AI085090 (T.A.C.), 5R01AI119918 (to H.C.O), NIDDK grant No. P30DK056338 (to L.B.), T32 AI007512 (O.T.B.), the Rao Chakravorti Family Fund (H.C.O.), the Bunning Foundation (H.C.O., T.A.C.) and DBT/Wellcome Trust India Alliance Intermediate Fellowship IA/I/19/1/504276 (K.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement. L.B., T.A.C., A.A.-G. and R.R. are inventors on published US patent No. US10391131B2, submitted by The Brigham and Women’s Hospital, Inc. and Children’s Medical Center Corporation, that covers methods and compositions for the prevention and treatment of food allergy using microbial treatments. T.A.C, E.S.-V., A.A.-G. and R.R. have pending patent applications related to the use of probiotics in enforcing oral tolerance in food allergy (Nos. 62/798,224). L.B and T.A.C. and R.R. are Co-founders of and/or have equity in Paretobio. The other authors declare no competing interests.
Supplementary Materials. Fig S1 – S7; Table S1.
References
- Abdel-Gadir A, Schneider L, Casini A, Charbonnier LM, Little SV, Harrington T, Umetsu DT, Rachid R, and Chatila TA (2018). Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy 48, 825–836. [Europe PMC free article] [Abstract] [Google Scholar]
- Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, Wang L, Li N, Crestani E, Spielman S, et al. (2019). Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med 25, 1164–1174. [Europe PMC free article] [Abstract] [Google Scholar]
- Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Dejardin F, Sparwasser T, Berard M, Cerf-Bensussan N, and Eberl G (2019). A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 50, 1276–1288 e1275. [Abstract] [Google Scholar]
- Apostolou I, and von Boehmer H (2004). In vivo instruction of suppressor commitment in naive T cells. J Exp Med 199, 1401–1408. [Europe PMC free article] [Abstract] [Google Scholar]
- Azhar M, Yin M, Bommireddy R, Duffy JJ, Yang J, Pawlowski SA, Boivin GP, Engle SJ, Sanford LP, Grisham C, et al. (2009). Generation of mice with a conditional allele for transforming growth factor beta 1 gene. Genesis 47, 423–431. [Europe PMC free article] [Abstract] [Google Scholar]
- Bach JF (2002). The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347, 911–920. [Abstract] [Google Scholar]
- Bach JF (2018). The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18, 105–120. [Abstract] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, and Greenleaf WJ (2015). ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol 109, 21 29 21–21 29 29. [Europe PMC free article] [Abstract] [Google Scholar]
- Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, and Oettgen HC (2013). Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal Immunol 6, 740–750. [Europe PMC free article] [Abstract] [Google Scholar]
- Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, and Oettgen HC (2014). Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity 41, 141–151. [Europe PMC free article] [Abstract] [Google Scholar]
- Charbonnier LM, Cui Y, Stephen-Victor E, Harb H, Lopez D, Bleesing JJ, Garcia-Lloret M, Chen K, Ozen A, Carmeliet P, et al. (2019). Functional Reprogramming of Regulatory T cells in the absence of Foxp3. Nat Immunol (in press). [Europe PMC free article] [Abstract] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764. [Europe PMC free article] [Abstract] [Google Scholar]
- Cuende J, Lienart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, Huygens C, Colau D, Somja J, Delvenne P, et al. (2015). Monoclonal antibodies against GARP/TGF-beta1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Science translational medicine 7, 284ra256. [Abstract] [Google Scholar]
- Edwards JP, Hand TW, Morais da Fonseca D, Glass DD, Belkaid Y, and Shevach EM (2016). The GARP/Latent TGF-beta1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance. Eur J Immunol 46, 1480–1489. [Europe PMC free article] [Abstract] [Google Scholar]
- Esterhazy D, Loschko J, London M, Jove V, Oliveira TY, and Mucida D (2016). Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat Immunol 17, 545–555. [Europe PMC free article] [Abstract] [Google Scholar]
- Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, et al. (2005). TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol 174, 5987–5993. [Europe PMC free article] [Abstract] [Google Scholar]
- Gorelik L, and Flavell RA (2000). Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181. [Abstract] [Google Scholar]
- Grant CR, Liberal R, Mieli-Vergani G, Vergani D, and Longhi MS (2015). Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet-unanswered questions. Autoimmun Rev 14, 105–116. [Abstract] [Google Scholar]
- Grutzkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, and Henz BM (2004). LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry. Part A : the journal of the International Society for Analytical Cytology 61, 62–68. [Abstract] [Google Scholar]
- Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, and Li MO (2011). Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity 34, 396–408. [Europe PMC free article] [Abstract] [Google Scholar]
- Hall BE, Zheng C, Swaim WD, Cho A, Nagineni CN, Eckhaus MA, Flanders KC, Ambudkar IS, Baum BJ, and Kulkarni AB (2010). Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Laboratory investigation; a journal of technical methods and pathology 90, 543–555. [Europe PMC free article] [Abstract] [Google Scholar]
- Harb H, Amarasekera M, Ashley S, Tulic MK, Pfefferle PI, Potaczek DP, Martino D, Kesper DA, Prescott SL, and Renz H (2015). Epigenetic Regulation in Early Childhood: A Miniaturized and Validated Method to Assess Histone Acetylation. Int Arch Allergy Immunol 168, 173–181. [Abstract] [Google Scholar]
- Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, and Williams CB (2009). A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol 182, 3461–3468. [Europe PMC free article] [Abstract] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. (2007). Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39, 311–318. [Abstract] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Molecular cell 38, 576–589. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, and Grusby MJ (1996). Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity 4, 313–319. [Abstract] [Google Scholar]
- Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, Wang S, and Chen W (2017). Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 46, 660–674. [Abstract] [Google Scholar]
- Kotlarz D, Marquardt B, Baroy T, Lee WS, Konnikova L, Hollizeck S, Magg T, Lehle AS, Walz C, Borggraefe I, et al. (2018). Human TGF-beta1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat Genet 50, 344–348. [Europe PMC free article] [Abstract] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nature methods 9, 357–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Li MO, Wan YY, and Flavell RA (2007). T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26, 579–591. [Abstract] [Google Scholar]
- Lienart S, Merceron R, Vanderaa C, Lambert F, Colau D, Stockis J, van der Woning B, De Haard H, Saunders M, Coulie PG, et al. (2018). Structural basis of latent TGF-beta1 presentation and activation by GARP on human regulatory T cells. Science 362, 952–956. [Abstract] [Google Scholar]
- Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, and Chatila TA (2005). Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol 116, 1106–1115. [Abstract] [Google Scholar]
- Mani V, Bromley SK, Aijo T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-beta to precondition naive CD8(+) T cells for tissue-resident memory fate. Science 366. [Europe PMC free article] [Abstract] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, and Rudensky AY (2005). TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201, 1061–1067. [Europe PMC free article] [Abstract] [Google Scholar]
- McCarron MJ, and Marie JC (2014). TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest 124, 4375–4386. [Europe PMC free article] [Abstract] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, and Curotto de Lafaille MA (2005). Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 115, 1923–1933. [Europe PMC free article] [Abstract] [Google Scholar]
- Murphy KM, Heimberger AB, and Loh DY (1990). Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250, 1720–1723. [Abstract] [Google Scholar]
- Nasrallah R, Imianowski CJ, Bossini-Castillo L, Grant FM, Dogan M, Placek L, Kozhaya L, Kuo P, Sadiyah F, Whiteside SK, et al. (2020). A distal enhancer at risk locus 11q13.5 promotes suppression of colitis by Treg cells. Nature 583, 447–452. [Europe PMC free article] [Abstract] [Google Scholar]
- Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, and Chatila TA (2015). Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 42, 512–523. [Europe PMC free article] [Abstract] [Google Scholar]
- Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, Chehoud C, Kuczynski J, DeSantis T, Warrington J, et al. (2013). A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol 131, 201–212. [Europe PMC free article] [Abstract] [Google Scholar]
- Noval Rivas M, and Chatila TA (2016). Regulatory T cells in allergic diseases. J Allergy Clin Immunol 138, 639–652. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. (2015). MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993. [Abstract] [Google Scholar]
- Platts-Mills TA (2015). The allergy epidemics: 1870–2010. J Allergy Clin Immunol 136, 3–13. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, and Kiela PR (2012). Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 189, 3878–3893. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramirez F, Dundar F, Diehl S, Gruning BA, and Manke T (2014). deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42, W187–191. [Europe PMC free article] [Abstract] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [Europe PMC free article] [Abstract] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, and Mesirov JP (2011). Integrative genomics viewer. Nat Biotechnol 29, 24–26. [Europe PMC free article] [Abstract] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr., et al. (2008). Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558. [Abstract] [Google Scholar]
- Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, and Roers A (2008). Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res 17, 307–315. [Europe PMC free article] [Abstract] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. (2015). MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997. [Europe PMC free article] [Abstract] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, and et al. (1992). Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699. [Europe PMC free article] [Abstract] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1, 4. [Europe PMC free article] [Abstract] [Google Scholar]
- Stephen-Victor E, Crestani E, and Chatila TA (2020). Dietary and Microbial Determinants in Food Allergy. Immunity 53, 277–289. [Europe PMC free article] [Abstract] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, and Belkaid Y (2007). Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204, 1775–1785. [Europe PMC free article] [Abstract] [Google Scholar]
- Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, Mathias C, Kim HY, Umetsu DT, Oettgen HC, and Chatila TA (2010). In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol 125, 1128–1136 e1128. [Europe PMC free article] [Abstract] [Google Scholar]
- Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, and Shevach EM (2009). GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A 106, 13445–13450. [Europe PMC free article] [Abstract] [Google Scholar]
- Travis MA, and Sheppard D (2014). TGF-beta activation and function in immunity. Annu Rev Immunol 32, 51–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Voehringer D, Wu D, Liang HE, and Locksley RM (2009). Efficient generation of long-distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC biotechnology 9, 69. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu D, Luo Y, Guo W, Niu Q, Xue T, Yang F, Sun X, Chen S, Liu Y, Liu J, et al. (2017). Lkb1 maintains Treg cell lineage identity. Nature communications 8, 15876. [Europe PMC free article] [Abstract] [Google Scholar]
- Yeh HW, Hsu EC, Lee SS, Lang YD, Lin YC, Chang CY, Lee SY, Gu DL, Shih JH, Ho CM, et al. (2018). PSPC1 mediates TGF-beta1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nature cell biology 20, 479–491. [Abstract] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, and Bluestone JA (2008). Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med 205, 1983–1991. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.immuni.2020.10.002
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1074761320304143/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Pathogenic mechanisms in the evolution of food allergy.
Immunol Rev, 326(1):219-226, 17 Sep 2024
Cited by: 0 articles | PMID: 39285835 | PMCID: PMC11488529
Review Free full text in Europe PMC
A review of CD4+ T cell differentiation and diversity in dogs.
Vet Immunol Immunopathol, 275:110816, 21 Aug 2024
Cited by: 0 articles | PMID: 39173398
Review
Oral tolerance to dietary antigens and Foxp3<sup>+</sup> regulatory T cells.
Immunol Rev, 326(1):8-16, 25 Jul 2024
Cited by: 0 articles | PMID: 39054615
Review
SIRPG promotes lung squamous cell carcinoma pathogenesis via M1 macrophages: a multi-omics study integrating data and Mendelian randomization.
Front Oncol, 14:1392417, 04 Jun 2024
Cited by: 0 articles | PMID: 38894865 | PMCID: PMC11183323
Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion.
J Inflamm Res, 17:2697-2710, 01 May 2024
Cited by: 1 article | PMID: 38707955 | PMCID: PMC11070170
Review Free full text in Europe PMC
Go to all (54) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enforced ROR(gamma)t expression in haematopoietic stem cells increases regulatory T cell number, which reduces immunoreactivity and attenuates hypersensitivity in vivo.
Asian Pac J Allergy Immunol, 29(1):86-93, 01 Mar 2011
Cited by: 4 articles | PMID: 21560493
Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy.
Immunity, 42(3):512-523, 10 Mar 2015
Cited by: 206 articles | PMID: 25769611 | PMCID: PMC4366316
Dysregulated development of IL-17- and IL-21-expressing follicular helper T cells and increased germinal center formation in the absence of RORγt.
FASEB J, 30(2):761-774, 23 Oct 2015
Cited by: 18 articles | PMID: 26499265
Dietary and Microbial Determinants in Food Allergy.
Immunity, 53(2):277-289, 01 Aug 2020
Cited by: 29 articles | PMID: 32814026 | PMCID: PMC7467210
Review Free full text in Europe PMC
Funding
Funders who supported this work.
DBT/Wellcome Trust India Alliance (1)
Parsing the regulatory role of topoisomerase 1 in immune system
Dr Kushagra Bansal, Jawaharlal Nehru Centre For Advanced Scientific Research
Grant ID: IA/I/19/1/504276
NIAID NIH HHS (4)
Grant ID: R01 AI085090
Grant ID: R01 AI119918
Grant ID: R01 AI126915
Grant ID: T32 AI007512
NIDDK NIH HHS (2)
Grant ID: P30 DK034854
Grant ID: P30 DK056338