Abstract
Background
In Germany, public interest in a vegan diet is steadily growing. There are, however, no current data on the macro- and micronutrient status of vegans.Methods
In a cross-sectional study entitled "The Risks and Benefits of a Vegan Diet" (RBVD), we investigated the dietary intake, basic laboratory parameters, vitamin status, and trace-element status of 36 vegans and 36 persons on an omnivorous diet. Each group consisted of 18 men and 18 women aged 30-60.Results
Nearly all the vegans and one-third of the persons on a mixed diet had consumed supplements in the previous 4 weeks. Vegans and nonvegans had similar energy intake but differed in the intake of both macronutrients (e.g., dietary fiber) and micronutrients (e.g., vitamins B12, B2, D, E, and K, as well as folate, iodine, and iron). There were no intergroup differences in the biomarkers of vitamin B12, vitamin D, or iron status. The ferritin values and blood counts indicated iron deficiency in four vegans and three non-vegans. Measurements in 24-hour urine samples revealed lower calcium excretion and markedly lower iodine excretion in vegans compared to non-vegans; in one-third of the vegans, iodine excretion was lower than the WHO threshold value (<20 μg/L) for severe iodine deficiency.Conclusion
Vitamin B12 status was similarly good in vegans and non-vegans, even though the vegans consumed very little dietary B12. This may be due to the high rate of supplementation. The findings imply a need to also assure adequate iodine intake in the population, especially among persons on a vegan diet.Free full text

Vitamin and Mineral Status in a Vegan Diet
Abstract
Background
In Germany, public interest in a vegan diet is steadily growing. There are, however, no current data on the macro- and micronutrient status of vegans.
Methods
In a cross-sectional study entitled “The Risks and Benefits of a Vegan Diet” (RBVD), we investigated the dietary intake, basic laboratory parameters, vitamin status, and trace-element status of 36 vegans and 36 persons on an omnivorous diet. Each group consisted of 18 men and 18 women aged 30–60.
Results
Nearly all the vegans and one-third of the persons on a mixed diet had consumed supplements in the previous 4 weeks. Vegans and non-vegans had similar energy intake but differed in the intake of both macronutrients (e.g., dietary fiber) and micronutrients (e.g., vitamins B12, B2, D, E, and K, as well as folate, iodine, and iron). There were no intergroup differences in the biomarkers of vitamin B12, vitamin D, or iron status. The ferritin values and blood counts indicated iron deficiency in four vegans and three non-vegans. Measurements in 24-hour urine samples revealed lower calcium excretion and markedly lower iodine excretion in vegans compared to non-vegans; in one-third of the vegans, iodine excretion was lower than the WHO threshold value (<20 µg/L) for severe iodine deficiency.
Conclusion
Vitamin B12 status was similarly good in vegans and non-vegans, even though the vegans consumed very little dietary B12. This may be due to the high rate of supplementation. The findings imply a need to also assure adequate iodine intake in the population, especially among persons on a vegan diet.
In recent years, the interest in a vegan diet, avoiding all foods of animal origin, has been growing steadily in Germany. The results of market research surveys indicate that currently approximately 6 million German citizens follow a vegetarian diet (vegetarians) and almost 1 million a vegan diet (vegans) (1, 2). Data of the 7-day Adventist Health Study from the US describe positive effects of these types of diets against the development of obesity, hypertension (lacto-ovo vegetarians: relative risk [RR] 0.45; 95% confidence intervals: [0.44; 0.47]; vegans: RR 0.25 [0.22; 0.28]); diabetes (lacto-ovo vegetarians RR: 0.39 [0.36; 0.42]; vegans RR: 0.22 [0.18; 0.28]) (3) and cardiovascular mortality in males (lacto-ovo vegetarians RR 0.77 [0.59; 0.99]; vegans RR 0.58 [0.38; 0.89]) (4, 5). In addition, a recent review with meta-analysis has shown that a vegetarian diet is associated with a reduced risk of ischemic heart disease (RR: 0.75 [0.68; 0.82]) and cancer (RR: 0.92 [0.87; 0.98]), and a vegan diet with a decreased risk of cancer (RR: 0.85 [0.75; 0.95]) compared to an omnivorous diet, even after adjusting for key confounding factors, such as smoking and body mass index (6). Thus, a meat-free diet would be desirable from the perspective of the health of the entire population. Furthermore, reduced consumption of foods of animal origin could contribute to ensuring food security in the future (7) and combating climate change (8).
However, risks associated with a purely vegan diet are under discussion as well. In its vegan diet position paper, the German Nutrition Society (DGE, Deutsche Gesellschaft für Ernährung) describes the following substances as critical nutrients: vitamin B12, vitamin B2 (riboflavin) and vitamin D, protein (essential amino acids), long-chain n-3 fatty acids, as well as calcium and the trace elements iron, iodine, zinc, and selenium (9). By contrast, a vegan diet is expected to ensure a good supply of vitamin C, vitamin E, thiamine and folate, the minerals magnesium and potassium, as well as dietary fiber and secondary plant compounds (9, 10). The lower intake of saturated fatty acids and cholesterol is also regarded as beneficial (10). Up-to-date data on the intake of micronutrients and macronutrients among vegans in Germany are currently not available. Thus, the aim of this cross-sectional study of the German Federal Institute for Risk Assessment (BfR, Bundesinstitut für Risikobewertung) was to provide first insights into the current micronutrient status in a vegan diet compared to an omnivorous diet.
Methods
Study design
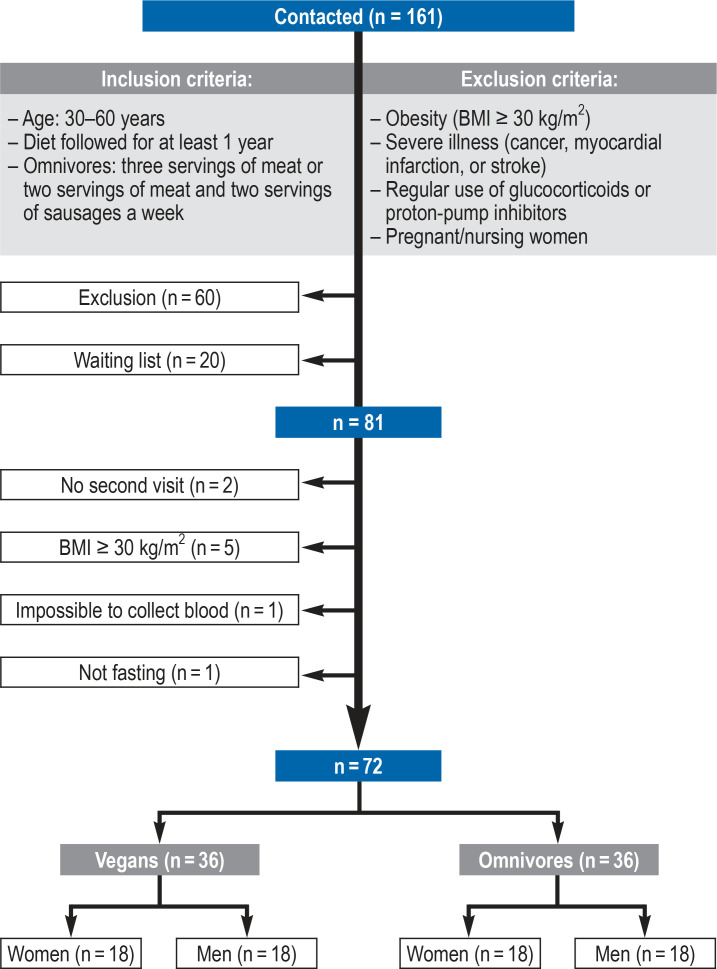
The 72 participants of the study “Risks and Benefits of a Vegan Diet“ (RBVD) were recruited in Berlin at the BfR in the period from January to July 2017 (figure 1) (11). The sample size for this study is based on the power calculation for the primary research question (bone health in vegans compared to omnivores). The observed differences in bone health are part of a further planned publication. The study was approved by the Ethics Committee of Charité—Universitätsmedizin Berlin (no. EA4/121/16).
Dietary habits were recorded using three-day weighed food protocol. With the help of the German Nutrient Database (BLS, Bundeslebensmittelschlüssel) Version 3.02, the mean daily intake of macronutrients and micronutrients was calculated (12). Information about age, educational attainment, and lifestyle factors was collected using tablet-based questionnaires. Height and body weight, waist circumference, and blood pressure were measured using a standardized method (eMethods, eTable 1).
eTable 1
| Sample material | Measured by: | Biomarker | Method | Additional information |
| Serum | Saarland University, Homburg, Germany | Vitamin B12 Holotranscobalamin Folate | Electrochemiluminescence immunoassay Chemiluminescent microparticle immunoassay Competitive immunoassay | |
| EDTA plasma | Martin-Luther University, Halle, Germany | Parathyroid hormone | Commercial ELISA test kits | The test measures intact, i.e. biologically active PTH. |
| Serum | Potsdam University, Nuthetal, Germany | Selenium Selenoprotein P | Inductively coupled plasma—tandem mass spectrometry; For quality control, reference serum and Seronorm were also tested. ELISA kit | Kopp JF, Mülle, SM, Pohl G, Lossow K, Kipp AP, Schwerdtle T: A quick and simple method for the determination of six trace elements in mammalian serum samples using ICP-MS/MS. J Trace Elem Med Biol 2019; 54: 221–5. |
| EDTA plasma | Medical laboratory Labor 28 GmbH, Berlin, Germany | Hemoglobin Hematocrit Erythrocytes Leucocytes Lymphocytes Platelets HbA1 c | SLS Photometric Hemoglobin detection (XN) Impedance measurement with hydrodynamic focusing; Fluorescence flow cytometry (XN) Turbidimetric inhibition immunoassay (XN) | www.labor28.de |
| NaF blood | Medical laboratory Labor 28 GmbH, Berlin, Germany | Glucose Homocysteine | Hexokinase/G6p DH testing High performance liquid chromatography with fluorescence detector | www.labor28.de |
| Serum | Medical laboratory Labor 28 GmbH, Berlin, Germany | GGT GOT GPT Creatinine hsCRP Ferritin Zinc | Enzyme International Federation of Clinical Chemistry and Laboratory Medicine Enzymatic method, nephelometry Electrochemiluminescence immunoassay Flame atomic absorption spectrometry | www.labor28.de |
| EDTA-Plasma | Bevital AS, Bergen, Norway | Methylmalonic acid | Gas chromatography–mass spectrometry | Windelberg A, Arseth O, Kvalheim G, Ueland PM: Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography–mass spectrometry. Clin Chem 2005; 51: 2103–9. |
| Vitamin D3 and D2 Vitamin A Alpha-tocopherol Gamma-tocopherol Vitamin K | Liquid chromatography–mass spectrometry/ mass spectrometry | Midttun Ø, Ueland PM: Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2011; 25: 1942–8. Midttun Ø, McCann A, Aarseth O, et al.: Combined measurement of 6 fat-soluble vitamins and 26 water-soluble functional vitamin markers and amino acids in 50 μL of serum or plasma by high-throughput mass spectrometry. Anal Chem 2011; 88: 10427–436. | ||
| B1 Thiamin | Liquid chromatography–mass spectrometry/ mass spectrometry | McCann A, Midttun Ø, Whitfield KC, et al.: Comparable performance characteristics of plasma thiamine and erythrocyte thiamine diphosphate in response to thiamine fortification in rural cambodian women. Nutrients 2017; 9: pii: E676. | ||
| B2 riboflavin B3 nicotinamide B6 pyridoxal 5‘-phosphate | Liquid chromatography—mass spectrometry/ mass spectrometry | Midttun Ø, Hustad S, Ueland PM: Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom,2009; 23: 1371–9. | ||
| 24-hour urine collection | Medical laboratory Labor 28 GmbH, Berlin, Germany | Calcium Creatinine | Flame atomic absorption spectrometry (AAS NovAA®350) Photometry | www.labor28.de |
| 24-hour urine collection | ETH Zurich, Zurich, Switzerland | Iodine | Modified Sandell–Kolthoff reaction | Pino S, Fang S, Braverman L: Ammonium persulfate: a safe alternative oxidising reagent for measuring urinary iodine. Clin Chem 1996; 42: 239–43. |
GOT, glutamic oxaloacetic transaminase; GPT, glutamic oxaloacetic transaminase; hsCRP, high-sensitivity C-reactive protein; SLS, sodium lauryl sulfate
Determination of the micronutrient status using biomarkers
From all participants in the study, 60 mL of blood was obtained. On the same day, a differential blood count was performed and lipid, HbA1c, glucose, liver enzyme, creatinine, homocysteine, (highly sensitive) C-reactive protein, ferritin, and zinc levels were determined in a certified routine laboratory (Labor 28 GmbH, Berlin, Germany). In a 24-hour urine sample, urine creatine and calcium concentrations were determined. All other biochemical analyses were performed on samples stored at a temperature of -80 °C (etable 1).
The vitamin B12 indicator (4cB12), calculated from the concentrations of holotranscobalamin, vitamin B12, homocysteine, and methylmalonic acid, was used to assess the vitamin B12 status (13).
Statistical analysis
The statistical software suite SAS Enterprise Guide Version 7.13 was used for the analysis of the data (eMethods).
Results
Characteristics of study participants
36 vegans and 36 omnivores aged between 30 and 57 years participated in the study. The vegan participants had followed their diet between 1.6 years and 20.2 years. General characteristics of the study participants and important lifestyle factors are summarized in Table 1. Almost all vegans and a third of the omnivores had taken dietary supplements within the preceding four weeks (Figure 2, eTables 2 and and3).3). A comparison of basic biochemical data revealed significantly lower concentrations of total cholesterol and LDL cholesterol among vegans compared to omnivores (table 3).
Table 1
| Parameter | Vegan | Omnivorous | p value |
| Study size, n Female Male | 36 18 18 | 36 18 18 | |
| Age (years) | 37.5 (32.5–44.0) | 38.5 (32.0–46.0) | 0.75 |
| Duration of vegan diet (years) | 4.8 (3.1–8.7) | ||
| Educational attainment Low (no qualifications) Moderate (vocational training) High (university / university of applied sciences) | 0 (0) 11 (30.6) 25 (69.4) | 1 (2.8) 11 (30.6) 24 (66.6) | 0.60 |
| Lifestyle factors | |||
| Physical activity (h/week) | 2.8 (0.9–3.8) | 2.3 (1.2–4.1) | 0.69 |
| Walking (h/week) | 7.0 (5.0–12.0) | 7.0 (3.5–11.8) | 0.15 |
| Never smoker Ex-smoker Smoker | 24 (66.7) 8 (22.2) 4 (11.1) | 21 (58.3) 6 (16.7) 9 (25.0) | 0.30 |
| Anthropometry | |||
| Body mass index (kg/m2) | 22.9 ± 3.2 | 24.0 ± 2.1 | 0.08 |
| Waist circumference men (cm) Waist circumference women (cm) | 84.5 ± 8.9 73.1 ± 6.9 | 86.0 ± 6.1 77.2 ± 6.2 | 0.560 0.07 |
| Blood pressure | |||
| Systolic blood pressure (mm Hg) Diastolic blood pressure (mm Hg) | 111.2 ± 11.0 69.8 ± 7.7 | 114.7 ± 11.9 73.0 ± 7.1 | 0.210 0.07 |
| Supplement intake | 35 (97.2) | 12 (33.3) | < 0.0001 0.0001 |
All results presented as median (interquartile range), mean ± standard deviation or number (percentage), p value for Mann–Whitney U test, t-test, chi-square test/Fisher’s exact test
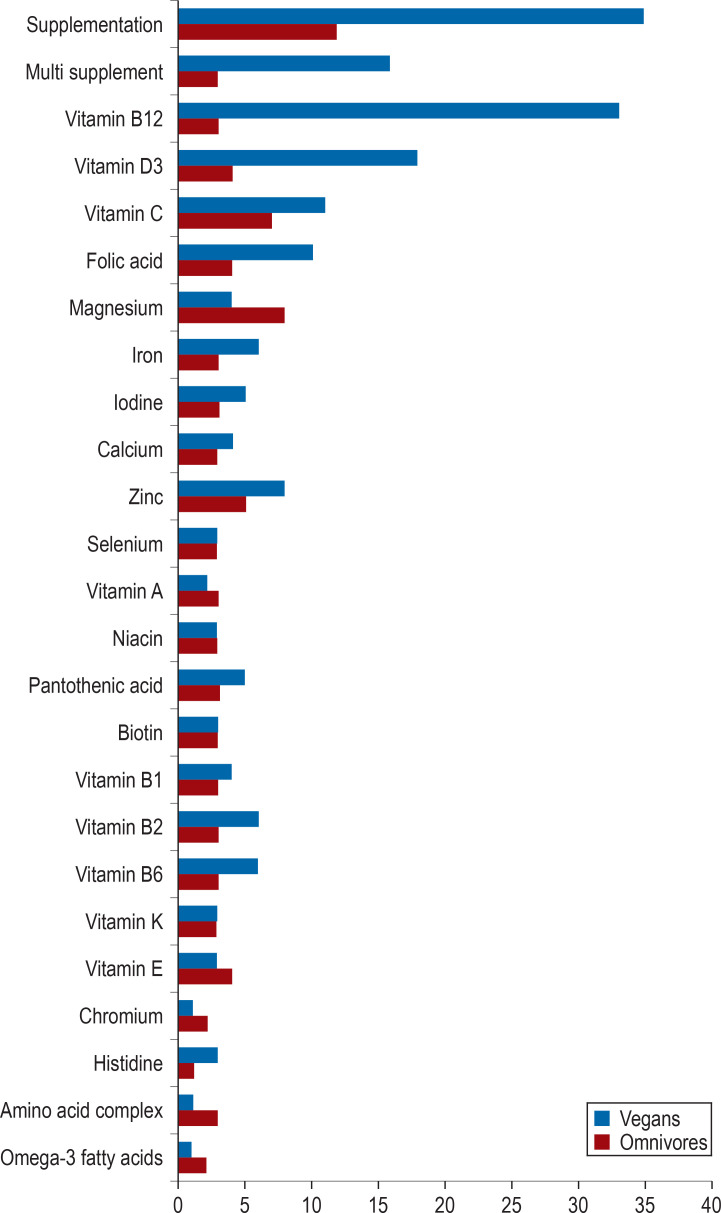
Supplement use among the participants of the cross-sectional RBVD study within the preceding four weeks
Table 3
| Parameter | Vegans | Omnivores | p | Reference intervals |
| Hemoglobin (g/dL) | 13.6 ± 1.2 | 13.8 ± 1.3 | 0.47 | F: 12.0–15.4; M: 13.5–17.2 |
| Hematocrit (%) | 40.6 ± 3.0 | 41.2 ± 3.2 | 0.42 | F: 35.5–45.0; M: 39.5–50.5 |
| Red blood cells (T/L) | 4.5 ± 0.5 | 4.7 ± 0.3 | 0.29 | F: 3.9–5.15; M: 4.3–5.75 |
| MCV (fl) | 91 (88–92) | 89 (85–91) | 0.09 | 80–99 |
| MCHC (g/dL) | 33.4 ± 0.9 | 33.4 ± 1.2 | 0.89 | 31.5–36.0 |
| Leucocytes (G/L) | 4.7 (4.1–6.4) | 5.2 (4.6–6.5) | 0.15 | 3.6–10.2 |
| Lymphocytes (%) | 31 ± 10 | 34 ± 8 | 0.18 | 20–44 |
| Platelets (G/L) | 215 ± 70 | 239 ± 49 | 0.11 | 150–370 |
| Total protein (g/dL) | 6.8 ± 0.4 | 7.0 ± 0.3 | 0.21 | 6.2–8.3 |
| hsCRP (mg/L) | 0.39 (0.21–0.88) | 0.63 (0.24–1.74) | 0.25 | <1.0 |
| Glucose (mg/dL) | 81 (78–87) | 83 (77–90) | 0.41 | 55–100 |
| HbA1c (%) | 5.1 (5.0–5.2) | 5.2 (5.1–5.4) | 0.09 | <6.5 |
| GGT (U/L) | 15 (12–20) | 18 (14–24) | 0.07 | F: <42; M: <71 |
| AST (U/L) | 23 (19–25) | 22 (18–27) | 0.65 | F: <35; M: <50 |
| ALT (U/L) | 21 (17–27) | 19 (15–28) | 0.57 | F: <35; M: <50 |
| Creatinine (mg/dL) | 0.82 ± 0.15 | 0.89 ± 0.15 | 0.042 | F: <0.95; M: <1.17 |
| Thyroid-stimulating hormone (TSH) (mU/L) | 2.1 ± 0.9 | 2.4 ± 1.1 | 0.34 | 0.27–4.2 |
| Parathyroid hormone (pg/mL) | 53 (40–66) | 51 (27–58) | 0.16 | 15–65 |
| Iron (µg/dL) | 89 ± 31 | 106 ± 39 | 0.053 | 33–193 |
| Ferritin (ng/mL) | 60 (31–84) | 69 (32–114) | 0.23 | F: 15–150; M: 30–400 |
| Cholesterol (mg/dL) | 157 (137–180) | 204 (179–223) | <0.0001 | <200 |
| LDL cholesterol (mg/dL) | 87 (69–97) | 116 (94–136) | 0.001 | |
| HDL cholesterol (mg/dL) | 57 (51–72) | 62 (52–81) | 0.21 | > 40 |
| Triglycerides (mg/dL) | 71 (53–91) | 85 (52–121) | 0.26 | <200 |
| Vitamin B12 (pg/mL) | 458 (295–758) | 363 (308–494) | 0.12 | 197–771 |
| Holotranscobalmin (pmol/L) | 89 (59–205) | 84 (68–100) | 0.35 | <35 |
| Homocysteine (µmol/L) | 8.6 (6.7–11.3) | 8.8 (7.3–10.5) | 0.90 | 5–15 |
| Methylmalonic acid (µmol/L) | 0.17 (0.15–0.22) | 0.18 (0.16–0.21) | 0.62 | <0.26 |
| 4cB12*1 | 0.54 (0.07–1.24) | 0.42 (0.19–0.70) | 0.47 | *1 |
| 25-hydroxyvitamin D (nmol/L) | 68.6 (21.5–88.1) | 45.4 (34.6–68.6) | 0.34 | 20–150 |
| Folate (ng/mL) | 10.9 (7.7–12.8) | 7.8 (6.4–11.2) | 0.03 | 3.9–26.8 |
| Vitamin A (µmol/L) | 1.8 ± 0.3 | 2.1 ± 0.5 | 0.003 | 1.46–2.84 |
| Vitamin A/cholesterol (µmol/mmol) | 0.45 (0.36–0.50) | 0.36 (0.33–0.52) | 0.19 | |
| Alpha-tocopherol (µmol/L) | 28.7 (26.0–34.0) | 34.8 (28.7–41.5) | 0.003 | 18.9–38.8 |
| Alpha-tocopherol/cholesterol (µmo/mmol) | 7.11 (6.51–7.43) | 6.72 (6.08–7.23) | 0.06 | |
| Vitamin K1 (nmol/L) | 1.55 (1.30–2.23) | 0.78 (0.54–1.13) | <0.0001 | 0.1–8.7 |
| Vitamin B1 thiamine (nmol/L) | 7.5 (5.8–8.6) | 6.4 (5.0–7.9) | 0.15 | 7–16 |
| Vitamin B2 riboflavin (nmol/L) | 6.0 (4.4–10.7) | 9.1 (6.8–11.8) | 0.03 | 5–38 |
| Vitamin B3 nicotinamide (nmol/L) | 190 (161–239) | 243 (194–299) | 0.01 | 92–388 |
| Vitamin B6 pyridoxal 5‘-phosphate (nmol/L) | 67 (49–89) | 79 (47–100) | 0.62 | 17–102 |
| Selenium (µg/L) | 68 (60–82) | 77 (68–84) | 0.11 | |
| Selenoprotein P (mg/L) | 3.3 (2.6–4.5) | 5.0 (4.2–5.5) | <0.0001 | |
| Zinc (µg/dL) | 80 ± 12 | 87 ± 13 | 0.008 | 60–120 |
| Iodine (µg/L)*2 | 28 (18–42) | 74 (42–102) | <0.0001 | >100*3 |
| Calcium (mg/L)*2 | 56 (37–73) | 86 (49–166) | 0.004 |
All results reported as median (interquartile range) or mean ± standard deviation; p values according to Mann–Whitney U test or t-test
*1 4cB12, vitamin B12 indicator, combing the four preceding markers, adjusted for age;
(>1.5 increased B12; -0.5 to 1.5 adequate supply; <-0.5 to -2.5 undersupply; <-2.5 deficiency)
*2 Excretion in 24-h urine sample
*3 WHO cut-off value for undersupply; F, women; M, men
eTable 2
| Parameter | Vegans (n = = 36) 36) | Omnivores (n = = 36) 36) | p |
| Vitamins (mg) | |||
| Retinol equ. (µg) | 0.00 (0.00–0.00) | 0.00 (0.00–85.71) | 0.06 |
| Vitamin B12 (µg) | 82.14 (3.86–286.43) | 0.00 (0.00–0.11) | <0.0001 |
| Vitamin D (µg) | 5.00 (0.00–1250.00) | 0.00 (0.00–1.04) | 0.07 |
| Folate (µg) | 0.00 (0.00–85.71) | 0.00 (0.00–41.43) | 0.71 |
| Vitamin B1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.15) | 0.30 |
| Vitamin B2 | 0.00 (0.00–0.00) | 0.00 (0.00–0.13) | 0.76 |
| Vitamin B3 | 0.00 (0.00–0.00) | 0.00 (0.00–1.93) | 0.14 |
| Vitamin B6 | 0.00 (0.00–0.00) | 0.00 (0.00–0.20) | 0.81 |
| Vitamin C | 0.00 (0.00–34.29) | 25.71 (0.00–420.00) | 0.04 |
| Vitamin E | 0.00 (0.00–0.00) | 0.00 (0.00–2.57) | 0.04 |
| Vitamin K | 0.00 (0.00–0.00) | 0.00 (0.00–1.50) | 0.18 |
| Pantothenic acid | 0.00 (0.00–0.00) | 0.00 (0.00–0.40) | 0.46 |
| Minerals (mg) | |||
| Calcium | 0.00 (0.00–0.00) | 0.00 (0.00–17.14) | 0.25 |
| Zinc | 0.00 (0.00–0.00) | 0.00 (0.00–3.04) | 0.23 |
| Iodine | 0.00 (0.00–0.00) | 0.00 (0.00–1.25) | 0.30 |
| Iron | 0.00 (0.00–0.00) | 0.00 (0.00–0.20) | 0.64 |
All values as median and interquartile range (Q1–Q3), p values according to Mann–Whitney U test
eTable 3
| Supplement | n | Vegans | n | Omnivores |
| Vitamin B12 | Vitamin B12 (pg/mL) | Vitamin B12 (pg/mL) | ||
| Yes No | 33 3 | 523 (312–759) 286 (257–456) | 3 33 | 317 (224–521) 362 (310–488) |
| Vitamin B12 | 4cB12 | 4cB12 | ||
| Yes No | 33 3 | 0.57 (0.09–1.25) 0.08 (−0.17–0.43) | 3 33 | 0.24 (−0.73–0.69)0.42 (0.20–0.70) |
| Vitamin D | 25-hydroxyvitamin D (nmol/L) | 25-hydroxyvitamin D (nmol/L) | ||
| Yes No | 18 18 | 88 (76–97) 24 (19–60) | 4 32 | 66 (46–79) 44 (35–69) |
| Folate | Folate (ng/mL) | Folate (ng/mL) | ||
| Yes No | 10 26 | 10.9 (8.5–19.6) 10.9 (7.6–12.3) | 4 32 | 14.3 (9.5–18.5) 7.5 (6.2–10.6) |
| Vitamin B1 | Thiamine (nmol/L) | Thiamine (nmol/L) | ||
| Yes No | 4 32 | 3.7 (2.4–5.5) 2.1 (1.6–2.8) | 3 33 | 2.4 (2.2–5.0) 1.8 (1.4–2.4) |
| Vitamin B2 | Riboflavin (nmol/L) | Riboflavin (nmol/L) | ||
| Yes No | 6 30 | 8.2 (5.9–18.1) 5.6 (4.3–10.2) | 3 33 | 13.2 (12.2–33.5) 8.5 (6.8–10.2) |
| Vitamin B3 | Nicotinamide (nmol/L) | Nicotinamide (nmol/L) | ||
| Yes No | 3 33 | 234 (123–306) 188 (162–228) | 3 33 | 252 (245–282) 242 (193–305) |
| Vitamin B6 | Pyridoxal 5‘-phosphate (nmol/L) | Pyridoxal 5‘-phosphate (nmol/L) | ||
| Yes No | 6 30 | 92 (54–154) 67 (47–84) | 3 33 | 132 (62–218) 78 (46–95) |
| Vitamin E | Alpha-tocopherol (µmol/L) | Alpha-tocopherol (µmol/L) | ||
| Yes No | 3 33 | 32.3 (27.2–34.8) 28.5 (25.8–33.9) | 4 32 | 37.6 (35.5–40.6) 33.2 (28.5–41.5) |
| Vitamin K | Vitamin K1 (nmol/L) | Vitamin K1 (nmol/L) | ||
| Yes No | 3 33 | 1.54 (1.40–1.62) 1.55 (1.29–2.24) | 3 33 | 1.60 (0.97–1.70) 0.75 (0.53–1.08) |
| Calcium* | Calcium (mg/L) | Calcium (mg/L) | ||
| Yes No | 4 32 | 49 (34–42) 57 (35–77) | 3 33 | 75 (42–328) 87 (50–164) |
| Zinc | Zinc (µg/dL) | Zinc (µg/dL) | ||
| Yes No | 5 28 | 85 (81–92) 77 (71–89) | 5 31 | 89 (85–91) 83 (81–94) |
| Selenium | Selenium (µg/L) | Selenium (µg/L) | ||
| Yes No | 3 33 | 74 (68–101) 67 (58–81) | 3 33 | 82 (70–88) 77 (67–82) |
| Selenium | Selenoprotein P(mg/L) | Selenoprotein P (mg/L) | ||
| Yes No | 3 33 | 5.1 (3.4–6.2) 3.0 (2.5–3.8) | 3 33 | 5.2 (4.9–5.9) 5.0 (4.1–5.5) |
| Iodine* | Iodine (µg/L) | Iodine (µg/L) | ||
| Yes No | 5 31 | 46 (36–56) 27 (17–36) | 3 33 | 49 (34–166) 78 (43–100) |
| Iron | Ferritin (ng/mL) | Ferritin (ng/mL) | ||
| Yes No | 6 30 | 86 (39–110) 56 (30–82) | 3 33 | 49 (31–64) 74 (33–121) |
All values as median and interquartile range (Q1–Q3); *excretion in 24-hour urine
Macro- and micronutrient intake
Despite the almost identical energy intake, differences between vegans and omnivores were observed with respect to both macronutrients and micronutrients. Of note is the considerably higher intake of dietary fiber, vitamin E, vitamin K, and folate as well as iron and the very low intake of vitamin B12, vitamin D, and iodine among vegans compared to omnivores (table 2).
Table 2
| Parameter | Vegans | Omnivores | p | D-A-CH recommen- dation* |
| Energy (kcal) | 2 270 (1 800–2 762) | 2 386 (2 081–2 737) | 0.32 | |
| Dietary fiber (g) | 46 (34–56) | 24 (19–30) | <0.0001 | |
| Protein (g) | 72 (55–92) | 86 (71–107) | 0.02 | |
| Carbohydrates (g) | 259 (212–371) | 230 (199–291) | 0.12 | |
| Sugar (g) | 54 (37–69) | 47 (35–69) | 0.48 | |
| Fat (g) | 86 (64–111) | 104 (88–143) | 0.004 | |
| Vitamins (mg) | ||||
| Retinol equ (µg) | 1 841 (1 161–2 609) | 1 390 (1 020–1 910) | 0.20 | |
| Vitamin B12 (µg) | 0.29 (0.14–0.85) | 5.22 (4.15–7.96) | <0.0001 | 3.0 |
| Vitamin D (µg) | 0.94 (0.29–1.85) | 2.53 (1.86–4.29) | <0.0001 | 20 |
| Folate (µg) | 446 (311–607) | 296 (249–343) | 0.0005 | 300 |
| Vitamin B1 | 1.72 (1.26–2.05) | 1.28 (1.09–1.68) | 0.049 | F: 1.0 M: 1.2 |
| Vitamin B2 | 1.47 (0.96–2.11) | 1.98 (1.56–2.26) | 0.01 | F: 1.0–1.1 M: 1.3–1.4 |
| Vitamin B3 | 14.2 (11.6–19.4) | 17.7 (14.4–22.3) | 0.02 | 12–14 |
| Vitamin B6 | 2.22 (1.68–2.69) | 1.75 (1.41–2.08) | 0.02 | 1.2–1.5 |
| Vitamin C | 173 (108–276) | 131 (98–174) | 0.12 | 95–110 |
| Vitamin E | 25.9 (16.1–37.6) | 13.4 (11.1–18.6) | <0.0001 | 12–14 |
| Vitamin K | 0.27 (0.17–0.43) | 0.10 (0.07–0.18) | <0.0001 | 0.06–0.08 |
| Pantothenic acid | 4.35 (3.46–5.65) | 5.21 (4.30–6.99) | 0.02 | 6 |
| Minerals (mg) | ||||
| Calcium | 899 (650–1 257) | 1 049 (822–1 457) | 0.04 | 1 000 000 |
| Zinc | 11.4 (8.8–13.4) | 12.3 (10.2–16.3) | 0.046 | 7–10 |
| Iodine | 0.08 (0.05–0.10) | 0.12 (0.08–0.17) | 0.002 | 0.15–0.18 |
| Iron | 22.0 (15.5–26.4) | 14.0 (11.5–17.0) | 0.0001 | 10–15 |
All values as median and interquartile range (Q1–Q3), p values according to Mann–Whitney U test.
*D-A-CH reference value: common reference values of the specialist societies of the three D-A-CH countries (Germany [D], Austria [A], and Switzerland [CH])
F, women; M, men
Micronutrient status (blood and urine)
Among vegans, lower concentrations of vitamin B2, vitamin B3, vitamin E (alpha-tocopherol), vitamin A, selenoprotein P, and zinc in blood as well as a reduced excretion of iodine and calcium in 24-hour urine samples compared to omnivores was observed. By contrast, folate and vitamin K1 blood levels were higher among vegans. Yet, no differences between vegans and omnivores were found with respect to median vitamin B12, 25-hydroxy vitamin D and ferritin concentrations (table 3). In four vegans and three omnivores, however, signs of latent to manifest iron deficiency (lower ferritin levels and blood count changes) were observed. With respect to the vitamin B12 status, the B12 indicator (4cB12) revealed a mild deficiency in two vegans and one omnivore, as well as increased levels in four vegans. The Spearman correlation coefficient for the association between the duration of vegan diet and 4cB12 was 0.30 (p = 0.07).
Riboflavin levels below the reference range were measured in 13 vegans (36%) and 5 omnivores (14%).
Parathyroid hormone (PTH) was measured as an important parameter of calcium, phosphate, and vitamin D metabolism. Ten vegans and three omnivores showed elevated PTH levels (>65 pg/mL). The comparison of calcium excretion found that the excretion in vegans with elevated PTH levels was lower compared to vegans without elevated PTH levels (p = 0.02). There was no correlation between duration of a vegan diet and calcium excretion (r = -0.02, p = 0.88 according to Spearman). With regard to 25-hydroxy vitamin D levels, plasma concentrations of <30 nmol/L (<12 ng/mL) were measured in 12 vegans and 8 omnivores, while plasma levels of <50 nmol/L (<20 ng/mL) were measured in 15 vegans and 19 omnivores. Supplement users showed higher 25-hydroxy vitamin D plasma concentrations (etable 3).
Iodine excretion was lower in vegans compared to omnivores. Only 8% of vegans and 25% of omnivores achieved iodine excretion of ≥100 µg/L. 31% of the vegans excreted less than 20 µg/L. When viewed alone, the screening parameter thyroid-stimulating hormone (TSH) was found abnormal in two vegans and two omnivores with values of >4mU/L.
Discussion
The focus of our study was on vitamin B12, vitamin B2 and vitamin D as well as calcium and the trace elements iron, iodine, zinc, and selenium, since these have been critically discussed with respect to a vegan diet.
Studies on the nutritional status of vegans are very rare (14–18). In addition, most of these studies only collected data on the intake of macronutrients and micronutrients using dietary food records. Only two of these studies—Elorinne et al. from Finnland and Schüpbach et al. from Switzerland—also looked at specific blood parameters (16, 18) (etable 4).
eTable 4
| Study characteristics | Results macronutrient and micronutrient intake (vegans compared to omnivores) | ||||||
| Higher intake | Lower intake | No difference | |||||
| Clarys et al. (2014) (15) | |||||||
| Country: | Belgium | Dietary fiberIron | Alcohol Calcium Cholesterol Energy (kcal) MUFA | ProteinPUFA Salt SFA Total fat | Carbohydrates Sugar | ||
| Vegans/omnivores: | n = = 104 104 / / n n = = 155 155 | ||||||
| Design: | Cross-sectional | ||||||
| Survey method: | FFQ | ||||||
| Kristensen et al. (2015) (17) | |||||||
| Country: | Denmark | Dietary fiberIron Energy (kJ)* 2 Folate Potassium Magnesium PUFA | SaltVitamin B1 Vitamin B6 Vitamin C Vitamin E | Beta-carotenoidsCalcium Cholesterol Iodine Carbohydrates*1 MUFA Phosphate Proteins Selenium SFA | Total fatTrans fatty acids Vitamin A Vitamin B12 Vitamin B2 Vitamin B3 Vitamin D Zinc Sugar | Energy (kJ)*1Carbohydrates*2 | |
| Vegans/omnivores: | n = = 70 70 / / n n = = 1257 1257 | ||||||
| Design: | Cross-sectional | ||||||
| Survey method: | 4-day weighed food protocol | ||||||
| Schüpbach et al. (2017 (18) | |||||||
| Country: | Switzerland | Dietary fiberIron Folic acid Potassium Carbohydrates Magnesium Pantothenic acid | PUFAVitamin B1 Vitamin B6 Vitamin C Vitamin E equ. Sugar | CalciumCholesterol Protein SFA Vitamin B12 Vitamin D | Chlorine Energy (kcal) MUFA Sodium Phosphorus Retinol equ. | Total fatVitamin B2 Vitamin B3 Zinc | |
| Vegans/omnivores: | n = = 53 53 / / n n = = 100 100 | ||||||
| Design: | Cross-sectional | ||||||
| Survey method: | 3-day weighed food protocol | ||||||
| Elorinne et al. (2016) (16) | |||||||
| Country: | Finnland | Dietary fiberIron Folate Carbohydrates | CholesterolProteins Selenium SFA Vitamin B12 | Vitamin B2Vitamin B3 Vitamin D Zinc | Beta-carotenoidsCalcium Energy (MJ) MUFA PUFA | Total fatVitamin A Vitamin B1 Vitamin C Vitamin E | |
| Vegans/omnivores: | n = = 22 22 / / n n = = 19 19 | ||||||
| Design: | Cross-sectional | ||||||
| Survey method: | 3-day weighed food protocol | ||||||
| Allès et al. (2017) (14) | |||||||
| Country: | France | Dietary fiberIron Folate Iodine Potassium Carbohydrates Copper Magnesium | Manganese MUFA PUFA Vitamin A Vitamin B1 Vitamin B6 Vitamin C Vitamin E Vitamin K | Alcohol Calcium Cholesterol Sodium Phosphate Proteins Selenium | SFATotal fat Vitamin B12 Vitamin B2 Vitamin B3 Vitamin D Zinc | Pantothenic acid | |
| Vegans/omnivores: | n = = 789 789 / / n n = = 90664 90664 | ||||||
| Design: | Cross-sectional | ||||||
| Survey method: | 3-day weighed food protocol | ||||||
| Study characteristics | Results macronutrient and micronutrient intake (vegans compared to omnivores) | ||||||
| Increased levels | Reduced levels | No difference | |||||
| Schüpbach et al. (2017) (18) | |||||||
| Country: | Switzerland | FolateMagnesium Vitamin B1 Vitamin C | Iodine (urine)Niacin Vitamin A Vitamin E | Zinc | Beta-carotenoidsBiotin Ferritin Pantothenic acid | SeleniumVitamin B12 Vitamin B2 Vitamin B6 | |
| Vegans/omnivores: | n = = 53 53 / / n n = = 100 100 | ||||||
| Design: | Cross-sectional | ||||||
| Elorinne et al. (2016) (16) | |||||||
| Country: | Finnland | DaidzeinFerulic acid Genistein Caffeic acid | Vanillic acid | Beta-carotenoidsCholesterol Ferritin Iodine (urine) | SeleniumVitamin B12 Vitamin D Vitamin E | Folate Homocysteine | |
| Vegans/omnivores: | n = = 22 22 / / n n = = 19 19 | ||||||
| Design: | Cross-sectional | ||||||
*1 in females;*2 in males
FFQ, Food Frequency Questionnaire; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids
Most vegans are aware that a vegan diet is associated with the risk of vitamin B12 deficiency and vitamin B12 is by far their most frequently taken supplement. Based on the vitamin B12 indicator findings, our study did not observe an increased risk of vitamin B12 deficiency among the vegan participants. Most likely, this is due to the high rate of vitamin B12 supplementation among vegans (92%). However, the proportion of dietary supplement users in our study appears to be high compared to those in recent studies from Germany (19, 20) (74% and 81% supplementation rate, respectively) and Denmark (17) (2/3 supplementation rate); only the data of the Finnish cross-sectional study are comparable (supplementation rate of 91%) (16). On the other hand, increased B12 indicator values were found in four vegan participants. Against the backdrop of recent studies about the association between the intake of vitamin B12 supplements and an increased lung cancer risk (21, 22), the intake of vitamin B12 supplements, which was previously regarded as safe, may need to be re-evaluated.
Vitamin B2 is present in larger amounts in animal products; in addition, its absorption from foods of plant origin is lower. In line with our results, recently conducted cross-sectional studies (14, 16, 17) have shown that the absorbed amounts of vitamin B2 tend to be lower in vegans. In the Swiss study (18), vitamin B2 deficiency was diagnosed based on B2 blood levels in one quarter of vegans and 14% der omnivores. However, little is known as yet about the clinical relevance of B2 levels below the cut-off value; further studies are needed to shed light on this question.
Most of the required amount of vitamin D is produced by sun-exposure–dependent endogenous synthesis and only a minor proportion is contributed by nutrition. Since the intake of vitamin D is mainly from animal products, the lower vitamin D uptake among vegans is not surprising. With a vitamin D supplementation rate of 50% among vegans, the rate in our study is rather high compared to other studies (19, 20). Comparable data are only known from the Finnish study which found that serum levels of vitamin D in vegans were 34% lower compared to omnivores, even though in the Finnish study the vitamin D supplementation rate among vegans was with 68% even higher than in our study (16). Without supplementation, a significantly greater proportion of vegans had subnormal 25-hydroxy vitamin D levels compared to omnivores, highlighting the importance of supplementation, especially in a vegan diet.
For many years, calcium deficiency due to the lack of intake of dairy products was regarded as a key risk in vegans (23). Recent studies on calcium uptake have shown conflicting results, with significant differences between vegans and omnivores in some studies (14, 15, 18) and similar calcium intake amounts in others ([16] and in our study). The observation that calcium intake among vegans is higher in recent years compared to older studies is usually attributed to the intake of fortified foodstuffs. However, the evaluation of calcium intake is also more reliable when it includes the corresponding biomarkers. In this context, the lower calcium excretion and the increased PTH levels of nearly every third vegan participant in our study may be interpreted as evidence of a physiological response to low calcium intake.
Especially in the evaluation of iron intake, the importance of using biomarkers is obvious. The bioavailability of iron depends very much on the source of iron, but also on the simultaneous intake of certain secondary plant compounds or of vitamin C. Divalent heme iron from animal products is absorbed two to three times better than trivalent iron from plants. Vitamin C facilitates the absorption of iron, while the simultaneous intake of phytic acid (for example from pulses and grains) or of polyphenols (tea or coffee) reduce the absorption of iron. Iron deficiency is one of the risks associated with a vegan or plant-based diet which is commonly mentioned (9). Signs of iron deficiency were noted in 11% of the vegan participants, which is in line with the results of the two comparable cross-sectional studies from Finnland and Switzerland (16, 18). However, we also observed insufficient iron intake in 8% of the omnivores which is comparable to the findings in the Swiss study (18).
Corresponding to the lower intake of iodine according to the weighed food protocols, iodine excretion in urine was far below the WHO cut-off value for undersupply of iodine (100 µg/L) in three-quarters of the omnivores and almost all vegans (24). Only 5 vegans supplemented iodine (Figure 2, eTable 3). In line with the findings of low iodine intake (16, 17) and iodine undersupply (16, 18, 25) in other recent studies, the results of our study suggest that it is challenging to ensure adequate iodine intake in vegans as there seems to be a lack of awareness of this potential deficiency. Individual monitoring of iodine intake based on iodine excretion is usually not practicable. In line with the literature (26), our study also showed that TSH is not a very sensitive marker—in only four participants with significantly decreased iodine excretion, TSH levels were found increased. Since iodine deficiency is a major cause for the development of goiter, it would need to be considered whether regular clinical assessments of the thyroid should be performed by ultrasound scans (18, 25). Due to the limited availability of natural iodine sources for vegans, it may also be appropriate to recommend iodine supplementation.
In contrast to the study by Schüpbach et al. which found that almost half of the vegans were deficient in zinc (18), we observed lowered zinc concentrations (<60 µg/dL) only in two vegans in our study.
Since selenium has numerous effects on the immune system, thyroid function and the cardiovascular system, and probably also influences carcinogenesis (27, 28), adequate supply of selenium should be ensured. In our study, we used selenoprotein P to assess the selenium status, in addition to the total blood selenium concentrations. As a selenium transport protein, selenoprotein P appears to be well suited as a biomarker to assess the selenium status, especially in patients with low selenium intake (29). Vegans showed lower selenoprotein P levels compared to the omnivores in our study, but also compared to the selenoprotein P levels in the largest European study on nutrition and cancer (The European Prospective Investigation into Cancer and Nutrition, EPIC) in which a representative number of selenoprotein P measurements was performed (30). The results indicate a lower selenium intake in vegans compared to omnivores and are consistent with the results of the Finnish cross-sectional study (16). Most studies, however, used the plasma concentration of selenium, a parameter for which we found no difference in our study. Based on the current recommendations for selenium intake, considering 60–70 µg/d as adequate (31), a normal selenium intake is assumed with serum concentrations of >50 µg/L. Selenium supplementation carries the risk of overdosing. According to EFSA, an intake of 300 µg selenium per day is considered acceptable in adults (32).
While vitamin K intake was generally good in both groups, vegans were found to have higher concentrations of vitamin K1 compared to omnivores. Further studies are needed to better understand this finding. Here, the discussion focuses on the positive effect of higher vitamin K1 concentrations on bone health as well as a lower risk of type 2 diabetes and cardiovascular disease (33– 35).
A special strength of our study is the excellent comparability between vegans and omnivores which was achieved by matching the subjects for age and sex, by the short recruitment period, and by the inclusion criterion of a BMI below 30 kg/m2. Furthermore, our study is notable for its elaborate dietary assessment, using three-day weighed food protocols, and for measuring a wide range of biomarkers. Limitations of our study include: With 72 participants, it is a rather small cross-sectional study with local data collection in the Berlin area. In addition, participants were mainly selected through postings on notice boards (convenience sample). Therefore, the possibility that these participants were particularly health-conscious cannot be excluded. However, since the same recruitment strategy was used for vegans and omnivores and a BMI ≥ 30 kg/m2 was chosen as an exclusion criterion, it can be assumed that the level of health consciousness was similar in both groups. Thus, no major differences between the two groups were present with regard to lifestyle characteristics. Consequently, the results of our study provide first insights into the current vitamin and mineral status in vegans versus omnivores in the German population.
Further studies, preferably with longitudinal design and a larger number of participants, are needed to, on the one hand, obtain up-to-date information about the nutritional and health status of a vegan population and, on the other hand, evaluate potential long-term health risks and protective effects.
eTable 5
| Potentially critical vitamins and minerals in a vegan diet | Food items of plant origin with high contents |
| Vitamin D | Some mushrooms |
| Vitamin B2 | Nuts, oilseeds, pulses, various types of vegetables (e.g. broccoli, kale), and whole grains |
| Calcium | Vegetables (e.g. broccoli, kale, rocket salad), nuts, pulses, tofu |
| Iron | Pulses, nuts, oilseeds, whole grains, and various types of vegetable (e.g. spinach, salsifies), berries |
| Iodine | Iodized table salt and food prepared with it, algae* |
| Zinc | Whole grains, pulses, nuts, oilseeds |
| Selenium | Cabbage (e.g. broccoli, white cabbage), bulb vegetables (e.g. garlic, onions), mushrooms, asparagus, pulses |
| Vitamin B12 | – |
This table was modified from to Richter et al. (9). It should be noted that by the consumption of the various food items adequate intake of the respective nutrients is not necessarily ensured.
*Algae may contain iodine in amounts that, when consumed in great quantities, exceed the maximum tolerable intake of iodine per day.
Supplementary Material
eMethods
Tthe participants of the present (RBVD) study were individuals who had responded to advertisements in (organic/vegan) supermarkets and had contacted the German Federal Institute for Risk Assessment (BfR) via phone or e-mail (n = 161) (figure 1). Following an initial screening by phone, 36 vegans and 36 omnivores (sex-matched and age-matched [to 3 years]) were included in the study (11). The study participants visited the study center twice. During the initial visit, informed consent was obtained and the method of recording the daily food intake (three-day weighed food records) was explained. Also, the container for a 24-h urine collection was handed out to each participant. During the second visit, information about lifestyle factors and the intake of supplements was obtained, besides a physical examination and fasting blood collection.
Determination of basic characteristics, lifestyle factors and dietary data
Information about age, educational attainment and lifestyle factors was collected using tablet-based questionnaires. Trained staff members performed anthropometric and blood pressure measurements. Physical activity was determined using the questionnaire of the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam study and comprises the total of the average hours per week spent with cycling, sports, or gardening (12). Dietary habits were determined between the first and the second visit, using three-day weighed food protocols on two week days and one weekend day. Participants received a comprehensive introduction, a nutrition diary, and an electronic kitchen scale. Participants were instructed to document for all foodstuffs and beverages detailed information about the time and the place of consumption, brand name, exact product name, packaging, condition at the time of purchase, organic yes/no, weighed quantity and remaining quantity, if any, on the specified days. In addition, participants were asked about their intake of supplements and medications. After completion of the study, the data of the nutrition diaries were entered into the software EAT (University of Paderborn, Version 3.5.5) which assigns a code of the German Nutrient Food Code and Data Base (BLS, Bundeslebensmittelschlüssel, version 3.02) to each food item and calculates the mean daily intake (12).
Power of the study
The sample size was calculated based on the assumption of a clinically relevant difference in bone health of at least 5% (estimated based on differences in the means of broadband ultrasound attenuation [BUA]) between vegans and omnivores. At a significance level of 5% and a power of 80%, a total of 72 participants (36 vegans, 36 omnivores) was required (G*power, independent-samples t-test).
Statistical analysis
The statistical software suite SAS Enterprise Guide Version 7.13 (SAS Institute, Cary, N.C., USA) was used for the analysis of the data. All results were reported either as mean and standard deviation or median and interquartile range, separately for the two diets evaluated. Categorical variables were reported as percentages. Accordingly, the Student’s t-test or the Mann–Whitney U test were used for continuous variables, while the chi-square test was used for categorical variables. Since the two groups matched very well in important characteristics, including age, sex, BMI, energy intake, education, and the recruitment period was short (almost uniform distribution in both groups over the months), no multivariable analyses were performed.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Acknowledgement
We thank all participants for their participation in the study. We also thank Elektra Polychronidou for her great commitment in the recruitment of the participants and the conduct of the study. We thank Corinna Genrich and Christel Rozycki for their fast processing of the biospecimens. We extend our thanks to Dr. Mark Lohmann and his expert group for their support in developing the lifestyle questionnaire and Dr. Oliver Lindtner and his expert group for their support in collecting the nutritional data using weighed food protocols. Our special thanks goes to Clarissa Lage-Barbosa and Marjolein Haftenberger (Robert Koch Institute) for their support in analyzing the weighed food protocols.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
Articles from Deutsches Ärzteblatt International are provided here courtesy of Deutscher Arzte-Verlag GmbH
Full text links
Read article at publisher's site: https://doi.org/10.3238/arztebl.2020.0575
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7779846?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3238/arztebl.2020.0575
Article citations
Association between dietary vitamin D intake and low muscle mass in US adults: results from NHANES 2011-2018.
Front Nutr, 11:1471641, 30 Oct 2024
Cited by: 0 articles | PMID: 39539378 | PMCID: PMC11559427
Internal exposure to heat-induced food contaminants in omnivores, vegans and strict raw food eaters: biomarkers of exposure to 2- and 3-monochloropropanediol (urinary excretion) and glycidol (hemoglobin adduct N-2,3-dihydroxypropyl-Val).
Arch Toxicol, 01 Oct 2024
Cited by: 1 article | PMID: 39352551
The Relevance of Plant-Based Diets and Micronutrient Supplementation for Body Composition: Data from the VeggieNutri Cross-Sectional Study.
Nutrients, 16(19):3322, 30 Sep 2024
Cited by: 0 articles | PMID: 39408288 | PMCID: PMC11478620
Nutritional analysis of vegan recipes: From social media to plate.
Food Sci Nutr, 12(10):7657-7670, 02 Aug 2024
Cited by: 0 articles | PMID: 39479681 | PMCID: PMC11521697
Unraveling the Evolutionary Diet Mismatch and Its Contribution to the Deterioration of Body Composition.
Metabolites, 14(7):379, 07 Jul 2024
Cited by: 0 articles | PMID: 39057702 | PMCID: PMC11279030
Review Free full text in Europe PMC
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland.
Eur J Nutr, 56(1):283-293, 26 Oct 2015
Cited by: 126 articles | PMID: 26502280
A cross-sectional study of nutritional status in healthy, young, physically-active German omnivores, vegetarians and vegans reveals adequate vitamin B12 status in supplemented vegans.
Ann Med, 55(2):2269969, 18 Oct 2023
Cited by: 7 articles | PMID: 37851870 | PMCID: PMC10586079
Intake of micronutrients and fatty acids of vegetarian, vegan, and omnivorous children (1-3 years) in Germany (VeChi Diet Study).
Eur J Nutr, 61(3):1507-1520, 02 Dec 2021
Cited by: 16 articles | PMID: 34855006 | PMCID: PMC8921058
[Vegetarian and vegan diets and their impact on health].
Rev Med Suisse, 15(667):1849-1853, 01 Oct 2019
Cited by: 4 articles | PMID: 31617971
Review