Abstract
Introduction
Knowledge is limited on the virologic course of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, particularly the time taken for viral clearance and the optimal time to discontinue isolation. This study aims to identify the clinical and demographic factors influencing the time taken for viral clearance in patients with COVID-19 to determine the optimal isolation period.Methods
This two-center retrospective observational cohort study was conducted between March 1 and June 31, 2020. Patients with COVID-19, which was confirmed by real-time reverse transcription polymerase chain reaction, were included. Data were extracted from medical records. The positive duration, which was defined as the period from the day of symptom onset to the negative conversion day, was assessed using a generalized linear model.Results
We included 63 patients. The mean positive duration was 20 days. The positive duration was significantly shorter for patients younger than 30 years of age and those between 30 and 60 years of age than for patients older than 60 years of age. We observed a more scattered distribution of the positive duration in older patients than in younger patients.Conclusions
Younger patients who recovered from COVID-19 took less time to clear SARS-CoV-2 than older patients; thus, a classification of the isolation periods based on age could be considered. A uniform viral clearance period for older patients may be difficult to determine because of biases such as underlying medical conditions. Further surveillance measures are recommended to determine the viral clearance time and the optimal isolation period.Free full text

Factors associated with viral clearance periods from patients with COVID-19: A retrospective observational cohort study
Abstract
Introduction
Knowledge is limited on the virologic course of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, particularly the time taken for viral clearance and the optimal time to discontinue isolation. This study aims to identify the clinical and demographic factors influencing the time taken for viral clearance in patients with COVID-19 to determine the optimal isolation period.
Methods
This two-center retrospective observational cohort study was conducted between March 1 and June 31, 2020. Patients with COVID-19, which was confirmed by real-time reverse transcription polymerase chain reaction, were included. Data were extracted from medical records. The positive duration, which was defined as the period from the day of symptom onset to the negative conversion day, was assessed using a generalized linear model.
Results
We included 63 patients. The mean positive duration was 20 days. The positive duration was significantly shorter for patients younger than 30 years of age and those between 30 and 60 years of age than for patients older than 60 years of age. We observed a more scattered distribution of the positive duration in older patients than in younger patients.
Conclusions
Younger patients who recovered from COVID-19 took less time to clear SARS-CoV-2 than older patients; thus, a classification of the isolation periods based on age could be considered. A uniform viral clearance period for older patients may be difficult to determine because of biases such as underlying medical conditions. Further surveillance measures are recommended to determine the viral clearance time and the optimal isolation period.
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an emerging infectious disease that has spread rapidly worldwide, thus causing major concerns about transmission. COVID-19 is transmitted by inhalation or contact with droplets from an infected individual [1]. To prevent transmission, the isolation of infected patients is recommended. The Centers for Disease Control and Prevention in the United States recommends that patients discontinue isolation three days after the fever has receded and respiratory symptoms have improved and 10 days after the onset of symptoms once the viral load has decreased [2]. Cheng et al. [3] reported that they found no epidemiological evidence of transmission approximately one week after the infection of an individual but did not provide molecular evidence to support these findings.
Recently, an increasing number of studies have reported on the clinical course of SARS-CoV-2. Comorbid conditions such as hypertension, chronic obstructive pulmonary disease, and diabetes are recognized as risk factors for severe COVID-19 [4,5]. By contrast, knowledge about the virologic course of SARS-CoV-2 infection, particularly the time taken for viral clearance and the optimal time to discontinue isolation, is far more limited. In the current study, we aimed to identify the clinical characteristics and factors that influence viral clearance from patients with COVID-19 and to determine the positive duration on an individual basis.
2. Material and methods
2.1. Study design and setting
This double-center retrospective observational cohort study was conducted at Nara Medical University and Nara Prefecture General Medical Center in Nara, Japan. These medical centers are hospitals designated to treat patients with SARS-CoV-2 pneumonia. Almost all of the patients who tested positive for SARS-CoV-2 in the Nara Prefecture were transferred to these hospitals. We retrospectively analyzed data from all patients who were diagnosed as positive for SARS-CoV-2 between March 1, 2020, and June 31, 2020. This study was approved by the Ethics Committee of Nara Medical University (Project identification code: No. 2726). The requirement to obtain informed consent was waived because this study was retrospective.
2.2. Sample collection
Nasopharyngeal samples were collected from all study participants. The samples were collected by the medical personnel of the two medical centers. Total RNA was extracted from each specimen by using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) or Maxwell RSC Viral Total Nucleic Acid Purification Kit (Promega Corporation, Wisconsin, USA). Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) assays were conducted using primers and probes targeting the ORF1ab, N, S, or E genes and a positive reference gene. The reaction system and amplification conditions were set, and test results were assessed according to the manufacturer’s specifications (F. Hoffmann-La Roche, Ltd., Switzerland), and the protocol developed by the National Institute of Infectious Diseases, Japan [6], was followed. A SARS-CoV-2 infection was confirmed by a positive SARS-CoV-2 result from the qRT-PCR assay.
2.3. Data collection
We retrospectively reviewed the medical records of the patients and collected data on the following variables: age, sex, body mass index, smoking history, chronic medical history (chronic pulmonary disease, diabetes, hypertension, and cardiovascular disease), angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) medication history, symptoms (fever, cough, sputum, dyspnea, joint pain and muscle pain, and loss of smell and taste), radiological examinations on admission, treatment regimen (need for oxygen or intubation, antiviral agents, antibacterial agents, and steroids), onset day, first qRT-PCR positive day, last qRT-PCR positive day, and negative conversion day. When two consecutive samples were negative for SARS-CoV-2 using the qRT-PCR testing method, the day on which the first sample was collected was determined to be the negative conversion day. The time to diagnosis was defined as the period from the day of symptom onset to the first qRT-PCR positive day. The positive duration was defined as the period from the day of symptom onset to the negative conversion day. The negative conversion lag was defined as the period from the last qRT-PCR positive day to the qRT-PCR negative conversion day. Symptoms were classified into three categories: mild symptoms (no need for oxygen), moderate symptoms (need for oxygen), and severe symptoms (need for intubation). Oxygen administration was initiated when the SpO2 in patients with COVID-19 was less than 90–93%, and the need for intubation was decided by an infectious disease physician and an intensive care physician. A diagnosis of pneumonia was made by two infectious disease physicians and was confirmed using chest radiography or computed tomography.
2.4. Statistical analysis
We calculated the positive duration by using a generalized linear model with a canonical link. The independent variables included age group, sex, smoking history, chronic pulmonary disease, diabetes, hypertension, fever, negative conversion lag, radiological findings on admission, and treatment. We performed two-tailed statistical tests and considered p < 0.05 statistically significant. All statistical analyses were performed using SPSS for Windows, Version 26.0 (IBM Corp, Armonk, NY, USA). There were no missing data.
3. Results
3.1. Participant characteristics
A total of 85 patients with COVID-19 were admitted to the participating hospitals: 18 were asymptomatic, two died during treatment, and two were transferred to isolation facilities after admission. We included the remaining 63 patients in the analysis, comprising four children and 59 adults. The clinical and demographic characteristics of the participants are shown in Table 1 . The mean age of the study participants was 47 years (range, 3–90 years), and 38 (60%) were male. The most common underlying medical conditions were hypertension (17%) and diabetes (6%). Moreover, 21 patients (33%) had a history of smoking. The mean age of the 16 participants who had underlying medical conditions was 65 years (range, 46–86 years). Two patients had an ACEIs medication history, and four had taken ARBs. The most common symptoms were fever (68%), cough (52%), and loss of taste and smell (44%). Sixteen percent of the patients received antiviral therapy (favipiravir), and 14% received antibacterial therapy. The median negative conversion lag was 3 days.
Table 1
Clinical and demographic characteristics of patients with coronavirus disease (N = 63).
| Variable | |
|---|---|
| Age in years, mean ± SDa | 47 ± 19.9 |
| Age > 60 years, n (%) | 15 (24) |
| Sex (male), n (%) | 38 (60) |
| BMIb, mean ± SDa | 23.1 ± 4.95 |
| Comorbidities, n (%) | |
| Hypertension | 11 (17) |
| Diabetes mellitus | 4 (6) |
| Cardiovascular disease | 3 (5) |
| Chronic pulmonary disease | 3 (5) |
| Clinical presentation, n (%) | |
| Fever | 43 (68) |
| Cough | 33 (52) |
| Loss of taste and smell | 28 (44) |
| Joint pain and muscle pain | 17 (27) |
| Dyspnea | 10 (16) |
| Sputum | 8 (13) |
| Pneumonia | 26 (41) |
| Symptom severity, n (%) | |
| Mild symptoms | 52 (83) |
| Moderate symptoms | 7 (11) |
| Severe symptoms | 4 (6) |
| Treatment, n (%) | |
| Antiviral therapy | 10 (16) |
| Antibacterial therapy | 9 (14) |
| Steroids | 2 (3) |
| Positive duration, days, mean ± SDa | 20 ± 9.2 |
3.2. Time to diagnosis and positive duration distribution according to age
The time to diagnosis was significantly shorter in participants younger than 60 years of age than in those older than 60 years of age (median difference, 95% confidence interval [CI]: -7.2 days, −10.2 to −4.3 days for those younger than 30 years of age; −5.9 days, −8.4 to −3.4 days for those between 30 and 60 years of age).
The distribution of the positive duration by age group is shown in Fig. 1 . The means and standard deviations of the positive durations of patients younger than 30 years of age, those between 30 and 60 years of age, and those older than 60 years of age were 15.3 ± 4.1 days, 20.1 ± 7.3 days, and 26.5 ± 13.5 days, respectively. In older patients, the positive duration had a scattered distribution.
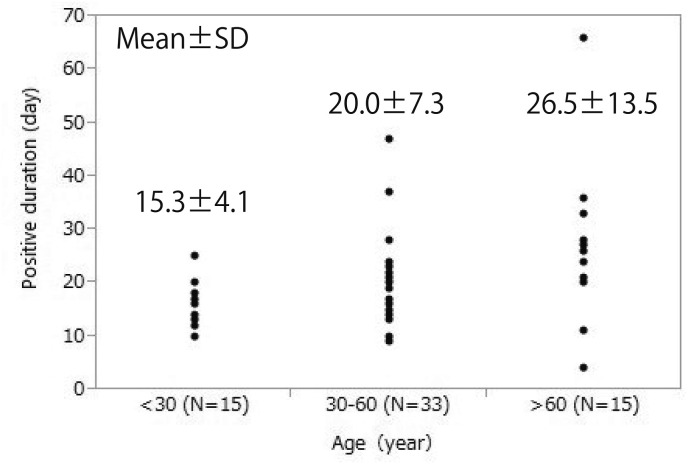
Positive duration in study participants by age group, This figure shows the relationship between positive duration and age. Positive duration was 15.3 ± 4.1 days (mean ± standard deviation) in patients under 30 years of age, 20.0 ± 7.3 days in patients 30–60 years of age, and 26.5 ± 13.5 days in those over 60 years of age, showing more scattered results in older patients.
3.3. Factors affecting the positive duration
Table 2 summarizes the results of the generalized linear model analysis of factors associated with the positive duration. The median positive duration was 20 days (range, 4–66 days). The positive duration was significantly shorter in participants younger than 60 years of age than in those older than 60 years of age (median difference, 95% confidence interval [CI]: -9.5 days, −15.7 to −3.3 days for those younger than 30 years of age; −6.0 days, −11.3 to −0.7 days for those aged 30–60 years of age). Additionally, the positive duration was significantly shorter in patients with mild symptoms than in those with severe symptoms (median difference, 95% CI: -16.5 days, −26.5 to −6.4 days). Patients with diabetes and fever had a shorter positive duration (median difference, 95% CI: -9.5 days, −18.0 to −1.0 days and −2.7 days, −7.3 to 1.9 days, respectively). By contrast, patients with hypertension had a longer positive duration (median difference, 95% CI: 5.6 days, 0.02–11.3 days). Table 3 shows the association between hypertension and ACEIs or ARBs medication histories and positive duration. The mean positive duration among patients with hypertension and an ACEIs medication history was 29.5 days, and 18 days in those between 30 and 60 years of age. In contrast, the mean positive duration among patients with hypertension and an ARBs medication history was 21.3 days and 46 days in those older than 60 years, respectively. These results showed a trend in which positive duration in patients who had taken ACEIs was shorter than the average positive duration of the corresponding age groups while positive duration was longer in patients who had taken ARBs. The variance inflation factor was less than two for all variables considered, thus suggesting that there was little effect due to multicollinearity.
Table 2
Results of multivariate generalized linear model analysis of factors associated with the positive duration in patients with coronavirus disease (N = 63).
| Variable | Β | Standard error | 95% Wald confidence interval | P value | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| Age, < 30 years | −9.552 | 3.1576 | −15.741 | −3.364 | 0.002 |
| Age, 30–60 years | −6.031 | 2.6923 | −11.308 | −0.754 | 0.025 |
| Age, > 60 years | Ref. | – | – | – | – |
| Male | −0.772 | 2.0970 | −4.882 | 3.338 | 0.713 |
| Fever | −2.724 | 2.3737 | −7.377 | 1.928 | 0.251 |
| Mild symptoms | −16.542 | 5.1249 | −26.586 | −6.497 | 0.001 |
| Moderate symptoms | −12.560 | 5.2900 | −22.928 | −2.192 | 0.018 |
| Severe symptoms | Ref. | – | – | – | – |
| Pneumonia | −1.063 | 2.4311 | −5.828 | 3.702 | 0.662 |
| Chronic pulmonary disease | 2.180 | 4.5719 | −6.781 | 11.141 | 0.634 |
| Diabetes | −9.529 | 4.3315 | −18.018 | −1.039 | 0.028 |
| Hypertension | 5.695 | 2.8944 | 0.022 | 11.368 | 0.049 |
| Smoking history | −2.686 | 2.1709 | −6.941 | 1.569 | 0.216 |
| Negative conversion lag | −0.185 | 0.2130 | −0.603 | 0.232 | 0.385 |
| Intercept | 45.198 | 6.3939 | 32.666 | 57.730 | <0.001 |
Table 3
Association between the positive duration in patients with hypertension and ACEIs and ARBs medication histories (N = 63).
| Variable | Hypertension | ACEIs intake | ARBs intake | N | Mean duration (days) | SDa |
|---|---|---|---|---|---|---|
| Age, < 30 years | No | No | No | 15 | 15.3 | 4.08 |
| Age, 30–60 years | No | No | No | 27 | 18.7 | 4.63 |
| Yes | No | No | 2 | 29.5 | 10.6 | |
| Yes | Yes | No | 2 | 18 | 5.65 | |
| Yes | No | Yes | 2 | 30 | 24.0 | |
| Age, > 60 years | No | No | No | 10 | 24.1 | 5.91 |
| Yes | No | No | 3 | 21.3 | 16.1 | |
| Yes | No | Yes | 2 | 46.5 | 27.5 |
4. Discussion
Studies have reported that patients with COVID-19 shed SARS-CoV-2 RNA for 10–20 days [4,7]. Currently, the required isolation period in Japan is a minimum of 10 days after the onset of symptoms and 72 h after their improvement. There is also limited data on the time taken for SARS-CoV-2 clearance in infected patients and the factors that influence this duration. Hattori et al. [8] reported that an older age is associated with a longer duration of SARS-CoV-2 RNA detection; however, this report lacked multivariate analysis and an evaluation of the differences based on age. Thus, little is known about the factors that influence the positive duration.
To the best of our knowledge, our study is the first to evaluate the positive duration of SARS-CoV-2 infection by using multivariate analysis. It revealed that an older age was associated with a significantly longer positive duration. Patients younger than 30 years of age had a considerably shorter positive duration than patients older than 60 years of age. Zou et al. [9] reported that the viral RNA shedding pattern of patients with COVID-19 resembles that of patients with influenza. We speculate that an older age slows down viral clearance from the nasopharynx, similar to that in influenza [10], thus resulting in a longer positive duration period in older patients. Moreover, our study showed a delayed diagnosis in older patients compared with that in younger patients. It might have been too difficult for elderly patients to access the social system to obtain a diagnosis in the first wave of COVID-19.
We observed a scattered distribution for the positive duration with increasing age, especially in patients older than 60 years of age. In our study, an 80-year-old female patient with hypertension, cerebral infarction, atrial fibrillation, and aortic dissection continued to have positive qRT-PCR results for 66 days. By contrast, an 86-year-old male patient with diabetes and hypertension had positive qRT-PCR results for only four days. Considering that older patients with COVID-19 are more likely to have underlying diseases, such as diabetes, hypertension, and chronic pulmonary disease, than younger patients with COVID-19 [11], these factors may influence viral clearance from the nasopharyngeal cavities. In our study, diabetes was associated with a shorter positive duration; by contrast, hypertension was associated with a longer positive duration. According to Trump et al. [12], hypertension delayed viral clearance, and antihypertensive treatment with ARBs, but not ACEIs, is associated with delayed viral clearance. Our report also shows the same association, but more surveillance is needed because the number of patients with ACEIs and ARBs medication histories in our study was low. Although it is unclear why diabetes is associated with a shorter positive duration, more large-scale surveillance is needed to assess the risk factors accurately.
The variability in the positive duration with increasing age, especially in patients older than 60 years of age makes it difficult to determine standard viral clearance periods in this group of patients. Therefore, in this group of patients, a uniform isolation period may be difficult to determine.
The duration of viral RNA shedding in patients with influenza has been shown to be shorter in asymptomatic individuals [13]. By contrast, we observed that patients with COVID-19 without fever or pneumonia had a longer positive duration than patients with fever and pneumonia. However, in view of the small sample size and the exclusion of the 18 asymptomatic patients who were not admitted to the hospital, we were unable to determine whether individuals with asymptomatic infection have a longer positive duration.
The primary limitation of our study is that we could not distinguish between viable and nonviable viruses because the qRT-PCR method used herein detects both viable and nonviable viral particles. Therefore, a positive nasopharyngeal swab result does not necessarily indicate an infectious virus. However, the duration of viral shedding is likely to be strongly correlated with the positive duration. Our results suggest that older age can be a factor in prolonging virus shedding. A second limitation of this study is that the qRT-PCR method used may not have been uniform because this study was conducted in two hospitals. Hence, there may have been differences in the sensitivity of SARS-CoV-2 detection by qRT-PCR between the two hospitals, which, in turn, may have resulted in measurement errors. A third limitation is the small sample size. Because our region experienced a small COVID-19 epidemic compared to that seen in urban areas such as Tokyo, we were limited in the number of participants that could be recruited for our study.
5. Conclusion
In conclusion, our results suggest that viral clearance periods from patients with COVID-19 can depend on age; thus, a classification of the isolation periods based on age could be considered. A uniform viral clearance period for older patients may be difficult to determine because of biases such as underlying medical conditions. Further surveillance and molecular studies, as well as larger cohort studies, are recommended to determine the viral clearance time and the optimal isolation period.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship statement
Nobuyasand Hirai wrote the manuscript; Yuichi Nishioka, Takahiro Sekine, Yuji Nishihara, Nao Okuda, Tomoko Nishimura, Hiroyuki Fujikura, Natsuko Imakita, Tatsuya Fukumori, Masatoshi Sato, Koichi Maeda, and Taku Ogawa treated the patients and collected data on the samples; Naokuni Hishiya, Yuki Suzuki, Ryuichi Nakano, and Hisakazu Yano conducted the laboratory work; Yuichi Nishioka and Tomoaki Imamura interpreted the results statistically; Kei Kasahara contributed to the writing of the manuscript. All authors contributed to the writing of the final manuscript and the management or administration of this study.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jiac.2021.02.015
Read article for free, from open access legal sources, via Unpaywall:
http://www.jiac-j.com/article/S1341321X21000556/pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.jiac.2021.02.015
Article citations
Comparison of time to negative conversion of SARS-CoV-2 between young and elderly among asymptomatic and mild COVID-19 patients: a cohort study from a national containment center.
Front Med (Lausanne), 11:1217849, 18 Mar 2024
Cited by: 0 articles | PMID: 38562375 | PMCID: PMC10983848
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.
NPJ Sci Food, 8(1):19, 30 Mar 2024
Cited by: 2 articles | PMID: 38555403 | PMCID: PMC10981760
Review Free full text in Europe PMC
Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Vaccination for Negative Conversion Time of Nucleic Acid in Nonsevere COVID-19 Patients Infected by SARS-CoV-2 Omicron Variant.
Int J Clin Pract, 2023:9576855, 25 Sep 2023
Cited by: 3 articles | PMID: 37790860 | PMCID: PMC10545465
'Dynamic zero-COVID' policy and viral clearance during an omicron wave in Tianjin, China: a city-wide retrospective observational study.
BMJ Open, 12(12):e066359, 15 Dec 2022
Cited by: 6 articles | PMID: 36521897 | PMCID: PMC9755905
Impact of Remdesivir on SARS-CoV-2 Clearance in a Real-Life Setting: A Matched-Cohort Study.
Drug Des Devel Ther, 16:3645-3654, 19 Oct 2022
Cited by: 2 articles | PMID: 36268521 | PMCID: PMC9578770
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea.
JAMA Pediatr, 175(1):73-80, 01 Jan 2021
Cited by: 120 articles | PMID: 32857112 | PMCID: PMC7455883
Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19.
Int J Infect Dis, 105:463-469, 27 Feb 2021
Cited by: 19 articles | PMID: 33647508 | PMCID: PMC7910140
Comparison of Severe Acute Respiratory Syndrome Coronavirus 2 Screening Using Reverse Transcriptase-Quantitative Polymerase Chain Reaction or CRISPR-Based Assays in Asymptomatic College Students.
JAMA Netw Open, 4(2):e2037129, 01 Feb 2021
Cited by: 9 articles | PMID: 33570576 | PMCID: PMC7879237
Prolonged SARS-CoV-2 detection and reversed RT-PCR results in mild or asymptomatic patients.
Infect Dis (Lond), 53(1):31-37, 16 Sep 2020
Cited by: 16 articles | PMID: 32935628





