Abstract
Free full text

Drug Repurposing Screen for Compounds Inhibiting the Cytopathic Effect of SARS-CoV-2
Abstract
Drug repurposing is a rapid approach to identify therapeutics for the treatment of emerging infectious diseases such as COVID-19. To address the urgent need for treatment options, we carried out a quantitative high-throughput screen using a SARS-CoV-2 cytopathic assay with a compound collection of 8,810 approved and investigational drugs, mechanism-based bioactive compounds, and natural products. Three hundred and nineteen compounds with anti-SARS-CoV-2 activities were identified and confirmed, including 91 approved drugs and 49 investigational drugs. The anti-SARS-CoV-2 activities of 230 of these confirmed compounds, of which 38 are approved drugs, have not been previously reported. Chlorprothixene, methotrimeprazine, and piperacetazine were the three most potent FDA-approved drugs with anti-SARS-CoV-2 activities. These three compounds have not been previously reported to have anti-SARS-CoV-2 activities, although their antiviral activities against SARS-CoV and Ebola virus have been reported. These results demonstrate that this comprehensive data set is a useful resource for drug repurposing efforts, including design of new drug combinations for clinical trials for SARS-CoV-2.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global health crisis. As of September 17, 2020, the global case report stands at 30 million, with a death toll of 942,989 (Dong et al., 2020). Only remdesivir, an investigational drug developed for Ebola virus, has been recently approved for treatment of hospitalized COVID-19 patient, though its therapeutic efficacy is mild (Eastman et al., 2020). Since an effective vaccine is currently unavailable for COVID-19, drug repurposing has received significant attention in the rapid search to fill this unmet therapeutic need.
The requirement of biosafety level 3 (BSL-3) containment laboratories for handling SARS-CoV-2 has limited the number of high throughput screening (HTS) laboratories that are capable of carrying out large scale compound screens using live SARS-CoV-2. Despite these challenges, several drug repurposing screens have been carried out using live SARS-CoV-2, showing promising results (Dittmar et al., 2020; Ellinger et al., 2020; Riva et al., 2020; Touret et al., 2020). Here we report a screening campaign against a collection of 8,810 approved and investigational drugs, mechanism-based bioactive compounds, and natural products, carried out in quantitative HTS (qHTS) format (Inglese et al., 2006). Compounds were screened at four concentrations in a SARS-CoV-2 cytopathic effect (CPE) assay in Vero E6 cells that were selected for high ACE2 expression, with an accompanying cytotoxicity counter-assay. The primary screen yielded 319 hits with confirmed anti-SARS-CoV-2 activity. The primary screening data have been made publicly available on the National Center for Advancing Translational Sciences (NCATS) OpenData Portal (https://opendata.ncats.nih.gov/covid19/index.html) (Brimacombe et al., 2020). We intend this manuscript as a companion to guide investigators in utilizing that data, and to present further details of qHTS with the SARS-CoV-2 CPE assay, including identification of top annotated hits.
Materials and Methods
Compounds and Compound Libraries
All compound libraries were assembled internally at NCATS. The NCATS pharmaceutical collection (NPC) contains 2,678 compounds, covering drugs approved by US FDA and foreign health agencies in European Union, United Kingdom, Japan, Canada, and Australia, as well as some clinical trialed experimental drugs (Huang et al., 2019). The NCATS Mechanism Interrogation Plate (MIPE) 5.0 library contains 2,480 mechanism based bioactive compounds, targeting more than 860 distinct mechanisms of action (Lin et al., 2019). The NCATS Pharmacologically Active Chemical Toolbox (NPACT) is a library of mechanistically defined molecules and natural products (5,099 compounds). Other small custom NCATS collections were also screened: anti-infective (752 compounds), kinase inhibitors (977 compounds), epigenetic modulators (335 compounds). A commercially available autophagy-focused screening library (Cayman #23537) was analyzed and 29 compounds that were not already present in our collections were purchased. All compounds were dissolved in DMSO to make 10 mM stock solutions, unless solubility was limiting, and was diluted four times at 1:5 ratio for the primary screens, and at 1:3 ratio for follow up assays at eight concentrations.
CPE Assay
A SARS-CoV-2 CPE assay was conducted in the BSL3 facilities at the contract research organization Southern Research (Birmingham, AL). Briefly, compounds were titrated in DMSO and acoustically dispensed into 384-well assay plates at 60 nL/well at NCATS, and provided to Southern Research. Cell culture media (MEM, 1% Pen/Strep/GlutaMax, 1% HEPES, 2% HI FBS) was dispensed at 5 µL/well into assay plates, and incubated at room temperature to allow for compound dissolution. Vero E6 African green monkey kidney epithelial cells (selected for high ACE2 expression) were inoculated with SARS-CoV-2 (USA_WA1/2020) at a multiplicity of infection (MOI) of 0.002 in media, and quickly dispensed into assay plates as 25 µL/well. The final cell density was 4,000 cells/well. Assay plates were incubated for 72 h at 37°C, 5% CO2, and 90% humidity. CellTiter-Glo (30 µL/well, Promega #G7573) was dispensed into the assay plates. Plates were incubated for 10 min at room temperature. Luminescence signal was measured on Perkin Elmer Envision or BMG CLARIOstar plate readers. An ATP content cytotoxicity counter-assay was conducted using the same protocol as the CPE assay, without the addition of SARS-CoV-2 virus.
Data Analysis
Results from the primary screen and confirmation screens were processed at NCATS using a software developed in-house (Wang et al., 2010). For the CPE assay, raw plate data were normalized with DMSO-only wells as 0% CPE rescue (negative signal control), and no-virus control wells as 100% CPE rescue (positive signal control). For the cytotoxicity assay, raw plate data were normalized with DMSO-only wells as 100% viability (positive signal control), and cells treated with hyamine (benzethonium chloride) control compound as 0% viability (negative signal control). The half-maximum effective values (EC50) and percent efficacy were obtained by fitting the concentration-response titration data to a four-parameter Hill equation. Compounds with >55% efficacy were selected for cherry-pick confirmation. The concentration-response curves of re-tested compounds were also plotted using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA). Results in the figures are expressed as mean ± standard deviation (SD).
Results
High Throughput Screening With SARS-CoV-2 CPE Assay
Our aims were two-fold in initiating this program. The first was to identify active compounds that may provide opportunities for repurposing, or identify mechanistic targets of interest. The second was to create a complete HTS reference dataset that can be shared openly with the scientific community for study of disease pathology and new therapeutics development. The CPE reduction assay format has been widely employed to screen for antiviral agents due to its ease of scalability for HTS (Heaton, 2017). In this assay, viral infection kills host cells, and the cell viability is used as a surrogate readout for viral infection and replication. In other words, compounds with anti-viral activities rescue cells from the cytopathic effect of SARS-CoV-2 (a gain-of-signal assay).
A total of 9,952 compounds were tested in the primary screen, but due to the overlapping composition of the libraries, a significant number of compounds were tested multiply. A total of 8,810 unique compounds in six compound libraries were tested in the primary screen including the NCATS Pharmaceutical Collection (NPC), NCATS Mechanism Interrogation Plate (MIPE), NCATS Pharmacologically Active Chemical Toolbox (NPACT), Epigenomic library, Autophagy library, and anti-infective library. These compounds contain 1,345 approved drugs (by the FDA, EMA, DPD), 751 compounds approved outside of those countries, 1,067 investigational drugs (tested in clinical trials), 1,057 pre-clinical compounds (tested in animals), and 4,472 bioactive compounds (tool compounds) (Figure 1A). By their mechanisms of action and clinical applications, these compounds are divided into diverse groups (Figure 1B).
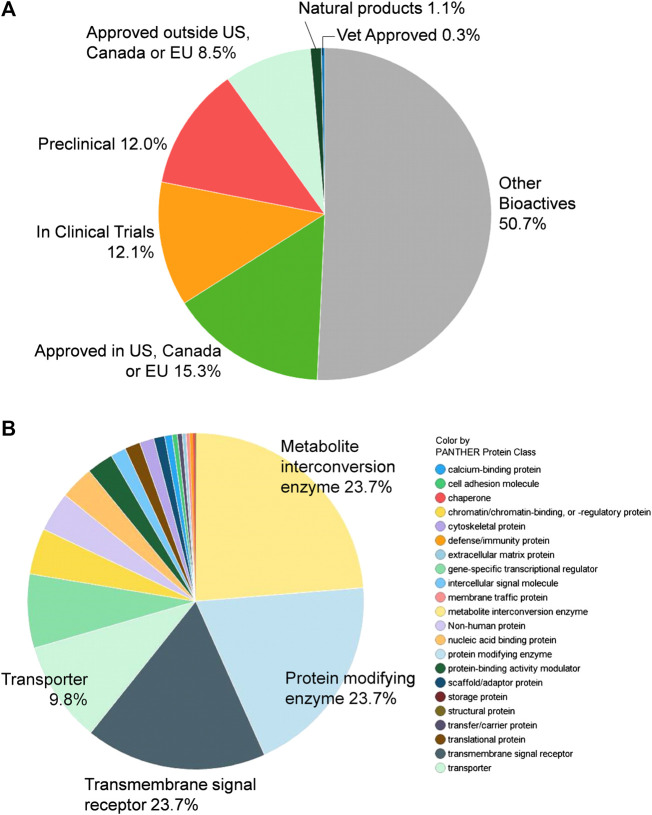
Compound library description. (A) By approval status: approved drugs (FDA and others), tested in clinical trials, or preclinical. (B) By mechanism of action.
The CPE assay performed well in the primary screen, with an average Z’ factor of 0.83 over 133 plates, from three batched runs (Figure 2A). Remdesivir concentration-response was included as a control for each screening run, and yielded consistent EC50 values of 4.56, 4.42 and 7.28 µM (Figure 2B). Using the criteria of >55% efficacy, 380 compounds were selected as the primary screen hits, out of which, 319 compounds were confirmed using 8-point, 1:3 titration, in duplicate. Among these primary hits, 89 of 319 had previously reported activity against SARS-CoV-2, including reports of live virus assays, enzymatic assays, or virtual screening, while 230 were novel hits from this qHTS (Table 1, Supplementary Table S1). In the following sections, these newly identified SARS-CoV-2 CPE-protective compounds are further descriped.
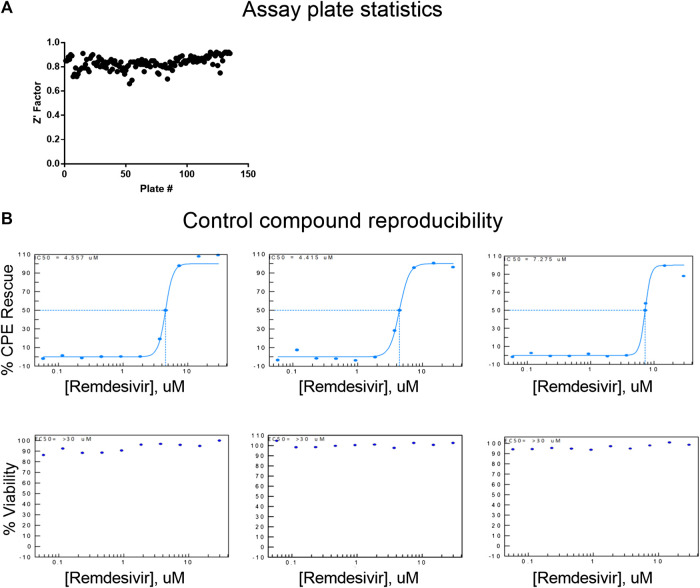
Assay reproducibility. (A) Assay plate statistics showing Z′ factors across all 133 384-well plates in the primary screen. (B) Concentration-response curve fittings for remdesivir in four independent runs for primary screens and hit confirmation. EC50 values of 4.56, 4.42, 7.28, and 5.17 µM of remdesivir in the CPE assay demonstrate day-to-day reproducibility of the assay.
TABLE 1
Top confirmed anti-SARS-CoV-2 compounds.
| Sample ID | Sample name | CPE EC50 (uM) | CPE % efficacy | Cytotox CC50 (uM) | % Cytotox | Previous reports against CoVs | Approval status | MOA |
|---|---|---|---|---|---|---|---|---|
| Viral target | ||||||||
| NCGC00686694 | Remdesivir | 10.0 | 133.1 | N/A | <30 | Clinical (Beigel et al., 2020) | FDA | RdRP inhibitor |
| Autophagy modulators | ||||||||
| NCGC00387732 | VPS34-IN1 | 0.63 | 103.0 | 10.0 | −76.5 | None | Bioactive | Autophagy modulator |
| NCGC00344081 | STF-62247 | 1.1 | 107.1 | 11.2 | −56.6 | None | Preclinical | Autophagy modulator; Renal cell growth inhibition |
| NCGC00507892 | VPS34 Inhibitor 1 | 1.4 | 98.3 | N/A | <30 | None | Preclinical | Autophagy modulator |
| GPCR modulators | ||||||||
| NCGC00346896 | MCOPPB | 3.5 | 85.6 | N/A | <30 | None | Preclinical | ORL1 (OP4, NOP) agonists |
| NCGC00370950 | GW 803430 | 3.5 | 93.3 | N/A | <30 | None | Bioactive | Melanin-concentrating hormone receptor 1 antagonist |
| NCGC00017063 | Amodiaquine dihydrochloride | 4.0 | 87.2 | N/A | <30 | In vitro live virus (Ianevski et al., 2020) | FDA | Histamine receptor antagonist |
| NCGC00485045 | N-Methylspiperone hydrochloride | 4.5 | 80.0 | N/A | <30 | None | Clinical trial | Serotonin 2 (5-HT2) receptor antagonist |
| NCGC00016710 | Clemastine fumarate | 7.9 | 96.0 | N/A | <30 | Mpro assay (Vatansever et al., 2020) | FDA | Histamine receptor antagonist |
| NCGC00386477 | GMC 2-29 | 7.9 | 117.2 | N/A | <30 | None | Bioactive | 5-hydroxytryptamine receptor 1D antagonist |
| NCGC00378842 | Lu AE58054 hydrochloride | 10.0 | 97.2 | N/A | <30 | None | Clinical trial | Serotonin 6 (5-HT6) receptor antagonist |
| NCGC00013683 | Chlorprothixene | 10.0 | 104.4 | N/A | <30 | None | FDA | Dopamine receptor antagonist |
| NCGC00014482 | Methdilazine hydrochloride | 10.0 | 86.4 | N/A | <30 | Virtual: AI prediction (Grzybowski et al., 2020) | FDA | Antihistamine |
| NCGC00179370 | Methotrimeprazine maleate | 10.0 | 84.6 | N/A | <30 | None | FDA | Antagonist for adrenergic, dopamine, histamine, cholinergic and serotonin (5-hydroxytryptamine; 5-HT) receptors |
| NCGC00016642 | Piperacetazine | 10.0 | 103.7 | N/A | <30 | None | FDA | Dopamine receptor antagonist |
| NCGC00181913 | Difeterol | 10.0 | 113.4 | N/A | <30 | None | Approved outside of US | Antihistamine |
| NCGC00386484 | (R)-(-)-LY 426965 dihydrochloride | 10.0 | 110.7 | N/A | <30 | None | Bioactive | Serotonin 2b (5-HT2b) receptor modulator |
| NCGC00015608 | Loperamide hydrochloride | 10.0 | 98.6 | N/A | <30 | In vitro live virus (Jeon et al., 2020) | FDA | Opioid receptor agonist |
| NCGC00485321 | Naltrindole isothiocyanate hydrochloride | 10.0 | 114.7 | N/A | <30 | None | Bioactive | Delta opioid receptor antagonist |
| NCGC00165726 | AM1241 | 10.0 | 97.6 | N/A | <30 | None | Bioactive | Cannabinoid CB2 receptor agonist |
| NCGC00386703 | CpdD hydrochloride | 10.0 | 96.9 | N/A | <30 | None | Bioactive | Ghrelin receptor antagonist |
| NCGC00386219 | SB 271046 hydrochloride | 10.0 | 107.5 | N/A | <30 | None | Bioactive | Serotonin 6 (5-HT6) receptor antagonist |
| NCGC00386479 | GMC 2-113 | 10.0 | 129.7 | N/A | <30 | Virtual: RdRP (Dwivedy et al., 2020) | Bioactive | 5-hydroxytryptamine receptor 1D antagonist |
| Host protease inhibitors | ||||||||
| NCGC00386330 | Z-FA-FMK | 0.13 | 104.8 | N/A | <30 | Mpro assay, in vitro live virus (Zhu et al., 2020b) | Bioactive | Cathepsin L inhibitor |
| NCGC00485951 | VBY-825 | 0.14 | 97.8 | N/A | <30 | In vitro live virus (Riva et al., 2020) | Clinical trial | Cathepsin S inhibitor |
| NCGC00345807 | CAA-0225 | 0.20 | 99.3 | N/A | <30 | None | Preclinical | Cathepsin L inhibitors |
| NCGC00386232 | Cathepsin Inhibitor 1 | 0.25 | 114.4 | N/A | <30 | None | Bioactive | Cathepsin inhibitors |
| NCGC00163432 | Calpeptin | 0.50 | 111.7 | N/A | <30 | Mpro assay, in vitro live virus (Ma et al., 2020) | Preclinical | Calpain inhibitor |
| NCGC00485375 | Z-Gly-Leu-Phe-chloromethyl ketone | 1.3 | 87.2 | N/A | <30 | None | Bioactive | Granzyme B Inhibitor |
| NCGC00371151 | Balicatib | 2.0 | 100.3 | N/A | <30 | None | Clinical trial | Cruzipain (Trypanosoma cruzi) inhibitor |
| NCGC0016166 | Calpain Inhibitor I, ALLN | 2.0 | 111.1 | N/A | <30 | None | Bioactive | Calpain inhibitor |
| Kinase modulators | ||||||||
| NCGC00263093 | Apilimod | 0.023 | 104.4 | N/A | <30 | In vitro live virus (Riva et al., 2020) | Clinical trial | IL-12 Production inhibitor; PIKfyve inhibitor |
| NCGC00386313 | Berzosertib | 0.71 | 87.9 | 11.2 | -98.5 | None | Clinical trial | ATR Kinase inhibitor |
| NCGC00347280 | IKK-2 inhibitor VIII | 7.1 | 91.7 | N/A | <30 | None | Preclinical | IKK-2 (IKK-beta) inhibitor |
| NCGC00387166 | NSC 33994 | 8.9 | 107.6 | N/A | <30 | None | Bioactive | Jak2 inhibitor |
| NCGC00159456 | Imatinib | 10.0 | 119.0 | N/A | <30 | Clinical (Morales-Ortega et al., 2020) | FDA | Bcr-Abl kinase inhibitor; KIT inhibitor; PDGFR tyrosine kinase receptor inhibitor |
| Others | ||||||||
| NCGC00178090 | Pristimerin | 0.11 | 87.4 | 1.1 | −93.2 | SARS Mpro assay (Ryu et al., 2010) | Preclinical | Monoacylglycerol lipase (MGL) inhibitor |
| NCGC00385252 | alpha-l-Arabinopyranose | 2.4 | 104.0 | N/A | <30 | None | Bioactive | Induces Pbad promoter expression in E. coli |
| NCGC00351072 | ML414 | 3.2 | 79.6 | N/A | <30 | None | Bioactive | Oligosaccharyltransferase inhibitor |
| NCGC00379165 | IT1t dihydrochloride | 3.5 | 96.3 | N/A | <30 | None | Bioactive | CXCR4 inhibitor |
| NCGC00485648 | S-15176 difumarate salt | 3.8 | 127.4 | N/A | <30 | None | Bioactive | Oxidative stress inhibitor |
| NCGC00384450 | JTV519 Hemifumarate | 5.5 | 85.7 | N/A | <30 | None | Clinical trial | Ryanodine receptor (RyR) inhibitor |
| NCGC00253604 | Rescimetol | 8.9 | 81.8 | N/A | <30 | None | Approved outside of US | Antihypertensive agent |
| NCGC00164559 | Duloxetine hydrochloride | 10.0 | 90.0 | N/A | <30 | Mpro assay (Vatansever et al., 2020) | FDA | Norepinephrine reuptake inhibitor; Serotonin-norepinephrine reuptake inhibitor (SNRI) |
| NCGC00181168 | Trifluomeprazine 2-butenedioate | 10.0 | 90.2 | N/A | <30 | None | Bioactive | Antipsychotic agents |
| NCGC00169804 | Asteriscunolide D | 10.0 | 93.3 | N/A | <30 | None | Bioactive | Natural product |
| NCGC00485925 | Genz-123346 (free base) | 10.0 | 99.4 | N/A | <30 | In vitro live virus (Vitner et al., 2020) | Bioactive | Ceramide glucosyltransferase inhibitor |
| NCGC00015708 | Maprotiline hydrochloride | 10.0 | 103.7 | N/A | <30 | Virtual: Mpro docking (Chauhan, 2020) | FDA | Norepinephrine reputake inhibitor; tricyclic antidepressant |
| NCGC00168786 | Deserpidine | 10.0 | 84.7 | N/A | <30 | Virtual: NSP16 docking (Jiang et al., 2020) | FDA | Angiotensin converting enzyme inhibitor |
| NCGC00015096 | Amiodarone hydrochloride | 10.0 | 100.5 | N/A | <30 | Clinical (Castaldo et al., 2020) | FDA | Potassium channel blocker |
| NCGC00181088 | Melitracen hydrochloride | 10.0 | 97.1 | N/A | <30 | None | Approved outside of US | Antidepressive agents, tricyclic |
| NCGC00015428 | (+/-) -Fluoxetine | 10.0 | 115.8 | N/A | <30 | In vitro live virus (Zimniak et al., 2020) | FDA | Selective serotonin reuptake inhibitor (SSRI) |
| NCGC00018102 | Flunarizine | 10.0 | 94.1 | N/A | <30 | Virtual: Spike docking (Chernyshev, 2020) | Approved outside of US | Calcium channel blocker |
| NCGC00183024 | Proglumetacin | 10.0 | 87.6 | N/A | <30 | None | Approved outside of US | Cyclooxygenase inhibitor |
| NCGC00378760 | DMP 777 | 10.0 | 92.5 | N/A | <30 | None | Clinical trial | Leukocyte elastase inhibitor |
| NCGC00476094 | Dexanabinol | 10.0 | 110.8 | N/A | <30 | None | Clinical trial | NMDA antagonist |
91 Approved Drugs and 49 Investigational Drugs Protected Against Cytopathic Effect of SARS-CoV-2 Infection
There were 56 top confirmed hits with EC50 values of ≤10 µM and efficacy values of greater than 80% in the CPE assay, and with greater than 10-fold selectivity index (SI) between cytotoxicity and CPE assays (Table 1, Figure 3). When grouped by mechanism of action targets, 19 compounds were GPCR modulators, eight were host protease inhibitors, five were kinase modulators, and three were autophagy modulators (Figure 3). Interestingly, in the 56 top hits, remdesivir is only one that has a viral target as a known primary mechanism, whereas the known mechanisms of action of the other compounds are directed against host targets.
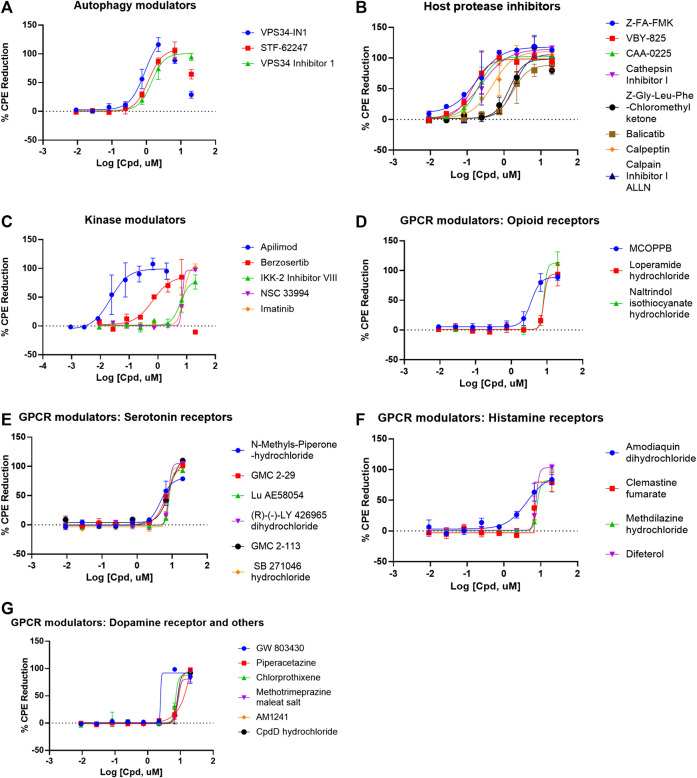
Compounds concentration-response curves in the CPE assay. (A) Autophagy modulators, (B) host protease inhibitors, (C) kinase modulators, (D) opioid receptor modulators, (E) serotonin receptor modulators, (F) histamine receptor modulators, and (G) dopamine and other GPCR receptor modulators. Berzosertib, VPS34-IN1, and STF-62247 showed bell-shaped concentration-responses due to cytotoxicity. No other compounds caused any reduction in viability in the cytotoxicity assay.
There have been several previous drug repurposing screens reported for SARS-CoV-2 in 2D cell culture infection models (Dittmar et al., 2020; Ellinger et al., 2020; Jeon et al., 2020; Riva et al., 2020; Touret et al., 2020; Weston et al., 2020). These screens had some compound overlap with our qHTS screen, particularly for the FDA approved drugs. We performed a literature search of our confirmed compounds and previous reports were noted in Table 1 and Supplementary Table S1. Three of the top 56 hits were novel and FDA approved. These hits are chlorprothixene, methotrimeprazine, and piperacetazine, which showed 10 µM potencies in the CPE assay. In order for a drug to be efficacious in vivo, the in vivo exposure at the site of infection (e.g. drug plasma concentration) would need to be higher than the in vitro potency (e.g. EC50). To help guide compound prioritization, the reported clinical plasma pharmacokinetic values of the top confirmed hits are summarized in Table 2. Of the top approved drugs that are active against SARS-CoV-2 in the CPE assay, only amiodarone HCl showed lower EC50 value in the CPE assay than plasma Cmax, whereas, remdesivir and imatinib showed EC50 values that were within 2-fold of plasma Cmax (Table 2).
TABLE 2
Reported human pharmacokinetic properties of FDA-approved top hits.
| Sample name | Cmax (ng/ml) | MW (g/mol) | Cmax (µM) | Elimination T1/2 | Dosing regimen | References |
|---|---|---|---|---|---|---|
| (+/-) -Fluoxetine | 15–55 | 309.33 | 0.05–0.18 | 1–3 days | Single dose 40 mg PO | Eli Lilly and Company (1987) |
| Amiodarone hydrochloride | 5,000–41,000 | 681.78 | 7.33–60.14 | 9–36 days | Single dose 5 mg/kg IV | Hospira (1995) |
| Amodiaquine dihydrochloride | 32 ± 3 | 464.8 | 0.069 | 5.2 ± 1.7 h | Single dose 600 mg PO | Winstanley et al. (1987) |
| Chlorprothixene | 430 ± 81 | 315.9 | 1.36 | 25.8 ± 13.6 h | Single dose 100 mg IV | Bagli et al. (1996) |
| Clemastine fumarate | 0.577 ± 0.252 | 460 | 0.0013 | 21.3 ± 11.6 h | Single dose 1.34 mg PO | Schran et al. (1996) |
| Deserpidine | 0.172 | 578.66 | 0.0003 | 42.9 ± 17.8 h | Single dose 0.25 mg PO | Zhang et al. (2009) |
| Duloxetine hydrochloride | 110 | 333.88 | 0.33 | 6.96–14.9 h | 60 mg BID PO | Knadler et al. (2011) |
| Imatinib | 3,395 ± 2,409 | 493.6 | 6.88 | 10–18.9 h | Single dose 600 mg PO | Peng et al. (2005) |
| Loperamide hydrochloride | 2 | 477 | 0.0042 | 9.1–14.4 h | Single dose 2 mg PO | Janssen Pharmaceutica Inc. (1998) |
| Maprotiline hydrochloride | 25 | 313.87 | 0.080 | 45 h | Single dose 75 mg PO | Maguire et al. (1980) |
| a Methdilazine hydrochloride | 332.9 | |||||
| Methotrimeprazine maleate | 3.44 | 444.6 | 0.0077 | 10.8 h | Single dose 25 mg PO | AA Pharma Inc. (2012) |
| a Piperacetazine | 410.6 | |||||
| Remdesivir | 4,420 | 602.58 | 7.34 | 1.05 h | Single dose 225 mg IV | Humeniuk et al. (2020) |
Cmax: maximum serum/plasma concentration; MW: molecular weight; Elimination T1/2: elimination half life; PO: per os (oral dosing); IV: intravenous.
Four drugs approved outside of the US were also identified as novel compounds with anti-SARS-CoV-2 effects: difeterol, rescimetol, melitracen HCl, and proglumetacin. Furthermore, we identified 7 novel clinical trial drugs with anti-SARS-CoV-2 activities: N-methylspiperone HCl, Lu AE58054 HCl, balicatib, berzosertib, JTV519 hemifumarate, DMP 777, and dexanabinol. In addition to the above novel hits, four drugs, approved by the FDA and elsewhere, methdilazine, maprotiline HCl, deserpidine, and flunarizine, were previously reported in virtual screens against SARS-CoV-2 targets without supporting biological data. Here, we report their activities against SARS-CoV-2 infection. In addition, we have confirmed 53 approved drugs with anti-SARS-CoV-2 effects that were reported previously (Table 1 and Supplementary Table S1). Together, our results demonstrate a comprehensive set of 91 approved drugs and 49 investigational drugs with anti-SARS-CoV-2 activity that can be considered for design of new clinical trials, especially drug combination therapies, to increase and improve treatment options for COVID-19.
Discussion
In contrast to the other reported drug repurposing screens for SARS-CoV-2 using a single drug concentration in the primary screens (Dittmar et al., 2020; Ellinger et al., 2020; Jeon et al., 2020; Riva et al., 2020; Touret et al., 2020; Weston et al., 2020), we have used a quantitative HTS (qHTS, concentration-response) method (Inglese et al., 2006) where four compound concentrations were used in the primary screen instead of a single compound concentration. We also assessed the cytotoxicity of each compound against Vero E6 cells (without virus infection) in parallel with the SARS-CoV-2 CPE screening. The concentration-response for each compound used in the primary screen can improve identification of positive hits, especially compounds with biphasic actions (bell-shaped curves) or screening errors. In addition, NCATS has more inclusive compound collections with drugs approved by regulatory agencies outside of the US, such as Canada, Europe and Japan, that were not previously screened in SARS-CoV-2 assays. We also screened a set of investigational drugs that have human clinical data for drug properties such as the mechanism(s) of action, pharmacokinetics, and drug toxicity, which could be leveraged to speed up drug development. The other bioactive compounds screened have drug targets and mechanisms of action that may be useful for further studies of disease pathophysiology and for potential drug development.
We identified 319 compounds with activity against SARS-CoV-2 CPE from a qHTS of 8,810 unique compounds. Among the top 56 hits identified with <10 µM EC50 values and >80% efficacies, the anti-SARS-CoV-2 activity of 37 of them has not been reported elsewhere. Of these novel top hits, three were FDA approved drugs with novel anti-SARS-CoV-2 activity. Chlorprothixene is a dopamine receptor antagonist, a classic antipsychotic agent approved for treatment of schizophrenia (Schrijver et al., 2016). Methotrimeprazine, also named as levomepromazine, is another tricyclic antipsychotic agent approved for psychotic disorders including schizophrenia, and manic-depressive syndromes (Sivaraman et al., 2010). Both chlorprothixene and methotrimeprazine were previously found to inhibit the SARS-CoV replication with EC50s around 10 µM (Barnard et al., 2008). Piperacetazine is also an older tricyclic antipsychotic drug approved for treatment of schizophrenia (Eslami Shahrbabaki et al., 2018). The antiviral effect of piperacetazine was found previously to block the Ebola viral entry with the EC50 of 9.68 µM (Kouznetsova et al., 2019).
We also confirmed the anti-SARS-CoV-2 activity of five compounds that were reported as virtual screening hits but had yet to be confirmed experimentally, including methdilazine by an AI prediction algorithm (Grzybowski et al., 2020), GMC 2-113 by a virtual screen of RNA dependent RNA polymerase (RdRP) (Dwivedy et al., 2020), maprotiline by a main protease docking (Chauhan, 2020), deserpidine by a NPS-16 docking (Jiang et al., 2020), and flunarizine by a spike protein docking screen (Chernyshev, 2020). Our data supports the utility of these emerging technologies and the field of AI for advancing drug development.
For in vitro screens of antiviral compounds, molecular target (mechanism) based assays and phenotypic assays are two major approaches. Common targets are viral enzymes such as viral protease, DNA and RNA polymerases, reverse transcriptase, and integrase. Development of assays targeting viral enzymes rely on viral enzyme expression, purification, assay development, and validation (Shyr et al., 2020). Alternatively, phenotypic assays involving live-virus infection are readily executed once the viruses are isolated from patients and viral replication in appropriate host cells is established. A common live virus infection assay is the measurement of CPE in virus infected host cells. There are two possibilities (fates) for the host cells after viral infection, including cytopathic infection (i.e. death of host cells) and persistent infection (Heaton, 2017). The CPE effect can be readily measured by the ATP content cell viability assay, which is robust and amenable for HTS. Due to the nature of the CPE assay, compounds that suppress CPE can act against any part of the virus infection cycle, including the binding of virus to the host cell receptor, entry into host cells, virus replication, viral assembly/budding, and virus reinfection of adjacent cells.
It is worth briefly reflecting on the limitations of the drug repurposing assay approach. A number of small molecules of interest for treating COVID-19 that are currently in clinical trials were not hits in our assay. For example, the TMPRSS2 inhibitors camostat and nafamstat are protease inhibitors approved in Japan for treating pancreatitis, and known to inhibit TMPRSS2 (Shrimp et al., 2020). While TMPRSS2 is reported to be a mediator of SARS-CoV-2 cell entry, Vero E6 cells do not express TMPRSS2, so this class of compound are not active in the Vero E6 assay. The drug efflux transporter P-glycoprotein (P-gp) can reduce cellular concentrations of test agents, and as a kidney epithelial cell line, Vero E6 cells likely expresses significant P-gp concentrations, which would reduce activity of P-gp substrates (Robey et al., 2018). Remdesivir itself is a substrate of Pgp (EMA, 2020), and is weaker against SARS-CoV-2 in assays using Vero E6 cells (EC50 > 1 µM) compared with Calu-3 or Huh7 cell lines (EC50 > 50 nM) (Stanford University, 2020). These examples highlight the need for careful interpretation and critical follow-up studies after initial high-throughput screening analyses. Furthermore, the list of compounds presented here are confirmed hits in a SARS-CoV-2 CPE assay, and will require considerable follow up work to determine their feasibility for translation to clinical use. A possible pipeline for follow up could be testing in more physiologically relevant 2D human cells using orthogonal assays, and 3D human in vitro respiratory tissue models. These results would require confirmation in animal efficacy models, as well as evaluation of human PK and tolerability of these compounds. Additionally, the hits identified in this screen could be further tested in pair-wise matrix combinations to identify synergistic combinations for potential cocktail treatments (Shinn et al., 2019).
Importantly, the comprehensive primary screen datasets of this study for approved and investigational drugs, and mechanism-based bioactive compounds have been made publicly available in real-time on the NCATS OpenData Portal (https://opendata.ncats.nih.gov/covid19/index.html) (Brimacombe et al., 2020). These datasets provide a wealth of quality live-virus data that is freely available to the research community for future studies and data mining with the aim of offering new therapeutics to treat COVID-19 patients efficiently and safely (Zhu et al., 2020a; Huang et al., 2020).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession numbers can be found in the article/Supplementary Material. Primary screen data can be found at http://opendata.ncats.nih.gov. Secondary screen data are uploaded in PubChem AIDs 1508605 and 1508606. All other data are available upon request.
Author Contributions
PS, ZI, and RTE prepared the assay ready plates. RB and LR conducted the CPE and cytotoxicity assays. PS, CK‐T, KMW, and SGM curated the compound libraries. CZC and MDH designed the experiments. CZC, BMB, and WZ wrote the manuscript. RH, MS, XH, HG, and TZ performed data analysis and data uploads. All authors provided critical reading of the manuscript.
Funding
This work was supported by the Intramural Research Program of National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD, United States.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.592737/full#supplementary-material.
References
- AA Pharma Inc (2012). Methotrimeprazine Maleate Tablets [Prescribing Information] [Online]. https://www.aapharma.ca/downloads/en/PIL/Methoprazine_PI.pdf Accessed 16 9, 2020).
- Bagli M., Rao M. L., Hoflich G., Kasper S., Langer M., Barlage U., et al. (1996). Pharmacokinetics of chlorprothixene after single intravenous and oral administration of three galenic preparations. Arzneimittelforschung 46 (3), 247–250. [Abstract] [Google Scholar]
- Barnard D. L., Day C. W., Bailey K., Heiner M., Montgomery R., Lauridsen L., et al. (2008). Is the anti-psychotic, 10-(3-(dimethylamino)propyl)phenothiazine (promazine), a potential drug with which to treat SARS infections?. Antivir. Res 79 (2), 105–113. 10.1016/j.antiviral.2007.12.005 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Beigel J. H., Tomashek K. M., Dodd L. E., Mehta A. K., Zingman B. S., Kalil A. C., et al. (2020). Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med 383 (19), 1813–1826. 10.1056/NEJMoa2007764 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brimacombe K. R., Zhao T., Eastman R. T., Hu X., Wang K., Backus M., et al. (2020). An OpenData portal to share COVID-19 drug repurposing data in real time. bioRxiv 19, 5046. 10.1101/2020.06.04.135046 [CrossRef] [Google Scholar]
- Castaldo N., Aimo A., Castiglione V., Padalino C., Emdin M., Tascini C. (2020). Safety and efficacy of Amiodarone in a patient with COVID-19. JACC (J. Am. Coll. Cardiol.) Case Rep 2 (9), 1307–1310. 10.1016/j.jaccas.2020.04.053 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chauhan N. (2020). Possible drug candidates for COVID-19. ChemRxiv 98, 5231. 10.26434/chemrxiv.11985231.v1 [CrossRef] [Google Scholar]
- Chernyshev A. (2020). Pharmaceutical targeting the envelope protein of SARS-CoV-2: the screening for inhibitors in approved drugs. ChemRxiv 28, 542. 10.26434/chemrxiv.12286421.v1 [CrossRef] [Google Scholar]
- Dittmar M., Lee J. S., Whig K., Segrist E., Li M., Jurado K., et al. (2020). Drug repurposing screens reveal FDA approved drugs active against SARS-Cov-2. bioRxiv 16, 1042. 10.1101/2020.06.19.161042 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dong E., Du H., Gardner L. (2020). An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis 20 (5), 533–534. 10.1016/S1473-3099(20)30120-1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dwivedy A., Mariadasse R., Ahmed M., Kar D., Jeyakanthan J., Biswal B. K. (2020). In silico characterization of the NiRAN domain of RNA-dependent RNA polymerase provides insights into a potential therapeutic target against SARS-CoV2. OSF Preprints 31, 219. 10.31219/osf.io/wd6zu [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Eastman R. T., Roth J. S., Brimacombe K. R., Simeonov A., Shen M., Patnaik S., et al. (2020). Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci 6 (5), 672–683. 10.1021/acscentsci.0c00489 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Eli Lilly and Company (1987). Prozac: Highlights of Prescribing Information [Online]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018936s091lbl.pdf. Accessed 169, 2020).
- Ellinger B., Bojkova D., Zaliani A., Cinatl J., Claussen C., Westhaus S., et al. (2020). Identification of inhibitors of SARS-CoV-2 in-vitro cellular toxicity in human (Caco-2) cells using a large scale drug repurposing collection. Res. Sq 23, 951. 10.21203/rs.3.rs-23951/v1 [CrossRef] [Google Scholar]
- EMA. (2020). Summary on compassionate use: Remdesivir gilead Editor Division H. M. [Google Scholar]
- Eslami Shahrbabaki M., Dehnavieh R., Vali L., Sharafkhani R. (2018). Chlorpromazine versus piperacetazine for schizophrenia. Cochr. Datab. Syst. Rev 10, CD011709. 10.1002/14651858.CD011709.pub2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Grzybowski B., Grynkiewicz G., Szymkuć S., Molga K., Wołos A., Gajewska E. P., et al. (2020). Suggestions for second-pass anti-COVID-19 drugs based on the Artificial Intelligence measures of molecular similarity, shape and pharmacophore distribution. ChemRxiv 84, 690. 10.26434/chemrxiv.12084690.v2 [CrossRef] [Google Scholar]
- Heaton N. S. (2017). Revisiting the concept of a cytopathic viral infection. PLoS Pathog 13 (7), e1006409. 10.1371/journal.ppat.1006409 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hospira (1995). Amiodaron HCl injection for intravenous use: highlights of prescribing information https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/075955s015lbl.pdf.
- Huang R., Xu M., Zhu H., Chen C. Z., Lee E. M., He S., et al. (2020). Massive-scale biological activity-based modeling identifies novel antiviral leads against SARS-CoV-2. bioRxiv 22, 3578. 10.1101/2020.07.27.223578 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Huang R., Zhu H., Shinn P., Ngan D., Ye L., Thakur A., et al. (2019). The NCATS pharmaceutical collection: a 10-year update. Drug Discov. Today 24 (12), 2341–2349. 10.1016/j.drudis.2019.09.019 [Abstract] [CrossRef] [Google Scholar]
- Humeniuk R., Mathias A., Cao H., Osinusi A., Shen G., Chng E., et al. (2020). Safety, tolerability, and pharmacokinetics of Remdesivir, an antiviral for treatment of COVID‐19, healthy subjects. Clin. Transl. Sci 383(19), 1813–1826. 10.1111/cts.12840 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ianevski A., Yao R., Fenstad M. H., Biza S., Zusinaite E., Reisberg T., et al. (2020). Potential antiviral options against SARS-CoV-2 infection. Viruses 12 (6), 642. 10.3390/v12060642 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Inglese J., Auld D. S., Jadhav A., Johnson R. L., Simeonov A., Yasgar A., et al. (2006). Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. USA 103 (31), 11473–11478. 10.1073/pnas.0604348103 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Janssen Pharmaceutica Inc. (1998). Imodium Capsules [Package Insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/017694s052lbl.pdf. Accessed 16 9, 2020).
- Jeon S., Ko M., Lee J., Choi I., Byun S. Y., Park S., et al. (2020). Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother 64 (7), e00819. 10.1128/AAC.00819-20 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jiang Y., Liu L., Manning M., Bonahoom M., Lotvola A., Yang Z.-Q. (2020). Repurposing therapeutics to identify novel inhibitors targeting 2'-O-Ribose Methyltransferase Nsp16 of SARS-CoV-2. ChemRxiv 25, 2965. 10.26434/chemrxiv.12252965.v1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Knadler M. P., Lobo E., Chappell J., Bergstrom R. (2011). Duloxetine. Clin. Pharmacokinet 50 (5), 281–294. 10.2165/11539240-000000000-00000 [Abstract] [CrossRef] [Google Scholar]
- Kouznetsova J., Sun W., Martínez-Romero C., Tawa G., Shinn P., Chen C. Z., et al. (2019). Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microb. Infect 3 (1), 1–7. 10.1038/emi.2014.88 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lin G. L., Wilson K. M., Ceribelli M., Stanton B. Z., Woo P. J., Kreimer S., et al. (2019). Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med 11 (519), eaaw0064. 10.1126/scitranslmed.aaw0064 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ma C., Sacco M. D., Hurst B., Townsend J. A., Hu Y., Szeto T., et al. (2020). Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res 30 (8), 678–692. 10.1038/s41422-020-0356-z [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Maguire K. P., Norman T. R., Burrows G. D., Scoggins B. A. (1980). An evaluation of maprotiline intravenous kinetics and comparison of two oral doses. Eur. J. Clin. Pharmacol 18 (3), 249–254. 10.1007/bf00563007 [Abstract] [CrossRef] [Google Scholar]
- Morales-Ortega A., Bernal-Bello D., Llarena-Barroso C., Frutos-Pérez B., Duarte-Millán M. Á., García de Viedma-García V., et al. (2020). Imatinib for COVID-19: a case report. Clin. Immunol 218, 108518. 10.1016/j.clim.2020.108518 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun 11 (1), 1620. 10.1038/s41467-020-15562-9 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Peng B., Lloyd P., Schran H. (2005). Clinical pharmacokinetics of imatinib. Clin. Pharmacokinet 44 (9), 879–894. 10.2165/00003088-200544090-00001 [Abstract] [CrossRef] [Google Scholar]
- Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., et al. (2020). Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586 (7827), 113–119. 10.1038/s41586-020-2577-1 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Robey R. W., Pluchino K. M., Hall M. D., Fojo A. T., Bates S. E., Gottesman M. M. (2018). Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18 (7), 452–464. 10.1038/s41568-018-0005-8 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ryu Y. B., Park S.-J., Kim Y. M., Lee J.-Y., Seo W. D., Chang J. S., et al. (2010). SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett 20 (6), 1873–1876. 10.1016/j.bmcl.2010.01.152 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schran H. F., Petryk L., Chang C.-T., O'Connor R., Gelbert M. B. (1996). The pharmacokinetics and bioavailability of clemastine and phenylpropanolamine in single-component and combination formulations. J. Clin. Pharmacol 36 (10), 911–922. 10.1002/j.1552-4604.1996.tb04758.x [Abstract] [CrossRef] [Google Scholar]
- Schrijver E. J. M., de Graaf K., de Vries O. J., Maier A. B., Nanayakkara P. W. B. (2016). Efficacy and safety of haloperidol for in-hospital delirium prevention and treatment: a systematic review of current evidence. Eur. J. Intern. Med 27, 14–23. 10.1016/j.ejim.2015.10.012 [Abstract] [CrossRef] [Google Scholar]
- Shinn P., Chen L., Ferrer M., Itkin Z., Klumpp-Thomas C., McKnight C., et al. (2019). High-throughput screening for drug combinations. Bioinform. Drug Discov 28, 11–35. 10.1126/scitranslmed.aaw0064 [Abstract] [CrossRef] [Google Scholar]
- Shrimp J. H., Kales S. C., Sanderson P. E., Simeonov A., Shen M., Hall M. D. (2020). An enzymatic TMPRSS2 assay for assessment of clinical candidates and discovery of inhibitors as potential treatment of COVID-19. ACS Pharmacol. Transl. Sci 3 (5), 997–1007. 10.1021/acsptsci.0c00106 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shyr Z. A., Gorshkov K., Chen C. Z., Zheng W. (2020). Drug discovery strategies for SARS-CoV-2. J. Pharmacol. Exp. Therapeut 375 (1), 127–138. 10.1124/jpet.120.000123 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sivaraman P., Rattehalli R. D., Jayaram M. B. (2010). Levomepromazine for schizophrenia. Cochr. Datab. Syst. Rev 10, CD007779. 10.1002/14651858.CD007779.pub2 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stanford University (2020). Coronavirus antiviral research database https://covdb.stanford.edu/search/?compound=Remdesivir&target=Polymerase. Accessed Aug 5, 2020). [Google Scholar]
- Touret F., Gilles M., Barral K., Nougairède A., van Helden J., Decroly E., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep 10 (1), 70143. 10.1038/s41598-020-70143-6 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vatansever E. C., Yang K., Kratch K. C., Drelich A., Cho C.-C., Mellot D. M., et al. (2020). Targeting the SARS-CoV-2 main protease to repurpose drugs for COVID-19. bioRxiv 11, 2235. 10.1101/2020.05.23.112235 [CrossRef] [Google Scholar]
- Vitner E. B., Avraham R., Achdout H., Tamir H., Agami A., Cherry L., et al. (2020). Antiviral activity of Glucosylceramide synthase inhibitors against SARS-CoV-2 and other RNA virus infections. bioRxiv 10, 3285. 10.1101/2020.05.18.103283 [CrossRef] [Google Scholar]
- Wang Y., Jadhav A., Southal N., Huang R., Nguyen D. T. (2010). A grid algorithm for high throughput fitting of dose-response curve data. Curr. Chem. Genom 4, 57–66. 10.2174/1875397301004010057 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weston S., Coleman C. M., Haupt R., Logue J., Matthews K., Frieman M. B. (2020). Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo . bioRxiv 84, 82. 10.1101/2020.03.25.008482 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Winstanley P., Edwards G., Orme M., Breckenridge A. (1987). The disposition of amodiaquine in man after oral administration. Br. J. Clin. Pharmacol 23 (1), 1–7. 10.1111/j.1365-2125.1987.tb03002.x [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang H., Zhong D., Zhang Z., Dai X., Chen X. (2009). Liquid chromatography/tandem mass spectrometry method for the quantification of deserpidine in human plasma: application to a pharmacokinetic study. J. Chromatogr. B 877 (27), 3221–3225. 10.1016/j.jchromb.2009.06.005 [Abstract] [CrossRef] [Google Scholar]
- Zhu H., Chen C. Z., Sakamuru S., Simeonov A., Hall M. D., Xia M., et al. (2020a). Mining of high throughput screening database reveals AP-1 and autophagy pathways as potential targets for COVID-19 therapeutics arXiv 2007.12242v1 [Europe PMC free article] [Abstract] [Google Scholar]
- Zhu W., Xu M., Chen C. Z., Guo H., Shen M., Hu X., et al. (2020b). Identification of SARS-CoV-2 3CL protease inhibitors by a quantitative high-throughput screening. ACS Pharmacol. Transl. Sci 5, 1008–1016. 10.1021/acsptsci.0c00108 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zimniak M., Kirschner L., Hilpert H., Seibel J., Bodem J. (2020). The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2. bioRxiv 15, 490. 10.1101/2020.06.14.150490 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Articles from Frontiers in Pharmacology are provided here courtesy of Frontiers Media SA
Full text links
Read article at publisher's site: https://doi.org/10.3389/fphar.2020.592737
Read article for free, from open access legal sources, via Unpaywall:
https://www.frontiersin.org/articles/10.3389/fphar.2020.592737/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102377280
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3389/fphar.2020.592737
Article citations
Hexestrol, an estrogen receptor agonist, inhibits Lassa virus entry.
J Virol, 98(7):e0071424, 29 May 2024
Cited by: 0 articles | PMID: 38809021
COVID-19 drug discovery and treatment options.
Nat Rev Microbiol, 22(7):391-407, 15 Apr 2024
Cited by: 7 articles | PMID: 38622352
Review
Binding Activity Classification of Anti-SARS-CoV-2 Molecules using Deep Learning Across Multiple Assays
Balkan Med J, 41(3):186-192, 11 Mar 2024
Cited by: 0 articles | PMID: 38462979 | PMCID: PMC11077922
Molecular docking as a tool for the discovery of novel insight about the role of acid sphingomyelinase inhibitors in SARS- CoV-2 infectivity.
BMC Public Health, 24(1):395, 06 Feb 2024
Cited by: 1 article | PMID: 38321448 | PMCID: PMC10848368
Review Free full text in Europe PMC
How Deep Learning in Antiviral Molecular Profiling Identified Anti-SARS-CoV-2 Inhibitors.
Biomedicines, 11(12):3134, 24 Nov 2023
Cited by: 1 article | PMID: 38137356 | PMCID: PMC10740425
Go to all (55) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Screening a Library of FDA-Approved and Bioactive Compounds for Antiviral Activity against SARS-CoV-2.
ACS Infect Dis, 7(8):2337-2351, 15 Jun 2021
Cited by: 22 articles | PMID: 34129317
Identification of SARS-CoV-2 3CL Protease Inhibitors by a Quantitative High-Throughput Screening.
ACS Pharmacol Transl Sci, 3(5):1008-1016, 04 Sep 2020
Cited by: 134 articles | PMID: 33062953 | PMCID: PMC7507806
Current Strategies of Antiviral Drug Discovery for COVID-19.
Front Mol Biosci, 8:671263, 13 May 2021
Cited by: 51 articles | PMID: 34055887 | PMCID: PMC8155633
Review Free full text in Europe PMC
Funding
Funders who supported this work.




