Abstract
Free full text

Long-Haul Post–COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome
Abstract
Major clinical centers in Sweden have witnessed an inflow of patients with chronic symptoms following initial outpatient care for coronavirus disease-2019 (COVID-19) infection, suggestive of postural orthostatic tachycardia syndrome. This report presents the first case series of 3 Swedish patients diagnosed with postural orthostatic tachycardia syndrome more than 3 months after the primary COVID-2019 infections. (Level of Difficulty: Intermediate.)
Central Illustration
INTRODUCTION
Postural orthostatic tachycardia syndrome (POTS) is the most prevalent chronic cardiovascular dysautonomia among young and middle-age individuals, predominantly women. It is characterized by chronic orthostatic intolerance, abnormal heart rate (HR) increase on standing, and deconditioning (1,2) (Table 1). The syndrome has been postulated to have post-viral autoimmune activation as a possible etiology (3).
Table 1
Diagnostic Criteria of POTS
| Sustained heart rate increment of not <30 beats/min or above 120 beats/min within 10 min of active standing or head-up tilt. For individuals who are younger than 19 yrs the required increment is at least 40 beats/min. |
| Absence of orthostatic hypotension (i.e., sustained systolic blood pressure drop of not <20 mm Hg). |
| Reproduction of spontaneous symptoms such as light-headedness, palpitations, tremulousness, generalized weakness, blurred vision, and fatigue. In some patients, tachycardia may evoke vasovagal syncope corresponding to spontaneous attacks from patient’s history. |
| History of chronic orthostatic intolerance and other typical POTS-associated symptoms (for at least 6 months [1]). |
| Absence of other conditions provoking sinus tachycardia such as anxiety disorders, hyperventilation, anemia, fever, pain, infection, dehydration, hyperthyroidism, pheochromocytoma, use of cardioactive drugs (sympathomimetics, anticholinergics). |
This table has been endorsed by the American Academy of Neurology, the American Autonomic Society, the American College of Cardiology, the American Heart Association, the European Federation of Autonomic Societies, the European Heart Rhythm Association, the European Society of Cardiology, and the Heart Rhythm Society.
Adopted with permission from Fedorowski (1).
POTS = postural orthostatic tachycardia syndrome.
In the acute phase, coronavirus disease-2019 (COVID-19) causes multiple complications including pneumonia, respiratory distress syndrome, liver injury, cardiac injury, and prothrombotic coagulopathy. Long-term consequences remain unknown (4). Recently, chronic (“long-haul”) symptoms following COVID-19 infections have been consistent with a POTS-like presentation (4). Here we present a series of 3 patients with chronic post-COVID-19 symptoms diagnosed as POTS.
Patient #1
In March 2020, a 42-year-old woman with history of allergic rhinitis and conjunctivitis developed flu-like symptoms including malaise, cough, fever, weakness, loss of appetite, myalgia, and loss of smell and taste. She did not seek medical attention and her condition improved progressively but, in May, symptoms recurred with the addition of abdominal pain and odynophagia. Chest computed tomography and laboratory testing were normal. A nasopharyngeal swab showed no evidence of COVID-19.
In July, debilitating symptoms of profound exhaustion with associated sinus tachycardia followed. Telemetry showed HR of 70 to 160 beats/min. Echocardiography was normal. Serology tests for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) were considered borderline. By September, she was found unable to stand more than 5 min. Additionally, she complained of palpitations, dizziness, heat, and exercise intolerance (Figure 1). During a head-up tilt test, her HR increased from 85 beats/min supine to 135 beats/min upright within 10 min (Figure 2). Active standing showed initial orthostatic hypotension (Figure 3), whereas Valsalva maneuver showed a hyperadrenergic response characteristic for POTS (Figure 4).
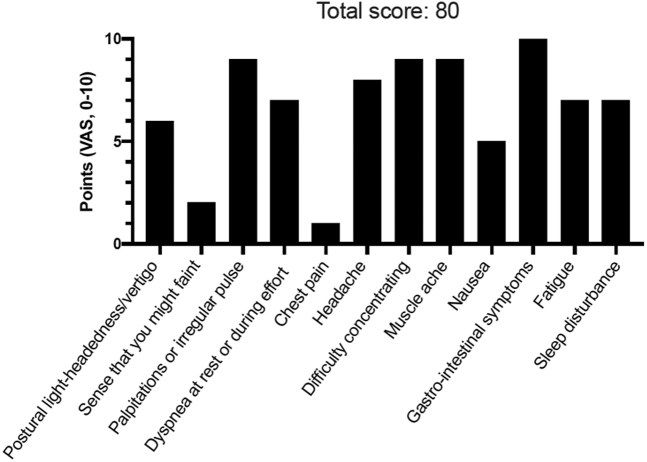
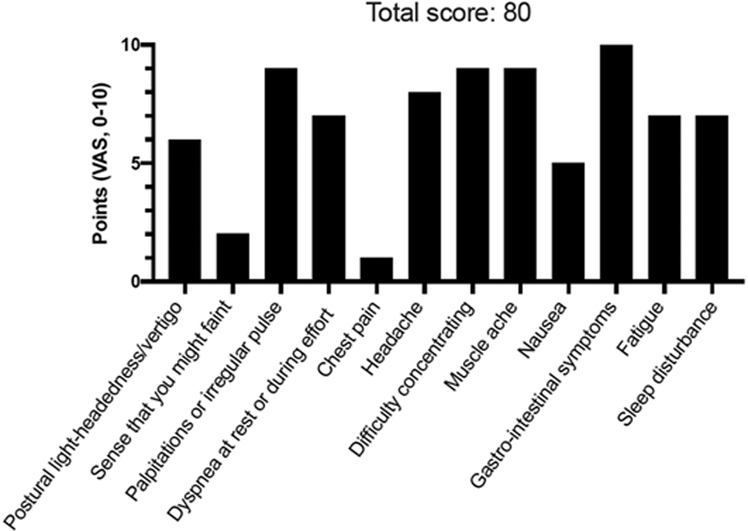
POTS Symptom Scoring in 42-Year-Old Woman
Patient #1 self-reported symptoms using a dedicated postural orthostatic tachycardia syndrome (POTS) symptom scoring questionnaire composed of 12 most commonly reported symptoms in POTS. Patients were asked to grade their symptoms using a visual analogue scale (VAS) ranging from 0 (no symptom) to 10 (worst possible). The maximum score is 120 points. A score >40 points likely indicates pathology.
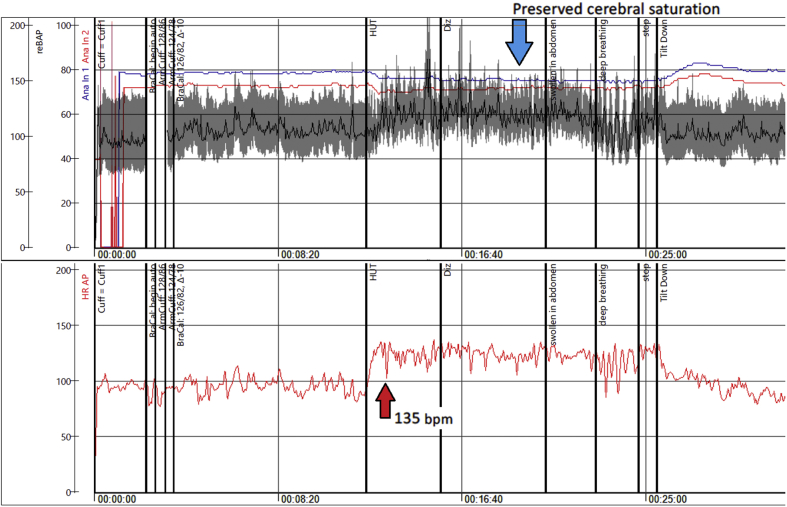
HUT Testing in 42-Year-Old Woman
Head-up tilt (HUT) test revealing post–coronavirus disease-2019 POTS in a 42-year-old woman (Patient #1), with red arrows indicating the marked increase in heart rate (HR) during orthostasis. bpm = beats/min; Diz = dizziness; POTS = postural orthostatic tachycardia syndrome.
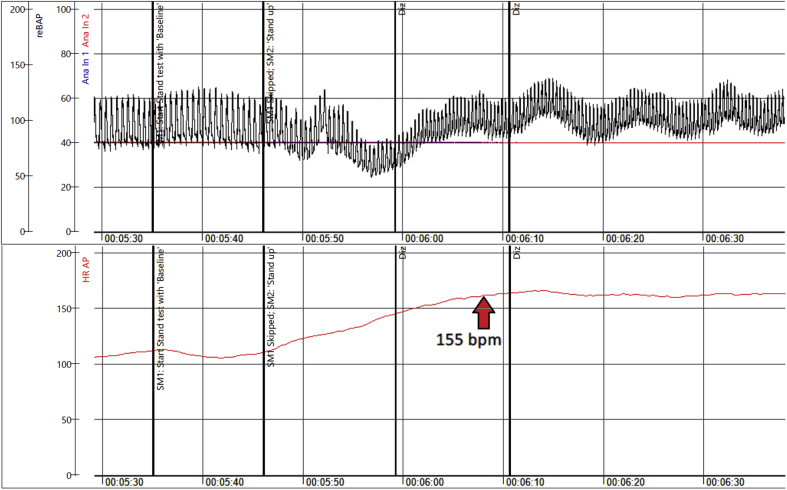
Active Standing in 42-Year-Old Woman
Active standing test demonstrating initial orthostatic hypotension and POTS in a 42-year-old woman (Patient #1) with long-haul post–coronavirus disease-2019 symptoms, with red arrow indicating the marked increase in heart rate during orthostasis. Abbreviations as in Figures 1 andand22.
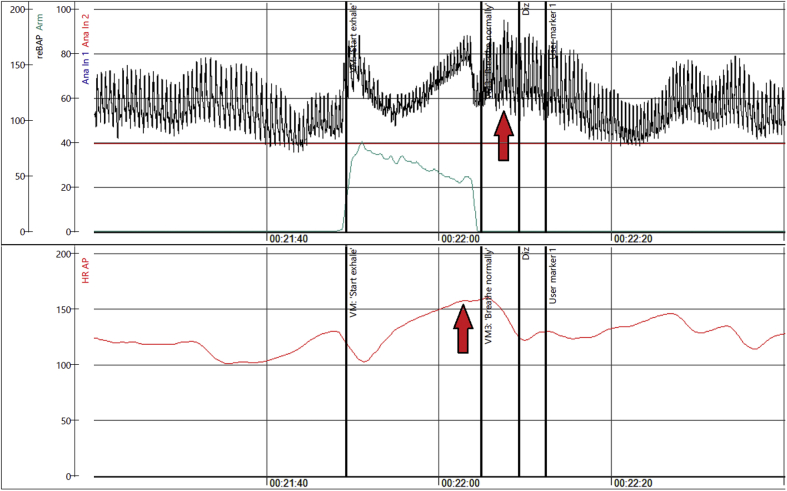
Valsalva Response in 42-Year-Old Woman
Hyperadrenergic Valsalva maneuver in a 42-year-old woman (Patient #1) with long-haul post–coronavirus disease 2019 symptoms, with red arrows indicating the marked increase in heart rate and blood pressure (hyperadrenergic response). Abbreviations as in Figure 2.
Ambulatory monitoring demonstrated a mean HR of 89 beats/min with episodic sinus tachycardia, maximum rate of 153 beats/min, while standing. Spirometry and cardiac magnetic resonance were normal. Autoimmunological screening of antiphospholipid antibodies, antinuclear antibodies, antineutrophil cytoplasmic antibody was normal. Twenty-four–hour blood pressure (BP) monitoring showed average BP of 112/74 mm Hg, preserved circadian profile, and lowest BP during daytime of 79/46 mm Hg.
Nonpharmacological measures including increased fluid intake, compression stockings, and avoidance of orthostatic triggers were recommended. She did not tolerate beta-blockers due to worsened orthostatic intolerance, and ivabradine 7.5 mg twice a day was started (Table 2) with substantial improvement, although the patient remains on sick leave.
Table 2
Proposed Treatment of POTS
| Drugs | Dosage | Side Effects | Precautions |
|---|---|---|---|
| Nonpharmacological treatments | |||
| Withdraw exacerbating medications | Stop drugs that decrease blood volume or directly increase heart rate | ||
| Increased oral water intake | Target 2–3 l/day | Frequent urination | |
| Increased oral NaCl intake | Target 8–10 g/day | Hypertension, peripheral edema | Buffered NaCl tablets can be used if this cannot be done with diet alone |
| Lower body compression garments | 20–40 mm Hg compression; focus on abdomen ± legs | Can be hot, tight, and itchy | |
| Exercise training | Aerobic: 30+ min 4 days/week with some leg resistance training | Will initially feel poorly and/or worse for up to 6 weeks | Initial recumbent exercise, such as a rowing machine, recumbent cycle, or swimming are preferred |
| Pharmacological treatments | |||
| Blood volume expanders | |||
| Fludrocortisone | 0.1–0.2 mg daily | Hypokalemia, edema, headache | Electrolytes should be monitored |
| Desmopressin (DDAVP) | 0.1–0.2 mg as needed | Hyponatremia, edema | Electrolytes should be monitored if used chronically |
| Acute IV saline | 2 l IV over 2–3 h | Venous thrombosis, infection | |
| Chronic IV saline | 2 l given IV once weekly | Infection risk of central venous catheters | Avoid long-term use and placement of central catheters |
| Erythropoietin | 10,000 IU weekly | Increased risk of cardiovascular death | Hematocrit should be monitored |
| Heart rate inhibitors | |||
| Propranolol | 10–20 mg orally up to 4× daily | Hypotension, bradycardia, bronchospasm | Can worsen asthma or exercise tolerance |
| Ivabradine | 2.5–7.5 mg orally twice daily | Headaches, palpitations, hypertension, visual disturbances | |
| Pyridostigmine | 30–60 mg orally up to 3× daily | Abdominal cramps, diarrhea | Can worsen asthma |
| Vasoconstrictors | |||
| Midodrine | 2.5–15 mg orally 3× daily | Headache, scalp tingling, hypertension | |
| Octreotide | Long-acting intramuscular injection 10–30 mg | Nausea, stomach cramps, diarrhea | |
| Methylphenidate | 10 mg orally 2× to 3× a day. Last dose should be avoided before bed | Tachycardia, insomnia, nausea, headache, dizziness | |
| Droxidopa | 100–600 mg 3× daily | Headache, nausea, hypertension, and tachycardia | Off-label use only |
| Sympatholytic drugs | |||
| Alpha2 adrenergic agonists, such as clonidine | 0.1–0.2 mg orally 2× to 3× daily or long-acting patch | Hypotension, fatigue, brain fog | |
| Methyldopa | 125–250 mg orally twice daily | Hypotension, fatigue, brain fog | |
| Other | |||
| Modafinil | 50–200 mg orally once or twice daily | Tachycardia | |
Adapted with permission from Miller and Raj (9).
DDAVP = desmopressin; IU = international units; IV = intravenous(ly); POTS = postural orthostatic tachycardia syndrome.
Patient #2
A 28-year-old woman developed COVID-19 symptoms in May 2020 with fever, dyspnea, chest pain, lightheadedness, and headache. Polymerase chain reaction testing was positive for SARS-CoV-2. Previous medical history included arthroscopic meniscectomy, tonsillectomy, and discectomy.
Due to persistent and progressive symptoms (chest pain, fatigue, vertigo, headache), she was hospitalized twice. Blood tests were normal except slight leukocytosis. Chest computed tomography excluded pulmonary embolism and viral pneumonia but revealed pericardial effusion. As pericarditis was suspected, prednisolone was started, and subsequently changed to colchicine and ibuprofen, with no effect on symptoms. Due to headache, nausea, and photophobia, viral meningitis was suspected. However, lumbar puncture and spine and brain magnetic resonance imaging scan were normal.
An active standing test demonstrated HR increase from 75 to 128 beats/min with concomitant slight increase in systolic BP (10 mm Hg) and pronounced orthostatic intolerance (dizziness, lightheadedness, tremor) (Figure 5).
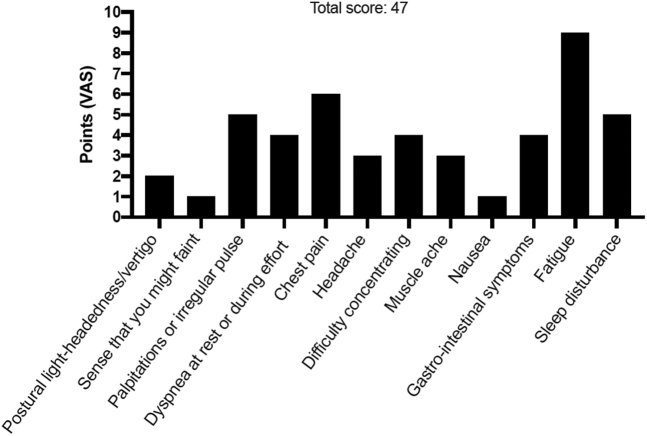
POTS Symptom Scoring in 28-Year-Old Woman
Patient #2 self-reported symptoms using a dedicated POTS symptom scoring questionnaire composed of 12 most commonly reported symptoms in POTS. Patients were asked to grade their symptoms using a VAS ranging from 0 (no symptom) to 10 (worst possible). The maximum score is 120 points. A score >40 points likely indicates pathology. Abbreviations as in Figure 1.
In September, she was referred to a tertiary center for post–COVID-19 follow-up and tested positive for SARS-CoV-2 immunoglobulin G antibodies. During a 6-min walking test peripheral oxygen desaturation was noted (from 99% to 89%) without any other findings.
Further evaluation with perfusion stress cardiac magnetic resonance, antinuclear antibodies, antineutrophil cytoplasmic antibody, metoxycatecholamines, and antiphospholipid autoantibodies was normal. POTS was confirmed by both active standing test and head-up tilt (symptomatic sinus tachycardia >130 beats/min).
Nonpharmacological recommendations included increased daily fluid and salt intake, as well as use of compression socks. She was prescribed propranolol 10 mg ×3 times a day (Table 2). After several weeks, she developed gastrointestinal symptoms, itching, and orbital edema. Because concomitant mast cell activation syndrome was suspected, she received H1 and H2 antihistamines but remains highly symptomatic and is on sick leave.
Patient #3
In May 2020, a 37-year-old man developed sore throat, fever, fatigue, muscle weakness, dry cough, and palpitations. He had a history of childhood sepsis and migraine. Polymerase chain reaction testing for SARS-CoV-2 and antibodies was negative on multiple occasions. Although not initially hospitalized, the patient was later admitted twice during the summer due to severe and persistent symptoms of extreme fatigue, muscle weakness, insomnia, palpitations, and “brain fog” with trouble concentrating.
A chest computed tomography revealed no signs of viral pneumonia. Myositis and neurological complications were initially suspected; however, the full-body magnetic resonance imaging (including brain and neck) was normal. Lumbar puncture, echocardiography, and laboratory work-up were normal. Skeletal muscle biopsy revealed minimal enrichment of inflammatory cells with unclear significance. Marked fluctuations in sinus rate (periodic increases >140 beats/min with minimal effort) were noted on telemetry. An active standing test demonstrated increase in sinus rate from 98 to 142 beats/min, whereas BP remained stable. POTS was confirmed.
He was referred to a tertiary center for post–COVID-19 follow-up. Additional tests were negative for antinuclear antibodies, antineutrophil cytoplasmic antibody, and metoxycatecholamines. Anticardiolipin and anti-beta-2-glycoprotein-2 immunoglobulin M were slightly elevated (9 E/ml, with values >30 diagnostic for antiphospholipid syndrome).
Recommendations were given to increase fluid and salt intake and use compression socks (Table 2). The patient received propranolol 10 mg ×3 times a day and pyridostigmine 10 mg ×3, the latter due to severe fatigue and muscle weakness. In addition to typical POTS symptoms he developed nausea, orbital edema, and gastrointestinal symptoms (Figure 6). Empirical treatment with H1 and H2 antihistamines was initiated due to suspected mast cell activation syndrome, with moderate clinical improvement. Despite up-titration of propranolol and pyridostigmine, he is still highly symptomatic and on sick leave.
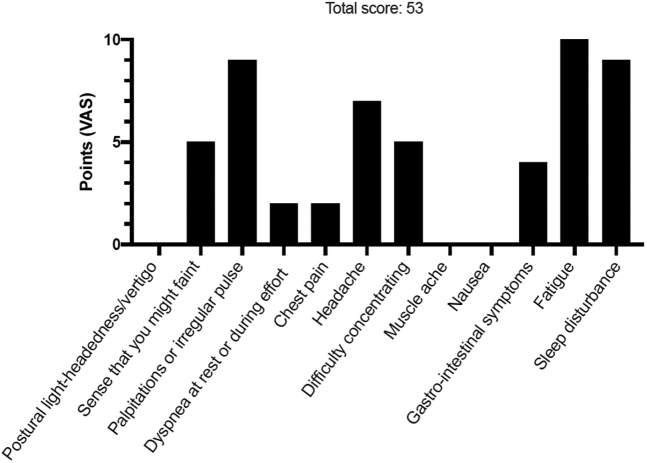
POTS Symptom Scoring in 37-Year-Old Man
Patient #3 self-reported symptoms using a dedicated POTS symptom scoring questionnaire composed of 12 most commonly reported symptoms in POTS. Patients were asked to grade their symptoms using a VAS ranging from 0 (no symptom) to 10 (worst possible). The maximum score is 120 points. A score >40 points likely indicates pathology. Abbreviations as in Figure 1.
Discussion
We describe 3 Swedish patients diagnosed with POTS-like symptoms following probable COVID-19 infections. Patients were diagnosed on the grounds of characteristic orthostatic tachycardia and chronic symptoms of orthostatic intolerance after exclusion of competing etiologies (Table 1).
POTS affects primarily women (≈80%) and is manifested by orthostatic tachycardia, in association with various symptoms including palpitations, dizziness, headache, fatigue, and blurred vision (1,2) (Table 3). The syndrome can be precipitated by viral illness or severe infection (5) in 30% to 50% of all patients. The mechanism of POTS is generally undetermined. Similarly, the mechanism of post–COVID-19 POTS remains unknown, although a chronic inflammatory or autoimmune response may be at play. Whereas few reports have been published (6,7), the number of patients affected by long-haul post–COVID-19 will likely grow.
Table 3
Typical Clinical Presentation of POTS
| Cardiovascular symptoms (pathognomonic) | |
| Cardiovascular system | Main: orthostatic intolerance, orthostatic tachycardia, palpitations, dizziness, lightheadedness, (pre-)syncope, exercise intolerance Other frequent symptoms: dyspnea, chest pain/discomfort, acrocyanosis, Raynaud phenomenon, venous pooling, limb edema |
| Noncardiovascular symptoms (accompanying) | |
| General symptoms | General deconditioning, chronic fatigue, exhaustion, heat intolerance, fever, debility, bedridden |
| Nervous system | Headache/migraine, mental clouding (“brain fog”), cognitive impairment, concentration problems, anxiety, tremulousness, light and sound sensitivity, blurred/tunnel vision, neuropathic pain (regional), sleeping disorders, involuntary movements |
| Musculoskeletal system | Muscle fatigue, weakness, muscle pain |
| Gastrointestinal system | Nausea, dysmotility, gastroparesis, constipation, diarrhea, abdominal pain, weight loss |
| Respiratory system | Hyperventilation, bronchial asthma, shortness of breath |
| Urogenital system | Bladder dysfunction, nocturia, polyuria |
| Skin | Petechiae, rashes, erythema, telangiectasias, abnormal sudomotor regulation, diaphoresis, pallor, flushing |
Adapted with permission from Fedorowski (1).
POTS = postural orthostatic tachycardia syndrome.
Negative SARS-CoV-2 test results do not exclude SARS-CoV-2 infection and ought to be interpreted with caution in the context of typical symptoms (8). In the differential diagnosis of POTS, it is important to consider and exclude other identifiable causes of sinus tachycardia such as dehydration, other infections, hyperthyroidism, cardiac disease, anxiety, anemia, metabolic disorders, chronic fatigue syndrome, or deconditioning (5) (Table 1).
Available management protocols for POTS (Table 2) aim at increasing intake of fluids (water) and salt, physical countermaneuvers, and individually adapted aerobic exercise in recumbent position (1,5,9) to help correct the physiological abnormalities. Pharmacotherapy includes volume expanders, vasoconstrictors, and HR regulators but patients may remain symptomatic and incapable of work.
Much remains unknown about the specific mechanisms responsible for the POTS-like symptoms in post–COVID-19 patients or how long these symptoms will last but chronic symptoms are expected in a subset of patients based on this initial clinical experience.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
Articles from JACC Case Reports are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101686415
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.jaccas.2021.01.009
Article citations
Long COVID in Children, Adults, and Vulnerable Populations: A Comprehensive Overview for an Integrated Approach.
Diseases, 12(5):95, 06 May 2024
Cited by: 1 article | PMID: 38785750 | PMCID: PMC11120262
Review Free full text in Europe PMC
Multidisciplinary Management Strategies for Long COVID: A Narrative Review.
Cureus, 16(5):e59478, 01 May 2024
Cited by: 0 articles | PMID: 38826995 | PMCID: PMC11142761
Review Free full text in Europe PMC
Gut microbiota composition is altered in postural orthostatic tachycardia syndrome and post-acute COVID-19 syndrome.
Sci Rep, 14(1):3389, 09 Feb 2024
Cited by: 1 article | PMID: 38336892 | PMCID: PMC10858216
Insights into postural orthostatic tachycardia syndrome after COVID-19 in pediatric patients.
World J Pediatr, 20(3):201-207, 16 Feb 2024
Cited by: 1 article | PMID: 38363488
Postural orthostatic tachycardia syndrome and other autonomic dysfunctions following COVID-19: Incidence, characteristics, and associated factors.
J Arrhythm, 40(2):230-236, 05 Feb 2024
Cited by: 0 articles | PMID: 38586859 | PMCID: PMC10995586
Go to all (93) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Postural orthostatic tachycardia syndrome as a sequela of COVID-19.
Heart Rhythm, 19(11):1880-1889, 16 Jul 2022
Cited by: 44 articles | PMID: 35853576 | PMCID: PMC9287587
Review Free full text in Europe PMC
Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Medicina (Kaunas), 58(1):28, 24 Dec 2021
Cited by: 38 articles | PMID: 35056336 | PMCID: PMC8778312
Post-acute sequelae of SARS-CoV-2 syndrome presenting as postural orthostatic tachycardia syndrome.
Clin Exp Emerg Med, 10(1):18-25, 30 Jan 2023
Cited by: 2 articles | PMID: 36718484 | PMCID: PMC10090716
Review Free full text in Europe PMC
Complete remission with histamine blocker in a patient with intractable hyperadrenergic postural orthostatic tachycardia syndrome secondary to long coronavirus disease syndrome.
J Hypertens, 42(5):928-932, 08 Mar 2024
Cited by: 0 articles | PMID: 38526146 | PMCID: PMC10990027




