Abstract
Free full text

Rhinocerebral Mucormycosis and COVID-19 Pneumonia
Associated Data
Abstract
As the coronavirus disease 2019 (COVID-19) pandemic is evolving, more complications associated with COVID-19 are emerging. In this case report, we present a case of rhinocerebral mucormycosis concurrent with COVID-19 pneumonia in a 41-year-old man with a history of type 1 diabetes mellitus (T1DM). COVID-19 pneumonia was diagnosed with reverse transcription-polymerase chain reaction (RT-PCR). He was promptly treated with steroids and hydroxychloroquine, as this was the recommended regional COVID-19 practice patterns at the time. He was treated with intravenous (IV) fluids and an insulin drip for his diabetic ketoacidosis (DKA), cefepime and IV abelcet, along with three surgical debridements for the rhinocerebral mucormycosis. The pneumonia resolved during the course of his stay in the hospital. With prompt diagnosis and treatment of rhinocerebral mucormycosis, the patient was cleared for discharge and was instructed to complete his course of treatment with coumadin and IV abelcet at home. Saprophytic fungi cause rhinocerebral mucormycosis, a rare opportunistic infection of the sinuses, nasal passages, oral cavity and brain. It usually occurs in patients with poorly controlled diabetes mellitus or those who are immunocompromised, which is again demonstrated in this case report. In the setting of COVID-19 pneumonia and an underlying condition, healthcare professionals should act promptly. In cases where mucormycosis infection is suspected, a prompt diagnosis and treatment should be started because of the angioinvasive character and rapid disease progression that contribute to the severity of the mucormycosis infection.
Introduction
Coronavirus disease 2019 (COVID-19) pneumonia has been frequently associated with symptoms such as shortness of breath, cough, loss of sense of smell, fever and fatigue. Patients with preexisting conditions such as hypertension, diabetes mellitus, or coronary artery disease are especially susceptible to compilations arising from COVID-19 pneumonia. Patients with poorly controlled diabetes mellitus or those that are immunocompromised are at an increased risk of developing mucormycosis. Our patient presented with COVID-19 pneumonia and had a history of poorly controlled diabetes mellitus, which led to the development of rhinocerebral mucormycosis. In an extensive literature search, there have not been any reported cases of rhinocerebral mucormycosis in the setting of COVID-19 pneumonia. However, there has been one reported case in which a patient with COVID-19 pneumonia developed pulmonary mucormycosis. They believed that this likely occurred because of the patient’s immunocompromised state [1].
Case Report
A 41-year-old Haitian Creole man with a past medical history of type 1 diabetes mellitus (T1DM) presented to an outside hospital with a 1-week history of loss of taste and cough. He complained of deep aching pain in his nose that radiated down to the throat. He rated his pain a 10 out of 10 on the pain scale. He stated that pain medications alleviated the pain and discomfort, but eating exacerbated it; thus, he was not eating. There was no nausea, vomiting, abdominal pain or diarrhea, and no weakness or numbness. Due to illness, he had not been taking his insulin and had not been checking his blood sugars.
He was not hypoxic but did have a dry cough. When examining the oral cavity, a black eschar was noted on the palate. The physical exam showed pupils equal, round and reactive to light (PERRL), and they were icteric. His neck was supple, and no adenopathy or rigidity was noted; cardiac exam revealed tachycardia with a regular rhythm and no rubs or gallops. On auscultation of the lungs, bilateral crackles and no wheezing was appreciated. His abdomen was soft, and no hepatosplenomegaly was present. Additionally, there were no signs of clubbing or edema. There were also many laboratory abnormalities, as listed in Table 1.
Table 1
| Blood plasma, serum | Reference range (normal) | SI reference intervals (normal) | |
|---|---|---|---|
| Sodium | 140 mEq/L | 135 - 145 mEq/L | 136 - 145 mmol/L |
| Potassium | 2.6 mEq/L (low) | 3.5 - 5.0 mEq/L | 3.5 - 5.0 mmol/L |
| Chloride | 106 mEq/L (high) | 95 - 105 mEq/L | 95 - 105 mmol/L |
| Bicarbonate | 4 mEq/L (low) | 22 - 28 mEq/L | 22 - 28 mmol/L |
| Glucose | 185 mg/dL (high) | 70 - 110 mg/dL | 3.8 - 6.1 mmol/L |
| BUN | 5 mg/dL (low) | 7 - 18 mg/dL | 0.18 - 0.48 mmol/L |
| Creatinine | 1.3 mg/dL (high) | 0.6 - 1.2 mg/dL | 53 - 106 mmol/L |
| Calcium | 8.6 mg/dL | 8.4 - 10.2 mg/dL | 2.1 - 2.8 mmol/L |
| Bilirubin | 0.46 mg/dL | 0.1 - 1.0 mg/dL | 2 - 17 µmol/L |
| Total protein | 6.8 g/dL | 6.0 - 7.8 g/dL | 60 - 70 g/L |
| Albumin | 1.7 g/dL (low) | 3.5 - 5.5 g/dL | 35 - 55 g/L |
| Alkaline phosphatase | 113 U/L (high) | 20 - 70 U/L | 20 - 70 U/L |
| AST | 53 U/L (high) | 8 - 20 U/L | 8 - 20 U/L |
| ALT | 38 U/L (high) | 8 - 20 U/L | 8 - 20 U/L |
| Magnesium | 2.1 mEq/L (high) | 1.5 - 2.0 mEq/L | 0.75 - 1.0 mmol/L |
| LDH | 330 U/L (high) | 45 - 90 U/L | 45 - 90 U/L |
| Ferritin | 3044 ng/mL (high) | 15 - 200 ng/mL | 15 - 200 µg/L |
| TSH | 1.8 µU/mL | 0.5 - 5.0 µU/mL | 0.5 - 5.0 mU/L |
| G6PD | 17.4 U/g | 5.5 - 20.5 U/g | 5.5 - 20.5 U/g |
COVID-19: coronavirus disease 2019; BUN: blood urea nitrogen; AST: aspartate aminotransferase; ALT: alanine transaminase; LDH: lactate dehydrogenase; TSH: thyroid-stimulating hormone; G6PD: glucose-6-phosphate dehydrogenase.
His vitals showed a temperature of 37.7 °C, heart rate of 115, respiratory rate of 19, blood pressure of 149/79 mm Hg and pulse oximetry 96%. A routine electrocardiogram (EKG) demonstrated sinus tachycardia. A chest X-ray was then ordered and revealed atelectasis and pneumonia on the left lobes more than the right lobes. A chest computed tomography (CT) scan was performed and revealed peripheral bilateral lung infiltrates and chronic sinusitis. A COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) was ordered, and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR returned positive for viral RNA, and he was diagnosed with COVID-19 pneumonia.
With no evidence of hypoxemia, treatment with steroids and hydroxychloroquine, which are immunosuppressants, was initiated. This treatment regimen was the regional COVID-19 practice patterns at the time. A complete workup also revealed that the patient was in diabetic ketoacidosis (DKA). He was subsequently treated with intravenous (IV) fluids and an insulin drip. His anion gap quickly closed, blood sugars returned to the normal range and significant hypokalemia was corrected. He was then transitioned to insulin glargine.
Once stabilized, he was transferred to our facility and was evaluated by infectious disease and the oral and maxillofacial surgery (OMFS) team. He was empirically started on cefepime and IV abelcet, which is amphotericin B complexed with two phospholipids. Initial debridement demonstrated evidence of mucormycosis. Subsequently, the patient was imaged with a CT scan and magnetic resonance imaging (MRI). Imaging revealed the disease’s extension into the sinuses and intracranial abscess in the infratemporal fossa with cavernous sinus enhancement. This can be seen in Figure 1.
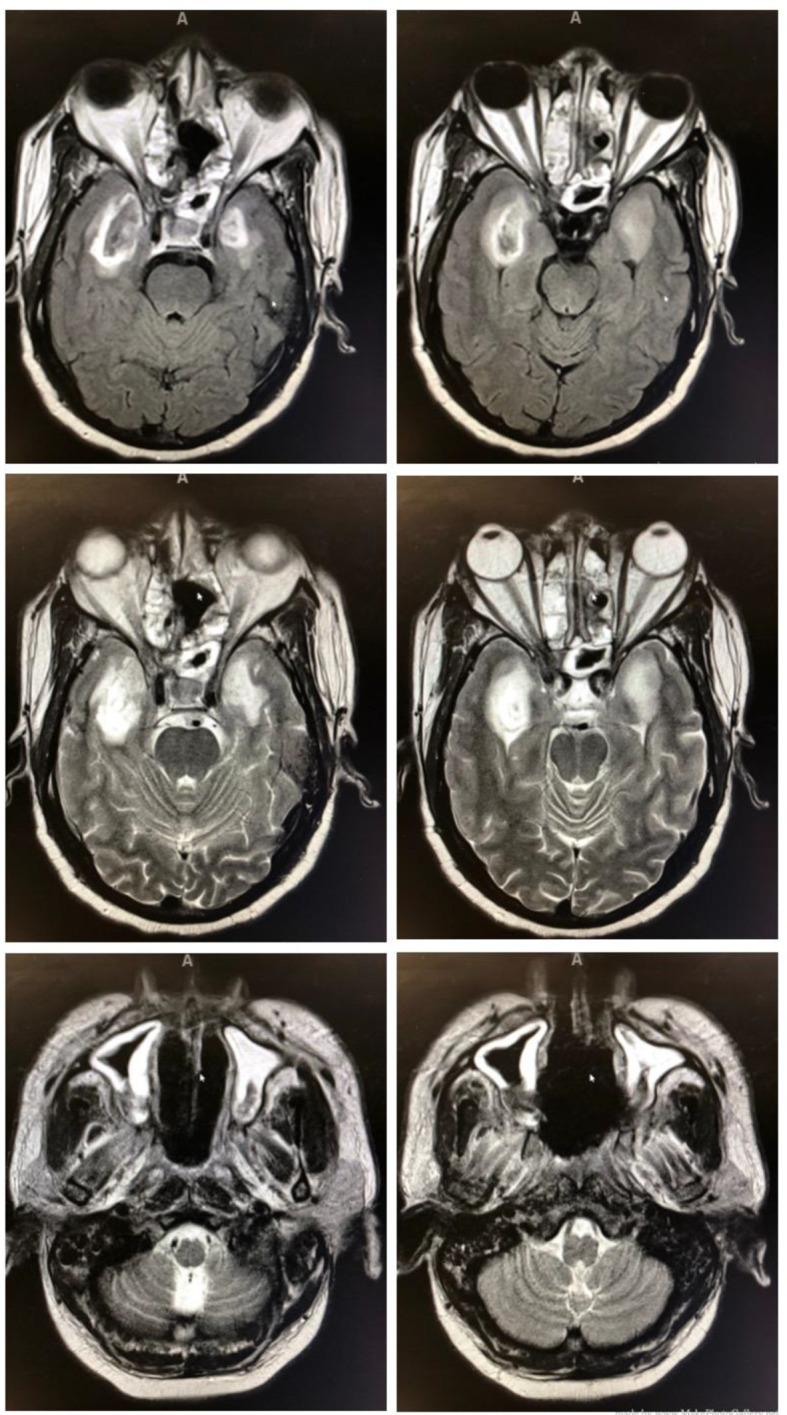
Intracranial abscess in the infratemporal fossa with cavernous sinus enhancement (top four images) and mucormycosis extension into the sinuses (bottom two images).
The first surgery’s operational findings showed extensive mucormycosis of the entire nasal mucosa involving the septum, bilateral inferior turbinate, middle turbinate, ethmoid sinuses and the maxillary sinus. A partial septectomy and bilateral maxillary antrostomy, total ethmoidectomy and sphenoidotomy to remove disease-ridden tissue was done. The bilateral inferior turbinates were also resected. Finally, the hard palate was also debrided, removing all of the necrotic mucosa.
A wide maxillary antrostomy, sphenoidotomy and ethmoidectomy with frontal recess opening was done, and a recommendation for a second debridement was noted. A repeat MRI demonstrated the disease’s progression intracranially and with the involvement of the right cavernous sinus with thrombophlebitis. He was started on IV heparin for the cavernous vein thrombosis.
Prior to his second surgery, a second COVID-19 RT-PCR returned negative. He no longer had any signs of COVID-19 pneumonia, and treatment was now focused on the rhinocerebral mucormycosis. The second surgery was performed 1 week after his first surgery. Surgery revealed widespread crusting in the cavity and was removed. Extensive granulations and some healthy mucosa were noted involving the nasal septal edges, maxillary antrostomy and sphenoid sinus. After removing the crusting, the mucosa was decongested with epinephrine soaked cottonoids.
Cultures were taken from the secretions within the right sphenoid sinus. Then using the Coblator, granulations were removed from the anterior face of the sphenoid. The right sphenoid sinus exhibited pathology extending to the lateral recess and was also removed. Some granulations around the nasal re-antrostomy site and edges of the septum were cauterized. The bone of the posterior wall of the right nasal sinus, adjacent to the sphenopalatine area, appeared to be disease-ridden with mucormycosis and was non-viable. A small area of the left maxillary sinus’s posterior wall was also seen to be ischemic and involved. After achieving hemostasis, Arista, a plant-based absorbable surgical hemostatic powder, was placed in the nasal cavity.
Mouth gag was now placed in where hard palate that was exposed. Debris and slough were removed from the hard palate edges. Following debridement, the bleeding along the mucosa was cauterized. The hard palate appeared to be involved and possibly nonviable. He continued monotherapy with IV abelcet as well as IV heparin. Two weeks later, he underwent a final debridement. During his hospital stay, and treatment for rhinocerebral mucormycosis, DKA and COVID-19 pneumonia wholly resolved. He was discharged to complete his therapy course of coumadin and IV abelcet.
Discussion
Fungi that belong to Mucorales cause infections known as mucormycosis. The infection site will determine the clinical presentation. This can manifest as cutaneous, pulmonary, sinusitis, gastrointestinal, or even dissemination. These fungi rarely affect the immunocompetent, but rather immunocompromised patients. This mainly occurs in those that are on hemodialysis, high-dose glucocorticoids, have trauma like extensive burns and with uncontrolled diabetes mellitus [2, 3].
In the normal functioning immune cells, the spores and hyphae are readily taken up and destroyed by mononuclear and polymorphonuclear phagocytes. When patients have a low phagocyte count, impaired phagocyte function, neutropenia, or poorly controlled diabetes mellitus, they become increasingly susceptible to invasive mucormycosis [3].
Rhizopus is the most common fungi found in patients that have mucormycosis in diabetic patients. Rhizopus is unable to sequester iron from iron-binding proteins [4]. In patients with poorly controlled diabetes mellitus, the chronically elevated blood glucose levels will lead to an impaired neutrophil function. In ketoacidosis, the hyperglycemia and acidic pH may lead to a defect in the motility and killing of bacteria and fungi by neutrophils. It is believed that as the pH becomes acidic, the iron-protein complexes dissociate, which allows for the fungal cells to use the increased free iron [4]. In patients with diabetes mellitus, rhinocerebral mucormycosis is noted to be the most common type [5].
Our patient had COVID-19 pneumonia, uncontrolled diabetes mellitus, which led to the development of rhinocerebral mucormycosis. In an extensive literature search, there was one reported COVID-19 pneumonia case that led to necrotizing pulmonary mucormycosis. Typically, this has only been documented in immunocompromised patients [1].
In rhinocerebral mucormycosis, the disease’s hallmark is attributed to tissue necrosis from angioinvasion and subsequent thrombosis. This presents as notoriously black, necrotic eschars. The fungi gain entry via inhalation into the paranasal sinuses and may ultimately spread to the sphenoid sinus, palate and cavernous sinus. Patients may complain of blurry vision, inflammation around the orbit, sinusitis, facial pain or numbness, headache, proptosis, ophthalmoplegia, or even periorbital cellulitis [3, 6].
To diagnose suspected mucormycosis, an extensive history, physical exam and imaging is crucially important. In diabetic patients, a typical finding on a cranial CT will show bone destruction. For additional sensitivity, a cranial MRI is indicated because the results will show any involvement of the brain, sinuses and orbit. With imaging, the staging will be noted in terms of sinus and cerebral involvement. A biopsy should be scheduled and sent to visualize under direct microscopy, culture in routine media at 30 and 37 °C, or PCR. Once that is established, you can send for susceptibility [2, 6].
Suspected and/or confirmed mucormycosis requires consultation with the surgical team [7]. It is crucial that there are clean margins during surgical debridement to stop the spread of the fungal infection. This acts as the immediate treatment. Biopsies obtained in the surgery can be sent for histopathology as well as microbiological diagnostics.
The widely accepted treatment of choice for mucormycosis is amphotericin B. When administering amphotericin B, it is essential to monitor kidney function due to its high nephrotoxicity incidence. When extensive disease occurs, second-line therapies may be contemplated. A combination therapy with echinocandins and amphotericin B is a second-line therapy that is recommended. When echinocandins are combined with amphotericin B, they add a polyene backbone, which increases the success of therapy. Some of the other second-line accepted antifungals include the triazoles, posaconazole and isavuconazole [2]. Triazoles inhibit the 14-α-demethylation, which leads to an increase in toxic 14-α-methylsterols that alters the fungal membrane’s permeability. Patients that are intolerant to amphotericin B are given posaconazoles. Isavuconazole has an extended spectrum. Because of this, it is the only antifungal used in the treatment of invasive mucormycosis [3].
Identification and treatment of any underlying condition can help assure a good prognosis in the presence of mucormycosis. Regarding uncontrolled diabetes mellitus, it is vital to correct the hyperglycemia and ketoacidosis [2]. According to Brunet, the prompt control of hyperglycemia and the reversal of ketoacidosis have led to the best outcomes [7]. Additionally, tapering immunosuppressive therapy may play an additional role in the treatment of mucormycosis.
Conclusion
The severity of mucormycosis infection is due to its rapid disease progression and angioinvasive character. Healthcare professionals should act promptly, especially in patients with poorly controlled diabetes mellitus with a suspicion of mucormycosis. A multidisciplinary approach should include the prompt diagnosis and treatment with antifungals, any appropriate surgical consultation and surgical treatment, plus the reversal of the underlying condition.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was taken from the patient for reporting this case.
Author Contributions
Kirill Alekseyev contributed to investigating, writing, review and editing. Lidiya Didenko contributed to investigating, writing, review and editing. Bilal Chaudhry contributed to investigating, writing, review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
Articles from Journal of Medical Cases are provided here courtesy of Elmer Press
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/105866960
Article citations
The Study of Clinical Profile of Patients With Mucormycosis During COVID-19 Pandemic in Tertiary Care Hospital.
Cureus, 15(10):e47065, 15 Oct 2023
Cited by: 0 articles | PMID: 38021649 | PMCID: PMC10651161
A Case Report of Invasive Mucormycosis in a COVID-19 Positive and Newly-Diagnosed Diabetic Patient.
J Educ Teach Emerg Med, 8(3):V10-V13, 31 Jul 2023
Cited by: 0 articles | PMID: 37575409 | PMCID: PMC10414977
Mucormycosis and Its Upsurge During COVID-19 Epidemic: An Updated Review.
Curr Microbiol, 80(10):322, 17 Aug 2023
Cited by: 3 articles | PMID: 37592083
Review
Clinicoradiological Profile of COVID-19-Associated Rhino-Orbital Cerebral Mucormycosis with a Focus on Computed Tomography: A Clinical Case Series and Review.
Am J Trop Med Hyg, 109(3):600-607, 24 Jul 2023
Cited by: 0 articles | PMID: 37487562 | PMCID: PMC10484265
COVID-19-Associated Rhino-Orbital Mucormycosis in a Tertiary Health Care Center in Odisha, India.
Cureus, 15(8):e43811, 20 Aug 2023
Cited by: 1 article | PMID: 37731437 | PMCID: PMC10508707
Go to all (83) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Diabetic ketoacidosis and coronavirus disease 2019-associated mucormycosis: a case report.
J Med Case Rep, 16(1):400, 31 Oct 2022
Cited by: 1 article | PMID: 36316719 | PMCID: PMC9624001
When Worlds Collide: An Interesting Case of Rhinocerebral Mucormycosis Exacerbated by COVID-19 and Diabetic Ketoacidosis Complicated by Intraorbital Hematoma.
Cureus, 14(1):e21203, 13 Jan 2022
Cited by: 1 article | PMID: 35186519 | PMCID: PMC8844255
Invasive rhinocerebral mucormycosis: Imaging the temporal evolution of disease in post COVID-19 case with diabetes: A case report.
World J Radiol, 15(7):234-240, 01 Jul 2023
Cited by: 0 articles | PMID: 37545647 | PMCID: PMC10401400
Rhinocerebral mucormycosis in a patient with type 1 diabetes presenting as toothache: a case report from Himalayan region of India.
BMJ Case Rep, 2013:bcr2013200811, 30 Oct 2013
Cited by: 3 articles | PMID: 24172773 | PMCID: PMC3822077
Review Free full text in Europe PMC



