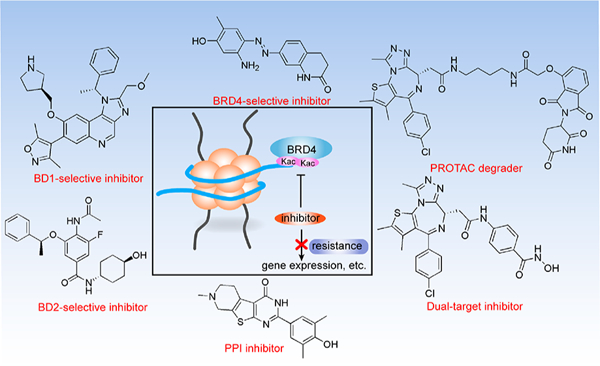
Abstract
Free full text

Targeting Bromodomain and Extraterminal Proteins for Drug Discovery: From Current Progress to Technological Development
Abstract
Bromodomain and extraterminal (BET) proteins bind acetylated lysine residues in histones and nonhistone proteins via tandem bromodomains and regulate chromatin dynamics, cellular processes, and disease procession. Thus targeting BET proteins is a promising strategy for treating various diseases, especially malignant tumors and chronic inflammation. Many pan-BET small-molecule inhibitors have been described, and some of them are in clinical evaluation. Nevertheless, the limited clinical efflcacy of the current BET inhibitors is also evident and has inspired the development of new technologies to improve their clinical outcomes and minimize unwanted side effects. In this Review, we summarize the latest protein characteristics and biological functions of BRD4 as an example of BET proteins, analyze the clinical development status and preclinical resistance mechanisms, and discuss recent advances in BRD4-selective inhibitors, dual-target BET inhibitors, proteolysis targeting chimera degraders, and protein−protein interaction inhibitors.
1. INTRODUCTION
In humans, 61 bromodomains, each composed of ~110 amino acids forming four antiparallel α helices (αZ, αA, αB, and αC) and two hydrophobic (ZA and BC) loops, in 46 different proteins have been described.1 Among these, bromodomain and extraterminal (BET) proteins constitute a unique group with four family members, bromodomain-containing protein 4 (BRD4), BRD3, BRD2, and testis-specific BRDT, featuring the presence of two N-terminal tandem bromodomains (BD1 and BD2) and an ET (extraterminal) domain (Figure 1A).2,3 Notably, BRD4 and BRDT contain an extended C-terminal motif (CTM) not found in BRD2 and BRD3. BET proteins are epigenetic “readers” binding specifically to N-ε-acetyllysine (Kac) residues in histones and nonhistone proteins via their BD1 and BD2 bromodomains to engage in chromatin dynamics and transcriptional regulation.4,5 However, the molecular distinction underlying the functional redundancy and unique aspects of each BET protein, with respect to their acetylated histone and nonhistone protein recognition, remains elusive.6 Here we summarize the structural and functional characteristics of BRD4, which is the most understood member of the BET family, as an example of BET proteins.
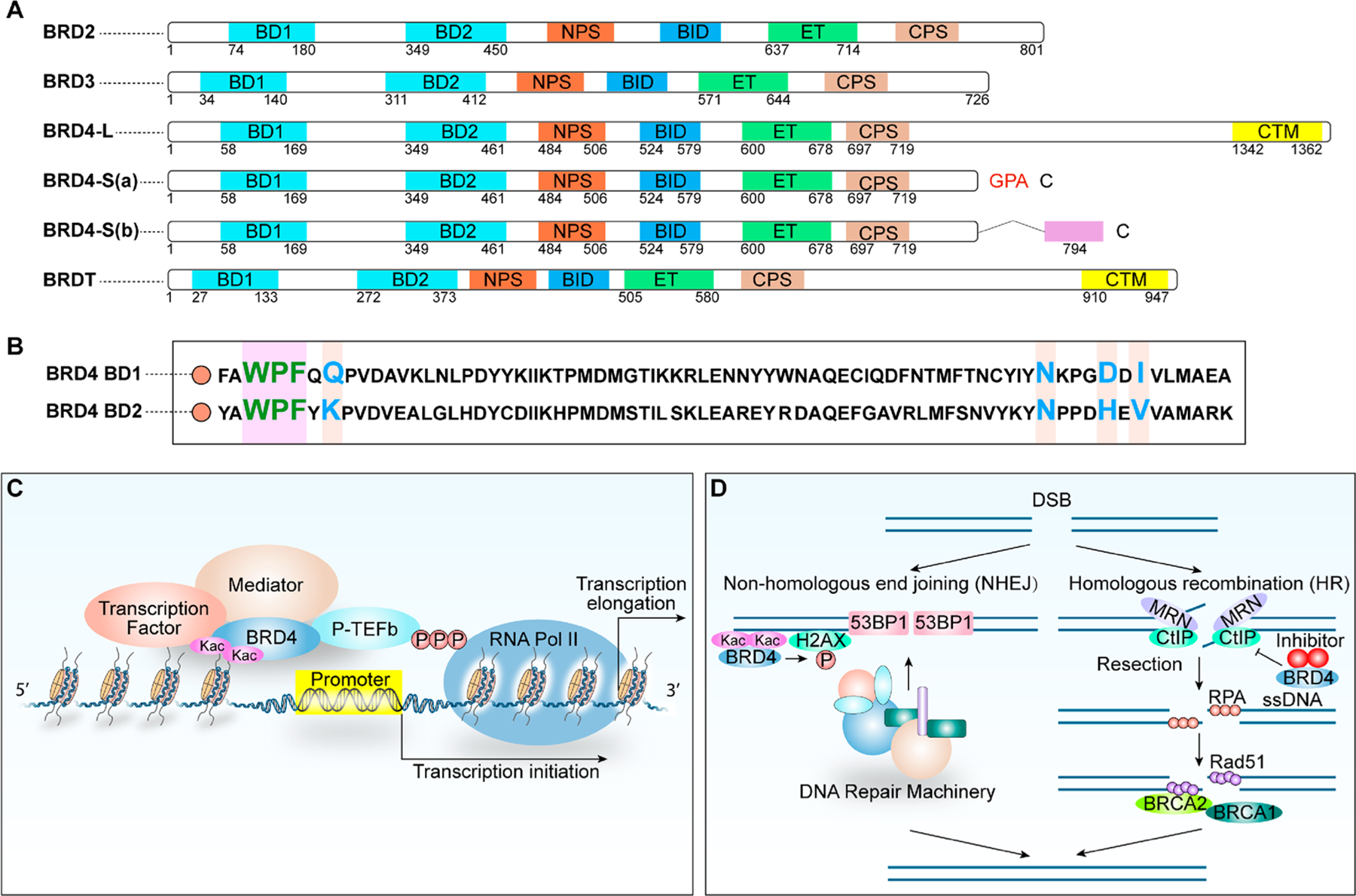
(A) Domain organization of human BET proteins. (B) Conserved sequence of BRD4 BD1 and BRD4 BD2. Key varying residues are marked in different colors. (C) Recruitment of P-TEFb facilitates BRD4-enhanced Pol II elongation. BRD4 binds to Kac sites on histones and recruits BRD4 interactors and the P-TEFb complex, leading to enhanced transcriptional elongation by Ser2-phosphorylated Pol II. (D) BRD4 function in DNA damage response in both NHEJ and HR pathways.
BRD4 has three characterized protein isoforms with different C-terminal amino acid residues one long (L) and two short isoforms, S(a) and S(b) resulting from alternative mRNA splicing.7 Amino acids 1−719 are common among the three isoforms, including two highly conserved N-terminal tandem bromodomains (BD1 and BD2), the N-terminal cluster of phosphorylation sites (NPS), the basic residue-enriched interaction domain (BID), the extraterminal (ET) domain, and the C-terminal cluster of phosphorylation sites (CPS; see Figure 1A). Interestingly, the conformation of BD2 in a closed or open configuration is controlled by the downstream NPS, whose extent of phosphorylation modulated by casein kinase II (CK2)8,9 and protein phosphatase 2A (PP2A)10,11 regulates BRD4 association with different transcriptional regulators such as p53, AP-1, NF-κB, and EN1.7,10,12 Although BET bromodomains are structurally conserved in general, the ZA and BC loops between BD1 and BD2 are slightly different in sequence and length, thus contributing to the context-specific binding of acetylated lysine residues (Figure 1B). Sequence differences between BD1 and BD2 provide a structural basis for designing selective inhibitors of BD1 or BD2. Besides sequence variation, BD1 and BD2 also exhibit functional differences in which BD1 primarily serves as a chromatin-binding module and BD2 is typically used for factor recruitment,13 hence partly accounting for BD1 being indispensable for steady-state gene expression and both BD1 and BD2 being seemingly required for the acute gene response induced by inflammatory stimulation.14 In addition, the conserved BID and phospho-NPS each interact directly with p53 but with different functional outcomes in regulating p53 binding to its target sequences.8 The ET domain of BRD4 interacts with select chromatin regulators via specific structural contacts.15 A unique CTM present in BRD4-L but absent in BRD4-S(a) and BRD4-S(b) functions to recruit transcriptional regulators, such as positive transcription elongation factor b (P-TEFb)16,17 and human papillomavirus (HPV)-encoded E2 protein that is capable of inhibiting AP-1-dependent HPV transcription via specific E2-binding sites.10 Both BRD4-L and BRD4-S(a) can induce liquid−liquid phase separation (LLPS) in vitro and in the cell to mediate transcription complex assembly and regulate gene transcription.18–20 Recent research reveals that the expression level of BRD4-S(a), rather than BRD4-L, is closely related to the number and size of BRD4 puncta formed in the cell.20 An oncogenic function of BRD4-S(a) and a tumor-suppressive activity of BRD4-L recently revealed in breast cancer development further indicate the importance of target selectivity in anti-BRD4 drug development.7
BRD4 interacts with both cyclin T1 (cycT1) and cyclin-dependent kinase 9 (CDK9) subunits of the dimeric P-TEFb complex8,16,21 and recruits transcription factors such as MYC,22,23 p53,8,24 AP-1,7,8,10 NF-κB,10,25 TWIST,26 SNAIL,27 EN1,7 YAP1,28,29 TEAD4,7 and NF-YA7 to facilitate RNA polymerase II (Pol II)-dependent transcription (Figure 1C).13,30,31 BRD4 is also implicated in DNA repair to control genome stability and cancer development. The role of BRD4 in the DNA damage response is not limited to nonhomologous end joining (NHEJ)32 and also involves homologous recombination (HR)33 (Figure 1D). BRD4 is associated with R-loop formation, which enhances double-strand break (DSB) formation and engages in the activation of the NHEJ pathway implicated in immunoglobulin class switch recombination32,33 and oncogenic gene rearrangement.34 In contrast, the inhibition of BRD4 induces the loss of C-terminal binding protein-interacting protein (CtIP) involved in DNA end resection and single-strand DNA (ssDNA) generation, resulting in a decrease in the HR competency.35 The involvement of BRD4 in cancer initiation and progression can be attributed to chromosomal translocation leading to the formation of oncogenic fusion drivers (e.g., BRD4-NUT)36 through epigenetic control via the recruitment of cell-type-specific transcriptional regulators37 or by modulating the activity8 or recruitment of cell-cycle-promoting factors such as CDK4/6.38 In NUT midline carcinoma (NMC), BRD4-NUT fusion oncoprotein induces the hyperacetylation of chromatin and the formation of a unique nuclear subcompartment that squelches key transcription components or chromatin regulators, leading to the inhibition of transcription initiation by RNA Pol II.39 Another study identified CDK9 as a kinase implicated in BRD4 hyperphosphorylation that contributes to its oncogenic activity,40 consistent with the finding of enhanced BRD4 phosphorylation due to the reduced PP2A activity during triple-negative breast cancer progression.11 In addition, BRD4 is also involved in blastic plasmacytoid dendritic cell neoplasm (BPDCN),41,42 Cornelia-de-Lange-like syndrome,43 and fragile X syndrome.44 Expanded research on BRD4 protein isoforms with gain- and loss-of-function approaches conducted in various breast cancer cells and mouse models, in conjunction with genome-wide transcriptome and binding profiling, unequivocally establishes the opposing functions of BRD4 isoforms in breast cancer and significantly enhances our understanding of gene targets and pathways associated with cancer progression.7
Over the past decade, numerous pan-BET inhibitors have been generated, and some of them are currently in clinical trials, such as OTX-015, CPI-0610, and I-BET762.45 Although small-molecule pan-BET inhibitors show promising effects in clinical evaluation, there are still problems and challenges to overcome, such as the moderate clinical efflcacy and the occurrence of preclinical resistance.46 Encouragingly, with recent advances in pharmaceutical chemistry and chemical biology, new technologies that facilitate the development of more selective inhibitors or other new-generation inhibitors targeting specific functional aspects of BET proteins have been used to assuage some clinical concerns.
2. CURRENT CLINICAL DEVELOPMENT STATUS AND OBSERVATIONS
In 2017, pan-BET inhibitors available at that time were reviewed in detail regarding their chemical types, activities, and therapeutic potentials.45 At present, some pan-BET inhibitors are in clinical trials for treating different cancers (Table 1). However, the clinical advancement of most pan-BET inhibitors is still in the early stages and mainly focuses on cancer treatment. The results of clinical trials published to date raise several key issues to be considered. First, some inhibitors display toxicity during clinical trials, including dose-limiting toxicity (DLT). The most common adverse effects are thrombocytopenia, diarrhea, fatigue, vomiting, anemia, and hyperbilirubinemia. A commonly observed DLT is thrombocytopenia with undamaged platelet function. Reduced platelet counts were reversible and could be restored within 1 week after drug withdrawal.46 In addition, complications and immunodeficiency are potential undesirable reactions to the use of pan-BET inhibitors.47 The phase I trial of BAY1238097 (NCT02369029) was terminated early due to severe toxic effects, and INCB057643 (NCT02711137) was also discontinued because of safety concerns. Second, several inhibitors exhibited much lower antitumor activity than those observed in preclinical trials. In a dose study of CPI-0610 given to 44 patients with relapsed or refractory lymphoma, only 2 showed a complete response, and 1 had a partial response. In addition, studies with OTX-015 (NCT02698176 and NCT02296476), as reported for I-BET151 (NCT02630251), were forced to terminate because of its limited efflcacy in solid tumors. Pan-BET inhibitors show a significant survival advantage in tumor models, which are often abrogated in transplants of drug-resistant cells.48 The main reason for the lack of clinical efflcacy is likely due to a failure to identify the right group of patients most likely to benefit from pan-BET inhibitor treatment, resulting in ineffectual dosing before DLT occurs. Moreover, modest outcomes may be partly attributed to the development of resistance, diminishing the clinical response to pan-BET inhibitors.49
Table 1.
Pan-BET Inhibitors in Cancer-Related Clinical Trialsa
| drug | sponsor | phase | indication | NUT identifier | status |
|---|---|---|---|---|---|
| I-BET762 | GlaxoSmithKline | I | NMC, other solid tumors | NCT01587703 | completed |
| I | CRPC | NCT03150056 | active, not recruiting | ||
| I | NCT02706535 | completed | |||
| I | solid tumors, lymphomas | NCT03925428 | withdrawn | ||
| NMC | NCT03702036 | no longer available | |||
| II | hematological malignancies | NCT01943851 | completed | ||
| II | HR+/HER2− advanced or metastatic breast cancer | NCT02964507 | active, not recruiting | ||
| II | solid tumor | NCT03266159 | withdrawn | ||
| National Cancer Institute | I/II | NUT carcinoma | NCT04116359 | withdrawn | |
| OTX015 | Merck Sharp & Dohme Corp | I | NMC, TNBC, NSCLC, CRPC | NCT02698176 | terminated |
| I | AML, DLBCL | NCT02698189 | active, not recruiting | ||
| Oncoethix GmbH | I | NMC, TNBC, NSCLC, CRPC | NCT02259114 | completed | |
| I/II | AML | NCT02303782 | withdrawn | ||
| I | AML, DLBCL, ALL, MM | NCT01713582 | completed | ||
| II | GBM | NCT02296476 | terminated | ||
| TEN-010 | Hoffmann-La Roche | I | AML, myelodysplastic syndromes | NCT02308761 | completed |
| I | solid tumors, AST | NCT01987362 | completed | ||
| Bay1238097 | Bayer | I | solid tumors, lymphoma | NCT02369029 | terminated |
| CPI-0610 | University of Texas Southwestern Medical Center | II | malignant peripheral nerve sheath tumors | NCT02986919 | withdrawn |
| Constellation Pharmaceuticals | I | previously treated MM | NCT02157636 | completed | |
| I | progressive lymphoma | NCT01949883 | completed | ||
| II | myelofibrosis, MDS/MPN, U | NCT02158858 | recruiting | ||
| I-BET151 | GlaxoSmithKline | I | metastatic, unresectable solid tumors | NCT02630251 | terminated |
| INCB054329 | Incyte Corporation | I/II | advanced-stage cancer | NCT02431260 | terminated |
| metastatic renal cell carcinoma | NCT03896815 | no longer available | |||
| INCB057643 | Incyte Corporation | I/II | advanced-stage cancer | NCT02711137 | terminated |
| I | primary myelofibrosis, secondary myelofibrosis | NCT04279847 | not yet recruiting | ||
| I/II | solid tumors, advanced malignancies, metastatic cancer | NCT02959437 | active, not recruiting | ||
| ADZ5153 | AstraZeneca | I | malignant solid tumors, lymphoma, ovarian cancer | NCT03205176 | recruiting |
| Acerta Pharma BV|AstraZeneca | I | NHL, DLBCL, non-Hodgkin’s lymphoma | NCT03527147 | recruiting | |
| ABBV-075 | AbbVie | I | AST | NCT02391480 | completed |
| ABBV-744 | I | CRPC, AML | NCT03360006 | recruiting | |
| BMS-986158 | Dana-Farber Cancer Institute | I | solid tumor, lymphoma, brain tumor (pediatric) | NCT03936465 | recruiting |
| Bristol-Myers Squibb | I/II | AST | NCT02419417 | recruiting | |
| PLX51107 | M.D. Anderson Cancer Center | I | AML, myelodysplastic syndrome, MDS/MPN, U | NCT04022785 | recruiting |
| Plexxikon | I | solid tumors, AML, myelodysplastic syndrome, NHL | NCT02683395 | terminated | |
| BI 894999 | Boehringer Ingelheim | I | neoplasms | NCT02516553 | recruiting |
| ZEN003694 | Zenith Epigenetics | I/II | MCRPC | NCT04145375 | enrolling by invitation |
| I | TNBC | NCT03901469 | recruiting | ||
| II | MCRPC | NCT02705469 | completed | ||
| I/II | MCRPC | NCT02711956 | active, not recruiting | ||
| GS-5829 | Gilead Sciences | I/II | metastatic CRPC | NCT02607228 | completed |
| I | solid tumors, lymphomas | NCT02392611 | completed | ||
| FT-1101 | Forma Therapeutics | I | AML, MDS | NCT02543879 | completed |
3. RESISTANCE MECHANISMS
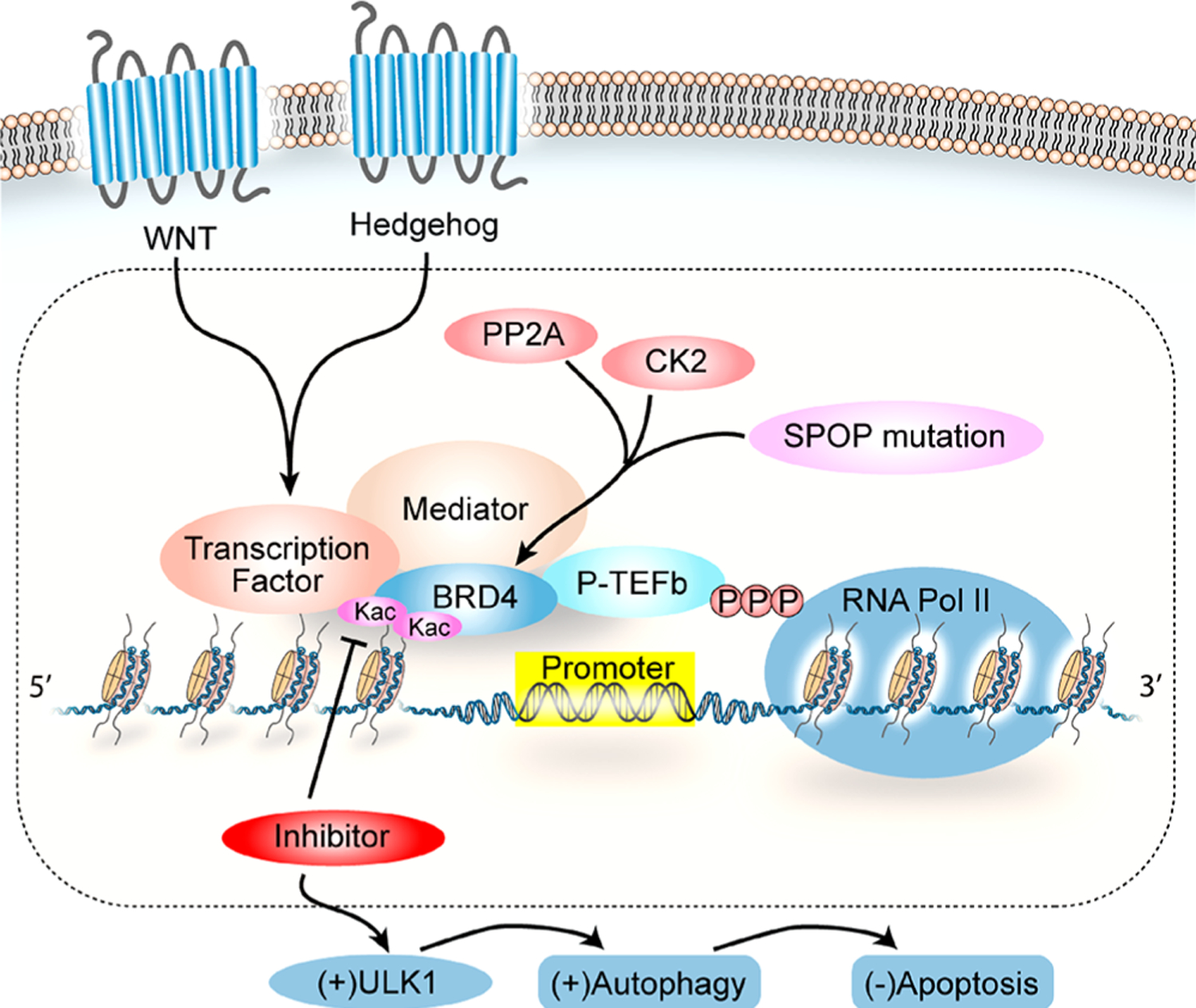
In related research of BRD4, problems with preclinical drug resistance have attracted significant attention. In drug resistance, the activation of alternative or compensatory pathways such as WNT/β-catenin and hedgehog signaling can maintain MYC expression independently of BRD4 (Figure 2).48–50 In pan-BET inhibitor-resistant leukemia cells, genome-wide BRD4 binding is decreased upon BET inhibitor treatment, but alternative binding by β-catenin to key oncogenic sites or nearby WNT-responsive enhancers maintains MYC expression.48 In addition, the activation of the hedgehog signaling pathway is another mechanism to restore MYC expression through altered transcription programming.49
The phosphorylation of BRD4, which is crucial for chromatin binding and factor recruitment,8 also has a great effect on the drug resistance, as shown in triple-negative breast cancer (TNBC) cells.11,12 Significantly increased BRD4 phosphorylation (or hyperphosphorylation) due to reduced PP2A activity results in a stronger association of BRD4 with the MED1 component of the mediator complex that recruits RNA Pol II to the promoter region, thereby promoting bromodomain-independent gene activation via an alternative protein−protein (i.e., BRD4−mediator) recruitment mechanism.11,51 BRD4 hyperphosphorylation has also been observed in NMC to support its oncogenic activity.40
Furthermore, adapted apoptotic signaling through AMPK/ULK1-mediated autophagy represents another resistance mechanism to BRD4 inhibitors in certain JQ1-resistant acute myelogenous leukemia (AML) cells, where reduced apoptosis is achieved by JQ1-induced prosurvival autophagy, as the pharmacological inhibition of AMPK leads to diminished ULK1-mediated autophagy and increased apoptosis.52
Conceivably, the activity of the E3 ubiquitin ligase adaptor SPOP (speckle-type POZ protein) and deubiquitinase DUB3 that directly modulate the BRD4 protein stability may potentially account for the drug resistance, as a deficiency in SPOP binding to BRD4 results in the enhanced BRD4-dependent expression of RAC1 GTPase and the activation of cholesterol biosynthetic enzymes and AKT-mTORC1 to mediate intrinsic resistance.53–55 Likewise, a mutation in NCOR2 that negatively regulates DUB3 gene transcription frequently observed in castration-resistant prostate cancer (CRPC) leads to enhanced DUB3 and BRD4 protein levels,56 thus contributing to resistance to BRD4 inhibitor treatment. Moreover, a mutation in PI3KCA has also been identified as a resistance determinant to BRD4 inhibitors based on whole-genome shRNA dropout screens performed in 77 breast cancer cell lines.57
Notably, in preclinical models, the complexity of drug resistance with diverse factors and pathways involved greatly diminishes the clinical responses. It is indeed a significant challenge to overcome resistance to BET inhibitors, and no compounds targeting specific gene mutation have thus far been reported.
4. DEVELOPMENT OF NEXT-GENERATION INHIBITORS
Poor and short-term clinical responses have become a significant issue in the use of pan-BET small-molecule inhibitors for the clinical treatment of cancer. Thus higher and stricter requirements for the development and rational use of inhibitors are essential. New technologies have been developed to facilitate the discovery of novel inhibitors, such as BD1-, BD2-, or BRD4-selective inhibitors, BET proteolysis targeting chimeras (PROTACs), dual-target inhibitors, and protein−protein interaction (PPI) inhibitors. Among these inhibitors, pan-BET inhibitors show no selectivity against specific BET protein isoforms or bromodomains, BD1- and BD2-selective inhibitors imply pan-BET activity toward BRD2/3/4/T but with selectivity for specific bromodomains, and BRD4-selective inhibitors mean target preference for BRD4 rather than BRD2/3/T but without bromodomain selectivity. A recent report depicting functional differences between BD1 and BD2 highlights the potential for developing BD1-specific inhibitors in cancer therapy and BD2-specific inhibitors in immunoinflammation that may be more favorable compared with nonselective BET bromodomain targeting.14 Dual-target inhibitors with bimolecular binding to two different proteins, such as dual BET/kinase inhibitors, display either synergistic effects or synthetic lethality that may reduce the drug resistance frequently seen with monotherapy during cancer treatment. PROTACs targeting BET proteins that allow the prompt removal of a large amount of BET proteins in the cell show strong antitumor activity in preclinical studies. Undoubtedly, PPI inhibitors blocking BRD4 interaction with other proteins offer another line of new approaches to drug discovery and cancer treatment. The development of these more selective BET inhibitors with alternative mechanisms of action is of vital importance to overcome the limited clinical efflcacy and off-target toxicities often associated with the use of pan-BET inhibitors.
4.1. BD1-Selective Inhibitors.
BD1 and BD2 each consist of four antiparallel α-helixes (αZ, αA, αB, and αC) with different lengths linked by two hydrophobic loops (ZA and BC) that together form a hydrophobic Kac recognition pocket. Amino acid differences in the binding pockets of BD1 and BD2 lead to narrower binding regions and differences in the polarity and hydrophobicity of BD2. Poor selectivity among BET proteins and between BD1 and BD2 has incurred unwanted side effects and reduced the efflcacy of BET bromodomain inhibitors in clinical trials.58 Thus selectively targeting only BD1 or BD2 in BRD4 can provide better specificity and avoid unnecessary bioreactions triggered by the other domain inhibition as well as poor clinical evaluation due to the concurrent targeting of both BD1 and BD2 with differences in functions and structures. Gilan et al. have developed respective BD1- and BD2-specific inhibitors for better disease treatment and efflcacy based on the functional and structural differences between BD1 and BD2.14 Because BD1 and BD2 within the same protein are structurally and functionally more diverse compared with the analogous BD1 or BD2 found in different BET proteins,2 it is in fact easier to develop inhibitors selective for BD1 or BD2 than for individual BET family members.
Many BD1-selective inhibitors have been discovered, including Olinone (1)59,60 and MS436 (2),60 with the 3D crystal structure of the latter with BRD4-BD1 also shown on the right (Figure 3A). ZL0580 (3) (IC50 = 163 nM), a BRD4 BD1-selective inhibitor, was discovered by structure-guided drug design.61 Overlay analysis reveals that 3 can access the Kac-binding pocket in BD1 with binding notably different from that of JQ1. Compound 3 induces the epigenetic suppression of HIV in multiple in vitro and ex vivo models. Another BD1-selective inhibitor, LT052 (4),62 with more than 100-fold selectivity for BRD4-BD1 over BRD4-BD2 and exhibiting a stable level in liver microsomal metabolic analysis inhibits monosodium urate (MSU)-induced apoptosis in human THP-1 monocytic cells and improves symptoms of acute gouty arthritis via BRD4/NF-κB/NLRP3 inflammasome signaling pathways. Chromone derivatives ZL0513 (5) and ZL0516 (6) both show high binding afflnity for BD1 with a value of 67 and 84 nM, respectively. In vivo pharmacokinetics (PK) profiles between compounds 5 and 6 are similar (half life 3 to 4 h, maximum concentration ~600 ng/mL, AUC ~5000 ng·h/mL), and the oral bioavailability of compounds 5 and 6 of around 35−38% (20 mg/kg) is generally acceptable in rats.63 GSK778 (7), identified by the functional and structural comparison between BD1 and BD2 showing 130 times higher afflnity for BD1 than BD2, inhibits not only the growth and vitality of human cancer cell lines but also the clone-forming ability of human primary AML cells.14 Further chemical modification of compound 7 leads to another potent, selective, and cell-permeable inhibitor of BD1 (GSK789 (8)), displaying more than 1000-fold selectivity for BD1 over BD2 and achieving a submicromolar inhibition of monocyte chemoattractant protein-1 (MCP-1) release in human whole blood (pIC50 = 6.5).64 Starting with I-BET151 guided by structural information leads to imidazoquinoline compound 9 with 150-fold BD1 selectivity over BD2.65 Compound 9 reduces the production of IL-6 and MCP-1 in LPS-stimulated human peripheral blood mononuclear cells (PBMCs) and whole blood and suppresses MV4–11 lymphoblast leukemia cell growth (pIC50 = 7.0), indicating that BD1-selective inhibitor compound 9 is sufflcient to maintain the anti-inflammatory and antiproliferative activity of pan-BET inhibition. Compound 10, another BD1-selective inhibitor (28 times higher afflnity for BRD4-BD1 than BRD4-BD2), displays similar selectivity against the BD2 of BRD2 and BRD3 (25 and 33 times, respectively).66 This study also reveals that the underlying BD1 selectivity lies in the flexibility of the BD1-conserved YNKP motif and the substitution of structured waters in the Kac-binding site, which, unlike typical pan-BET inhibitor-suppressed MYC expression, leads to enhanced MYC expression in compound 10-treated MM.1S multiple myeloid cells. This unexpected result needs to be further investigated.
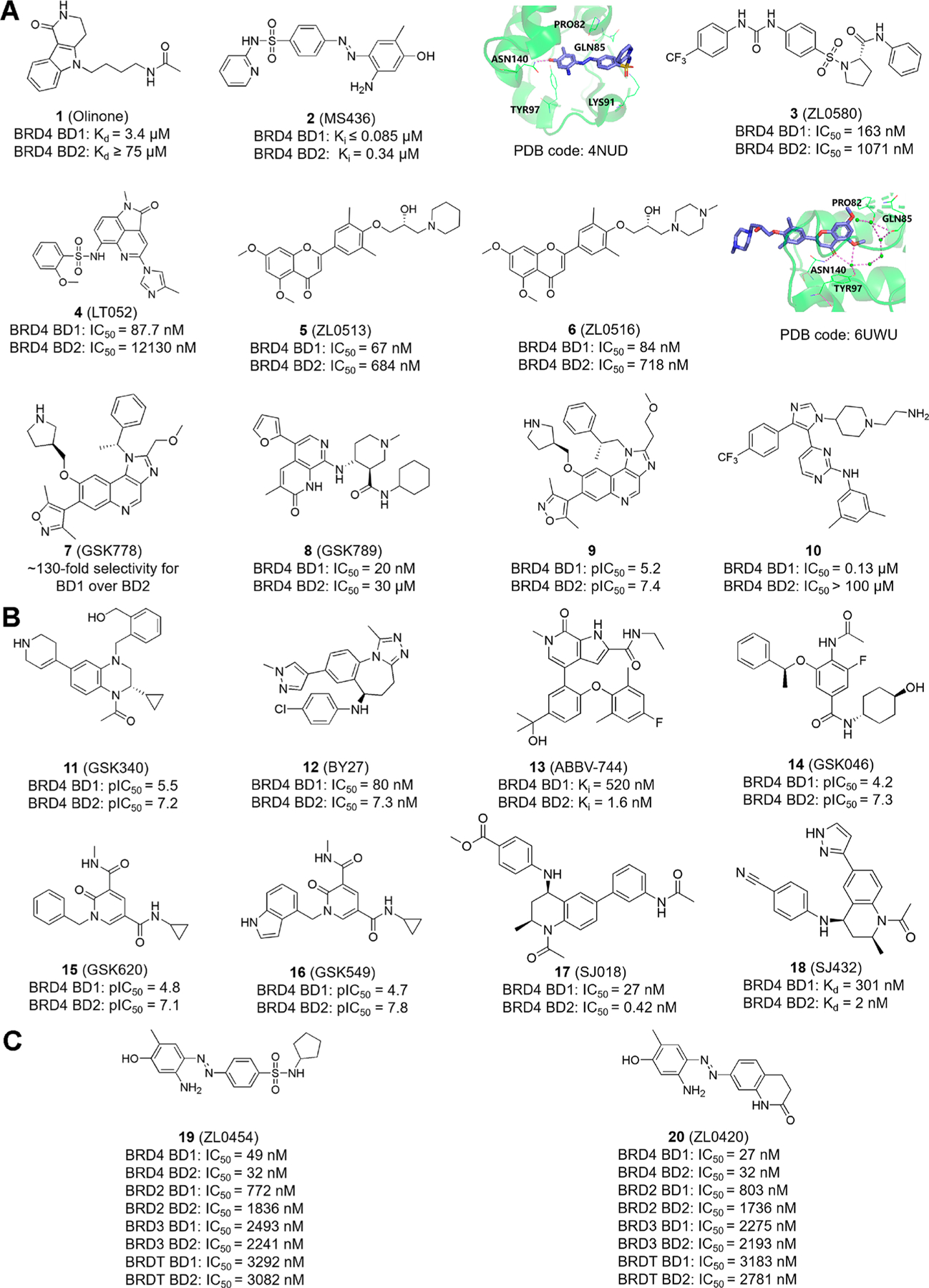
Reported novel selective inhibitors 1−20. (A) Chemical structures of BRD4 BD1-selective inhibitors. The docking pose of compounds 2 and 6 with BRD4−BD1 in ribbon representation is shown on the right. Key residues, including Pro82, Gln85, Lys91, Tyr97, and Asn140, are shown in green sticks, and the amide ketone of compound 2 interacts with the critical conserved residue Asn140 to form a hydrogen bond. Similarly, in the docking pose of compound 6, key residues Asn140, Tyr97, Gln85, and Pro82 are green. The carbonyl O atom of compound 6 interacts with the conserved residue Asn140, forming a direct H bond, and with Tyr97, forming an indirect hydrogen bond mediated by a water molecule. (B) Reported BRD4 BD2-selective inhibitors. (C) Chemical structures of BRD4-selective inhibitors.
4.2. BD2-Selective Inhibitors.
Representative BD2-selective inhibitors identified to date are shown in Figure 3B. The tetrahydroquinoxaline derivative GSK340 (11) with BD2 selectivity displays good solubility in both thermodynamic and kinetic solubility assays.67 In addition, compound 11 is cell-permeable and reduces the release of the MCP-1 cytokine in human PBMCs and whole blood. BY27 (12) is a potent BD2-selective inhibitor with 7 times higher selectivity for BD2 over BD1.68 In MV4–11 cells, compound 12 reduced the c-MYC protein level and increased the p21 expression in a dose-dependent manner. Compared with I-BET762, which caused 11% weight loss at 60 mg/kg, compound 12 is less toxic (exhibiting 7% weight loss at 150 mg/kg) at high doses and has 67% inhibition of tumor growth in a MV4–11 mouse xenograft model. Currently, ABBV-744 (13),69 a highly effective inhibitor of the BD2 domain with more than 300 times higher selectivity for BD2 over BD1, is synthesized and shows moderate preclinical PK profiles, including a moderate to high volume of distribution, a 2.0−4.4 h half life, and moderate clearance.70 The structural modification of an ABBV-075 analog leads to compound 13. 2,6-Dimethylphenyl ether in compound 13 contributes to BD2 selectivity, with a tertiary alcohol on the central phenyl ring suppressing the activity against BRD4-BD1 and a fluorine atom on the phenyl ether improving the pharmacokinetic properties of compound 13. In a myelodysplastic SKM-1 cell-derived xenograft mouse model, compound 13 displays 83% tumor growth inhibition with minimal (2%) weight loss over 21 days at a dose of 18.8 mg/kg, whereas ABBV-075 has similar tumor growth inhibition with 7% weight loss at the maximally tolerated dose of 1 mg/kg, suggesting that more selective BET inhibitors may improve the tolerability, albeit modestly. Compound 13 is currently in phase I clinical evaluation. GSK046 (iBET-BD2, 14), although less effective than 8 in the human cancer cell lines examined, exhibits more than 300 times higher selectivity for BD2 over BD1 and appears to ameliorate inflammatory disease in preclinical models.14 A recent study shows that compound 14 has physicochemical and pharmacokinetic properties suitable for use in vivo, and it retains the ability to effectively inhibit the release of MCP-1 cytokines (pIC50 = 7.5) in the cellular and whole blood environment.71 Interestingly, the inhibition of BD1 or BD2 leads to the suppression of MCP-1 despite the fact that these two types of inhibitors have distinct selectivity profiles. Another study applying a template hopping approach based on the orthogonal template (compound 14) leads to the synthesis of compounds GSK620 (15) and GSK549 (16) with high selectivity and low levels of inhibition against indicators of hepatoxicity.72 SJ018 (17) and SJ432 (18), two tetrahydroquinoline analogs, inhibit the expression of MYC and downstream targets but display no rebound effects, as compared with JQ1 in pediatric tumor cell lines, suggesting that BD2-selective inhibitors are effective candidates for the design of antitumor drugs for MYC-driven pediatric malignancies.73
4.3. BRD4-Selective Inhibitors.
Besides BD1- and BD2-selective inhibitors, the specific targeting of BRD4 rather than other BET proteins is also emerging. The ubiquitously expressed BRD2, BRD3, and BRD4 have a broad range of functions and participate in housekeeping and lineage-specific gene regulation in somatic and progenitor/stem cells by interacting with distinct partner proteins. BRD2 associates with E2F transcription factors to regulate E2F-dependent cell differentiation and proliferation genes and interacts with RNA Pol II to engage in transcription elongation in embryonic kidney cells through hyperacetylated nucleosomes due to the lack of a CTM.74–76 Similar to BRD2, BRD3 is mainly involved in the regulation of gene transcription related to the development of embryonic stem cells, but unlike BRD4, it interacts with RNA Pol II in a P-TEFb-independent manner.77 The germ-cell-specific BRDT associates with multiple interactors during spermatogenesis and oogenesis.78,79 Mice treated with the pan-BET inhibitor JQ1 had a reduced spermatozoa number and reduced motility, indicating that BRDT could be an ideal target for contraceptive drug development.80,81 Considering the intricate physiological functions of BET family proteins, it is important to improve the target selectivity of BRD4 inhibitors in specific biological processes and disease progression without incurring unintended side effects, especially in the treatment of cancer and inflammation.58 Two BRD4-selective inhibitors reported in recent years are shown in Figure 3C. ZL0454 (19) and ZL0420 (20) are two BRD4-selective inhibitors displaying 30−60-fold selectivity over BRD2, 50−90-fold selectivity over BRD3, and 70−120-fold selectivity over BRDT.82 Molecular docking shows that compounds 19 and 20 occupy the Kac-binding pocket of BRD4 in a similar way. In vivo experiments further found that 19 and 20 almost completely blocked the TLR3 (toll-like receptor 3)-dependent expression of innate immune genes, including ISG54, ISG56, IL-8, and Groβ in human primary small airway epithelial cells (hSAECs). Given the shared functions between BRD4 and the other BET family proteins yet to be completely defined, it is difflcult to predict what types of diseases are most suited for BRD4-selective targeting. Nevertheless, therapy with fewer unintended targets has the potential to improve the clinical outcomes.
4.4. Dual Pharmacophores.
In recent years, multitarget approaches targeting different proteins and inhibiting kinases have received increasing attention in anticancer drug development.83 A previous study that reported that the CDK inhibitor Dinaciclib could interact with the Kac-binding pocket of BRDT provided the first indication that kinase inhibitors might be used to rationally design a new generation of BRD4 inhibitors.84 Since then, on the basis of the idea that polypharmacological inhibitors with multitargets would have a more robust action, many dual BET/kinase inhibitors have been described.85,86 Some of the recently discovered dual-target inhibitors are shown in Figure 4.
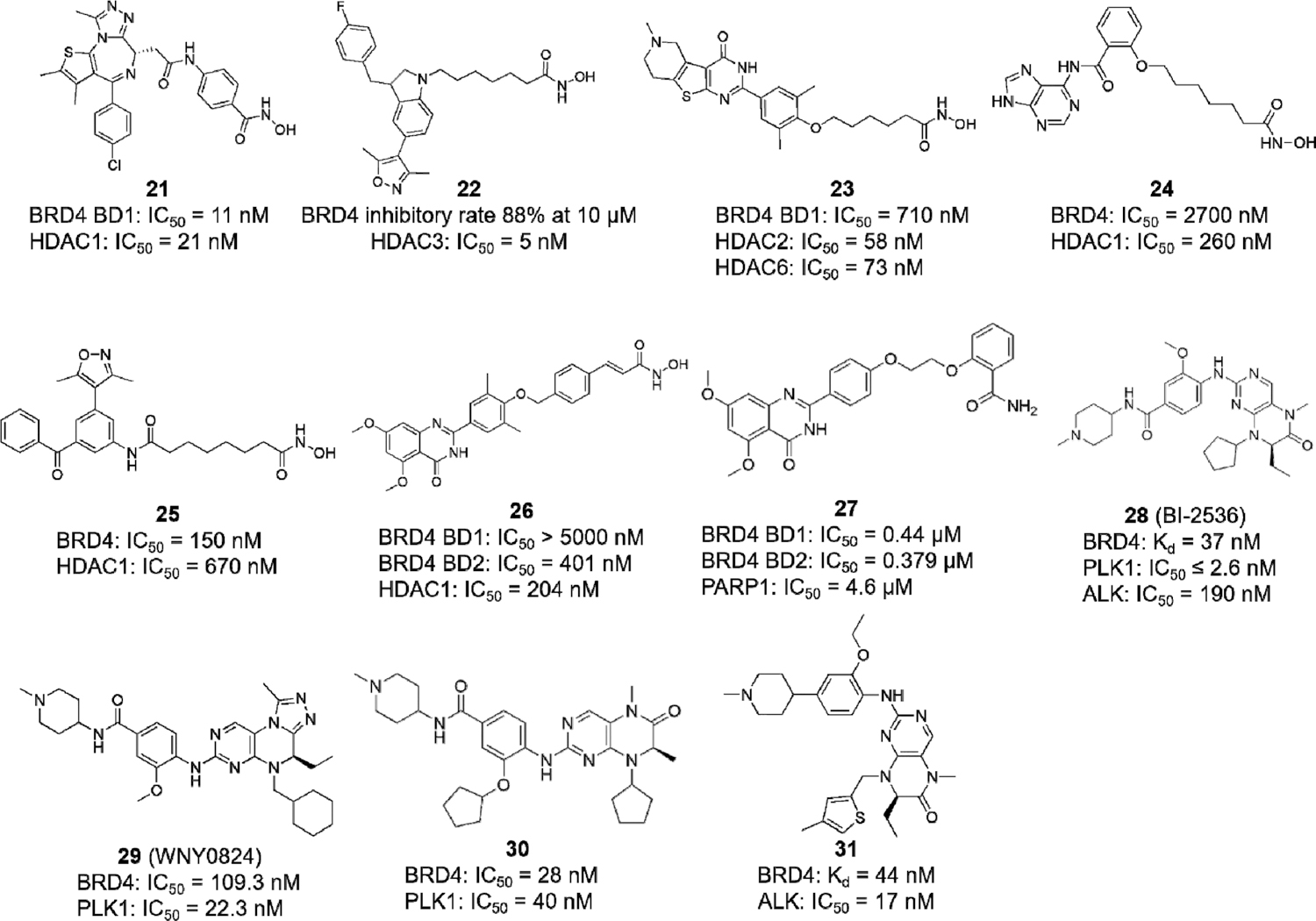
Histone deacetylases (HDACs) reduce the acetylation level of lysine residues where BET proteins recognize and promote factor recruitment to targeted gene loci, thereby regulating gene transcription.2,87–89 It is logical to cotarget BET proteins and HDACs for the more efflcient repression of oncogenic gene transcription and tumor cell proliferation.90,91 Compound 21, a highly potent dual BET/HDAC inhibitor, was reported with balanced activity against BRD4 BD1 and HDAC1.92 Compound 22 shows the inhibition of BRD4 at a rate of 88% at 10 μM and strong HDAC3 inhibition with an IC50 value of 5 nM.93 Compound 23, another dual BRD4/HDAC inhibitor, induces autophagic cell death in colorectal cancer (CRC) cells at nanomolar levels.90 Compound 24 induces HL-60 AML cell death with c-MYC-inhibitory activity and suppresses cell growth in BET inhibitor-resistant cells.94 Compounds 25 and 26 also exhibit potent antiproliferative activity in human leukemia cell lines.95,96
Because BRD4 plays a critical role in DNA repair (Figure 1D), the inhibition of BRD4 shows synthetic lethality with poly-(ADP-ribose) polymerase-1 (PARP1) inhibitors, resulting in HR deficiency and providing a strategy for cancer treatment.35,97 Compound 27, designed with the conception of synthetic lethality, is the first dual BRD4/PARP1 inhibitor reported that triggers breast cancer cell apoptosis and induces cell-cycle arrest in the G1 phase.98 A recent study reports that polo-like kinase 1 (PLK1) upregulates BRD4-controlled MYC transcription and protein stability, with MYC, in turn, enhancing the PLK1 gene transcription, thus forming a PLK1-MYC feed-forward circuit in double-hit lymphoma (DHL) cells.99 Via the chemical modification of the pre-existing dual BRD4/PLK1 inhibitor BI-2536 (28), the dual BET/PLK1 inhibitor WNY0824 (29) was shown to suppress CRPC cell proliferation as well as the tumor growth in an enzalutamide-resistant CRPC xenograft mouse model by blocking BRD4-dependent androgen-receptor- and MYC-driven transcription programs.100 Fine-tuning the selectivity of inhibitors can also be achieved through structural guidance. Compound 30 is identified as a well-balanced dual BRD4/PLK1 inhibitor, illustrating that the methylated amide and the cyclopentyl group of the compound 28 scaffold are important for maintaining bromodomain-binding activity but are less critical for PLK1-inhibitory activity.101 Another study also chose compound 28 as the starting compound but changed PLK1 inhibition to anaplastic lymphoma kinase (ALK) targeting with the derived inhibitor compound 31, maintaining the BRD4-inhibitory activity but shifting the kinase selectivity from diminished PLK1 inhibition to enhanced ALK inhibition, which shows the remarkable suppression of ALKF1174L- and BRD4-dependent MYCN-driven gene transcription in pediatric neuroblastoma cells.102 These strategies provide new directions for the development of novel dual-target inhibitors via the chemical modification of existing pharmacophores targeting, respectively, BET proteins and kinases. However, this new line of research also poses significant challenges in how to properly balance the selectivity between BET proteins and specific kinases in designing dual-target inhibitors with enhanced antitumor activity. A report documenting that PLK inhibition, rather than BRD4 inhibition, is the main action for the anticancer activity of compound 28 in MV4–11 cells may provide some mechanistic hints about this specific compound.103
It should be mentioned that many dual-target inhibitors were discovered through their unexpected BET-targeting activity. For instance, TG101209 and BI-2536, originally reported as JAK2 inhibitor and PLK1 inhibitor, respectively, were found to have strong inhibitory potential against BRD4 through binding to the Kac pocket, providing a rationale for the development of dual-target inhibitors.104 As the products of a new technology, dual-target inhibitors display great potential in cancer therapy, even though they are still in the preclinical stage. Combining two pharmacophores cotargeting BET proteins and HDACs based on their pharmacological activity and safety evaluation, for example, 21 synthesized by linking (+)-JQ1 to HDAC inhibitors SAHA and CI-944,92 has become a popular trend in the discovery of dual-target inhibitors.105 For dual-target inhibitors that have already been developed, structural modification may result in new inhibitors with different biological activities, such as compounds 29, 30, and 31, optimized from compound 28.96,102
Additional kinase inhibitors to be considered include those involved in the PI3K-AKT-mTOR pathway that regulates MYC expression, stability, post-translational modification, and MYC protein synthesis106 as well as CDK9, which phosphorylates the Pol II CTM at Ser2, resulting in the stimulation of transcription elongation, and is associated with BRD4 hyperphosphorylation in NMC.40,83,107 CK2, which phosphorylates BRD4, implicated in chromatin targeting and factor recruitment,8 is another promising candidate for the future development of potent dual-target inhibitors for cancer therapy.
4.5. PROTACs.
PROTACs, initially proposed by Deshaies and colleagues,108 are an effective way to inactivate protein function via compound-mediated proteolysis. PROTACs targeting BET proteins have distinct pharmacological effects compared with the corresponding small-molecule inhibitors because they can disrupt the function of the entire protein rather than just the bromodomain activity.46,109 A sustained loss of BET proteins has superior antiproliferative effects in vitro while showing better anticancer activity in vivo with no obvious toxicity in a mouse tumor regression study.110
The currently developed BET degraders use ligand binding to the cereblon (CRBN) or von Hipple-Lindau (VHL) component of an E3 ubiquitin ligase. Compounds 32−38 are CRBN-based degraders, whereas compounds 39−45 are based on the VHL component (Figure 5A). Among them, dBET1 (32) containing an eight-atom linker is the first degrader displaying remarkable selectivity in depleting BET proteins with more potent inhibition compared with JQ1 against MV4–11 cells, DHL-4 lymphoma cells, and primary blasts from leukemia patients.111 ARV-825 (33) with a different linker type also achieves an effective degradation of BRD4 (DC50 < 1 nM), resulting in strong and lasting c-MYC inhibition in comparison with pan-BET inhibitors JQ1 and OTX015.112 BETd-246 (34) induces BET protein degradation at low nanomolar concentrations within an hour in TNBC cells by downregulating many proliferation/apoptosis-related genes, leading to growth inhibition and apoptosis.113 Another degrader, BETd-260 (35), shows picomolar potency (IC50 = 51 pM) in inhibiting RS4–11 lymphoblastic leukemia cell growth.110
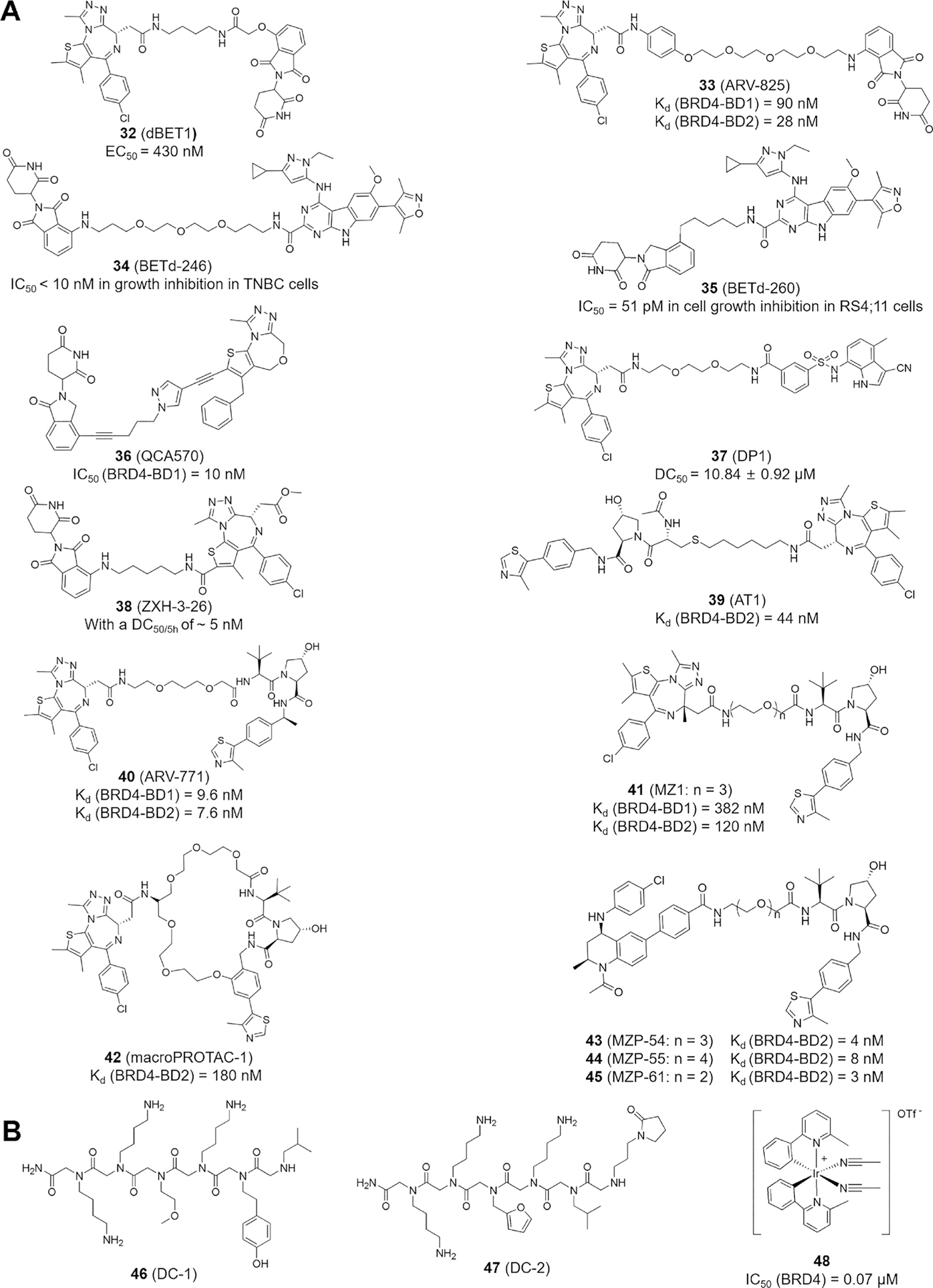
Reported PROTAC degraders and PPI inhibitors. (A) PROTAC degraders based on CRL4CRBN (32−38) and CRL2VHL E3 ligase (39−45). (B) Chemical structures of 46−48.
QCA570 (36) was designed and synthesized based on the 1,4-oxazepine structure with more than 1000 times higher potency compared with 33. Significantly, in both the MV4–11 and RS4–11 acute leukemia xenograft models, compound 36 shows complete and long-lasting tumor regression at 5 mg/kg without apparent toxicity.114 A recent study highlights that DP1 (37) not only achieves rapid, stable, and persistent BRD4 degradation in SU-DHL-4 lymphoma cells but also has significant inhibitory effects on immunodeficient mice with xenotransplanted lymphoma cells.115 Cellular degradation assays showed that ZXH-3–26 (38) exhibits a DC50/5h of ~5 nM and BRD4 selectivity without inducing significant inhibition or degradation of BRD2 and BRD3,116 a result similar to that observed with AT1 (39) displaying BRD4-selective depletion but negligible activity against BRD2 and BRD3 at 1 to 3 μM of 39 treatment.117 ARV-771 (40), a small-molecule pan-BET degrader, reduces the protein levels of androgen receptor (AR) signaling in a CRPC mouse xenotransplantation model, providing the first reported BET degrader activity against a solid tumor.118 Compound 40 also presents superior pharmacological properties compared with those of 33 in mantle cell lymphoma (MCL) Mino cells.119 MZ1 (41), a JQ1-based BRD4 degrader, exhibits BRD4-targeting preference in cell-based assays but comparable in vitro binding afflnities to individually purified BRD2, BRD3, and BRD4 bromodomains measured by isothermal calorimetry (ITC), suggesting that MZ1-induced proximity and accessible surface lysine residues on BRD4, versus BRD2 and BRD3, might be differentially contacted by the VHL E3 ligase module in vivo and are presumably regulated by BET interactors that are unique to each family member.120 Adding a cyclizing linker to compound 40 leads to a macrocyclic PROTAC, macroPROTAC-1 (42), with cellular activity similar to that of compound 41, thus providing a new strategy for drug design.121
The targeting effects between triazolodiazepine JQ1 and tetrahydroquinoline I-BET762 and the optimal length of linkers connecting the BET-binding ligand JQ1 or I-BET762 to the VHL ligand VH032 for degrader design were examined by synthesizing a series of I-BET762-derived degraders, including MZP-54 (43), MZP-55 (44), and MZP-61 (45),122 for comparison with MZ1-related compounds, with the results showing that JQ1-based PROTACs are more effective at inducing BET protein degradation, even though JQ1 alone is not as potent as I-BET762 in BET protein binding. This parallel comparison indicates that stronger inhibitors may not necessarily produce more effective PROTAC degraders, and the linker length is critical in PROTAC design. It is important to note that PROTAC binding to incidental or unintended targets through substrate or E3 ligase association does not guarantee substrate degradation.123 These BET degraders are effective in inhibiting cancer cell proliferation in vivo and in vitro. In mice, compounds 34, 35, and 36 show good tolerance without obvious toxicity.124
BET-targeting PROTACs are still in the preclinical stage, and thus their therapeutic potential is presently unknown. For the current degraders, the main challenge is to improve the bioavailability index through precise modification without significantly increasing the molecular size. More selective and tissue-specific degraders are also needed for clinical trials. A strategy involving antibody−PROTAC conjugation provides a proof of concept for tissue-specific BRD4 degradation.125 A trastuzumab−PROTAC conjugate (Ab-PROTAC) with blocked degradation activity by an antibody linker that can be selectively released to produce an active PROTAC demonstrates the significant potential for tissue-specific target degradation. It should be mentioned that targeting BET proteins for degradation is not limited to ubiquitination. The lysosomal/autophagy pathway provides cargo-specific degradation through a different molecular mechanism.126
4.6. Targeting the Protein−Protein Interaction Network.
The PPI forms signal nodes and hubs in normal cells and cancer cells and transmits pathophysiological cues along molecular networks to promote tumorigenesis, migration, invasion, and metastasis.127 Therefore, blocking the PPI is an effective way to inactivate network function and signal transmission. DC-1 (46) and DC-2 (47), two peptoids (i.e., N-substituted oligoglycines; Figure 5B) isolated from a combinatorial compound library of ~38 500 peptoids,128 bind specifically to phospho-NPS in human BRD4, which provides an effective strategy to inhibit the phosphorylation-dependent gene-specific function of BRD4 and factor-regulated transcription programs.10,12 The iridium(III) transition-metal-based irreversible PPI inhibitor 48 blocks BRD4-BD1 but not BRD4-BD2, binding to a tetraacetylated histone H4 peptide with IC50 = 70 nM, and suppresses melanoma cell growth in vitro and mouse xenograft tumor growth in vivo by inhibiting BRD4-driven MYC and BCL-2 gene transcription.129 Clearly, PPI inhibitors provide another promising strategy for BRD4-targeted disease treatment.
Compared with other technologies, targeting the PPI associated with BRD4 is less reported, in part due to the challenge in identifying the in vivo targets and the mechanisms of action as well as the difflculty in establishing effective screening systems.130 Encouragingly, targeting phospho-NPS in BRD4, as illustrated with compounds 46 and 47, has been shown to be a promising and effective way to inhibit BRD4 association with specific transcription factors without blocking the chromatin-binding activity of BRD4, providing a rationale for targeting nonbromodomain surfaces of BRD4 to block specific disease-associated pathways with minimal perturbation of normal BRD4 function.10 A unique interface formed between BRD4 and GATA4 interaction in cardiomyocytes that regulates the bioenergy homeostasis of the adult heart would be an interesting region for the future development of BRD4-targeting PPI inhibitors.131
5. CONCLUSIONS AND FUTURE PERSPECTIVES
In mouse tumor models, BD1-selective inhibition shows reduced proliferation, cell-cycle arrest, and apoptosis, as seen with pan-BET inhibitors, whereas BD2-selective inhibition is more effective in suppressing inflammation, metabolic diseases, and fibrosis by reducing the negative effects of enhancer reprogramming and minimizing the maladaptive transcription of disease-causing genes on potential enhancers.132 The development of BRD4-selective inhibitors is in an early stage. Considering the oncogenic role the BRD4 short isoform plays in the extracellular matrix (ECM) network during breast cancer progression7 and the potential active gene transcription through LLPS,20 selective targeting of the BRD4 short isoform without cross-inhibiting the tumor-suppressive function of the BRD4 long isoform is a critical strategy for future drug development. The advancement in BRD4 protein characterization also sheds new light on the design of selective inhibitors targeting a specific region of BRD4, such as phospho-NPS, which provides more selective interaction with cancer-associated transcription factors. Further development of inhibitors targeting other BET proteins could also be beneficial to disease treatment or birth control, such as BRDT-specific targeting for contraceptives. Conceivably, research on the structural understanding of BET proteins will contribute to the development of more selective inhibitors. A computational protein structure prediction is helpful to simulate the protein structure, with refinement making the model closer to the natural structure,133 thus allowing the exploration of whether or how small molecules bind to the protein through docking.134 All of these provide new methodologies for the further development of selective inhibitors.
At present, dual kinase/BRD4 inhibitors are still in the early stages. A rational design of dual kinase/BET inhibitors may be conceptually easier than designing bifunctional ligands targeting two different kinases due to the similarity of their catalytic pockets. It is also challenging to design dual-target inhibitors with balanced selectivity. Here computational methods are useful for predicting and identifying new ligands with dual activity by simulating the binding modes of ligands and using ligand-based and structure-based approaches. Moreover, the physical and chemical properties of dual-target inhibitors may be poor due to an increase in the relative molecular weight and structural complexity. How to balance the target selectivity of dual-target inhibitors to maximize the clinical efflcacy is another challenge. It is likely that partial coverage of the kinase and full coverage of BRD4 may achieve the maximum outcome.
The pharmacological effects of PROTACs are not limited to only protein loss but also affect the related subcellular localization and transcriptional regulation, thus improving the pharmacological efflcacy through this additional function. Another feature of PROTACs is that they require a relatively low dose to achieve pharmacological effects. This also reduces the possibility of off targets and thus improves the drug tolerance. In addition, PROTACs targeting BET proteins, in a way similar to gene knockout, can cause a more lasting loss of protein expression than other targeted inhibitors, namely, a more prolonged inhibitory effect.
The PPI is an important part of the functional exploration of BRD4. Besides the well-known bromodomain, the nonbromodomain PPI is also of great research value, especially the phospho region of BRD4 targeted by the existing peptoid inhibitors. Accordingly, PPI inhibitors, with in-depth mechanistic studies and chemical probing, have great therapeutic potential warranting further development.
All of these new technologies have shown great potential based on in vitro and in vivo bench studies. The primary challenge is to transfer the preclinical antitumor activity of compounds to clinical efflcacy. Altogether, the use of small-molecule inhibitors targeting BRD4 is expected to be effective for the treatment of a variety of cancers. Interdisciplinary cooperation among investigators is of great need to make groundbreaking drug discoveries in this exciting area of research.
ACKNOWLEDGMENTS
Funding for the authors’ research was sponsored in part by the National Natural Science Foundation of China, grant numbers 81922064 (to L.O.), 81874290 (to L.O.) and 81903502 (to J.Z.); the National Science and Technology Major Project of the Ministry of Science and Technology of China, grant number 2018ZX09735005 (to L.O.); the National Major Scientific and Technological Special Project for ‘Signficant New Drugs Development’, grant number 2018ZX09201018–021 (to L.O.); the Sichuan Science and Technology Program, grant number 2019YFS0003 (to L.O.); the Sichuan Science and Technology Program, grant number 2020YJ0091 (to J.L.); the China Postdoctoral Science Foundation, grant number 2020M673268 (to J.Z.); the U.S. National Institutes of Health (NIH), grant number 1RO1CA251698–01 (to C.-M.C.); and the Cancer Prevention and Research Institute of Texas (CPRIT), grant numbers RP180349 and RP190077 (to C.-M.C.).
ABBREVIATIONS USED
| ALK | anaplastic lymphoma kinase |
| ALL | acute lymphoblastic leukemia |
| AML | acute myelogenous leukemia |
| AR | androgen receptor |
| AST | advanced solid tumor |
| BET | bromodomain and extraterminal |
| BID | basic residue-enriched interaction domain |
| BPDCN | blastic plasmacytoid dendritic cell neoplasm |
| BRD4 | bromodomain-containing protein 4 |
| CDK9 | cyclin-dependent kinase 9 |
| CK2 | casein kinase II |
| CPS | C-terminal cluster of phosphorylation sites |
| CRBN | cereblon |
| CRC | colorectal cancer |
| CRPC | castration-resistant prostate cancer |
| CtIP | C-terminal binding protein-interacting protein |
| CTM | C-terminal motif |
| DHL | double-hit lymphoma |
| DLBCL | diffuse large B-cell lymphoma |
| DLT | dose-limiting toxicity |
| DSB | DNA double-strand break |
| ECM | extracellular matrix |
| ET | extraterminal |
| GBM | glioblastoma multiform |
| HDAC | histone deacetylase |
| HPV | human papillomavirus |
| HR | homologous recombination |
| hSAEC | human primary small airway epithelial cell |
| ITC | isothermal calorimetry |
| Kac | N-ε-acetyllysine |
| LLPS | liquid−liquid phase separation |
| MCL | mantle cell lymphoma |
| MCP-1 | monocyte chemoattractant protein-1 |
| MCRPC | metastatic castration-resistant prostate cancer |
| MDS | myelodysplastic syndrome |
| MM | multiple myeloma |
| MPN | U myeloproliferative neoplasm-unclassifiable |
| NHEJ | nonhomologous end joining |
| NHL | non-Hodgkin’s lymphoma |
| NMC | NUT midline carcinoma |
| NPS | N-terminal cluster of phosphorylation sites |
| NSCLC | nonsmall cell lung cancer |
| PARP1 | poly(ADP-ribose) polymerase-1 |
| PBMC | peripheral blood mononuclear cell |
| PK | pharmacokinetics |
| PLK1 | polo-like kinase 1 |
| PP2A | protein phosphatase 2A |
| PPI | protein−protein interaction |
| PROTAC | proteolysis targeting chimera |
| P-TEFb | positive transcription elongation factor b |
| SPOP | speckle-type POZ protein |
| TLP3 | toll-like receptor 3 |
| TNBC | triple-negative breast cancer |
| VHL | von Hipple-Lindau |
Biographies
Pan Tang received her B.S. degree in Pharmacy English from Shenyang Pharmaceutical University in 2018. She is currently a Master’s student at the State Key Laboratory of Biotherapy and Cancer Center, West China Hospital and Collaborative Innovation Center of Biotherapy, Sichuan University. Her research interest focuses on the anticancer mechanism of small-molecule compounds.
Jifa Zhang received his Ph.D. degree from Southwest Jiaotong University in 2018 and is currently a postdoctoral student at the State Key Laboratory of Biotherapy and Cancer Center, West China Hospital and Collaborative Innovation Center of Biotherapy, Sichuan University. His research interests focus on the discovery, optimization, and molecular mechanism of candidate small-molecule compounds with antitumor activity.
Jie Liu received his Ph.D. degree in Medicinal Chemistry from West China School of Pharmacy, Sichuan University in 2010. He is now a Professor at State Key Laboratory of Biotherapy, West China Hospital, Sichuan University. He has long been engaged in the application of transition-metal-mediated organic synthesis in small-molecule-targeted drugs and pharmacochemical biology.
Cheng-Ming Chiang received his Ph.D. from the Department of Biochemistry, University of Rochester in 1991. He has been an Assistant Professor in the Department of Biochemistry at the University of Illinois Urbana−Champaign (1995−2000), an Associate Professor in the Department of Biochemistry at Case Western Reserve University in Cleveland, Ohio (2000−2007), and a Professor at Simmons Comprehensive Cancer Center, Department of Biochemistry, and Department of Pharmacology at the University of Texas Southwestern Medical Center (2007−present). His research interests primarily focus on transcription mechanisms, gene regulation, epigenetic control, chromatin dynamics, posttranslational modification, human papillomavirus, cancer, and therapeutics targeting phospho-BRD4 and BET proteins.
Liang Ouyang received his Ph.D. degree in Medicinal Chemistry from West China School of Pharmacy, Sichuan University, in 2010. He is now Professor at State Key Laboratory of Biotherapy, West China Hospital, Sichuan University. He has long-time interests in the design and synthesis of drugs based on original targeting of autophagy, basic research on the application of lead compound screening, the development of new drug target recognition methods and drug discovery, and taking advantage of interdisciplinary approaches including systems biology, structural biology, computer-aided drug design, medicinal chemistry, chemical biology, and molecular biology.
Contributor Information
Pan Tang, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University, Chengdu 610041, China.
Jifa Zhang, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University, Chengdu 610041, China.
Jie Liu, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University, Chengdu 610041, China.
Cheng-Ming Chiang, Simmons Comprehensive Cancer Center, Department of Pharmacology, and Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, Texas 75390, United States.
Liang Ouyang, State Key Laboratory of Biotherapy and Cancer Center, National Clinical Research Center for Geriatrics, West China Hospital of Sichuan University, Chengdu 610041, China.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1021/acs.jmedchem.0c01487
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8106541
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/100666646
Article citations
An updated patent review of BRD4 degraders.
Expert Opin Ther Pat, 34(10):929-951, 04 Sep 2024
Cited by: 0 articles | PMID: 39219068
Review
Combining Data-Driven and Structure-Based Approaches in Designing Dual PARP1-BRD4 Inhibitors for Breast Cancer Treatment.
J Chem Inf Model, 64(19):7725-7742, 18 Sep 2024
Cited by: 0 articles | PMID: 39292752 | PMCID: PMC11480993
BRD4: an effective target for organ fibrosis.
Biomark Res, 12(1):92, 30 Aug 2024
Cited by: 0 articles | PMID: 39215370 | PMCID: PMC11365212
Review Free full text in Europe PMC
Application of PROTACs in Target Identification and Target Validation.
Acta Mater Med, 3(1):72-87, 01 Feb 2024
Cited by: 0 articles | PMID: 39373008 | PMCID: PMC11452161
IDR-targeting compounds suppress HPV genome replication via disruption of phospho-BRD4 association with DNA damage response factors.
Mol Cell, 84(2):202-220.e15, 15 Dec 2023
Cited by: 3 articles | PMID: 38103559
Go to all (32) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 44 of 44)
- (2 citations) ClinicalTrials.gov - NCT02630251
- (2 citations) ClinicalTrials.gov - NCT02369029
- (2 citations) ClinicalTrials.gov - NCT02711137
- (2 citations) ClinicalTrials.gov - NCT02698176
- (2 citations) ClinicalTrials.gov - NCT02296476
- (1 citation) ClinicalTrials.gov - NCT03527147
- (1 citation) ClinicalTrials.gov - NCT01943851
- (1 citation) ClinicalTrials.gov - NCT01587703
- (1 citation) ClinicalTrials.gov - NCT03936465
- (1 citation) ClinicalTrials.gov - NCT04116359
- (1 citation) ClinicalTrials.gov - NCT02392611
- (1 citation) ClinicalTrials.gov - NCT02964507
- (1 citation) ClinicalTrials.gov - NCT02308761
- (1 citation) ClinicalTrials.gov - NCT02959437
- (1 citation) ClinicalTrials.gov - NCT04145375
- (1 citation) ClinicalTrials.gov - NCT02157636
- (1 citation) ClinicalTrials.gov - NCT02711956
- (1 citation) ClinicalTrials.gov - NCT02391480
- (1 citation) ClinicalTrials.gov - NCT03901469
- (1 citation) ClinicalTrials.gov - NCT03925428
- (1 citation) ClinicalTrials.gov - NCT02698189
- (1 citation) ClinicalTrials.gov - NCT01949883
- (1 citation) ClinicalTrials.gov - NCT03896815
- (1 citation) ClinicalTrials.gov - NCT02543879
- (1 citation) ClinicalTrials.gov - NCT03150056
- (1 citation) ClinicalTrials.gov - NCT02706535
- (1 citation) ClinicalTrials.gov - NCT02259114
- (1 citation) ClinicalTrials.gov - NCT04022785
- (1 citation) ClinicalTrials.gov - NCT02705469
- (1 citation) ClinicalTrials.gov - NCT02683395
- (1 citation) ClinicalTrials.gov - NCT03360006
- (1 citation) ClinicalTrials.gov - NCT02158858
- (1 citation) ClinicalTrials.gov - NCT04279847
- (1 citation) ClinicalTrials.gov - NCT02419417
- (1 citation) ClinicalTrials.gov - NCT01987362
- (1 citation) ClinicalTrials.gov - NCT02516553
- (1 citation) ClinicalTrials.gov - NCT03266159
- (1 citation) ClinicalTrials.gov - NCT02303782
- (1 citation) ClinicalTrials.gov - NCT02607228
- (1 citation) ClinicalTrials.gov - NCT03702036
- (1 citation) ClinicalTrials.gov - NCT02986919
- (1 citation) ClinicalTrials.gov - NCT02431260
- (1 citation) ClinicalTrials.gov - NCT03205176
- (1 citation) ClinicalTrials.gov - NCT01713582
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Covalent-Fragment Screening of BRD4 Identifies a Ligandable Site Orthogonal to the Acetyl-Lysine Binding Sites.
ACS Chem Biol, 15(4):1036-1049, 23 Mar 2020
Cited by: 18 articles | PMID: 32149490 | PMCID: PMC7271629
Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer.
Nature, 578(7794):306-310, 22 Jan 2020
Cited by: 162 articles | PMID: 31969702
BET Bromodomain as a Target of Epigenetic Therapy.
Chem Pharm Bull (Tokyo), 64(6):540-547, 01 Jan 2016
Cited by: 27 articles | PMID: 27250788
Review
Targeting the epigenetic reader "BET" as a therapeutic strategy for cancer.
Bioorg Chem, 140:106833, 04 Sep 2023
Cited by: 1 article | PMID: 37683545
Review
Funding
Funders who supported this work.
Cancer Prevention and Research Institute of Texas (2)
Grant ID: RP190077
Grant ID: RP180349
China Postdoctoral Science Foundation (1)
Grant ID: 2020M673268
Department of Science and Technology of Sichuan Province (1)
Grant ID: 20YYJC3921
NCI NIH HHS (1)
Grant ID: R01 CA251698
National Institutes of Health (1)
Grant ID: 1RO1CA251698-01
National Natural Science Foundation of China (4)
Grant ID: 81874290
Grant ID: 81903502
Grant ID: 81673290
Grant ID: 81922064