Abstract
Objective
The aim of this is to provide an updated review of the literature and to report our institutional experience with this rare gynecologic malignancy.Methods
The medical records of patients with diagnosis of non-Hodgkin lymphoma of the female genital tract from 1980 to 2013 at the Yale-New Haven Hospital were reviewed retrospectively. Histological classification and staging were determined by the World Health Organization and Ann Arbor systems, respectively. Kaplan-Meier was used to calculate the survival.Results
There were 36 patients with diagnosis of non-Hodgkin lymphoma of the female genital tract and followed for a median of 61 months (0-361 months). The median age of diagnosis was 44 years (19-87 years), and 76% (n = 28) were classified as stage IV.Of these, 4 patients were asymptomatic on presentation, and 13 were identified incidentally during surgery/radiography (n = 9), on prenatal ultrasound (n = 1), and on Papanicolaou test (n = 3). The location of the disease included the ovary (n = 6), uterine corpus and cervix (n= 9), vagina (n = 1), a pelvic mass (n = 7), isolated pelvic/para-aortic lymph nodes (n = 3), and/or multiple sites (n = 9). There were 6 cases that were concomitant with other gynecologic malignancies.Diffuse large B-cell lymphoma (n= 18) was the most common histologic type. A total of 28 patients underwent surgery. Combination chemotherapy was used in 34 patients, with concomitant radiation therapy in 7 and stem cell transplantation in 3. A total of 5 patients had recurrent disease.The overall median survival from the diagnosis of lymphoma was 70 months (0.3-361 months) with a 91% 1-year survival, 86% 5-year survival, and a 79% 10-year survival.Conclusions
Our report is the largest published single-institution experience of this disease. It demonstrates a more favorable prognosis and proposes that with early diagnosis and appropriate therapy, radical gynecologic surgery can be avoided.Free full text

Institutional Review of Primary Non-Hodgkins Lymphoma of the Female Genital Tract a 33-year experience
Abstract
Objective
Provide an updated review of the literature and report our institutional experience with this rare gynecologic malignancy.
Methods
Medical records of patients diagnosed with non-Hodgkin's lymphoma (NHL) of the female genital tract from 1980-2013 at Yale-New Haven Hospital were reviewed retrospectively. Histological classification and staging were determined by the World Health Organization (WHO) and Ann Arbor systems, respectively. Kaplan-Meier was used to calculate survival.
Results
There were 36 patients that were diagnosed with NHL of the female genital tract and followed for a median of 61 months (0-361). Median age of diagnosis was 44 years (19-87), and 76% (28) were stage IV.
Of these, four patients were asymptomatic on presentation and thirteen were identified incidentally during surgery/radiographically (9), on prenatal ultrasound (1), and on PAP smear (3). Location of the disease included the ovary (6), uterine corpus and cervix (9), vagina (1), a pelvic mass (7), isolated pelvic/paraaortic lymph nodes (3), and/or multiple sites (9). There were six cases that were concomitant with other gynecologic malignancies.
Diffuse large B-cell lymphoma (DLBCL) (18) was the most common histologic type. A total of 28 patients underwent surgery. Combination chemotherapy was used with 34 patients, with concomitant radiation therapy in seven and stem cell transplantation in three. A total of five patients had recurrent disease.
Overall median survival from diagnosis of lymphoma was 70 months (0.3361) with a 91% 1-year survival, 86% 5-year survival and a 79% 10-year survival.
Conclusions
Our report is the largest published single-institution experience of this disease. It demonstrates a more favorable prognosis and proposes that with early diagnosis and appropriate therapy, radical gynecologic surgery can be avoided.
Introduction
Non-Hodgkin's lymphoma (NHL) arising in the gynecologic tract is exceedingly rare. While primary extranodal lymphoma accounts for 20% to 34% of all cases, depending on the criteria,[1] only 0.2% to 1.1% of these lymphomas present with primary involvement of the gynecologic tract [2-4]. Based on the year 2000 census count, 165 cases of primary pelvic, or genital tract, NHL are expected to be diagnosed annually in the United States [5, 6]. However, as many as 2500 female genital tract lymphomas are estimated to occur annually as part of disseminated disease (reported as 7%-30% in the literature), or as secondary genital tract NHL [4, 5, 7]. Lymphoma involving of the uterus and ovaries is often documented at the time of autopsy in women dying from advanced disease, demonstating late dissemination of disease to these organs [8, 9].
NHL encompasses B-cell and T-cell lymphomas, each of which is further classified into histologic sub-categories. The B-cell phenotype is the predominant lymphoma of the genital tract. B-cell lymphomas comprise 80% in the lymphomas of the Western hemisphere, respond better to traditional treatment and have a better prognosis than T-cell lymphomas [4, 5].
Female genital tract NHL includes lymphoma occurring in the ovary, uterus, cervix, vagina, and the vulva. Lymphoma involving the pelvic organs can be segregated into primary or secondary lymphoma. The distinction between primary and secondary genital tract lymphoma is of critical importance since it impacts treatment and prognosis. Primary disease involves localized NHL, which presumably has arisen in the gynecologic tract, whereas secondary disease is NHL involving the gynecologic organs as part of systemic disease [8]. Most lymphomas arise from areas of concentrated lymphoid tissue i.e., lymph nodes, spleen, gut-associated lymphoid tissue and bone marrow [5]. Lymphocytes are able to circulate throughout the body enabling the possibility of displaced primary tumors. Diagnostic imaging, pathology and bone marrow biopsy assist in the identification of primary lymphoma. However, once the disease is disseminated, the distinction between primary and secondary lymphoma is difficult.
Primary genital tract NHL, as an entity, has been widely debated in the literature [2, 3, 7, 9, 10]. While some reports refute such an entity, several reports describe ovarian lymphomas treated with surgical resection alone without subsequent chemotherapy. These patients had no evidence of systemic involvement and were disease-free with prolonged clinical follow-up [11].
The presence of normal lymphoid tissue in the ovaries and uterus is similarly controversial [12]. In 1993, Monterroso, et al identified small numbers of lymphocytes in the ovaries, surrounding blood vessels in the hilus and within or surrounding the corpora lutea, supporting findings of Woodruff, et al in 1963 [7]. These findings provide support for the hypothesis that though rare, malignant lymphoma can arise in the ovary.
In this report we describe the clinical and pathologic findings of patients presenting with primary genital tract lymphoma. We will also show how the initial correct diagnosis and appropriate lymphoma differentiation is essential in order to provide appropriate therapy and avoid radical gynecologic surgery.
Materials and Methods
A retrospective chart review was performed on patients diagnosed with genital tract NHL, or lymphoma of the gynecologic tract, between the years of 1980 and 2012 at Yale-New Haven Hospital (YNHH). The Medical records, including diagnostic imaging, pathology reports and obituaries were reviewed. Information regarding the patient's age, presenting symptoms, location of disease, staging, therapy and clinical follow-up were collected. The histological classification was based on the World Health Organization (WHO) and staging was based on the Ann Arbor system[13, 14]. The Ann Arbor staging system was developed in 1971 for Hodgkin's lymphoma and later adapted for staging of non-Hodgkin's lymphomas. The stage is determined by the number and location of the tumor sites (nodal and extranodal), and presence of systemic symptoms.
All pathology specimens were obtained from patients at Yale-New Haven hospital and reviewed by an on-site pathologist. This study was approved by the hospital institutional review board.
Results
We identified thirty-six patients diagnosed with a female genital tract non-Hodgkin's lymphoma (NHL) from 1980 to 2013. Patients ranged from 19 to 87 years of age, with a median of 44 years of age at the time of the diagnosis. Median follow-up was 61 months (range 0-361). The overall median survival from diagnosis of genital tract lymphoma was 70 months (range 0.3-361) with a 91% 1-year survival, 86% 5-year survival and 79% 10-year survival. A total of nineteen patients are alive at the time of this report, seven are lost to follow-up and ten are deceased. Of those nineteen, sixteen patients have no evidence of disease (NED) with a median survival of 97.5 months (range 4-361) and median follow-up time of 97.5 months (range 4-361). There are three that are alive with disease (AWD) and have a median survival of 1 month (range 0.5-75) and median follow-up time of 1 month (range 0.5-75). There are four patients that are dead of disease (DOD) with a median survival of 12 months (range 8-59) and median follow-up time of 12 months (range 8-59). In one patient, death was attributed to her more aggressive synchronous cancer (uterine serous cancer) at 19 months from the time of diagnosis.
There were seventeen patients that had lymphoma found at multiple sites, with seven of those patients having lymphoma limited to pelvic organs. Pelvic organ sites of tumor included: vagina, cervix, uterus, fallopian tubes, ovaries, pelvic lymph nodes or multiple sites. The median survival rate for these patients, with multiple sites involved at the time of diagnosis, was 59 months (range 0-361). The highest median survival rate of 184 months (range 109-361) was found amongst patients with both vaginal and cervical lymphoma (n=3). There were six patients that had primary ovarian and/or fallopian tube NHL. Their median survival rate was 48.5 months (range 10-166) with recurrences in five. In addition, there were six patients that had other site involvement at the time of genital tract lymphoma diagnosis, including the hip, liver, mandible, axilla, neck, bladder, breast, bowel, rectum, mesentery and omentum. For the latter patients, their median survival was 48 months (range 0-263) and median follow-up time was 40 months (range 0-263).
Diffuse large B-cell lymphoma (DLBCL) (18), was the most common NHL histology found amongst our patient population. Other histological NHLs found included: Burkitt (5), follicular (4), mixed DLBCL and follicular (2), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (1), mantle cell (1), marginal zone (1), plasma cell neoplasm (subtype: extraosseus plasmacytoma) (1), NHL not otherwise specified (NOS) (1), peripheral T-cell lymphoma NOS (1). Median survival for subtypes with n>2 were: DLBCL 54.5 months (range 0-361), Burkitt lymphoma 85 months (range 19-155), and follicular 67 months (range 4-166). Immunophenotypic data revealed all tumors expressing one or more pan-B-cell antigens (CD 20, CD19, CD 10, BCL 2, BCL 6). All cases tested negative for CD3, CD5, CD15, CD23, ER/PR and cyclin D1.
A total of twenty-eight patients underwent surgery. Not suspecting lymphoma, most patients underwent surgical staging for gynecologic malignancies (total hysterectomy, pelvic and paraaortic lymph node dissection, omentectomy) in addition to tumor debulking, bowel resection, vaginectomy. Others underwent a total abdominal hysterectomy with or without bilateral salpingoopherectomy. A few had removal of a pelvic mass with unilateral salpingoopherectomy plus/minus wedge resection of contralateral ovary. One patient underwent a cervical cone for the finding of lymphoma on PAP, and subsequently has not required further treatment.
The postoperative treatment for genital tract lymphomas included regimens of chemotherapy with or without radiation therapy, radiation therapy alone and stem cell transplantation. Chemotherapy regimens primarily included CHOP (cyclophosphamide, adriamycin, vincristine, prednisone), rituximab (Rituxan), hyper CVAD (fractionated cyclophosphamide, vincristine, adriamycin, dexamethasone, methotrexate, leucovorin, cytorabine) and single agent bleomycin.
Of the treatment modalities, median survival was found to be highest with the five patients undergoing surgery with concomitant radiation and chemotherapy; these patients had DLBCL (3), follicular (1) and peripheral T-cell NOS (1). Their median survival was 75 months (range 12-361). Of the two patients who underwent surgery, chemotherapy, and stem cell transplant, they had a median survival of 160 months (range 154-166) with a median follow-up of 160 months (range 154-166). Both patients are alive and free of disease.
There were five patients who experienced a recurrence of NHL in the gynecologic organs, all of which involved the ovary. The sites of recurrence included bone, lung, brain, cervical lymph nodes, skin and the manubrium. The median time to recurrence was 11.5 months (range 7-112). The histology included DLBCL and follicular. Of these, four were Stage IV with one Stage I transformed to Stage IV. Surgical management included TAH/BSO (+/- debulking) for four patients with biopsy for one patient. Chemotherapy was rituximab/cyclophosphamide/doxorubicin hydrochloride (Hydroxydaunomycin)/vincristine sulfate (Oncovin)/prednisone (R-CHOP) (5), ifosfamide/carboplatin/etoposide (ICE) (2) and carmustine (BCNU)/etoposide/cytarabine (Ara-C)/melphelan (BEAM) (1) in addition to palliative radiation therapy for one.
Discussion
NHL of the gynecologic organs is a rare occurrence. The clinical work-up leading to the appropriate diagnosis is also challenging. Patients tend to be asymptomatic or have non-specific abdominal symptoms including bloating, abdominal pressure, and discomfort. Constitutional symptoms generally seen with NHL patients, including fever, night sweats and weight loss occurred, but were only reported in 17% of patients with ovarian lymphomas in another series.8 Our findings mirror these reported values with 11% of patients being asymptomatic, 24% of patients presenting with vague abdominal complaints and only 14% of patients presenting with constitutional symptoms.
Given the vague symptoms that patients with NHL of the gynecologic organs present with or the lack thereof, radiologic or imaging studies has been also evaluated as a diagnostic method. Although a prospective diagnosis of a gynecologic lymphoma is often difficult to make, there are a few imaging features that are suggestive of lymphoma. Although ultrasound or CT are frequently non-specific for a solid gynecologic mass, MRI has superior contrast resolution that enables visualization of underlying zonal architecture (1). Lymphoma of the cervix (2, 3) corpus (4-7) or vagina (8) often enlarges and preserves the zonal architecture of the organ (Figures 2 and and3).3). On T1 weighted scans, lymphoma is hypointense whereas on T2 weighted scans, it is usually of mild to moderate signal intensity. Additionally, if IV contrast is administered, the mass is typically hypovascular. Primary ovarian lymphoma does not preserve the internal structure of the ovary (Figure 4). Ovarian lymphoma often presents with a large ovarian mass, with an average size of 10 cm (9). The presence of large, bilateral, homogeneous ovarian masses is a clue to the diagnosis of ovarian lymphoma (10). Advanced disease is present in more than half of the cases, with spread to the peritoneal cavity, thus making the distinction from the far more common epithelial ovarian cancer very difficult (11, 12). Imaging modalities used on our patient population failed to identify features specific for genital tract lymphoma.

CT scan shows enlargement of the corpus with nodularity (arrow) secondary to a primary lymphoma of the corpus uterus.
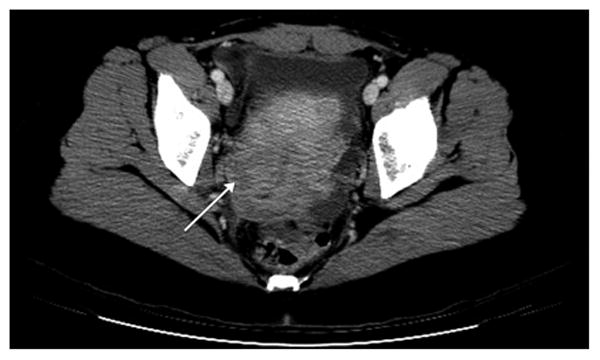
CT scan shows a low density region in cervical stroma (arrow) with preservation of the canal consistent with a primary cervical lymphoma.

T2 weighted axial MRI scan shows heterogeneous mass replacing left ovary (long arrow) that was a primary ovarian lymphoma. Adjacent normal right ovary is seen (short arrow).
The median age at which malignant ovarian lymphomas initially present is reported to be 41.8 years for patients with primary disease versus 33 years for patients with secondary disease [8]. Patients in our cohort included all NHL of gynecologic organs and tended to present slightly older, with a median age of 44 (ranging from 19 to 87). The older median age at diagnosis is likely secondary to a proportionally larger number of follicular and follicular subtypes, most common in older patients, while DLBCL is most common in the 35 to 45 year age group [5]. In addition, the primary site of lymphoma may have also played a role in the somewhat older median age of diagnosis, as uterine, vaginal and vulvar lymphomas are inclined to occur in older women [15, 16]. Studies suggest that primary uterine lymphomas occur in postmenopausal patients but can occur occasionally in women in their 20s or 30s [17, 18]. Cervical lymphomas also tend to present in premenopausal women [19]. The majority of our patients had multiple sites affected.
Subclassification of genital tract NHL is performed according to the WHO Formulation, which requires incisional or excisional biopsies for an accurate diagnosis. Fine-needle aspiration biopsies do not provide sufficient information regarding cell-to-cell architectural organization [13]. In regards to NHL subtypes, our findings support previous evidence of DLBCL NHL lymphoma being the most common type of NHL of the gynecologic organs [8, 10, 20]. We also identified Burkitt and follicular lymphoma to be more common in our cohort of patients (25%). Follicular lymphoma alone was present in four patients and mixed follicular & DLBCL presented in two. This appears to be a contrast to prior studies, which had reported follicular lymphomas to be very rare in the gynecologic tract [2, 7].
In our series, as with other studies, patients were surgically staged. The reason for this is that pathologists often miss the diagnosis at the time of frozen section. This is a difficult diagnosis to make, particularly for specialty pathologists who do not deal with lymphomas. This is primarily because the patient's presentation or diagnostic imaging suggested a possible genital tract malignancy. In addition, it may also be difficult to make the diagnosis of NHL with frozen sections when synchronous cancers are present. Until final histological classification was complete, patients would have to be surgically managed as if they had any other genital tract malignancy such as epithelial ovarian cancer. For example, a frozen section sent on one of our patients was read initially as an angiomyxoma. It wasn't until final histology was present, that it was revealed that she had genital tract NHL.
The impact of complete surgical staging is not clear, especially in the setting of synchronous tumors. The clinical manifestation of reproductive tract NHL is very similar to other gynecologic malignancies, including epithelial ovarian cancer and endometrial adenocarcinoma. It is difficult to differentiate lymphoma versus a female genital tract primary disease prior to surgery and thus tumor debulking is often performed. Even pathologic evaluation of these tumors can be challenging. Vang et al. reporting on three patients with endometrial carcinoma and NHL of the female genital tract and stated that the definitive diagnosis between poorly differentiated carcinoma and NHL can be difficult (many lymphomas can be suspected at the time of frozen section diagnosis if performed, which is important as without any suspicion, no fresh tissue will be available for flow cytometry and molecular studies) and requires the use of specific immunohistochemical studies i.e., cytokeratins to distinguish between them [11, 17].
The definitive diagnosis is often not definitive of NHL until postoperatively, as it requires flow cytometry, cytogenetics, immunohistochemistry, and molecular diagnostic evaluation to establish the diagnosis. Flow cytometry detects monoclonality in B cells. Immunohistochemisty helps determine if the tumor is a B-cell lymphoma (CD20+), T-cell lymphoma (CD3+) or a carcinoma (keratin+). Further screening panels are available for sub-classification of the B-cell lymphomas.
Based on our findings, our recommendations would be that approaches can be taken based on findings of a frozen section suggesting NHL: 1) if there is a high suspicion of a synchronous gynecologic malignancy, one can continue with staging and debulking 2) if there is a low suspicion of a gynecologic malignancy, one can wait for the final diagnosis to decide further therapy and surgical management.
The prognosis for genital tract NHL lymphoma arising in the gynecologic tract is reported to be variable. Survival rates depend on patient age, stage, whether the disease is localized vs disseminated, bilaterality of lesions, histology, the presence of systemic symptoms, acute onset of disease and elevated lactate dehydrogenase levels. Patients diagnosed with NHL subtypes other than B-cell, tend to have a worse prognosis [5, 8, 21]. Overall median survival in our patients was 70 months (range 0-361) with a 91% 1-year survival and 86% 5-year survival, which is better than observed in earlier reports. 64% of our cohort is disease free up to 361 months from diagnosis.
After diagnosis, survival rates also depend largely on treatment modalities. This series is consistent with a prior study demonstrating that surgery followed by adjuvant chemotherapy provides the best survival outcome in patients with ovarian lymphoma [21]. Currently, lymphoma confined/localized to the ovaries that is treated with surgery and appropriate chemotherapy has a similar prognosis to that of nodal NHL with 75% of patients achieving long term survival [22]. In 1983, Osborne et al. described a cohort of 42 patients with a 5-year survival for FIGO stages IA, IB and II, combined to be 62% after surgical management of ovarian lymphoma alone [9]. However, other literature reports very poor prognosis for genital tract lymphoma prior to the introduction of modern chemotherapy [7]. Myeloablative and nonmyeloablative stem cell transplantation has shown to further improve survival in NHL, in particular, mantle cell lymphoma [5].
Dimopoulos et al. reported a 15-year survival rate of 57%, which was higher than prior studies of 57%, and stressed the importance of choosing up-to-date combination chemotherapy regimens, some of which were not available for earlier studies, that were appropriate for NHL [22]. Fox et al. have reported a mean 60 months survival time for patients with Ann Arbor Stage IIE who underwent surgical debulking with subsequent chemotherapy [21].
Limitations
The medical treatment of lymphoma including chemotherapy regimens, has greatly changed between the time span of the study from 1980-2013. Although most patients did undergo similar chemotherapy regimens, it was not uniform amongst the population. This could in turn affect the survival and outcomes for a given time period.
In addition, lymphoma is generally a medical disease that is treated medically not surgically. Few patients can be diagnosed by simple biopsies. Eleven patients had no surgery to minimal surgery (biopsy, exam under anesthesia with proctoscopy and cystoscopy, cervical cone, pelvic mass removal, unilateral salpingo-oophorectomy). Their median survival was 19 months (range 1-184). A total of 23 patients underwent total abdominal hysterectomy with or without surgical staging and debulking and their median survival and follow-up was 75 months (range 0.33-361). Given the presentation of our patients and general inability to determine the definitive diagnosis from other gynecologic malignancies prior to surgery with surgical staging, it is difficult to ascertain what affect surgery has on the prognosis of these patients.
Genital tract NHL is a rare disease, and similar to other rare diseases, prospective chart review is difficult to perform. Therefore, our study design was retrospective chart review and with it comes the limitations of data accessibility.
Footnotes
The authors of this study have contributed in the diagnosis, treatment, management and review of these patients.
Financial disclosures: There are no financial disclosures
Conflict of Interest Statement: There were no conflicts of interest during this study or during the writing of this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/igc.0000000000000201
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8139417
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/igc.0000000000000201
Article citations
Vaginal involvement as a rare extranodal manifestation in advanced-stage follicular lymphoma: a case report and literature review.
Ann Hematol, 103(11):4773-4776, 23 Aug 2024
Cited by: 0 articles | PMID: 39177796
Review
Lymphomas of the Vulva: A Review of the MITO Rare Cancer Group.
Cancers (Basel), 16(11):2102, 31 May 2024
Cited by: 0 articles | PMID: 38893221 | PMCID: PMC11171216
Review Free full text in Europe PMC
Primary lymphoma of the female genital tract masquerading as gynecological malignancy.
BMC Womens Health, 24(1):247, 18 Apr 2024
Cited by: 1 article | PMID: 38637800 | PMCID: PMC11025207
Dedifferentiated Endometrial Carcinoma: A Rare Aggressive Neoplasm-Clinical, Morphological and Immunohistochemical Features.
Cancers (Basel), 15(21):5155, 26 Oct 2023
Cited by: 1 article | PMID: 37958329 | PMCID: PMC10647464
Review Free full text in Europe PMC
Primary diffuse large B-cell tumor of the uterus: a case report.
Front Med (Lausanne), 10:1234861, 13 Jul 2023
Cited by: 1 article | PMID: 37521344 | PMCID: PMC10373583
Go to all (20) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[NON-HODGKIN'S LYMPHOMA OF THE FEMALE GENITAL SYSTEM--A LITERATURE REVIEW].
Akush Ginekol (Sofiia), 54(3):17-23, 01 Jan 2015
Cited by: 2 articles | PMID: 26137775
Review
Stage I-IIE primary non-Hodgkin's lymphoma of the testis: results of a prospective trial by the GOELAMS Study Group.
Clin Lymphoma, 3(3):167-172, 01 Dec 2002
Cited by: 17 articles | PMID: 12521394
Primary lymphoma of the female genital tract masquerading as gynecological malignancy.
BMC Womens Health, 24(1):247, 18 Apr 2024
Cited by: 1 article | PMID: 38637800 | PMCID: PMC11025207
Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma.
J Clin Oncol, 21(11):2115-2122, 01 Jun 2003
Cited by: 80 articles | PMID: 12775737
Review






