Abstract
Free full text

Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus
Abstract
Increased risk of premature cardiovascular disease (CVD) is well recognized in systemic lupus erythematosus (SLE). Aberrant type I-Interferon (IFN)-neutrophil interactions contribute to this enhanced CVD risk. In lupus animal models, the Janus kinase (JAK) inhibitor tofacitinib improves clinical features, immune dysregulation and vascular dysfunction. We conducted a randomized, double-blind, placebo-controlled clinical trial of tofacitinib in SLE subjects (ClinicalTrials.gov NCT02535689). In this study, 30 subjects are randomized to tofacitinib (5 mg twice daily) or placebo in 2:1 block. The primary outcome of this study is safety and tolerability of tofacitinib. The secondary outcomes include clinical response and mechanistic studies. The tofacitinib is found to be safe in SLE meeting study’s primary endpoint. We also show that tofacitinib improves cardiometabolic and immunologic parameters associated with the premature atherosclerosis in SLE. Tofacitinib improves high-density lipoprotein cholesterol levels (p
mg twice daily) or placebo in 2:1 block. The primary outcome of this study is safety and tolerability of tofacitinib. The secondary outcomes include clinical response and mechanistic studies. The tofacitinib is found to be safe in SLE meeting study’s primary endpoint. We also show that tofacitinib improves cardiometabolic and immunologic parameters associated with the premature atherosclerosis in SLE. Tofacitinib improves high-density lipoprotein cholesterol levels (p =
= 0.0006, CI 95%: 4.12, 13.32) and particle number (p
0.0006, CI 95%: 4.12, 13.32) and particle number (p =
= 0.0008, CI 95%: 1.58, 5.33); lecithin: cholesterol acyltransferase concentration (p
0.0008, CI 95%: 1.58, 5.33); lecithin: cholesterol acyltransferase concentration (p =
= 0.024, CI 95%: 1.1, −26.5), cholesterol efflux capacity (p
0.024, CI 95%: 1.1, −26.5), cholesterol efflux capacity (p =
= 0.08, CI 95%: −0.01, 0.24), improvements in arterial stiffness and endothelium-dependent vasorelaxation and decrease in type I IFN gene signature, low-density granulocytes and circulating NETs. Some of these improvements are more robust in subjects with STAT4 risk allele.
0.08, CI 95%: −0.01, 0.24), improvements in arterial stiffness and endothelium-dependent vasorelaxation and decrease in type I IFN gene signature, low-density granulocytes and circulating NETs. Some of these improvements are more robust in subjects with STAT4 risk allele.
Introduction
The pathogenesis of systemic lupus erythematosus (SLE) involves dysregulation of multiple innate and adaptive immune pathways1. The role of the innate immune system, specifically type I interferons, low-density neutrophils, and neutrophil extracellular traps (NETs), is now recognized as a potential fundamental player in SLE pathogenesis and its associated vascular damage2. The risk of cardiovascular disease (CVD) and premature atherosclerosis is significantly increased in SLE, particularly in young women3,4. Efforts to modulate SLE vasculopathy, atherosclerosis development, and progression have been unsuccessful5.
The immune dysregulation characteristic of SLE has been proposed to play prominent roles in driving premature atherosclerosis6. Specifically, the type I-Interferon (IFN) pathway and a distinct subset of neutrophils called low-density granulocytes (LDGs) that display enhanced ability to form neutrophil extracellular traps (NETs) have been directly implicated in vascular damage, atherosclerotic plaque formation, and CVD progression7,8.
The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway is a fundamental signaling cascade used by cells to respond to a wide variety of cytokines and growth factors9. Many inflammatory cytokines implicated in SLE pathogenesis, including type I and II interferons (IFNs), signal through a JAK-STAT pathway10,11. JAK inhibitors (jakinibs) have displayed efficacy in various murine models of lupus12. Clinical trials using the jakinibs showed clinical efficacy in arthritis in patients with mild-to-moderate SLE13,14. Furthermore, tofacitinib modulated dysregulated neutrophil and type I IFN responses, endothelial dysfunction, and lipoprotein profiles in murine lupus12.
We hypothesized that mitigating the aberrant activation of innate immune pathways with a JAK inhibitor could lead to the improvement in cardiometabolic parameters and immune dysregulation associated with premature vascular damage in SLE. To this end, we conducted a phase Ib/IIa, double-blind, placebo-controlled clinical trial of tofacitinib in SLE subjects with mild-to-moderate disease. As the presence of the STAT4 risk allele (rs7574865) has been associated with more severe clinical phenotype and significantly increased risk of vascular disease in SLE, and since type I IFNs activate STAT4, we stratified subjects based on the presence (+) or absence (−) of STAT4 risk allele which has been suggested to increase the production of and sensitivity to type I IFNs in peripheral blood mononuclear cells of SLE patients, to investigate the effect(s) of these haplotypes on the clinical and immunologic response to tofacitinib15–17.
Here, we show that tofacitinib is safe and well-tolerated in subjects with mild-to-moderate SLE. There are no unexpected adverse events or worsening of SLE disease activity, with no severe adverse events, opportunistic infections, or thromboembolic events with the use of tofacitinib. The tofacitinib treatment improves high-density lipoprotein cholesterol levels and particle numbers, lecithin: cholesterol acyltransferase concentration, and cholesterol efflux capacity. It also improves arterial stiffness and endothelium-dependent vasorelaxation. The tofacitinib use significantly decreased the type I IFN gene signature, levels of low-density granulocytes, and circulating NETs. Some of these changes were more robust in SLE subjects with STAT4 risk allele. Long-term studies are needed to determine the efficacy of tofacitinib in CVD prevention in SLE.
Results
Safety and disease activity
Thirty SLE subjects were enrolled, all completed the study and were included in analyses. Baseline subject demographic and clinical characteristics stratified by their STAT4 risk allele status are shown in Table 1 and Supplementary Fig. 1. There were significantly more African American subjects in the STAT4 risk allele-negative subgroup in both tofacitinib and placebo groups (P =
= 0.002). The subjects with STAT4 risk allele were younger and mostly Hispanic, but these differences were not statistically significant. There were 71 adverse events: 43 in the tofacitinib group and 28 in the placebo group, with no serious AEs in the tofacitinib group; the differences in AE were not statistically significant (Table 2). Most of the AEs observed in the tofacitinib group were mild (16/43) and moderate (5/43) upper respiratory infections that either self-resolved or after treatment with oral antibiotics. No herpes zoster reactivation, BK viremia, or venous thromboembolic events were recorded. There were no clinical or statistically significant changes observed in the tofacitinib group compared to baseline measurements and to the placebo group in other laboratory safety parameters (Table 3). As compared to placebo in the tofacitinib group, the hemoglobin difference in change score was −0.33 (95% CI −0.33, −0.88) at day 56, −0.20 (95% CI, −0.81, 0.40) at day 84; white blood cell count difference in change score was −0.63 (95% CI −0.63, −1.46) at day 56, −0.52 (95% CI, −1.35, 0.30) at day 84; absolute neutrophil count difference in change score was −0.58 (95% CI −0.58, −1.35) at day 56, −0.21 (95% CI, −0.85, 0.43) at day 84; platelet count difference in change score was −15.36 (95% CI −15.36, −35.79) at day 56, 5.3 (95% CI, −12.33, 22.93) at day 84; serum AST difference in change score was 0.71 (95% CI, 0.71, −5.14) at day 56, −13.8 (95% CI, −34.91, 7.31) at day 84; and serum ALT difference in change score was 1.58 (95% CI, 1.58, −4.55) at day 56, −2.15 (95% CI, −8.86, 4.56) at day 84 (Table 3). None of these differences were statistically significant. None of the patients in either group met the disease flare criteria during the trial, and there were no new BILAG 2004 A or B scores. The baseline mean SLEDAI 2
0.002). The subjects with STAT4 risk allele were younger and mostly Hispanic, but these differences were not statistically significant. There were 71 adverse events: 43 in the tofacitinib group and 28 in the placebo group, with no serious AEs in the tofacitinib group; the differences in AE were not statistically significant (Table 2). Most of the AEs observed in the tofacitinib group were mild (16/43) and moderate (5/43) upper respiratory infections that either self-resolved or after treatment with oral antibiotics. No herpes zoster reactivation, BK viremia, or venous thromboembolic events were recorded. There were no clinical or statistically significant changes observed in the tofacitinib group compared to baseline measurements and to the placebo group in other laboratory safety parameters (Table 3). As compared to placebo in the tofacitinib group, the hemoglobin difference in change score was −0.33 (95% CI −0.33, −0.88) at day 56, −0.20 (95% CI, −0.81, 0.40) at day 84; white blood cell count difference in change score was −0.63 (95% CI −0.63, −1.46) at day 56, −0.52 (95% CI, −1.35, 0.30) at day 84; absolute neutrophil count difference in change score was −0.58 (95% CI −0.58, −1.35) at day 56, −0.21 (95% CI, −0.85, 0.43) at day 84; platelet count difference in change score was −15.36 (95% CI −15.36, −35.79) at day 56, 5.3 (95% CI, −12.33, 22.93) at day 84; serum AST difference in change score was 0.71 (95% CI, 0.71, −5.14) at day 56, −13.8 (95% CI, −34.91, 7.31) at day 84; and serum ALT difference in change score was 1.58 (95% CI, 1.58, −4.55) at day 56, −2.15 (95% CI, −8.86, 4.56) at day 84 (Table 3). None of these differences were statistically significant. None of the patients in either group met the disease flare criteria during the trial, and there were no new BILAG 2004 A or B scores. The baseline mean SLEDAI 2 K score in the tofacitinib group was 5.1
K score in the tofacitinib group was 5.1 ±
± 2.2 mean
2.2 mean ±
± standard deviation, compared to 5.5
standard deviation, compared to 5.5 ±
± 3.7 in the placebo group; the difference in change scores (tofacitinib vs. placebo) was 0.04 (95% CI: −1.04, 1.11) at day 56 and −0.72 (95% CI: −1.98, 0.53) at day 84. The baseline mean BILAG 2004 score in the tofacitinib group was 7.6
3.7 in the placebo group; the difference in change scores (tofacitinib vs. placebo) was 0.04 (95% CI: −1.04, 1.11) at day 56 and −0.72 (95% CI: −1.98, 0.53) at day 84. The baseline mean BILAG 2004 score in the tofacitinib group was 7.6 ±
± 4.6 mean
4.6 mean ±
± standard deviation, compared to 9.3
standard deviation, compared to 9.3 ±
± 4.3 in the placebo group; the difference in change scores (tofacitinib vs. placebo) was 1.56 (95% CI: −1.86, 4.98) at day 56 and −2.04 (95% CI: −4.96, 0.89) at day 84. The baseline mean PGA score in the tofacitinib group was 0.8
4.3 in the placebo group; the difference in change scores (tofacitinib vs. placebo) was 1.56 (95% CI: −1.86, 4.98) at day 56 and −2.04 (95% CI: −4.96, 0.89) at day 84. The baseline mean PGA score in the tofacitinib group was 0.8 ±
± 0.8 (mean
0.8 (mean ±
± standard deviation), compared to 1.2
standard deviation), compared to 1.2 ±
± 0.9 in the placebo group; the difference in change in scores (tofacitinib vs. placebo) was 0.18 (95% CI: −0.28, 0.64) at day 56 and 0.23 (95% CI: −0.30, 0.76) at day 84. None of these differences were statistically significant. The SLE serological disease activity (complement C3 and C4 levels) and the patient-reported outcomes (SF-36) were similar at baseline visit and did not have a significant difference in change scores at day 56 or day 84 (Table 4). There was a numerical increase in anti-ds-DNA antibody titers in both groups during the study, but these increases were not statistically significant. This study was not powered to assess clinical efficacy.
0.9 in the placebo group; the difference in change in scores (tofacitinib vs. placebo) was 0.18 (95% CI: −0.28, 0.64) at day 56 and 0.23 (95% CI: −0.30, 0.76) at day 84. None of these differences were statistically significant. The SLE serological disease activity (complement C3 and C4 levels) and the patient-reported outcomes (SF-36) were similar at baseline visit and did not have a significant difference in change scores at day 56 or day 84 (Table 4). There was a numerical increase in anti-ds-DNA antibody titers in both groups during the study, but these increases were not statistically significant. This study was not powered to assess clinical efficacy.
Table 1
Baseline demographic, clinical, and vascular characteristics of SLE subjects enrolled in the trial based on their STAT4 risk allele status.
| Tofacitinib | Placebo | Total N = = 30) 30) | P value at baseline | |||
|---|---|---|---|---|---|---|
STAT4 risk allele present (n = = 10) 10) | STAT4 risk allele absent (n = = 10) 10) | STAT4 risk allele present (n = = 5) 5) | STAT4 risk allele absent (n = = 5) 5) | |||
Age (years) mean ± ± SD SD | 44 ± ± 16.9 16.9 | 53.8 ± ± 13.9 13.9 | 35.6 ± ± 15.9 15.9 | 44.4 ± ± 7.2 7.2 | 45.9 ± ± 15.2 15.2 | 0.16 |
| Race/ethnicity, N (%) | ||||||
| African American | 1 (10%) | 8 (80%) | 0 (0%) | 4 (80%) | 13 (43.3%) | 0.002** |
| Asian | 0 (0%) | 1 (10%) | 0 (0%) | 1 (20%) | 2 (6.7%) | |
| Caucasian | 2 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (6.7%) | |
| Hispanic | 7 (70%) | 1 (10%) | 5 (100%) | 0 (0%) | 13 (43.3%) | |
| Gender, N (%) | 0.70 | |||||
| Female | 8 (80%) | 9 (90%) | 5 (100%) | 4 (80%) | 26 (86.7%) | |
| Male | 2 (20%) | 1 (10%) | 0 (0%) | 1 (20%) | 4 (13.3%) | |
Disease duration, mean ± ± SD SD | 10.8 ± ± 11.61 11.61 | 15.6 ± ± 11.42 11.42 | 9.8 ± ± 12.44 12.44 | 16.2 ± ± 9.68 9.68 | 13.1 ± ± 11.1 11.1 | 0.65 |
SLEDAI 2 K, mean K, mean ± ± SD SD | 5.2 ± ± 2.7 2.7 | 4.9 ± ± 1.79 1.79 | 5.6 ± ± 4.34 4.34 | 5.4 ± ± 3.44 3.44 | 5.2 ± ± 2.7 2.7 | 0.97 |
| Prednisone, N (%) | 6 (60%) | 3 (30%) | 2 (40%) | 3 (60%) | 14 (46.7%) | 0.52 |
Prednisone dose, mean ± ± SD SD | 5.0 ± ± 2.7 2.7 | 5.0 ± ± 4.3 4.3 | 7.5 ± ± 3.5 3.5 | 6.67 ± ± 2.9 2.9 | 5.7 ± ± 3.0 3.0 | 0.73 |
| HCQ, N (%) | 9 (90%) | 10 (100%) | 5 (100%) | 5 (100%) | 29 (96.67%) | 0.56 |
Rt CAVI, mean ± ± SD SD | 7.37 ± ± 1.08 1.08 | 8.24 ± ± 1.49 1.49 | 6.9 ± ± 1.62 1.62 | 7.1 ± ± 1.02 1.02 | 7.54 ± ± 1.36 1.36 | 0.22 |
| PWV, m/s | 7.46 ± ± 1.61 1.61 | 7.4 ± ± 2.82 2.82 | 7.22 ± ± 2.15 2.15 | 6.98 ± ± 0.7 0.7 | 7.32 ± ± 2.00 2.00 | 0.98 |
| Ln RHI | 0.43 ± ± 0.35 0.35 | 0.68 ± ± 0.25 0.25 | 0.83 ± ± 0.13 0.13 | 0.88 ± ± 0.28 0.28 | 0.65 ± ± 0.32 0.32 | 0.026* |
| Anti-dsDNA antibody, N (%) | 1 (10%) | 3 (30%) | 2 (40%) | 1 (20%) | 7 (23.33%) | 0.56 |
| ACA IgG, N (%) | 4 (40%) | 1 (10%) | 0 (0%) | 1 (20%) | 6 (20%) | 0.22 |
| ACA IgM, N (%) | 6 (60%) | 1 (10%) | 2 (40%) | 0 (0%) | 9 (30%) | 0.04* |
| Lupus anticoagulant, N (%) | 5 (50%) | 4 (40%) | 2 (40%) | 1 (20%) | 12 (40%) | 0.74 |
| History of lupus nephritis, N (%) | 2 (20%) | 3 (30%) | 1 (20%) | 0 (0%) | 6 (20%) | 0.60 |
SLEDAI 2 K
K
 Systemic Lupus Erythematosus Disease Activity Index 2000, HCQ
Systemic Lupus Erythematosus Disease Activity Index 2000, HCQ
 hydroxychloroquine, Rt. CAVI
hydroxychloroquine, Rt. CAVI right cardioankle vascular index, PWV, pulse wave velocity, Ln RHI
right cardioankle vascular index, PWV, pulse wave velocity, Ln RHI
 Log Reactive Hyperemia Index, Anti-ds-DNA
Log Reactive Hyperemia Index, Anti-ds-DNA
 anti-double-stranded DNA antibody, ACA IgG anticardiolipin antibody IgG, ACA IgM anticardiolipin antibody IgM.
anti-double-stranded DNA antibody, ACA IgG anticardiolipin antibody IgG, ACA IgM anticardiolipin antibody IgM.
*p <
< 0.05, **p<0.01.
0.05, **p<0.01.
The baseline variables were compared using the mean values using analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables.
Two-tailed tests were used where appropriate.
No adjustments were made for multiple comparisons.
Table 2
Adverse event frequency and severity.
| Body system preferred term severity | Tofacitinib adverse events = = 43 43 | Placebo adverse events = = 28 28 | Total adverse events = = 71 71 | P value |
|---|---|---|---|---|
| Any event | 0.09 | |||
| Mild | 32 (74.4%) | 23 (82.1%) | 55 (77.5%) | |
| Moderate | 11 (25.6%) | 3 (10.7%) | 14 (19.7%) | |
| Severe | 0 (0%) | 2 (7.1%) | 2 (2.8%) | |
| Cardiac disorders | NA | |||
| Mild | 1 (2.3%) | 0 (0.0%) | 1 (1.4%) | |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Eye disorders | 1.00 | |||
| Mild | 1 (2.3%) | 3 (10.7%) | 4 (5.6%) | |
| Moderate | 0 (0.0%) | 1 (3.6%) | 1 (1.4%) | |
| Severe | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| Gastrointestinal disorders | 1.00 | |||
| Mild | 4 (9.3%) | 4 (14.3%) | 8 (11.3%) | |
| Moderate | 2 (4.6%) | 1 (3.6%) | 3 (4.2%) | |
| Severe | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| General disorders | NA | |||
| Mild | 2 (4.6%) | 1 (3.6%) | 3 (4.2%) | |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Infections | 0.55 | |||
| Mild | 16 (37.2%) | 4 (14.3%) | 20 (28.2%) | |
| Moderate | 5 (11.6%) | 0 (0.0%) | 5 (7.0%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Investigations (clinical laboratory abnormalities) | 1.00 | |||
| Mild | 2 (4.7%) | 2 (7.1%) | 4 (5.6%) | |
| Moderate | 1 (2.3%) | 0 (0%) | 1 (1.4%) | |
| Severe | 0 (0%) | 1 (3.6%) | 1 (1.4%) | |
| Metabolism disorders | NA | |||
| Mild | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Moderate | 1 (2.3%) | 0 (0.0%) | 1 (1.4%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Musculoskeletal disorders | NA | |||
| Mild | 1 (2.3%) | 2 (7.1%) | 3 (4.2%) | |
| Moderate | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Nervous system disorders | 0.42 | |||
| Mild | 4 (9.3%) | 7 (25.0%) | 11 (15.5%) | |
| Moderate | 1 (2.3%) | 0 (0.0%) | 1 (1.4%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Renal and urinary disorders | NA | |||
| Mild | 1 (2.3%) | 0 (0.0%) | 1 (1.4%) | |
| Moderate | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Respiratory disorders | NA | |||
| Mild | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Moderate | 0 (0.0%) | 1 (3.6%) | 1 (1.4%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Skin disorders | NA | |||
| Mild | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Moderate | 1 (2.3%) | 0 (0.0%) | 1 (1.4%) | |
| Severe | 0 (0%) | 0 (0.0%) | 0 (0.0%) | |
| Vascular disorders | NA | |||
| Mild | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Severe | 0 (0.0%) | 1 (3.6%) | 1 (1.4%) |
Fisher’s exact test was used to calculate the P values and compare the incidents of the adverse events between tofacitinib and placebo. Two-tailed tests were used where appropriate.
No adjustments were made for multiple comparisons.
Table 3
Longitudinal follow-up of laboratory values during the trial.
Tofacitinib (n = = 20) 20) | Placebo (n = = 10) 10) | P value baseline | P value D56, difference in change scores (95% CI) | P value D84, difference in change scores (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome variable | Day 1 | Day 56 | Day 84 | Day 1 | Day 56 | Day 84 | |||
mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | ||||
| WBC, K/mcL | 5.3 ± ± 1.6 1.6 | 5.2 ± ± 1.7 1.7 | 4.8 ± ± 1.5 1.5 | 4.8 ± ± 1.1 1.1 | 5.3 ± ± 1.6 1.6 | 4.9 ± ± 1.1 1.1 | 0.32 | 0.13, −0.63 (−0.63, −1.46) | 0.19, −0.52 (−1.35, 0.3) |
| Absolute neutrophil counts, K/uL | 3 ± ± 1.3 1.3 | 2.9 ± ± 1.3 1.3 | 2.7 ± ± 1.1 1.1 | 2.7 ± ± 0.9 0.9 | 3.2 ± ± 1.3 1.3 | 2.8 ± ± 0.7 0.7 | 0.48 | 0.14, −0.58 (−0.58, −1.35) | 0.5, −0.21 (−0.85, 0.43) |
| Hemoglobin, g/dL | 12.8 ± ± 1.2 1.2 | 12.6 ± ± 1.2 1.2 | 12.4 ± ± 1.4 1.4 | 12.9 ± ± 1.5 1.5 | 13 ± ± 1.9 1.9 | 12.8 ± ± 2 2 | 0.8 | 0.24, −0.33 (−0.33, −0.88) | 0.5, −0.20 (−0.81, 0.40) |
| Platelet, K/mcL | 224 ± ± 72.5 72.5 | 221.6 ± ± 74.9 74.9 | 231.5 ± ± 68.4 68.4 | 213.4 ± ± 57.7 57.7 | 225.9 ± ± 67.1 67.1 | 213.7 ± ± 54.2 54.2 | 0.69 | 0.13, −15.36 (−15.36, −35.79) | 0.54, 5.3 (−12.33, 22.93) |
| ESR, mm/h | 22 ± ± 19.2 19.2 | 19.4 ± ± 17.2 17.2 | 22.6 ± ± 17.6 17.6 | 25.9 ± ± 24.3 24.3 | 25.7 ± ± 26.2 26.2 | 25.7 ± ± 25.6 25.6 | 0.63 | 0.2, −3.74 (−3.74, −9.63) | 0.77, 0.64 (−3.82, 5.10) |
| CRP, mg/L* | 4.6 ± ± 7.7 7.7 | 2.3 ± ± 3.2 3.2 | 3.2 ± ± 3.4 3.4 | 2.7 ± ± 4 4 | 3.3 ± ± 5.1 5.1 | 4 ± ± 8.2 8.2 | 0.36 | 0.24, 0.59 (0.23, 1.46) | 0.84, 0.95 (0.56, 1.60) |
| C3, mg/dL | 106.6 ± ± 26.1 26.1 | 100.4 ± ± 21.6 21.6 | 107.4 ± ± 24.1 24.1 | 108.8 ± ± 28.6 28.6 | 106.8 ± ± 29.7 29.7 | 108.5 ± ± 32.2 32.2 | 0.84 | 0.34, −4.48 (−4.48, −13.96) | 0.85, 0.82 (−7.76, 9.39) |
| C4, mg/dL | 22 ± ± 10.7 10.7 | 19.8 ± ± 10 10 | 21.1 ± ± 9.8 9.8 | 19.4 ± ± 8.2 8.2 | 18.8 ± ± 7.4 7.4 | 19 ± ± 8.5 8.5 | 0.51 | 0.32, −1.17 (−1.17, −3.53) | 0.88, −0.13 (−1.95, 1.69) |
| Anti dsDNA, IU/mL** | 98.6 ± ± 81.7 81.7 | 127.4 ± ± 85.9 85.9 | 157.2 ± ± 115.7 115.7 | 55.0 ± ± 21.7 21.7 | 61.3 ± ± 21.6 21.6 | 99.7 ± ± 70.7 70.7 | 0.41 | 0.14, 40.08 (−20.71, 100.87) | 0.96, 3.46 (−163.58, 170.50) |
| AST, u/L | 20.6 ± ± 5.3 5.3 | 22.4 ± ± 6 6 | 21.5 ± ± 5.6 5.6 | 21.7 ± ± 4.5 4.5 | 22.2 ± ± 9.2 9.2 | 35.1 ± ± 45.7 45.7 | 0.56 | 0.8, 0.71 (0.71, −5.14) | 0.19, −13.8 (−34.91, 7.31) |
| ALT, u/L | 17.7 ± ± 9 9 | 20.1 ± ± 11.5 11.5 | 19.6 ± ± 10.6 10.6 | 19.7 ± ± 8.6 8.6 | 20.3 ± ± 14.3 14.3 | 23.1 ± ± 13 13 | 0.56 | 0.6, 1.58 (1.58, −4.55) | 0.52, −2.15 (−8.86, 4.56) |
| Cholesterol, mg/dL | 170.5 ± ± 31.3 31.3 | 184.9 ± ± 45.3 45.3 | 170.6 ± ± 33.7 33.7 | 148.4 ± ± 23.2 23.2 | 147.2 ± ± 26.1 26.1 | 149.6 ± ± 31.6 31.6 | 0.06 | 0.25, 9.55 (−6.96, 26.06) | 0.91, −0.77 (−15.14, 13.61) |
| Triglycerides, mg/dL* | 108.7 ± ± 46.6 46.6 | 115.8 ± ± 107.7 107.7 | 107 ± ± 65.7 65.7 | 79.8 ± ± 29.9 29.9 | 84.5 ± ± 43.3 43.3 | 90.7 ± ± 44.2 44.2 | 0.09 | 0.97, 0.99 (0.68, 1.46) | 0.78, 0.95 (0.68, 1.34) |
| HDL, mg/dL | 56.5 ± ± 17.5 17.5 | 64.2 ± ± 18.8 18.8 | 56.5 ± ± 19.7 19.7 | 52.2 ± ± 16.4 16.4 | 51.4 ± ± 15.6 15.6 | 51.5 ± ± 19.3 19.3 | 0.53 | 0.0006, 8.72 (4.12, 13.32) | 0.92, 0.22 (−3.94, 4.38) |
| LDL, mg/dL | 92.2 ± ± 30 30 | 94.2 ± ± 31.6 31.6 | 92.8 ± ± 27.8 27.8 | 80.2 ± ± 23.4 23.4 | 79 ± ± 25.2 25.2 | 80.2 ± ± 24.2 24.2 | 0.28 | 0.29, 6.68 (−5.98, 19.33) | 0.61, 2.56 (−7.61, 12.72) |
| HDL particle number, mcmol/L | 32.1 ± ± 6.5 6.5 | 34.7 ± ± 6.1 6.1 | 31.2 ± ± 6.4 6.4 | 28.9 ± ± 5.6 5.6 | 28.4 ± ± 5.6 5.6 | 28.1 ± ± 7 7 | 0.2 | 0.0008, 3.46 (1.58, 5.33) | 0.98, 0.02 (−2.09, 2.14) |
| HDL size, nm | 9.4 ± ± 0.5 0.5 | 9.6 ± ± 0.6 0.6 | 9.5 ± ± 0.5 0.5 | 9.4 ± ± 0.6 0.6 | 9.5 ± ± 0.5 0.5 | 9.5 ± ± 0.5 0.5 | 0.86 | 0.85, 0.02 (−0.23, 0.28) | 0.37, −0.09 (−0.31, 0.12) |
| LDL particle number, nmol/L | 1024.8 ± ± 396.9 396.9 | 1055.5 ± ± 556.5 556.5 | 1053.3 ± ± 433 433 | 898.4 ± ± 286.8 286.8 | 869 ± ± 309.7 309.7 | 931.1 ± ± 372.9 372.9 | 0.23 | 0.71, 25.76 (−172.31, 223.82) | 0.95, −4.66 (−168.02, 158.71) |
| LDL size, nm | 20.7 ± ± 0.5 0.5 | 20.9 ± ± 0.7 0.7 | 20.7 ± ± 0.7 0.7 | 20.7 ± ± 0.6 0.6 | 20.7 ± ± 0.5 0.5 | 20.5 ± ± 0.7 0.7 | 0.89 | 0.28, 0.2 (−0.17, 0.57) | 0.29, 0.17 (−0.16, 0.50) |
| VLDL particle number, nmol/L | 43.9 ± ± 20 20 | 50.5 ± ± 40.1 40.1 | 44.1 ± ± 39.5 39.5 | 31.4 ± ± 18.3 18.3 | 34.7 ± ± 26.5 26.5 | 39.2 ± ± 25.4 25.4 | 0.11 | 0.58, 8.14 (−21.7, 37.98) | 0.83, −3.1 (−31.87, 25.68) |
| VLDL size, nm* | 48.9 ± ± 4.8 4.8 | 47.8 ± ± 5.6 5.6 | 49.1 ± ± 7.6 7.6 | 46 ± ± 7.1 7.1 | 47.3 ± ± 4.5 4.5 | 45.6 ± ± 4.8 4.8 | 0.15 | 0.70, 0.98 (0.9, 1.07) | 0.45, 1.04 (0.93, 1.16) |
| Glucose, mmol/l | 5 ± ± 0.7 0.7 | 4.9 ± ± 0.7 0.7 | 5.2 ± ± 1 1 | 4.9 ± ± 0.4 0.4 | 4.9 ± ± 0.6 0.6 | 5 ± ± 1.1 1.1 | 0.57 | 0.18, −0.16 (−0.16, −0.4) | 0.97, −0.007 (−0.57, 0.55) |
| Insulin, Pmol/L | 98.7 ± ± 42 42 | 88.3 ± ± 35.9 35.9 | 96.4 ± ± 38.7 38.7 | 143.1 ± ± 95.1 95.1 | 131.3 ± ± 104.4 104.4 | 204.3 ± ± 246.2 246.2 | 0.19 | 0.54, −12.19 (−52.40, 28.01) | 0.45, −29.12 (−106.62, 48.39) |
| HOMA2 IR | 1.8 ± ± 0.8 0.8 | 1.6 ± ± 0.7 0.7 | 1.8 ± ± 0.7 0.7 | 2.6 ± ± 1.7 1.7 | 2.4 ± ± 1.9 1.9 | 3.6 ± ± 4.2 4.2 | 0.21 | 0.51, −0.23 (−0.93, 0.48) | 0.38, −0.56 (−1.85, 0.74) |
WBC white blood cell, ANC absolute neutrophil count, ESR erythrocyte sedimentation rate, CRP
 C-reactive protein, C3 complement component 3, C4 complement component 4, anti-ds-DNA anti-double-stranded DNA antibody, AST
C-reactive protein, C3 complement component 3, C4 complement component 4, anti-ds-DNA anti-double-stranded DNA antibody, AST
 aspartate aminotransferase, ALT
aspartate aminotransferase, ALT
 alanine aminotransferase, HDL high-density lipoprotein, LDL low-density lipoprotein, VLDL
alanine aminotransferase, HDL high-density lipoprotein, LDL low-density lipoprotein, VLDL
 very-low-density lipoprotein, mcmol/L
very-low-density lipoprotein, mcmol/L
 micromoles per liter, nm nanometer, Pmol/L
micromoles per liter, nm nanometer, Pmol/L  picomole per liter, HOMA2 IR
picomole per liter, HOMA2 IR
 Homeostatic Model Assessment Index for Insulin Resistance.
Homeostatic Model Assessment Index for Insulin Resistance.
Baseline P values were calculated using the two-sample t tests. P values and the 95% confidence intervals for treatment group difference in change score from baseline to day 56 were calculated using either (1) linear mixed-effects models if there were multiple assessment time points between baseline and day 56 or (2) analysis of covariance models (ANCOVA) if the measures were collected only at baseline and day 56 during the treatment phase. For day 84, ANCOVA was used to compare the change scores between treatment groups. The STAT4 allele status and the baseline values were included as covariates in the linear mixed-effects models and ANCOVA.
*P values were calculated for the log-transformed data in order to meet the model normality assumptions. The estimates and the 95% confidence bounds were exponentiated back.
**Data are based on 5/20 subjects in the tofacitinib group and 3/10 subjects in the placebo group who had positive anti-ds-DNA results. Wilcoxon ranked-sum test was used to calculate the P values.
Two-tailed tests were used where appropriate.
No adjustments were made for multiple comparisons.
Table 4
Disease activity and patient-reported outcome measures.
Tofacitinib (n = = 20) 20) | Placebo (n = = 10) 10) | Difference in change (tofacitinib vs. placebo) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome variable | Day 1 | Day 56 | Day 84 | Day 1 | Day 56 | Day 84 | Day 1 | Day 56* | Day 84** |
mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | mean ± ± SD SD | P value | P value difference (95% CI) | P value difference (95% CI) | |
| SF-36 total | 110.3 ± ± 9.6 9.6 | 113.3 ± ± 9.5 9.5 | 111.9 ± ± 9.3 9.3 | 107.6 ± ± 8.4 8.4 | 111.5 ± ± 8.1 8.1 | 108 ± ± 9.8 9.8 | 0.46 | 0.91, −0.20 (−3.69, 3.29) | 0.31, 2.92 (−2.84, 8.69) |
| MD-Fatigue average | 1.6 ± ± 1.7 1.7 | 1.9 ± ± 2.2 2.2 | 2.1 ± ± 2.2 2.2 | 4.4 ± ± 2.7 2.7 | 2.2 ± ± 1.6 1.6 | 2.3 ± ± 2.2 2.2 | 0.001 | 0.016, 1.47 (0.30, 2.65) | 0.04, 1.71 (0.04, 3.36) |
| SLEDAI-2K | 5.1 ± ± 2.2 2.2 | 4.2 ± ± 2.3 2.3 | 3.9 ± ± 1.9 1.9 | 5.5 ± ± 3.7 3.7 | 4.5 ± ± 2.4 2.4 | 4.9 ± ± 2.9 2.9 | 0.68 | 0.95, 0.04 (−1.04, 1.11) | 0.25, −0.72 (−1.98, 0.53) |
| PGA | 0.8 ± ± 0.8 0.8 | 0.8 ± ± 0.9 0.9 | 0.9 ± ± 0.7 0.7 | 1.2 ± ± 0.9 0.9 | 0.9 ± ± 0.8 0.8 | 0.8 ± ± 0.8 0.8 | 0.21 | 0.43, 0.18 (−0.28, 0.64) | 0.38, 0.23 (−0.30, 0.76) |
| DAS-28-ESR | 2.7 ± ± 1.3 1.3 | 2.5 ± ± 1.2 1.2 | 2.5 ± ± 0.9 0.9 | 3.2 ± ± 2 2 | 2.9 ± ± 1.6 1.6 | 2.8 ± ± 1.3 1.3 | 0.44 | 0.73, −0.09 (−0.61, 0.43) | 0.81, 0.05 (−0.40, 0.51) |
| CLASI total activity | 2.6 ± ± 1.9 1.9 | 1.9 ± ± 1.7 1.7 | 2.1 ± ± 1.8 1.8 | 2.4 ± ± 1.7 1.7 | 2.1 ± ± 1.7 1.7 | 2.2 ± ± 1.5 1.5 | 0.83 | 0.35, −0.31 (−1.03, 0.42) | 0.54, −0.26 (−1.12, 0.60) |
| CLASI total damage*** | 1 ± ± 2.1 2.1 | 1.1 ± ± 2.2 2.2 | 1.1 ± ± 2.2 2.2 | 1 ± ± 2.2 2.2 | 0.9 ± ± 2.2 2.2 | 0.9 ± ± 2.2 2.2 | 1 | 0.46 | 0.46 |
| BILAG 2004† | 7.6 ± ± 4.6 4.6 | 6.2 ± ± 4.8 4.8 | 5.5 ± ± 3.9 3.9 | 9.3 ± ± 4.3 4.3 | 8.1 ± ± 5.5 5.5 | 7.9 ± ± 4.4 4.4 | 0.32 | 0.36, 1.56 (−1.86, 4.98) | 0.16, −2.04 (−4.96, 0.89) |
SF 36 Short Form Health Survey, MD-Fatigue Multidimensional Assessment of Fatigue Questionnaire, SLEDAI 2 K
K
 Systemic Lupus Erythematosus Disease Activity Index 2000, PGA
Systemic Lupus Erythematosus Disease Activity Index 2000, PGA
 Physician Global Assessment, DAS 28-ESR
Physician Global Assessment, DAS 28-ESR
 Disease Activity Score of the 28 joints with erythrocyte sedimentation rate, CLASI
Disease Activity Score of the 28 joints with erythrocyte sedimentation rate, CLASI
 Cutaneous Lupus Erythematosus Disease Area and Severity Index, BILAG 2004 British Isles Lupus Assessment Group Disease Activity Index.
Cutaneous Lupus Erythematosus Disease Area and Severity Index, BILAG 2004 British Isles Lupus Assessment Group Disease Activity Index.
*To calculate the P value and the estimated difference (95% confidence interval) in change from baseline (tofacitinib vs. placebo), the linear mixed-effects models were used to include all the other time points between baseline and day 56.
**To calculate the P value and the estimated difference (95% confidence interval) in change from baseline (tofacitinib vs. placebo), the analysis of covariance (ANCOVA) test was used to analyze data on day 84 only.
***Due to the violation of the normality assumption, the Wilcoxon ranked-sum test was used to assess the baseline data and to compare the change scores for day 56 and day 84 separately. The 95% confidence intervals were not provided.
†BILAG 2004 numerical scoring method: A =
= 12, B
12, B =
= 8, C
8, C =
= 1, D
1, D =
= 0, and E
0, and E =
= 0.
0.
Two-tailed tests were used where appropriate.
No adjustments were made for multiple comparisons.
Despite randomization, there were some differences between the tofacitinib and the placebo group at the baseline visit. The subjects on placebo reported a statistically significant higher degree of fatigue, as measured by MD-Fatigue scale at baseline, and a significant improvement in fatigue during the study. The subjects on placebo had higher baseline DAS-28-ESR and lower baseline anti-ds-DNA levels as compared to the tofacitinib group, but these were not statistically significant differences (Table 4). The safety and disease activity data of subjects on tofacitinib and placebo stratified based on presence or absence of STAT4 risk allele revealed non-statistically significant differences in some variables between groups (except for as noted elsewhere); the clinical relevance of these differences is uncertain and needs to be further explored in a larger sample (Supplementary Tables 1 and 2).
Tofacitinib modulated the type I IFN gene signature and pSTAT levels
To assess target engagement in subjects receiving tofacitinib, we measured phosphorylation of STAT1, STAT3, and STAT5. Tofacitinib use led to significant inhibition of pSTAT1 in CD4+ T cells at days 14 and 56 of the trial (P =
= 0.023; Fig. 1a). There was also a trend observed towards nonsignificant inhibition of pSTAT1 in subjects on a placebo that is of unknown significance and without any known plausible biological explanation. In contrast, pSTAT3, pSTAT5, and pSTAT1 levels were not significantly different in CD8+ T cells, CD20+ B cells or CD14+ monocytes between tofacitinib or placebo-treated patients (Fig. 1b and Supplementary Fig. 2a, b). Assessment of these parameters one month after drug discontinuation showed that the effects on pSTAT1 did not persist after drug was stopped.
0.023; Fig. 1a). There was also a trend observed towards nonsignificant inhibition of pSTAT1 in subjects on a placebo that is of unknown significance and without any known plausible biological explanation. In contrast, pSTAT3, pSTAT5, and pSTAT1 levels were not significantly different in CD8+ T cells, CD20+ B cells or CD14+ monocytes between tofacitinib or placebo-treated patients (Fig. 1b and Supplementary Fig. 2a, b). Assessment of these parameters one month after drug discontinuation showed that the effects on pSTAT1 did not persist after drug was stopped.
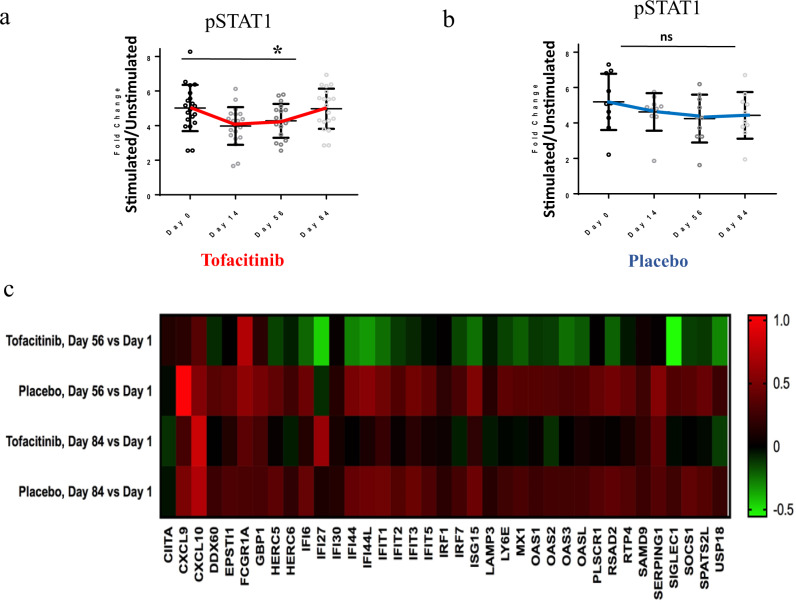
 +
+ T cells.
T cells.a Significant inhibition of pSTAT1 in patients on tofacitinib at day 56 (P =
= 0.023) with the return to baseline at day 84 n
0.023) with the return to baseline at day 84 n =
= 20 biologically independent samples. Data are presented as mean values
20 biologically independent samples. Data are presented as mean values +
+ /− SEM. A mixed linear model for repeated measures was used. b No significant change noted in patients on placebo. n
/− SEM. A mixed linear model for repeated measures was used. b No significant change noted in patients on placebo. n =
= 10 biologically independent samples. Data are presented as mean values
10 biologically independent samples. Data are presented as mean values +
+ /− SEM. A mixed linear model for repeated measures was used. c Tofacitinib decreases the type I IFN gene signature, gene expression in peripheral blood by Nanostring: Heatmap showing interferon-stimulated gene expression in peripheral blood by nanostring: Log2 mean fold change in expression of interferon-stimulated genes from baseline to day 56 and 84 in subjects on tofacitinib (n
/− SEM. A mixed linear model for repeated measures was used. c Tofacitinib decreases the type I IFN gene signature, gene expression in peripheral blood by Nanostring: Heatmap showing interferon-stimulated gene expression in peripheral blood by nanostring: Log2 mean fold change in expression of interferon-stimulated genes from baseline to day 56 and 84 in subjects on tofacitinib (n =
= 20 biologically independent samples) and placebo (n
20 biologically independent samples) and placebo (n =
= 10 biologically independent samples). Source data are provided as a Source Data file. Results shown as fold change in stimulated vs. unstimulated cell population. A mixed linear model for repeated measures was used. Unpaired two-tailed, t test were used where appropriate. No adjustments were made for multiple comparisons.
10 biologically independent samples). Source data are provided as a Source Data file. Results shown as fold change in stimulated vs. unstimulated cell population. A mixed linear model for repeated measures was used. Unpaired two-tailed, t test were used where appropriate. No adjustments were made for multiple comparisons.
We next assessed gene expression between groups at baseline, days 56 and 84 by whole blood RNAseq. In total, 90 genes were found to be twofold different (22 up and 68 down) by ANOVA comparison between tofacitinib and placebo treatment on day 56. Of the 68 genes that were significantly downregulated in the tofacitinib group at day 56, 19 were Interferon-Stimulated Genes (ISG). By day 84, the levels of most of these 19 ISGs had returned to pretreatment levels, but a few genes were still significantly downregulated (Fig. 1c and Supplementary Fig. 3). Nanostring was used to verify the impact of tofacitinib on the IFN signature (Fig. 1c). The IFN response gene score was not significantly different at baseline between subjects on tofacitinib and placebo. At day 56, the subjects on tofacitinib had a significant reduction in levels of ISGs in comparison to placebo (P =
= 0.01), which remained significant at the day 84 visit (P
0.01), which remained significant at the day 84 visit (P =
= 0.02) (Supplementary Fig. 4)18.
0.02) (Supplementary Fig. 4)18.
Tofacitinib modulated dysregulated neutrophil responses
The JAK/STAT pathway and cognate cytokines are crucial in neutrophil biology19. We examined the impact of tofacitinib on LDGs (a distinct subset of proinflammatory neutrophils elevated in SLE) and their ability to synthesize NETs. We found that there was a significant reduction in the percentage of LDGs in SLE subjects on tofacitinib compared to placebo at day 56 (P =
= 0.048) and the decrease was sustained at day 84 (P
0.048) and the decrease was sustained at day 84 (P =
= 0.014; Fig. 2a), whereas total neutrophils did not significantly decrease during active treatment (Fig. 2b and Table 4). There was also a concomitant increase in LDGs in the placebo group which partially explains this statistically significant difference. While the role of NETs in promoting inflammation and vascular damage in SLE has been previously described in vitro and in vivo, the effect of the STAT4 risk allele in NET formation is unknown7. We found that SLE subjects positive for the STAT4 risk allele had significantly higher levels of circulating NET complexes at baseline when compared to STAT4 risk allele-negative subjects (P
0.014; Fig. 2a), whereas total neutrophils did not significantly decrease during active treatment (Fig. 2b and Table 4). There was also a concomitant increase in LDGs in the placebo group which partially explains this statistically significant difference. While the role of NETs in promoting inflammation and vascular damage in SLE has been previously described in vitro and in vivo, the effect of the STAT4 risk allele in NET formation is unknown7. We found that SLE subjects positive for the STAT4 risk allele had significantly higher levels of circulating NET complexes at baseline when compared to STAT4 risk allele-negative subjects (P =
= 0.02) (Fig. 2c). The levels of circulating NET complexes significantly decreased compared to baseline in STAT4 risk allele-positive subjects that received tofacitinib (P
0.02) (Fig. 2c). The levels of circulating NET complexes significantly decreased compared to baseline in STAT4 risk allele-positive subjects that received tofacitinib (P =
= 0.037) (Fig. 2d). In contrast, subjects receiving placebo or those subjects receiving tofacitinib that were STAT4 risk allele-negative did not display decreases in circulating NET complexes (Fig. 2d). Overall, these results indicated that STAT4 risk allele-positive subjects displayed higher levels of circulating NETs and that tofacitinib modified features of SLE-associated neutrophil dysregulation in subjects positive for this genetic risk haplotype7. However, even though these differences were significant but there was an overlap between the groups and there was also a lower NET expression in placebo subjects at the baseline.
0.037) (Fig. 2d). In contrast, subjects receiving placebo or those subjects receiving tofacitinib that were STAT4 risk allele-negative did not display decreases in circulating NET complexes (Fig. 2d). Overall, these results indicated that STAT4 risk allele-positive subjects displayed higher levels of circulating NETs and that tofacitinib modified features of SLE-associated neutrophil dysregulation in subjects positive for this genetic risk haplotype7. However, even though these differences were significant but there was an overlap between the groups and there was also a lower NET expression in placebo subjects at the baseline.
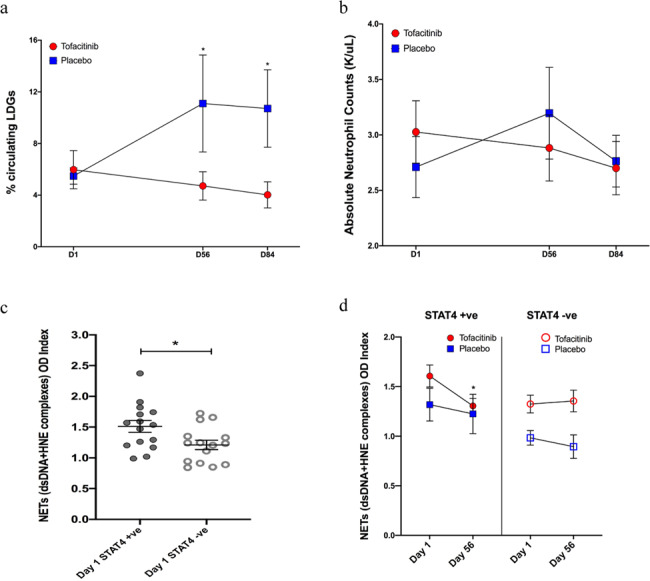
a Results represent changes in percentage of circulating LDGs: Subjects treated with tofacitinib vs. placebo. Significant decrease in LDGs in tofacitinib group P =
= 0.048 at day 56 and P
0.048 at day 56 and P =
= 0.014 at day 84 using unpaired t test. b Changes in absolute neutrophil counts. c Circulating NET levels at baseline: Individuals positive for STAT4 risk allele (each subject represented by closed circles) have enhanced circulating NET levels (assessed by human neutrophil elastase (HNE)-dsDNA complexes) then subjects who are STAT4 risk allele negative (each subject represented by open circles) P
0.014 at day 84 using unpaired t test. b Changes in absolute neutrophil counts. c Circulating NET levels at baseline: Individuals positive for STAT4 risk allele (each subject represented by closed circles) have enhanced circulating NET levels (assessed by human neutrophil elastase (HNE)-dsDNA complexes) then subjects who are STAT4 risk allele negative (each subject represented by open circles) P =
= 0.02 using unpaired t test. d Changes in circulating NET levels during the study: Patients who are STAT4 risk allele positive and receive tofacitinib display significant decrease in circulating NET complexes P
0.02 using unpaired t test. d Changes in circulating NET levels during the study: Patients who are STAT4 risk allele positive and receive tofacitinib display significant decrease in circulating NET complexes P =
= 0.037 using unpaired t test. Source data are provided as a Source Data file. All data represent mean
0.037 using unpaired t test. Source data are provided as a Source Data file. All data represent mean ±
± SEM, *P
SEM, *P <
< 0.05, and are based on tofacitinib n
0.05, and are based on tofacitinib n =
= 20, placebo n
20, placebo n =
= 10. Unpaired two-tailed, t test were used for all results. No adjustments were made for multiple comparisons.
10. Unpaired two-tailed, t test were used for all results. No adjustments were made for multiple comparisons.
Improvements in cardiometabolic parameters and vascular function
Since tofacitinib improved type I IFN signaling and neutrophil abnormalities associated with premature CVD, we determined if tofacitinib treatment affected plasma lipoprotein levels and function. There was a significant increase in HDL-C in all subjects in the tofacitinib group at the completion of the on-study drug phase (day 56) compared to baseline (P =
= 0.006) regardless of their STAT4 risk allele status (Fig. 3a and Supplementary Fig. 5b). Low-density lipoprotein levels and triglycerides were not affected (Supplemental Fig. 5a–d). Tofacitinib also significantly increased levels of large HDL particles, whereas other lipoprotein subfractions did not show significant changes (Table 4). At day 56, a significant increase in Lecithin–cholesterol acyltransferase (LCAT) concentration was detected in SLE subjects on tofacitinib that were STAT4 risk allele-positive (P
0.006) regardless of their STAT4 risk allele status (Fig. 3a and Supplementary Fig. 5b). Low-density lipoprotein levels and triglycerides were not affected (Supplemental Fig. 5a–d). Tofacitinib also significantly increased levels of large HDL particles, whereas other lipoprotein subfractions did not show significant changes (Table 4). At day 56, a significant increase in Lecithin–cholesterol acyltransferase (LCAT) concentration was detected in SLE subjects on tofacitinib that were STAT4 risk allele-positive (P =
= 0.024, 95% CI: 1.1–26.5) compared to the STAT4 risk allele-positive group on placebo or in subjects negative for this risk allele on tofacitinib (P
0.024, 95% CI: 1.1–26.5) compared to the STAT4 risk allele-positive group on placebo or in subjects negative for this risk allele on tofacitinib (P =
= 0.04, 95% CI: −18.2, 10.2) (Fig. 3b). In addition, at day 56, there was a statistically significant increase in cholesterol efflux capacity in SLE subjects treated with tofacitinib (P
0.04, 95% CI: −18.2, 10.2) (Fig. 3b). In addition, at day 56, there was a statistically significant increase in cholesterol efflux capacity in SLE subjects treated with tofacitinib (P =
= 0.002, 95% CI: 0.04–0.16) and a nonsignificant trend when compared to placebo (P
0.002, 95% CI: 0.04–0.16) and a nonsignificant trend when compared to placebo (P =
= 0.08, 95% CI: −0.01, 0.24) (Supplementary Fig. 5e). This effect was not dependent on the subject’s STAT4 risk allele status. No statistically significant differences in insulin resistance, as measured by HOMA-IR, were detected between placebo and tofacitinib-treated groups (P
0.08, 95% CI: −0.01, 0.24) (Supplementary Fig. 5e). This effect was not dependent on the subject’s STAT4 risk allele status. No statistically significant differences in insulin resistance, as measured by HOMA-IR, were detected between placebo and tofacitinib-treated groups (P =
= 0.51, 95% CI: −0.93, 0.48) (Table 3). Compared to the tofacitinib group, the placebo group had lower cholesterol and triglyceride values at the baseline, but these differences were not statistically significant and remained essentially unchanged throughout the study (Table 3).
0.51, 95% CI: −0.93, 0.48) (Table 3). Compared to the tofacitinib group, the placebo group had lower cholesterol and triglyceride values at the baseline, but these differences were not statistically significant and remained essentially unchanged throughout the study (Table 3).
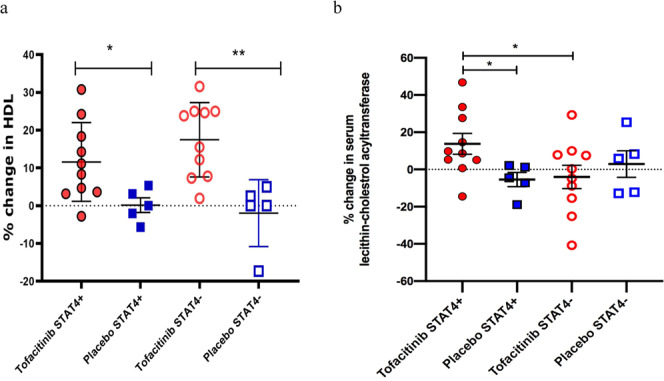
a Percent change in serum HDL-C at day 56 compared to day 1 based on STAT4 risk allele status (each circle and square represent individual subject): Results represent *P =
= 0.037 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs placebo and **P
0.037 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs placebo and **P =
= 0.002 STAT4 risk allele-negative subjects on tofacitinib vs placebo. Unpaired t test was used. b Percent change in Lecithin–cholesterol acyltransferase (LCAT) concentration at day 56 compared to day 1 based on STAT4 risk allele status (each circle and square represent individual subject): Results represent *P
0.002 STAT4 risk allele-negative subjects on tofacitinib vs placebo. Unpaired t test was used. b Percent change in Lecithin–cholesterol acyltransferase (LCAT) concentration at day 56 compared to day 1 based on STAT4 risk allele status (each circle and square represent individual subject): Results represent *P =
= 0.033 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs placebo and *P
0.033 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs placebo and *P =
= 0.044 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs STAT4 risk allele-negative subjects on tofacitinib. Kruskal–Wallis test (unpaired, nonparametric) was used. Source data are provided as a Source Data file. All results represent mean
0.044 for the difference between STAT4 risk allele-positive subjects on tofacitinib vs STAT4 risk allele-negative subjects on tofacitinib. Kruskal–Wallis test (unpaired, nonparametric) was used. Source data are provided as a Source Data file. All results represent mean ±
± SEM, *P
SEM, *P <
< 0.05 **P
0.05 **P <
< 0.01, and are based on tofacitinib n
0.01, and are based on tofacitinib n =
= 20, placebo n
20, placebo n =
= 10. Two-tailed tests were used where appropriate. No adjustments were made for multiple comparisons.
10. Two-tailed tests were used where appropriate. No adjustments were made for multiple comparisons.
Enhanced arterial stiffness and impairments in endothelium-dependent vasorelaxation have been described in association with vasculopathy and subclinical atherosclerosis in SLE20,21. Consistent with previous studies, the baseline values for various measures of arterial stiffness and impairments in endothelial dysfunction in SLE subjects were higher than the reference values for age-matched controls (Table 1)22,23. Patients in the tofacitinib arm had clinically significant decreases in arterial stiffness (as measured by CAVI and PWV) and improvement in endothelium-dependent vasorelaxation (as measured by EndoPAT RHI). Specifically, patients on tofacitinib had decreases in CAVI by mean ±
± standard error of the mean of −0.28
standard error of the mean of −0.28 ±
± 0.14 at day 56 and −0.14
0.14 at day 56 and −0.14 ±
± 0.19 at day 84 (Fig. 4a). PWV decreased only in the STAT4 risk allele-positive subjects on tofacitinib by −0.42
0.19 at day 84 (Fig. 4a). PWV decreased only in the STAT4 risk allele-positive subjects on tofacitinib by −0.42 m/s
m/s ±
± 0.58 at day 56, and −1.41
0.58 at day 56, and −1.41 m/s
m/s ±
± 0.92 at day 84, respectively, from baseline (Fig. 4b). Similarly, there was an improvement in RHI, in STAT4 risk allele-positive subjects on tofacitinib, by 0.18
0.92 at day 84, respectively, from baseline (Fig. 4b). Similarly, there was an improvement in RHI, in STAT4 risk allele-positive subjects on tofacitinib, by 0.18 ±
± 0.17 at day 56, and 0.23
0.17 at day 56, and 0.23 +
+ 0.09 at day 84 (Fig. 4c). In contrast, no improvements were observed in the placebo group. Overall, these results indicated that SLE subjects on short-term tofacitinib treatment exhibited improvements in cardiometabolic and vascular parameters previously associated with CV risk in SLE patients and in the general population. Importantly, some of these protective effects were more apparent in subjects bearing the STAT4 risk allele.
0.09 at day 84 (Fig. 4c). In contrast, no improvements were observed in the placebo group. Overall, these results indicated that SLE subjects on short-term tofacitinib treatment exhibited improvements in cardiometabolic and vascular parameters previously associated with CV risk in SLE patients and in the general population. Importantly, some of these protective effects were more apparent in subjects bearing the STAT4 risk allele.
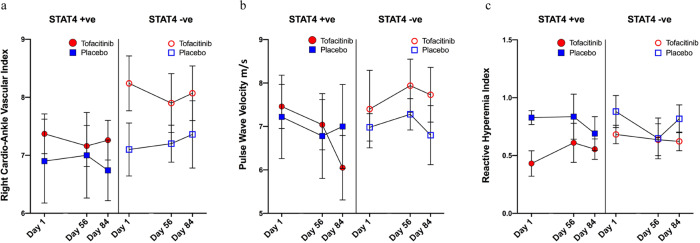
a Right cardioankle vascular index (CAVI): Results represent changes in CAVI in subjects on tofacitinib vs placebo in STAT4 risk allele-positive and -negative subjects during the trial. b Pulse wave velocity (PWV): Results represent changes in PWV in subjects on tofacitinib vs placebo in STAT4 risk allele-positive and -negative subjects during the trial. c Reactive hyperemia index (RHI): Results represent changes in RHI in subjects on tofacitinib vs placebo in STAT4 risk allele-positive and -negative subjects during the trial. Source data are provided as a Source Data file. All results represent mean ±
± SEM and are based on tofacitinib n
SEM and are based on tofacitinib n =
= 20, placebo n
20, placebo n =
= 10. The paired t test, Mann–Whitney U, or ANOVA were used for comparison where appropriate based on normality of distribution. Two-tailed tests were used where appropriate. No adjustments were made for multiple comparisons.
10. The paired t test, Mann–Whitney U, or ANOVA were used for comparison where appropriate based on normality of distribution. Two-tailed tests were used where appropriate. No adjustments were made for multiple comparisons.
Discussion
In this study, short-term use of tofacitinib in subjects with mild-to-moderate SLE was overall safe, well-tolerated, with no unexpected AEs, thromboembolic events or opportunistic infections. Our exploratory analyses showed that tofacitinib led to significant positive modulation of cardiometabolic and immunologic parameters previously linked to increased coronary atherosclerotic plaque, vascular inflammation, and abnormalities in blood vessel function in lupus and in the general population24,25. Premature atherosclerosis and vasculopathy leading to CV events significantly contribute to enhanced morbidity and mortality in SLE, a phenomenon not explained by traditional CV risk factors6,24. The observation that jakinibs may have a possible role in modulating SLE vasculopathy and potentially mitigating CV risk could have important implications in this patient population.
Previous work from our group and others has implicated a pathophysiologic alliance between type I IFNs, LDGs, and enhanced NET formation as a mechanism that promotes premature atherosclerosis and vasculopathy in SLE7,26–29. Tofacitinib significantly decreased the type I IFN gene signature in circulating immune cells and selectively modulated the numbers of circulating LDGs, but not total neutrophils, in association with significant decreases in levels of circulating NETs. The mechanism by which tofacitinib led to decreases in LDGs remains to be further characterized and may be related to inhibition of cytokine-specific effects on these cells in the bone marrow or tissues, perhaps promoting their death through apoptosis. Of interest, we found that individuals carrying the STAT4 risk allele had higher levels of circulating NETs, suggesting that certain polymorphisms associated with an enhanced risk for autoimmunity may promote the enhanced NET formation, a phenomenon recently reported for other autoimmunity risk SNPs30,31.
While the effect on type I IFN was expected, it remains unclear why LDGs were preferentially targeted by tofacitinib and why the response to the drug concerning NET inhibition was enhanced in the presence of the STAT4 risk allele. One scenario is that since type I IFNs activate STAT4, subjects bearing the STAT4 risk allele have increased priming of neutrophils resulting in increased NET formation. Our current data are consistent with this view and it appears that tofacitinib may have preferentially blocked this priming in subjects with the risk allele32. While tofacitinib affected type I IFN responses and markers of neutrophil dysregulation, the effects observed on other immune cell types or clinical activity in subjects with mild-to-moderate SLE were not significant. The exact role of type I IFN in the pathogenesis of SLE is still being defined and abrogating this pathway may not lead to decreases in disease manifestations, as evidence from recent clinical trials using interferon receptor blocker Anifrolumab and the plasmacytoid dendritic cells specific receptor antibody BIIB05933,34. Future studies on the effects of tofacitinib in larger patient groups, stratified by genetic risk and greater active disease, will be needed to assess alteration of both innate and adaptive immune parameters, and clinical disease activity.
SLE HDL has been described to be small particle size and with decreased cholesterol efflux capacity and LCAT activity driven at least in part by oxidation through enhanced NET formation7,35–37. Herein, we show that short-term use of tofacitinib in SLE led to significant improvements in the levels, size, and function of HDL. Importantly, tofacitinib led to improvements in arterial compliance and endothelial function, with the reversal of SLE-associated accelerated vascular aging. The improvement in aortic compliance as measured by PWV persisted during the washout phase possibly due to modulatory effects of tofacitinib on endothelial cells38. Even though these changes did not reach statistical significance (probably due to the small sample size) yet the extent of change in a short duration is impressive and requires further exploring. The mechanism of this finding of short-term tofacitinib exposure leading to improvements in arterial compliance and endothelial function remains speculative. Higher prevalence of the STAT4 risk allele has been associated with cardiovascular risk in SLE patients and atherosclerosis animal models16,39. In this study, HDL function (as measured by LCAT activity), arterial stiffness, and endothelium-dependent vasorelaxation improved in individuals on tofacitinib that were positive for STAT4 risk allele.
Future clinical trials will need to establish whether the positive effects on vascular function by jakinibs are disease-specific or are generalizable to other autoimmune disorders and, perhaps, even the general population. Indeed, addressing CV risk in a non-autoimmune disease population by using anti-inflammatory medications (canakinumab) has been reported to be successful25.
There was a nonsignificant trend toward lower absolute neutrophil counts in subjects on tofacitinib. As expected, there were more mild and moderate infections (mostly upper respiratory tract infections) in the tofacitinib group as compared to placebo. Some of the limitations of the current study include its short duration and a small number of subjects with mild-to-moderate disease. Being an early phase exploratory study, adjustments were not made for multiple comparisons. In addition, there were differences in the baseline between the groups and changes observed in the placebo group which may be partially responsible for some of the results observed in the secondary outcomes of this study. No concomitant immunosuppressive agents were allowed and therefore the safety profile in presence of additional drugs remains to be characterized. Nevertheless, using a drug that has a good safety profile and promotes beneficial effects on systemic inflammation, disease manifestations and CV risk prevention would be a highly desired target for SLE and other autoimmune diseases.
In summary, our study used a personalized approach in the context of SLE by stratifying patients according to STAT4 risk allele. Results from the current trial suggest the effect of JAK inhibition was more robust in subjects with STAT4 risk allele, which is associated with a more severe SLE, and an increased risk of CV events15,16. The JAK-STAT pathway is involved in the intracellular signaling of multiple cytokines; therefore, additional mechanistic studies are needed to better characterize the pathways responsible for findings in this study. Larger studies with longer follow-up can better assess if this personalized medicine approach of stratification by genetic risk allele leads to improved morbidity and mortality.
Methods
Study design and subjects
The study was approved by the National Institutes of Health (NIH) IRB (ClinicalTrials.gov NCT02535689). The study design and conduct complied with all relevant regulations regarding the use of human study participants and was conducted in accordance with the criteria set by the Declaration of Helsinki as authorized by the NIH Office of Human Subject Research. After written informed consent and determination of eligibility, subjects were randomized to tofacitinib (5 mg twice daily) or placebo in a 2:1 block. Thirty adult SLE subjects that met American College of Rheumatology (ACR) Revised Criteria for the Classification of SLE and had mild-to-severe disease activity (Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI 2
mg twice daily) or placebo in a 2:1 block. Thirty adult SLE subjects that met American College of Rheumatology (ACR) Revised Criteria for the Classification of SLE and had mild-to-severe disease activity (Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI 2 K) score between 2 and 14) were enrolled in an outpatient clinical research setting40. In each group, half of the subjects were homozygous or heterozygous for the STAT4 risk allele. Subjects took tofacitinib or placebo for 8 weeks and were followed for 4 additional weeks afterward (Supplementary Fig. 1). Eligible subjects were on stable doses of antimalarials (for 12 weeks prior to the screening visit) and/or oral glucocorticoids (for 4 weeks prior to the screening visit) (prednisone or equivalent <20
K) score between 2 and 14) were enrolled in an outpatient clinical research setting40. In each group, half of the subjects were homozygous or heterozygous for the STAT4 risk allele. Subjects took tofacitinib or placebo for 8 weeks and were followed for 4 additional weeks afterward (Supplementary Fig. 1). Eligible subjects were on stable doses of antimalarials (for 12 weeks prior to the screening visit) and/or oral glucocorticoids (for 4 weeks prior to the screening visit) (prednisone or equivalent <20 mg/day) but no immunosuppressants were allowed. The SLE disease activity was determined using SLEDAI 2
mg/day) but no immunosuppressants were allowed. The SLE disease activity was determined using SLEDAI 2 K, BILAG 2004, Disease Activity Score 28-Erythrocyte Sedimentation Rate (DAS-28-ESR), Physician Global Assessment (PGA) (Likert scale 0–3), and patient-reported outcomes (SF-36, Multidimensional Assessment of Fatigue questionnaire)41–46. The rates of adverse events (AEs, as defined by the National Cancer Institute (NCI), Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0) were recorded weekly. The primary outcome of the study was defined as comparing rates of adverse events and rates of SLE disease flares between the tofacitinib group and the placebo group. The SLE disease flare was defined as an increase in SLEDAI 2
K, BILAG 2004, Disease Activity Score 28-Erythrocyte Sedimentation Rate (DAS-28-ESR), Physician Global Assessment (PGA) (Likert scale 0–3), and patient-reported outcomes (SF-36, Multidimensional Assessment of Fatigue questionnaire)41–46. The rates of adverse events (AEs, as defined by the National Cancer Institute (NCI), Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0) were recorded weekly. The primary outcome of the study was defined as comparing rates of adverse events and rates of SLE disease flares between the tofacitinib group and the placebo group. The SLE disease flare was defined as an increase in SLEDAI 2 K score of ≥3 or an increase in PGA
K score of ≥3 or an increase in PGA >
> 1. The secondary outcomes included assessment of clinical response, effects on quality of life measures, and several exploratory mechanistic studies to evaluate the effect of the drug on immune dysregulation and cardiometabolic signatures associated with the development of premature cardiovascular disease (the original protocol, final protocol, and summary of changes are provided as part of the Supplementary files).
1. The secondary outcomes included assessment of clinical response, effects on quality of life measures, and several exploratory mechanistic studies to evaluate the effect of the drug on immune dysregulation and cardiometabolic signatures associated with the development of premature cardiovascular disease (the original protocol, final protocol, and summary of changes are provided as part of the Supplementary files).
Immunologic and hematologic parameters
Each subject had STAT4 risk allele genotyping prior to screening visit using commercially available TaqMan® Single Nucleotide Polymorphism Genotyping. Detailed reagents and methods for PBMC isolation, stimulation, and the fluorescent barcoding method used for the staining and multiplexed phosphoflow analysis are described in Supplementary Methods and elsewhere47. Pathway analysis and gene annotation were completed using Toppgene (toppgene.cchmc.org). Results were confirmed using NanoString (see Supplementary Methods). LDGs were classified as CD10+, CD15+, CD14lo48. Human neutrophil elastase (HNE)-DNA NET complexes were measured in plasma, as described49. Details are provided in Supplementary Methods.
Measurements of cardiovascular risk factors and vascular function
The homeostasis model assessment of insulin resistance (HOMA-IR) index =
= fasting glucose (mmol/l)
fasting glucose (mmol/l) ×
× fasting insulin (μU/ml)/22.5) was used to estimate insulin resistance50. Overnight fasting lipid profiles were performed at the NIH Clinical Center Central Laboratories. Lipoprotein particle concentration and diameters were measured using an automated Nuclear Magnetic Resonance Spectroscopy (NMR). High-density lipoprotein (HDL) cholesterol efflux capacity was measured using published methods and the macrophage cell line J77451. Human serum lecithin–cholesterol acyltransferase (LCAT) concentration was quantified by ELISA (BioVendor; Ashville, NC). Noninvasive vascular function studies included the cardioankle vascular index (CAVI), peripheral arterial tonometry (PAT; reactive hyperemia index (RHI), and pulse wave velocity (PWV)7. Details are provided in the Supplementary Methods.
fasting insulin (μU/ml)/22.5) was used to estimate insulin resistance50. Overnight fasting lipid profiles were performed at the NIH Clinical Center Central Laboratories. Lipoprotein particle concentration and diameters were measured using an automated Nuclear Magnetic Resonance Spectroscopy (NMR). High-density lipoprotein (HDL) cholesterol efflux capacity was measured using published methods and the macrophage cell line J77451. Human serum lecithin–cholesterol acyltransferase (LCAT) concentration was quantified by ELISA (BioVendor; Ashville, NC). Noninvasive vascular function studies included the cardioankle vascular index (CAVI), peripheral arterial tonometry (PAT; reactive hyperemia index (RHI), and pulse wave velocity (PWV)7. Details are provided in the Supplementary Methods.
Statistical analysis
The sample size chosen was based on what is commonly being used in similar studies and consistent with our experiences in early phase safety studies52,53. No formal power calculations were performed. Data from all randomized subjects were included in the analysis. The adverse events were summarized with frequency counts and percentages for each treatment group. To evaluate the treatment effect on the change from baseline to the end of the treatment period (day 56), a linear mixed-effects model approach was utilized to fit the longitudinal data on continuous safety parameters and clinical outcomes. The model included the baseline value, the STAT4 risk allele status, the treatment group, the categorical time point, and the treatment by time interaction as fixed effects. An unstructured variance-covariance matrix was used to account for the correlations among repeated measures. For variables assessed only at baseline and day 56 during the treatment period, the analysis of covariance (ANOCA) models was used including baseline, treatment, and the STAT4 risk allele status as the covariates. In addition, paired t test, Mann–Whitney U, or ANOVA were used for comparison where appropriate based on normality of distribution. Change scores from baseline to day 84 were analyzed separately using ANCOVA models. No adjustments for multiple comparisons were made to the P values due to the exploratory nature of the analysis. The first patient was enrolled on July 28, 2016 and the last patient was enrolled on September 18, 2017. All statistical analyses were performed with SAS software (version 9.4).
RNA sequencing analysis
Gene expression values were calculated with Partek Genomics Suite 6.6, which was also used for the principal components analysis (PCA) and one-way ANOVA. The ANOVA was performed on log2 transformed RPKM with a 0,1 offset.
NanoString data analysis
Assessment of the quality of the runs was done (Supplementary Methods). Data were combined, normalized, and analyzed in Microsoft Excel (Microsoft Corporation; Redmond, WA). JMP version 14 was used for further statistical analysis and plotting (SAS Corporation; Cary, NC). Synthetic DNA oligonucleotides of each of the 37 ISGs and 4 housekeeping genes were used as a calibration standard to check run and reagent lot consistency.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Pfizer Inc. provided study drug and placebo for this trial. The trial was funded by the Intramural Research Program, NIAMS/NIH. The drug and placebo were provided by Pfizer Inc. through an investigator-initiated research grant. The role of Pfizer was limited to providing study drug and placebo, they had no role in design or conduct of the study, reporting of results, or manuscript preparation.
Source data
Source Data(148K, xlsx)
Author contributions
S.A.H.: study concept, design, protocol development, conduct, analyses, and manuscript draft. S.G.: study design, protocol development, conduct, analyses, and manuscript review. M.D.: study conduct and manuscript review. E.P.: study conduct and manuscript review. Y.T-O.: study conduct and manuscript review. P.M.C.: performed experimental studies and manuscript review. X.W.: performed experimental studies and manuscript review. M.N.: performed experimental studies, results analyses, and manuscript review. M.P.P.: performed experimental studies and manuscript review. R.R.G.: performed experimental studies and manuscript review. X.L.: performed statistical analyses. A.J.B.: study conduct and manuscript review. I.O.-N.: study conduct. Z.M.: data management and manuscript review. Y.S.: study conduct. D.T.: study conduct and manuscript review. J.C.: performed experimental studies. A.B.: performed experimental studies and manuscript review. R.A.: performed experimental studies and manuscript review. F.C.: performed experimental studies and manuscript review. Y.K.: performed experimental studies and manuscript review. A.L.B.: performed experimental studies and manuscript review. H.Z.: performed experimental studies and manuscript review. R.S.: performed experimental studies and manuscript review. K.S.: performed experimental studies and manuscript review. W. L.T.: performed experimental studies. L.V.: performed experimental studies. N.G.: performed experimental studies. V.G.: performed experimental studies. S.L.: performed experimental studies, analyses, and manuscript review. S.R.B.: performed experimental studies, analyses, and manuscript review. M.M.: study design and manuscript review. P.G.: study design, performed experimental studies, and manuscript review. N.N.M.: performed experimental studies and manuscript review. A.T.R.: performed experimental studies, analyses, and manuscript review. B.D.: study concept, design, and manuscript review. J.J.O.: study concept, design, and manuscript review. M.G.: study conduct, analyses, and manuscript review. M.J.K.: study concept, design, conduct, analyses, manuscript draft, and review.
Data availability
RNA-Seq results have been deposited in the Gene Expression Omnibus (GEO) database; Geo Series Entry GSE139940. The study protocol and statistical analysis plan are submitted as part of the Supplementary File. In addition, the source data for fluorescent cell barcoding is provided as a separate file and can be accessed as referenced54. The RNA-Seq and fluorescent cell barcoding data are available without any material transfer and sharing agreement. Any other data are available after executing material transfer and sharing agreement as applicable under laws of the US Government. Please contact Sarfaraz A. Hasni, [email protected] for any data-sharing requests. Source data are provided with this paper.
Competing interests
The NIH and J.J.O.S. have a patent related to JAK inhibitors and receive royalties. The NIH and J.J.O.S. have had a collaborative agreement and development award (CRADA) with Pfizer that pertains to JAK inhibition and tofacitinib. The NIH and J.J.O.S. have an ongoing CRADA for new JAK inhibitors. The remaining authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Amit Saxena, Simon Skene and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/24/2021
In the original version of this Article, the given and family names of author John J. O'Shea were incorrectly tagged, leading to incorrect indexing. This error has now been corrected. The PDF and HTML versions displayed the name correctly at the time of publication.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23361-z.
References
Articles from Nature Communications are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41467-021-23361-z
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41467-021-23361-z.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/107230436
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41467-021-23361-z
Article citations
Systemic lupus erythematosus: pathogenesis and targeted therapy.
Mol Biomed, 5(1):54, 30 Oct 2024
Cited by: 0 articles | PMID: 39472388 | PMCID: PMC11522254
Review Free full text in Europe PMC
Tofacitinib for child-onset systemic lupus erythematosus.
Front Immunol, 15:1457821, 08 Oct 2024
Cited by: 0 articles | PMID: 39439798 | PMCID: PMC11493632
Neutrophils: a key component in ECMO-related acute organ injury.
Front Immunol, 15:1432018, 13 Sep 2024
Cited by: 0 articles | PMID: 39346902 | PMCID: PMC11427252
Review Free full text in Europe PMC
NETosis of psoriasis: a critical step in amplifying the inflammatory response.
Front Immunol, 15:1374934, 01 Aug 2024
Cited by: 0 articles | PMID: 39148738 | PMCID: PMC11324545
Review Free full text in Europe PMC
Interferon Inhibition in SLE: From Bench to Bedside.
Mediterr J Rheumatol, 35(suppl 2):354-364, 30 Jun 2024
Cited by: 0 articles | PMID: 39193183 | PMCID: PMC11345605
Review Free full text in Europe PMC
Go to all (81) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT02535689
SNPs
- (1 citation) dbSNP - rs7574865
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Safety, tolerability, and pharmacokinetics of single oral doses of tofacitinib, a Janus kinase inhibitor, in healthy volunteers.
Clin Pharmacol Drug Dev, 4(2):83-88, 05 Dec 2014
Cited by: 21 articles | PMID: 27128212
Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: Results from a dose-ranging phase 2 trial.
Br J Clin Pharmacol, 84(6):1136-1145, 23 Mar 2018
Cited by: 17 articles | PMID: 29377257 | PMCID: PMC5980553
Adverse drug events associated with 5mg versus 10mg Tofacitinib (Janus kinase inhibitor) twice daily for the treatment of autoimmune diseases: A systematic review and meta-analysis of randomized controlled trials.
Clin Rheumatol, 38(2):523-534, 22 Sep 2018
Cited by: 9 articles | PMID: 30242639
Review
Tofacitinib for the treatment of psoriasis and psoriatic arthritis.
G Ital Dermatol Venereol, 155(4):400-410, 29 Apr 2020
Cited by: 3 articles | PMID: 32348084
Review

 1
1 


