Abstract
Free full text

Latent Autoimmune Diabetes of Adults (LADA) Is Likely to Represent a Mixed Population of Autoimmune (Type 1) and Nonautoimmune (Type 2) Diabetes
Abstract
Latent autoimmune diabetes of adults (LADA) is typically defined as a new diabetes diagnosis after 35 years of age, presenting with clinical features of type 2 diabetes, in whom a type 1 diabetes–associated islet autoantibody is detected. Identifying autoimmune diabetes is important since the prognosis and optimal therapy differ. However, the existing LADA definition identifies a group with clinical and genetic features intermediate between typical type 1 and type 2 diabetes. It is unclear whether this is due to 1) true autoimmune diabetes with a milder phenotype at older onset ages that initially appears similar to type 2 diabetes but later requires insulin, 2) a disease syndrome where the pathophysiologies of type 1 and type 2 diabetes are both present in each patient, or 3) a heterogeneous group resulting from difficulties in classification. Herein, we suggest that difficulties in classification are a major component resulting from defining LADA using a diagnostic test—islet autoantibody measurement—with imperfect specificity applied in low-prevalence populations. This yields a heterogeneous group of true positives (autoimmune type 1 diabetes) and false positives (nonautoimmune type 2 diabetes). For clinicians, this means that islet autoantibody testing should not be undertaken in patients who do not have clinical features suggestive of autoimmune diabetes: in an adult without clinical features of type 1 diabetes, it is likely that a single positive antibody will represent a false-positive result. This is in contrast to patients with features suggestive of type 1 diabetes, where false-positive results will be rare. For researchers, this means that current definitions of LADA are not appropriate for the study of autoimmune diabetes in later life. Approaches that increase test specificity, or prior likelihood of autoimmune diabetes, are needed to avoid inclusion of participants who have nonautoimmune (type 2) diabetes. Improved classification will allow improved assignment of prognosis and therapy as well as an improved cohort in which to analyze and better understand the detailed pathophysiological components acting at onset and during disease progression in late-onset autoimmune diabetes.
Introduction
Latent autoimmune diabetes of adults (LADA) is typically defined as patients diagnosed with diabetes over 35 years of age presenting with clinical features of type 2 diabetes in whom a type 1 diabetes–associated autoantibody is detected. This identifies a group with clinical and genetic features that are intermediate between typical type 1 and type 2 diabetes, and the condition has been termed “slowly evolving immune-mediated diabetes” by the World Health Organization, under the category of hybrid forms of diabetes (1,2). The identification of this intermediate phenotype has led to the idea that autoimmune diabetes in middle age and old age typically progresses more slowly than autoimmune disease in children and young adults (1).
In this Perspective, we show how the definition of LADA predominantly using an imperfect diagnostic test, GAD65 autoantibody (GADA) measurement, in low-prevalence populations will result in a heterogeneous group of true positives (type 1 diabetes) and false positives (type 2 diabetes). This mixed group would explain how the clinical features (e.g., BMI, HbA1c, time to insulin treatment) of a group of individuals with LADA average out to be intermediate between the two subtypes, an alternative explanation of the intermediate phenotype observation. We explore how observations in descriptions of LADA more strongly support the presence of individuals with type 1 diabetes and type 2 diabetes rather than an intermediate condition. While we focus on GADA because of its predominant role in the definition of LADA, the issues described will affect any diagnostic test with imperfect specificity, including other islet autoantibody assays.
A Historical Perspective—LADA as an Intermediate Phenotype Between Typical Type 1 and Type 2 Diabetes
The recognition that pancreatic islet autoantibodies play a key role in defining the discrete and separate etiologies of type 1 and type 2 diabetes occurred in the 1970s (3,4). It was recognized that the islet cell autoantibodies (ICA) could be used to confirm a diagnosis of type 1 diabetes in children and young adults. Subsequent studies demonstrated that some older patients with initially noninsulin-dependent diabetes were also ICA positive and that this subgroup had more rapid progression to insulin (5–7). Following the discovery of insulin antibodies in 1983 (8), GADA was the second specific islet autoantibody to be recognized in 1990 (9). Subsequently, GADA were described in a minority of patients who were diagnosed after 30 years of age and thought to have type 2 diabetes, without initial insulin requirement (10,11). This group of patients was said to have LADA (11). These individuals were found to require insulin treatment earlier and more frequently than people diagnosed with diabetes at a similar age without GADA (12). However, it was far from the 100% rate of requiring insulin treatment seen in young subjects with positive antibodies who had typical type 1 diabetes (13). This led to the concept of LADA as adults with autoimmune diabetes and a less aggressive disease, with an intermediate phenotype between type 1 diabetes in children and type 2 diabetes in middle- and old-aged adults (1). The LADA definition was supported by genetic studies showing that there was an overrepresentation of HLA and non-HLA childhood type 1 diabetes susceptibility alleles as well as of type 2 diabetes susceptibility alleles in this group (1,14–16).
The definition of LADA in some cohorts includes autoimmunity defined by a number of different islet autoantibodies, but the vast majority of cases are identified by positive GADA alone because of other antibodies being less frequently tested and positive results infrequent in GADA-negative older adults (17,18). A number of studies have attempted to define the pathophysiology, epidemiology, and complication risk of these patients (1). This attempt has been limited by a lack of standardization of antibody tests used to define patients, particularly the titer deemed to represent positivity, though considerable effort has been undertaken to address this issue with improvement in assay performance in recent years. While recent reviews have recognized the heterogeneity of this condition, the broad concept of LADA as an intermediate form of autoimmune diabetes has persisted.
Measurable GADA Occur in Control Populations Without Diabetes: Detectable GADA Does Not Always Confirm Autoimmune Diabetes Etiology
Like many immunological tests, the presence of detectable GADA does not confirm the presence of disease; detectable GADA are present in people without diabetes (with the level dependant on the assay, population, and threshold used), with presence of GADA alone associated with low risk for development of type 1 diabetes (19–24). This can been termed “biological false-positive” or “diabetes-irrelevant” islet autoantibodies, with previous studies demonstrating these antibodies may have different epitope specificity (20). The term “biological false-positive” is used to describe detectable islet autoantibodies not associated with autoimmune disease; it does not usually imply a test error, and it is widely recognized that antibodies for many autoimmune conditions may be present in healthy people who do not have associated pathology or, in the majority of cases, go on to develop the associated disease (22,25–27).
The proportion of those who do not have autoimmune etiology diabetes who test positive for an antibody is determined by the test specificity, which will depend upon both the assay characteristics and the threshold chosen to define positivity. For example, an assay threshold yielding 95% specificity in similar control subjects would be expected to be positive in 5% of the population, including those with nonautoimmune (type 2 or monogenic) diabetes.
GADA assays prior to the last decade have not always been as technically reliable as more recent assays: median GADA specificity for the 39 laboratories participating in the 2010 Diabetes Autoantibody Standardization Program was 94%, with specificity ranging from 68% to 100% (20). This means that at that time, in a population without any autoimmune diabetes (such as those with true type 2 diabetes), using an assay with average specificity for that exercise, a median of 6% would be GAD islet antibody–positive, but this number will vary from 32% to <1% depending on the assay and threshold used. This alone may explain much of the heterogeneity in LADA prevalence and characteristics in reported literature: studies using an islet autoantibody assay and cutoff with limited diagnostic specificity will include many participants with positive islet autoantibodies who have diabetes that is not of autoimmune origin. In these cases, therefore, prevalence of LADA will be high, and characteristics will be less classical for type 1 diabetes when compared with studies using high-specificity assays. There has been a marked improvement in assay performance in recent years, with median specificity improving to 98.9% for participating laboratories in the 2018 Islet Autoantibody Standardization Program exercise; however, variation in performance persists (28).
The Implications of Positive Islet Autoantibody Will Be Very Different in Populations With High and Low Prevalence of Autoimmune Diabetes
The prevalence of autoimmune diabetes in the population tested with an islet autoantibody will markedly alter the implications of a positive result, even where a high-specificity assay is used. The positive predictive value (PPV) (the proportion [%] who have the disease when the test is positive) of a biochemical test can be dramatically different depending on the background prevalence of the disease it is aiming to detect. In cases where the disease prevalence is low, the PPV of a test will be lower. This supports the idea that those who test positive for GADA in a population with low prevalence of type 1 diabetes will be a mixture of true positives (type 1 diabetes) and false positives (type 2 diabetes). This is in marked contrast to populations with a high prior likelihood of type 1 diabetes, such as children and adolescents presenting with diabetes, where false positives will be low.
We illustrate this point by examining the proportions of true- and false-positive GADA results in two populations: one with 95% autoimmune etiology diabetes and one with 5% autoimmune etiology diabetes. Based on the median performance of the 2010 Diabetes Autoantibody Standardization Program, GADA had 94% specificity and 86% sensitivity for detecting autoimmune diabetes. Figure 1 shows how, in a population with high prevalence of autoimmune diabetes, out of 100 individuals we would anticipate 95 to be autoimmune and 86% of these will be GADA positive, but we would not expect any false positives (6% of 5 = 0.25). In contrast, for a population with low (5%) prevalence, out of 100 patients we would expect only 5 to be autoimmune and 4 of these GADA positive, but we would also anticipate a similar number of GADA-positive nonautoimmune patients (6% of 95 = ~6). While GADA assays have continued to improve in recent years, even with a high-performance assay, false-positive results will remain common where autoimmune diabetes is infrequent. Using the 2018 islet antibody program median performance (specificity of 98.9%, specificity 69%), with 5% prevalence of autoimmune diabetes, based on the same calculations 23% of those with a positive GADA will have a false-positive result.
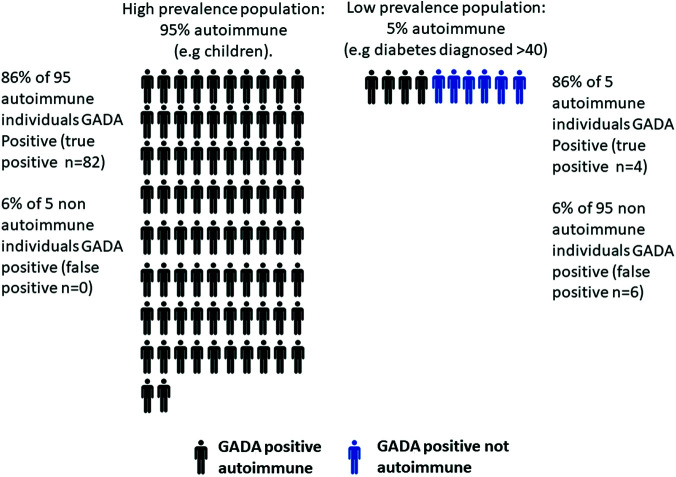
Proportion of GADA-positive individuals who have autoimmune etiology diabetes in a 95% and 5% prevalence population. Expected results from testing 100 participants, using median GADA assay performance from the 2010 Diabetes Autoantibody Standardization Program (assay specificity 94%, sensitivity 86%).
The PPV of a test of a given specificity and sensitivity, at a given disease prevalence, can be calculated with the following equation (29): PPV = (sensitivity × prevalence) / [(sensitivity × prevalence) + (1 – specificity) × (1 − prevalence)]. We can use this to calculate the PPV in populations with different prevalence of type 1 diabetes for assays with different performance (Fig. 2). If we use autoantibodies in a patient who is diagnosed with diabetes under the age of 20 years, when approximately 95% of patients will have type 1 diabetes, using this prevalence the PPV can be calculated to be >99% (Fig. 2) even when using a low-specificity assay and threshold. This means that in this setting, false-positive tests are rare (<1%). This explains why, in children where most diabetes is type 1, a positive islet autoantibodies test will confirm that the diagnosis is highly likely to be type 1 diabetes.
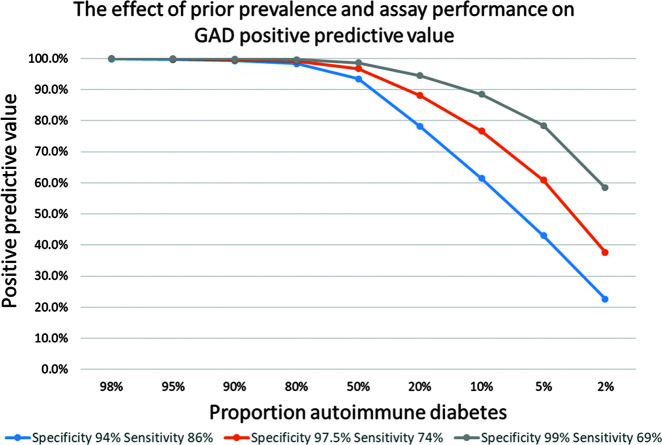
The effect of prior prevalence and assay performance on GADA PPV. Calculated as described in the text for GADA assays with the following characteristics: 94% specificity, 86% sensitivity (Diabetes Autoantibody Standardization Program 2010 median performance); 97.5% specificity, 74% sensitivity; and 99% specificity, 69% sensitivity (Diabetes Autoantibody Standardization Program 2018 median performance).
However, with increasing age, the number of patients with type 2 diabetes increases dramatically, such that in middle age (40–60 years), <5% of incident patients have type 1 diabetes (30). In this setting, false-positive results will be common, even with a high-specificity assay (Fig. 2). Therefore, the group of patients defined by positivity for GADA will consist of two subpopulations: adult-onset autoimmune diabetes (true positives) and adult-onset type 2 diabetes (false positives). In this case, the phenotype of the group will lie between the phenotype of type 1 and type 2 diabetes, with the proportion of those with autoimmune (type 1) and nonautoimmune (type 2) diabetes varying with assay performance and prior prevalence. The characteristics of those with positive GADA will therefore be somewhere between type 1 and type 2 diabetes, but this does not reflect a subgroup with a true intermediate phenotype but rather the average of the two subpopulations.
The Prevalence of LADA When Islet Autoantibody Specificity is Robustly Characterized Suggests That the Majority of Those Meeting Current Definitions of LADA Do Not Have Autoimmune Diabetes
An estimate of the proportion of a LADA population who are unlikely to have autoimmune etiology diabetes can be obtained by examining the extent to which the prevalence of antibody-positive individuals in a population with apparent type 2 diabetes exceeds the expected prevalence in a population without diabetes. Figure 3 shows the proportion of three populations testing positive for GADA using the same assay and laboratory: control subjects without diabetes, patients with new-onset clinically diagnosed type 2 diabetes, and those with long-standing type 2 diabetes with absence of early insulin requirement. This assay and threshold had 100% specificity in the 2018 Islet Autoantibody Standardization Program (n <100), but has 97.5% specificity in this much larger (n = 1,500) control sample, meaning 2.5% of those without autoimmune diabetes will test positive. In those with newly diagnosed type 2 diabetes and long-standing type 2 diabetes, respectively, 4.1% and 3.3% of participants were GADA-positive, only modestly higher than the expected 2.5% positive rate in those without autoimmune etiology diabetes. This is consistent with the majority of GADA-positive individuals with diabetes in these cohorts having diabetes that is not of autoimmune etiology, despite use of a high-performing modern assay. Previous large studies of LADA that have reported standardization program assay performance have been broadly consistent with this finding (18,31–33).
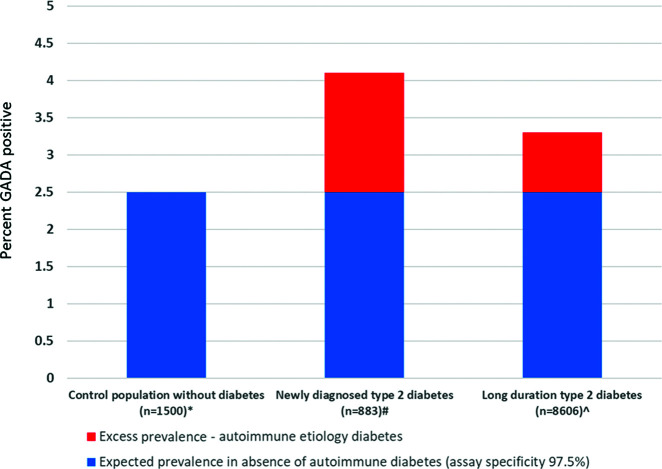
Excess prevalence of GADA in participants with clinically diagnosed diabetes in comparison with a control population without diabetes. GADA assessed using the RSR Limited (Cardiff, U.K.) bridging ELISA by the Blood Sciences Department of Royal Devon and Exeter Hospital, Exeter, U.K. A value >10 units was considered positive. *No known diabetes, HbA1c <48 mmol/mol, n = 1,500 (45). #Aged >18 years at diagnosis, clinical diagnosis of type 2 diabetes, median duration 3 months (A.G.J., StartRight Study Group, unpublished observation).  Age ≥35 years at diagnosis, clinical diagnosis of type 2 diabetes, absence of insulin requirement within 6 months of diagnosis (45).
Age ≥35 years at diagnosis, clinical diagnosis of type 2 diabetes, absence of insulin requirement within 6 months of diagnosis (45).
Studies of LADA Are More Consistent With a Heterogeneous Population of Type 1 and Type 2 Diabetes Rather Than a Single Intermediate Phenotype
We can test whether observations of patients with LADA fit more with a heterogeneous population of patients with type 1 and type 2 diabetes rather than a single intermediate phenotype. If this is the case, then factors altering the specificity and sensitivity of the antibody test, and altering prevalence of type 1 diabetes in the population tested, will alter the PPV of the antibody test and hence alter the proportion of false positives (patients with type 2 diabetes) within the cohort defined by positivity for the antibody test. The lower the prior prevalence, or lower the test threshold (and so specificity), the more false-positive (type 2 diabetes) and the fewer true-positive (type 1/autoimmune etiology) patients will be contributing to the phenotype. Hence, the combined cohort will be more like patients with type 2 diabetes—for example, to be older at diagnosis, have an increased BMI, and be less likely to progress to insulin. These predicted changes can be compared with the changes seen in cohorts of patients with LADA.
How Do Alterations in the Definition of Antibody Positivity Alter the Phenotype in LADA?
The specificity and sensitivity of autoantibody testing can be altered by changing the number of antibodies tested or the titer of GADA considered as positive. We would predict that with less-specific antibody tests (such as use of a low titer threshold and single positive islet auto-antibody), not only does the number of patients with LADA increase but also the phenotype moves to being more type 2 like.
Studies that have examined the relationship between titer and/or number of autoantibodies and clinical phenotype in late-onset initial noninsulin-requiring diabetes are summarized in Table 1. As shown in Table 1, a number of studies have shown that those who have low titers of a single autoantibody have clinical, biochemical, and genetic characteristics more similar to type 2 diabetes, in contrast to those with higher titers (13,18,32–38). In addition, low titers often become negative during follow-up, in contrast to those positive with high titers (39). Where late-onset autoimmune diabetes is defined by two positive autoantibodies (which will markedly increase specificity), a patient group is identified with very high rates of rapid insulin requirement and the clinical and genetic characteristics of young-onset type 1 diabetes (13,14).
Table 1
Summary of studies reporting the impact of differing GADA titers and/or numbers of positive islet autoantibodies on the prevalence and associated characteristics of LADA
| Study | n | Inclusion criteria | Antibody status and threshold | % of study population | PPV for insulin treatment | Age at diagnosis (years) | BMI (kg/m2) | C-peptide (nmol/L) | TG (mmol /L) |
|---|---|---|---|---|---|---|---|---|---|
| Turner 1997 (13) | 1,538* | Age 25–65 years, diagnosis of T2D | GADA and ICA neg | 88 | 14% at 6 years | — | — | — | — |
| GADA 20–60 units/L | 4.0 | 23% at 6 years | — | — | — | — | |||
| GADA ≥60 units/L | 7.1 | 63% at 6 years | — | — | — | — | |||
| GADA and ICA low titer | 4.3 | 77% at 6 years | — | — | — | — | |||
| GADA and ICA high titer | 3.0 | 89% at 6 years | — | — | — | -— | |||
| Tuomi 1999 (35) | 1,122 | Clinical diagnosis of T2D | GADA <5 RU | 90.8 | — | — | 27** | 0.62 | 2.0** |
| GADA 5–38 RU | 6.2 | — | — | 27** | 0.55 | 1.6** | |||
| GADA >38 RU | 3.0 | — | — | 25** | 0.27 | 1.5** | |||
| Davis 2000 (36) | 1,225 | Diagnosis of T2D at age >60; or no initial insulin | GADA neg | 96.3 | 11.2% at avg. 3 years | 61.1 | 29.6 | — | 1.9 |
| GADA 20–60 units/L | 1.4 | 17.6% at avg. 2.3 years | 58.4 | 27.4 | — | 2.0 | |||
| GADA >60 units/L | 2.3 | 46.4% at avg. 5.3 years | 59.7 | 27.0 | — | 1.3 | |||
| Genovese 2006 (37) | 881 | Diagnosis of T2D at age 40–70 years, hospital based | GADA neg (<3 units) | 95.4 | 20.2% at avg. 8.1 years | 52.0 | 29.2 | — | — |
| GADA 3–10 units | — | 42% at avg. 8.7 years*** | — | — | — | — | |||
| GADA >10 units | — | 73.9% at 8.7 years*** | — | — | — | — | |||
| GADA >3 units and IA-2A | 2.2 | 78.9% at 8.7 years*** | — | — | — | — | |||
| Buzzetti 2007 (34) | 4,248 | 35–75 years at diagnosis, not insulin treated, duration 6 months to 6 years | GADA neg | 95.5 | — | 55.5 | 29.9 | — | — |
| GADA pos <32 units | 2.3 | — | 51.1 | 28.4 | — | — | |||
| GADA pos >32 units | 2.2 | — | 49.1 | 26.2 | — | — | |||
| Maioli 2010 (38) | 5,568 | T2D age 35–70 years, no insulin 8 months post diagnosis; duration <5 years | GADA neg | 95.1 | — | 57.7 | 30.8 | — | 1.4 |
| GADA pos index <0.5 | 2.4 | — | 55.4 | 28.8 | — | 1.3 | |||
| GADA pos index >0.5 | 2.5 | — | 53.4 | 26.9 | — | 1.3 | |||
| Hawa 2013 (18) | 6,156 | Age 30–70 years, no insulin rx for 6 months from diagnosis | GADA neg | 90.2 | 13.2% at avg. 2.3 years | 54.9 | 30.9 | — | 2.0 |
| GADA 70–200 WHO units | 2.2 | 39.7% at avg. 2.5 years | 47.9 | 28.5 | — | 1.8 | |||
| GADA >200 WHO units | 6.5 | 54.6% at avg. 2.1 years | 47.0 | 26.7 | — | 1.2 | |||
| Zhou 2013 (33) | 4,880 | Onset age >30 years, no DKA, no insulin by 6 months, recruit by 1 year of dx | GADA neg | 94.1 | — | 51.4 | 24.8 | 0.64 | 1.8 |
| GADA 18–180 WHO units | 4.3 | — | 51.2 | 24.5 | 0.51 | 1.6 | |||
| GADA >180 WHO units | 1.6 | — | 48.1 | 22.3 | 0.32 | 1.2 | |||
| Maddaloni 2015 (32) | 17,072 | Onset age 30–70 years, no DKA, no insulin rx by 6 months of dx | GADA and IA-2A neg | 97.3 | — | 46.9 | 31.4 | — | — |
| GADA >10 units/mL | 2.6 | 21.8% at 5 years | 45.1 | 30.7 | — | — | |||
| GADA >10 units/mL and IA-2A 10 units/mL | 0.1 | — | 41.2 | 28.5 | — | — |
—, not reported; avg., average; DKA, diabetic ketoacidosis; IA-2A, islet antigen 2 antibody; neg, negative; pos, positive; PPV, positive predictive value; RU, relative unit; rx, prescription; T2D, type 2 diabetes; TG, triglyceride; WHO, World Health Organization.
In populations with a much higher prior prevalence of type 1 diabetes, such as children and young adults with diabetes, the impact of altering test specificity by using higher titers, or multiple positive islet autoantibodies, is modest (13,40,41). These findings are consistent with the influence of false-positive results being greater in low-prevalence populations (discussed below). In high-prevalence populations, even those with a weak positive antibody test will have a high probability of type 1 diabetes: test specificity will therefore be less critical to PPV and will have less effect on the characteristics of the test positive population (42).
How Do Differences in the Prevalence of Type 1 Diabetes in the Population in Which LADA Is Defined Alter the Phenotype?
Factors that alter the prevalence of the proportion of patients with type 1 diabetes will alter the PPV of the antibody test and hence the proportions of patients with type 1 and type 2 diabetes defined by antibody positivity. The easiest example is the age of the cohort that patients are taken from.
Type 2 diabetes is markedly more common with increased age; therefore, as demonstrated in Figs. 1 and and2,2, a positive islet antibody will be far less likely to be a false-positive result in younger people, where the prior probability of autoimmune diabetes is higher (30). This means the younger the population tested, the more type 1 like it is predicted to be. This has been seen in many studies; for example, in the UK Prospective Diabetes Study (UKPDS) of patients with a diagnosis of type 2 diabetes, young single-autoantibody–positive participants had rapid progression to insulin, in marked contrast to older participants (13).
Other clinical criteria that make type 1 diabetes more or less prevalent will also alter the PPV of a positive GAD islet autoantibody. For example, the frequently used exclusion of those patients treated with insulin within 6 months of diagnosis will reduce the number of true positives (type 1/autoimmune diabetes) and increase the number of false positives (type 2 diabetes), making the combined phenotype more type 2 like. This effect has been clearly seen in large series when looking at clinical criteria like age of onset, BMI, and time to insulin treatment, where selecting a subpopulation with lower prior probability of type 1 diabetes (for example, an older, more obese, or noninsulin-treated population) results in fewer who are GADA positive, and these participants will have characteristics mores similar to type 2 diabetes (18,38,43,44). This is also apparent when using genetic susceptibility to type 1 diabetes: patients with antibody-positive “type 2 diabetes” who lack genetic susceptibility to type 1 diabetes (and therefore have low prior probability) have low rates of early insulin requirement, in contrast to those at high genetic risk for type 1 diabetes (45).
Bimodality of GADA Titers and Differences in Epitope Specificity Support the Presence of “True” (Disease-Associated) and “False” (Disease-Irrelevant) Positive Islet Autoantibody Results
Antibody studies looking at both titer and epitope support LADA consisting of two subpopulations. A number of studies have reported a bimodal distribution of GADA titer, suggesting two subpopulations with low and high GADA titer (18,33,34,36,38). Recently, it has been shown that positive GADA that are not associated with disease have different epitope specificity and that in “LADA” different epitope specificities identify different subpopulations: patients who are GADA positive to standard full-length assays, but not to assays using GAD truncated to remove the N-terminus, have characteristics similar to antibody-negative patients with type 2 diabetes (20,46,47).
Definitions of Late-Onset Type 1 Diabetes That Are Independent of Islet Autoantibody Testing and Clinical Features Do Not Suggest an Intermediate Phenotype
Recent research has examined the characteristics of late-onset autoimmune diabetes defined by examining the characteristics of excess diabetes occurring in those who are genetically susceptible (48). This technique does not suffer from either the problem of false-positive islet antibody results or the problem of presupposing characteristics if a definition is based on clinical features. In marked contrast to autoimmune diabetes defined by GADA testing, older participants with type 1 diabetes caused by excess genetic risk appeared to have near universal early insulin requirement, with 89% treated with insulin within the first year of diagnosis and 11% developing ketoacidosis. When type 1 diabetes in later life is defined by the development of severe, near absolute insulin deficiency, the clinical phenotype is very similar to that defined by this genetic technique (49).
The Presence of a Biomarker That Can Occur in the Absence of Disease Should Not Define a Disease State
It is our opinion that the presence of islet autoantibodies in an individual from a population with low type 1 diabetes prevalence should not be considered to equate to a diagnosis of autoimmunity in that individual. Autoantibodies are a marker of autoimmunity and not the pathogenic agent (50). In other autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis, the presence of the highly associated autoantibody (which, like diabetes autoantibodies, may occur in healthy control populations) is not sufficient to make a diagnosis on its own, and other clinical, biomarker, or imaging criteria need to be met to make the diagnosis (27).
Practical Implications for Clinicians
For clinicians, the take-away message is that islet autoantibodies have far greater diagnostic utility when tested in patients with a clinical suspicion of type 1 diabetes. In those with clinical features suggestive of type 1 diabetes, a positive islet antibody result, using a modern validated assay, will usually confirm a diagnosis of autoimmune diabetes. However, in an adult without clinical features of type 1 diabetes, as we have shown, it is most likely that a single positive antibody will represent a false-positive result. When considering a result at diagnosis, prediction model approaches that combine islet antibodies with other features may offer a practical approach for a clinician to assess the predictive value of a positive autoantibody (51). Given the uncertainty at diagnosis in insulin-treated patients, C-peptide measurement, preferably >3–5 years after onset, is critical to establish treatment requirements where diabetes subtype is uncertain (52,53).
Implications for Researchers
To address any research question related to autoimmune diabetes in adults, it is essential that the population studied has autoimmune rather than type 2 diabetes. Where autoantibodies are used to define autoimmune diabetes, the performance of the assay used should be robustly demonstrated, as this is critical to interpretation of research findings (28). While modern islet autoantibody assay quality has increased, unusually high specificity is required where the prior probability of autoimmune diabetes is low. Therefore, if antibodies alone are used to define autoimmune diabetes in a low-risk population, such as adults with apparent type 2 diabetes, specificity should be increased. This could be achieved through requirement for multiple positive autoantibodies to define autoimmunity and/or through the use of assays with restricted epitope specificity (20,46). An alternative approach would be to use clinical features or other biomarkers (such as genetic risk scores or C-peptide) to increase prior probability of autoimmune diabetes, either alone or through prediction models that combine multiple features. Ultimately, the optimal approach will depend on the research question being addressed; for example, the use of clinical features or genetic risk to increase prior probability will not be appropriate for studies assessing these outcomes but may be appropriate for unrelated research questions.
Conclusions
Autoimmune diabetes in later life, and its diagnosis, is an important and challenging clinical problem. We have shown that observations in LADA, where autoimmune diabetes has been diagnosed on the basis of the presence of GAD islet autoantibodies in populations with low prevalence of type 1 diabetes, can be predominantly explained by the test identifying a mixture of true-positive (type 1 diabetes) patients and false-positive (type 2 diabetes) patients. Specifically, the intermediate phenotype of LADA will at least partly reflect a combination of two heterogeneous populations with very different phenotypes rather than a true intermediate subtype of diabetes. Improvement of the diagnostic approach by applying new findings from the field will greatly improve classification from the pioneering work that led to the first descriptions of LADA more than 25 years ago.
Article Information
Funding. A.G.J. is supported by a National Institute for Health Reseach (NIHR) Research Clinician Scientist award (CS-2015-15-018). T.J.M. is supported by an NIHR Senior Clinical Lecturer fellowship. B.M.S. and A.T.H. are supported by the NIHR Exeter Clinical Research Facility. W.H. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant U01-DK-063829 and JDRF grants 3-SRA-2019-827-S-B and 2-SRA-2020-964-S-B. A.T.H. is an NIHR and Wellcome Trust (098395/Z/12/Z) senior investigator.
The views given in this article do not necessarily represent those of the Wellcome Trust, the NIHR, the National Health Service, the Department of Health and Social Care, or the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care
2010;33:104–108 [Europe PMC free article] [Abstract] [Google Scholar]
8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care
2010;33:104–108 [Europe PMC free article] [Abstract] [Google Scholar]Articles from Diabetes Care are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/dc20-2834
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.org/care/article-pdf/44/6/1243/632473/dc202834.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/106220147
Article citations
The epidemiology of type 1 diabetes mellitus in older adults.
Nat Rev Endocrinol, 24 Oct 2024
Cited by: 0 articles | PMID: 39448829
Review
Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes.
Diabetologia, 67(9):1731-1759, 01 Sep 2024
Cited by: 1 article | PMID: 38910151 | PMCID: PMC11410955
Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes.
Diabetes Care, 47(8):1276-1298, 01 Aug 2024
Cited by: 1 article | PMID: 38912694
Review
Prevalence of latent autoimmune diabetes in adults and insulin resistance: a systematic review and meta-analysis.
Eur J Transl Myol, 34(3), 28 Aug 2024
Cited by: 0 articles | PMID: 39221599 | PMCID: PMC11487667
Polysaccharides from Medicinal Plants: Bridging Ancestral Knowledge with Contemporary Science.
Plants (Basel), 13(13):1721, 21 Jun 2024
Cited by: 0 articles | PMID: 38999561 | PMCID: PMC11243750
Review Free full text in Europe PMC
Go to all (30) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Latent autoimmune diabetes in youth shows greater autoimmunity than latent autoimmune diabetes in adults: Evidence from a nationwide, multicenter, cross-sectional study.
Pediatr Diabetes, 23(5):578-587, 17 May 2022
Cited by: 2 articles | PMID: 35451144
Autoantibody profile and epitope mapping in latent autoimmune diabetes in adults.
Ann N Y Acad Sci, 958:99-106, 01 Apr 2002
Cited by: 14 articles | PMID: 12021088
Latent Autoimmune Diabetes in Adults: A Review of Clinically Relevant Issues.
Adv Exp Med Biol, 1307:29-41, 01 Jan 2021
Cited by: 5 articles | PMID: 32424495
Review
Autoimmunity-Associated PTPN22 Polymorphisms in Latent Autoimmune Diabetes of the Adult Differ from Those of Type 1 Diabetes Patients.
Int Arch Allergy Immunol, 177(1):57-68, 12 Jun 2018
Cited by: 4 articles | PMID: 29895027
Funding
Funders who supported this work.
NIDDK NIH HHS (1)
Grant ID: U01 DK063829
National Institute for Health Research (NIHR) (2)
Grant ID: CS-2015-15-018
Preventing death and long term institutional care by developing a newborn screening strategy to Identify Neonatal Diabetes
Dr Timothy McDonald, Royal Devon and Exeter NHS Foundation Trust
Grant ID: ICA-SCL-2016-02-003
Wellcome Trust (1)
Grant ID: 098395/Z/12/Z




