Abstract
Free full text

Self-Assembling Influenza Nanoparticle Vaccines Elicit Broadly Neutralizing H1N1 Antibodies
Associated Data
Abstract
Influenza viruses pose a significant threat to the public and a burden on global health systems1,2. Each year, influenza vaccines must be rapidly produced to match circulating viruses, a process constrained by dated technology and vulnerable to unexpected strains emerging from humans and animal reservoirs. Here, we use knowledge of protein structure to design self-assembling nanoparticles that elicit more broad and potent immunity than traditional influenza vaccines. The viral hemagglutinin (HA) was genetically fused to ferritin, a protein that naturally forms nanoparticles composed of 24 identical polypeptides3. HA was inserted at the interface of adjacent subunits so that it spontaneously assembles and generates eight trimeric viral spikes on its surface. Immunization with this influenza nanoparticle vaccine elicited hemagglutination inhibition (HAI) antibody (ab) titers >10-fold higher than those from the licensed inactivated vaccine. Furthermore, it elicited neutralizing abs (nAbs) to two highly conserved vulnerable HA structures that are targets of universal vaccines, the stem4,5 and the receptor binding site (RBS) on the head6,7. Finally, abs elicited by a 1999 HA-nanoparticle vaccine neutralized H1N1 viruses from 1934 to 2007 and protected ferrets from an unmatched 2007 H1N1 virus challenge. This structure-based, self-assembling synthetic nanoparticle vaccine improves the potency and breadth of influenza virus immunity, and it provides a foundation for building broader vaccine protection against emerging influenza viruses and other pathogens.
Influenza outbreaks arise from viruses that evade human immunity. Advances in influenza virus structural biology, nanotechnology, and gene delivery offer new opportunities to develop improved vaccines that can confer more broadly protective immunity against diverse influenza viruses4–6,8,9. Among recent innovations, several natural proteins have shown the ability to form nanoparticles well-suited for antigen presentation and immune stimulation10. One such protein is ferritin, a ubiquitous iron storage protein that self-assembles into nanoparticles3. Though ferritin has been used to display exogenous peptides11, it has not been possible to display viral glycoproteins because of their complexity and requirements for trimerization. Additionally, recombinant ferritins made in prokaryotic cells were not subjected to mammalian glycosylation and other posttranslational modifications typical of viral proteins11–13. Structural analysis of ferritin suggested that it would be possible to insert a heterologous protein, specifically influenza virus HA, so that it could assume the physiologically relevant trimeric viral spike (Fig. 1a). Ferritin forms a nearly spherical particle composed of 24 subunits arranged with octahedral symmetry around a hollow interior. The symmetry includes eight three-fold axes on the surface. The aspartic acid (Asp) at residue 5 near the NH2 terminus is readily solvent accessible, and the distance (28 Å) between each Asp5 on the three-fold axis is almost identical to the distance between the central axes of each HA2 subunit of trimeric HA (Fig. 1a, right). We therefore hypothesized that HA would trimerize properly if inserted into this structure.
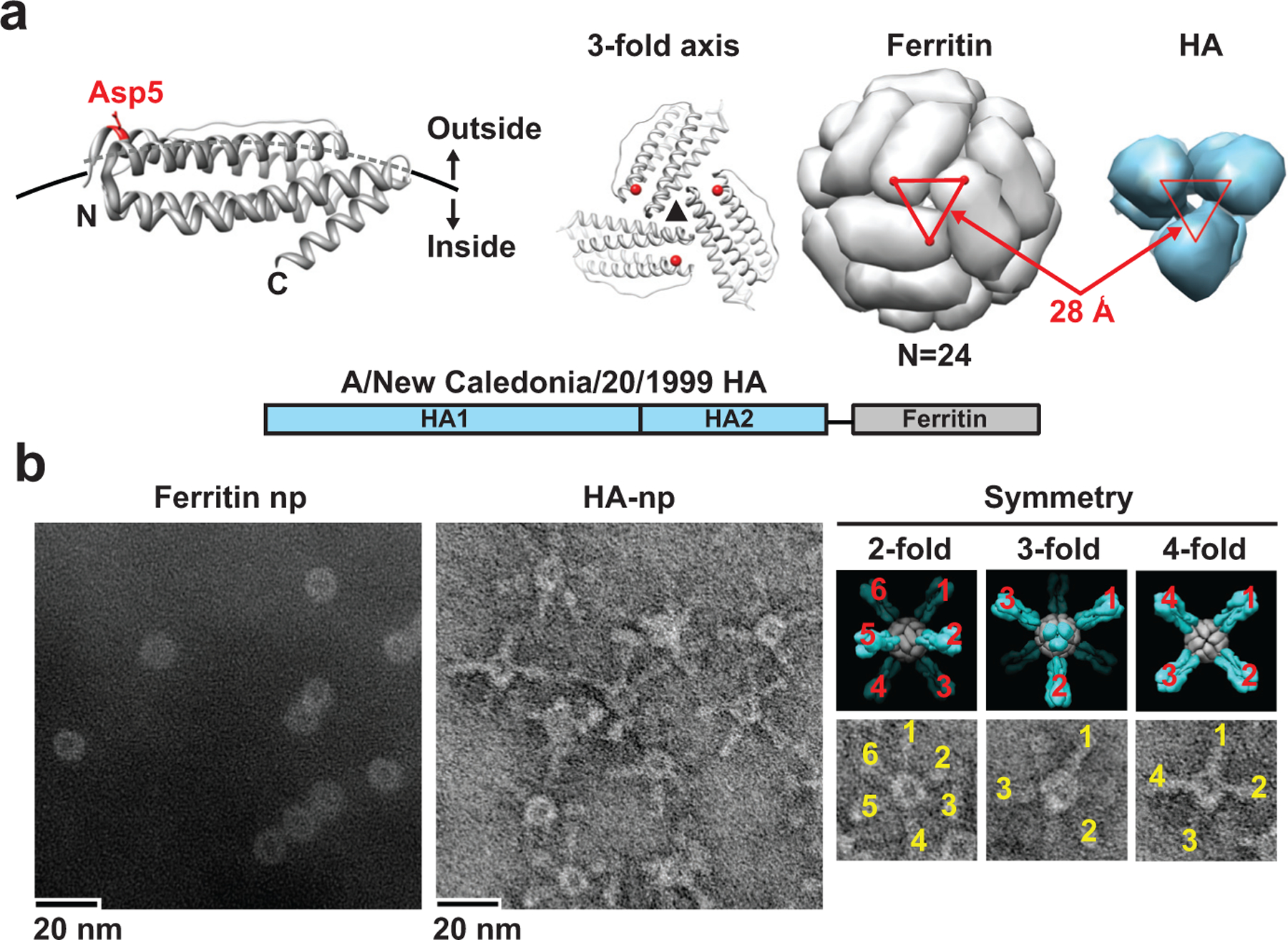
a, A subunit of H. pylori nonheme ferritin (PDB: 3bve) (left). The NH2- and COOH-termini are labeled as N and C, respectively. Three subunits surrounding a three-fold axis are shown (middle) and the Asp5 is colored in red. An assembled ferritin nanoparticle and an HA trimer (PDB: 3sm5) (viewed from membrane proximal end) (right). A triangle connecting the Asp 5 residues at the three-fold axis is shown in red. The same triangle is drawn on the HA trimer (right). A schematic representation of the HA-ferritin fusion protein is shown (bottom). b, Negatively stained TEM images of nanoparticles (np) (left and middle). Computational models and observed TEM image (right, top and bottom panels) representing octahedral 2-, 3- and 4-fold symmetries of HA-nanoparticles are shown as indicated. Visible HA spikes are numbered in the images.
To test this hypothesis, we genetically fused the ectodomain of A/New Caledonia/20/1999 (1999 NC) HA to Helicobacter (H.) pylori nonheme ferritin14 (Fig. 1a, bottom), a ferritin that diverges highly from its mammalian counterparts (Supplementary Fig. 1). This fusion protein was expressed in mammalian cells, and self-assembly of ferritin and HA-ferritin nanoparticles was confirmed by size exclusion chromatography and dynamic light scattering (Supplementary Fig. 2a, b). HA-ferritin also had the expected apparent molecular weight of 85 kDa (Supplementary Fig. 2c). While ferritin alone formed smooth spherical particles as visualized by transmission electron microscopy (TEM), HA-ferritin exhibited clearly visible spikes protruding from the spherical core (Fig. 1b, Ferritin np vs. HA-np). Remarkably, the placement of these spikes illustrated the octahedral symmetry of the HA-nanoparticle design. Octahedral two-, three- and four-fold axes were distinctly observed in the TEM image (Fig. 1b, right). These data demonstrated the formation of trimeric HA spikes on self-assembling nanoparticles.
To verify the antigenicity of the HA spikes on the nanoparticles, we analyzed their reactivity with an anti-HA head monoclonal ab (mAb) and a conformation-dependent mAb, CR6261, which recognizes a conserved structure on the HA stem4. The HA-nanoparticle binds to anti-head and anti-stem mAbs with affinities similar to trimeric HA or trivalent inactivated influenza vaccine (TIV) containing the same 1999 NC HA at equimolar concentrations of HA (Supplementary Fig. 3a). Analogous to trimeric HA, the HA-nanoparticle also blocked neutralization by CR6261 and another stem-directed mAb, F10 (ref. 5) (Supplementary Fig. 3b). These results confirmed that HA molecules on the HA-nanoparticle antigenically resembled the physiological HA viral spike.
To assess the immunogenicity of the HA-nanoparticle, mice were immunized twice with TIV or HA-nanoparticles with or without Ribi adjuvant. The HA-nanoparticles induced 1.6-fold higher HAI titers than TIV in the absence of adjuvant. While this increase did not reach statistical significance, HAI titers were 7.2-fold higher in animals receiving HA-nanoparticles when Ribi was used (Fig. 2a, left; p<0.0001), and a similar effect was observed in the neutralization and ELISA titers (Fig. 2a, middle and right; p<0.0001). For example, neutralization titers elicited by HA-nanoparticles as assessed by the concentration of ab needed to inhibit viral entry by 90% (IC90) were ~34 times higher than TIV (Fig. 2a, middle). We also evaluated whether a similar immune response can be induced by HA-nanoparticles with MF59, an adjuvant that has been used in humans15. Similarly high HAI and IC90 titers were observed in mice receiving MF59-adjuvanted HA-nanoparticles (4,608±512 and 47,140±22,561, respectively), and the titers were significantly higher than those induced by either non-adjuvanted HA-nanoparticles (p=0.0005 and 0.0311 for HAI and IC90, respectively) or MF59-adjuvanted TIV (p=0.0016 and 0.0388 for HAI and IC90, respectively) (Fig. 2b). These results demonstrated the feasibility of using the HA-nanoparticles with an adjuvant suitable for humans16. Because higher titers were observed using either adjuvant, further comparisons were performed with adjuvant (Ribi). Neutralization against a panel of H1N1 strains revealed not only increased potency, but also enhanced breadth, stimulated by HA-nanoparticles compared with TIV or trimeric HA (Fig. 2c). Neutralization against two unmatched, highly divergent H1N1 strains, A/Puerto Rico/8/1934 (1934 PR8) and A/Singapore/6/1986 (1986 Sing), was only observed in mice immunized with the HA-nanoparticles, and the titer against the contemporary strain A/Brisbane/59/2007 (2007 Bris) was more than 10-fold higher in mice immunized with HA-nanoparticles than with TIV (Fig. 2c).
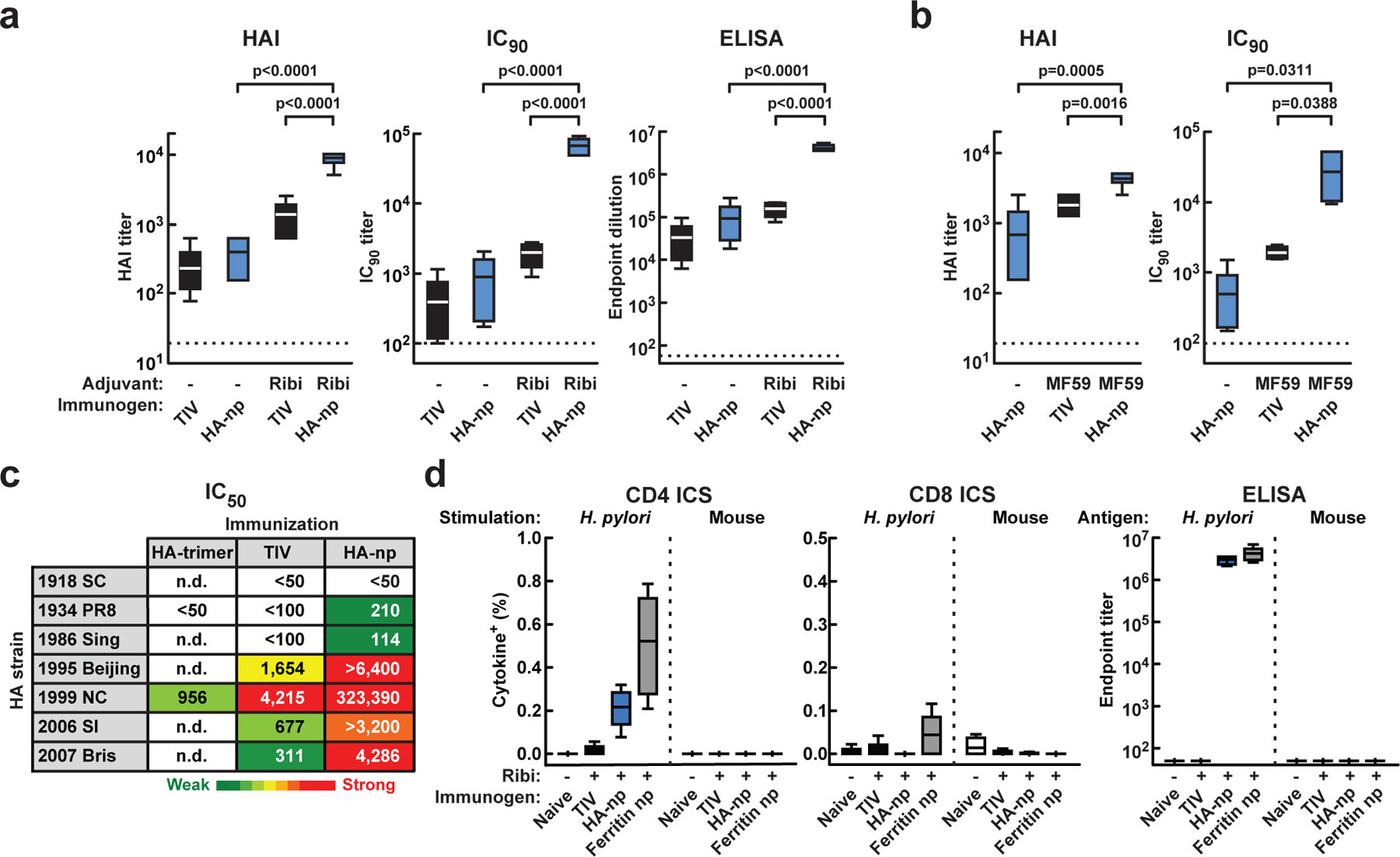
a, HAI (left), IC90 neutralization (middle), and anti-HA ab endpoint titers (right) after two immunizations of TIV or HA-nanoparticles with or without (−) Ribi. b, HAI (left) and IC90 (right) titers after two immunizations of TIV with MF59 or HA-nanoparticles with or without (−) MF59. Two of five mice immunized with MF59-adjuvanted HA-nanoparticles exhibited IC90 titer >51,200 and were plotted as 51,200. The data are presented as box-and-whiskers plots (boxed from lower to upper quartile with whiskers from minimum to maximum) with lines at the mean (n=5). c, Neutralization breadth of the immune sera (with Ribi). IC50 titers against a panel of H1N1 pseudotyped viruses were determined. Heat map is colored in gradient from green to yellow to red reflecting the neutralization strength. d, Cellular (left and middle) and humoral (right) immune responses against H. pylori and mouse ferritins. Cells expressing IFN-γ, TNFα, or IL-2 upon stimulation with peptides covering H. pylori or mouse ferritins were combined and plotted as cytokine+. The data are presented as box-and-whiskers plots with lines at the mean (n=5).
We next examined whether preexisting immunity to ferritin or to other HA subtypes would interfere with subsequent immunization using HA-nanoparticles. Mice pre-immunized with either H3 (A/Perth/16/09, 2009 Perth) HA-nanoparticles or empty ferritin nanoparticles generated substantial anti-H3 HA and/or anti-H. pylori ferritin ab responses (Supplementary Fig. 4a). They were then immunized with H1 (1999 NC) HA-nanoparticles. Comparable HAI, IC90 and ELISA titers against 1999 NC HA were observed in naïve animals as well as in groups pre-immunized with H3 HA-nanoparticles or empty ferritin nanoparticles (Supplementary Fig. 4b). These results indicated that preexisting anti-H. pylori ferritin immunity did not diminish the HA-specific ab response.
To address the concern that immunization with ferritin might abrogate immune tolerance and induce autoimmunity, we analyzed T-cell and ab responses against murine and H. pylori ferritins in HA-nanoparticle-immunized mice. While we found an increase in intracellular cytokine staining (ICS) of CD4+ T cells stimulated with H. pylori ferritin peptides (Fig. 2d, left), no increase in CD4+ or CD8+ ICS responses to murine ferritin peptides were observed (Fig. 2d, left and middle). In addition, abs to H. pylori ferritin, but not to mouse ferritin, were detected in the immune sera (Fig. 2d, right). We therefore found no evidence of immunity to autologous ferritin in mice. Moreover, the HA-nanoparticles are unlikely to affect iron homeostasis in vivo because of their minimal iron incorporation activity (Supplementary Fig. 5).
We next generated a trivalent vaccine comprising three, separately purified HA-nanoparticles formulated into a single vaccine dose, analogous to a standard TIV. The strains chosen were H1 (A/California/04/09, 2009 CA), H3 (2009 Perth) and influenza B (B/Florida/04/06, 2006 FL). All three HA-nanoparticles self-assembled and displayed morphology similar to 1999 NC HA-nanoparticles (Supplementary Fig. 6a). Immunogenicity of multispecific HA-nanoparticles (combination of three monospecific HA-nanoparticles) was compared to a seasonal TIV containing the same H1 and H3 strains and a mismatched influenza B (B/Brisbane/60/08). HAI titers against homologous H1N1 and H3N2 viruses were significantly increased in animals immunized with multispecific HA-nanoparticles relative to TIV (Supplementary Fig. 6b; p=0.0125 and 0.0036, respectively). When compared to animals immunized with the corresponding monospecific HA-nanoparticles, HAI titers against H1N1 and H3N2 viruses induced by multispecific HA-nanoparticles were comparable (Supplementary Fig. 6b). Therefore no substantial antigenic competition was observed with the multispecific HA-nanoparticle vaccine.
We next examined the immunogenicity of HA-nanoparticles (1999 NC) in ferrets. Three weeks after the first immunization, all ferrets receiving Ribi-adjuvanted HA-nanoparticles generated protective HAI titers against homologous virus (>40), while only 50% (3/6) of Ribi-adjuvanted TIV-immunized ferrets induced titers greater than 40 (Fig. 3a, left; p=0.0056). The same difference was also observed for both neutralization and ELISA titers (Fig. 3a, middle and right; p=0.0047 and p=0.0045, respectively), documenting the potency of HA-nanoparticles in a second species. After boosting, the HAI, IC90 and ELISA titers of the HA-nanoparticle-immune sera were ~10-fold higher than those of TIV-immune sera (Fig. 3a, left, middle and right; 457±185 vs. 5,760±1,541, p=0.0066; 598±229 vs. 5,515±1,074, p=0.0012; and 5,902±1,851 vs. 55,105±13,018, p=0.0038, respectively). Remarkably, a single immunization of HA-nanoparticles induced immune responses comparable to two immunizations of TIV (Fig. 3a).
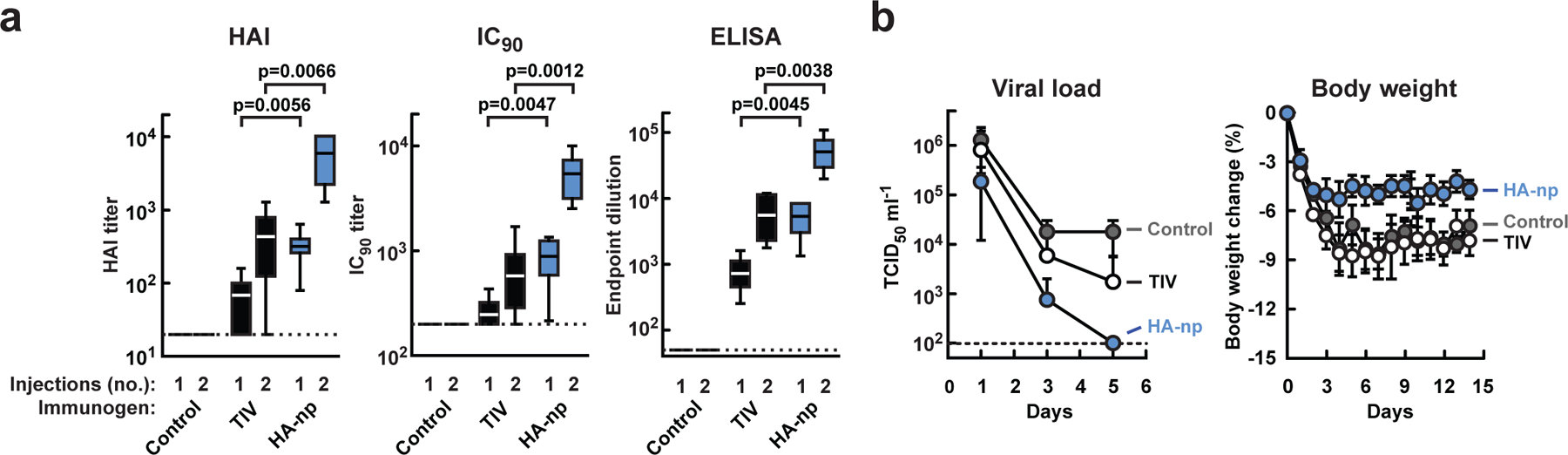
a, HAI (left), IC90 (middle), and anti-HA ab endpoint titers (right) against 1999 NC HA. Immune sera were collected after the first (1) and second (2) immunizations. The data are presented as box-and-whiskers plots with lines at the mean (n=6). b, Protection of immunized ferrets from 2007 Bris virus challenge. Challenge was performed with 106.5 EID50 via intranasal inoculation. Virus titers in the nasal washes were determined by TCID50 assay (left). The mean viral loads with s.d. at each time point were plotted (n=6). Change in body weight after virus challenge was monitored (right). Each data point represents the mean percent change in body weight from day 0 (pre-challenge) with s.e.m. (n=6).
To determine whether HA-nanoparticles could confer protection against an unmatched H1N1 virus, immunized ferrets were challenged with 2007 Bris virus, which had not yet evolved when 1999 NC circulated and required a different seasonal vaccine to confer protection in humans. Ferrets immunized with HA-nanoparticles showed a significant reduction in viral shedding beginning 1 day after challenge compared to the sham control group (Fig. 3b, left; p=0.0259). At the same time point, no significant reduction in viral shedding was seen in the TIV-immunized group (Fig. 3b, left; p=0.4665). In addition, HA-nanoparticle-immunized ferrets suffered less weight loss compared to the TIV-immunized and sham control animals (Fig. 3b, right), further demonstrating the protective efficacy of HA-nanoparticles.
Interestingly, unlike the TIV-immune sera which preferentially neutralized homologous 1999 NC and another closely related strain (A/Beijing/262/1995, 1995 Beijing), sera from HA-nanoparticle-immunized ferrets broadly neutralized heterologous 1986 Sing, 1995 Beijing, A/Solomon Islands/3/2006 (2006 SI) and 2007 Bris viruses (Fig. 4a, left). The recent identification of two classes of broadly nAbs (bnAbs) that target the highly conserved but vulnerable regions of HA suggests a potential pathway to develop influenza vaccines with broad coverage17. One class of bnAbs recognizes a hydrophobic groove on the HA stem and neutralizes virus by inhibiting membrane fusion4,5,18–21. The second class recognizes the RBS on the HA head and inhibits viral entry6,7,22. To determine whether the cross-reactivity across diverse H1N1 strains induced by HA-nanoparticles included nAbs to the HA stem epitope, ferret immune sera were pre-absorbed with cells expressing a stem mutant (ΔStem)8 HA to remove non-stem-directed abs and analyzed for binding to wild-type (WT) or ΔStem HA as previously described8. Stem-specific abs were detected in HA-nanoparticle-immunized ferrets (6/6) in greater frequency and magnitude than TIV-immunized ferrets (2/6) (Fig. 4b, left; p=0.0056). Moreover, binding of these pre-absorbed sera to HA was reduced by CR6261 (Fig. 4b, right; p=0.0019), further documenting the presence of abs targeting the same epitope as CR6261. The HAI titers against heterologous 2007 Bris virus were also significantly higher in ferrets immunized with HA-nanoparticles (6/6) than with TIV (3/6) (Fig. 4a, right; p=0.0054). Interestingly, HA-nanoparticle-immune sera have HAI responses against a highly divergent 1934 PR8 strain, with titers ≥40 in all ferrets. However, no HAI titers against 1934 PR8 were detected in TIV-immunized ferrets (Fig. 4a, right). These data suggested that the HA-nanoparticles might elicit another class of nAb directed towards the conserved RBS in the HA head. To dissect the specificity of the RBS-directed ab response, we generated an RBS mutant HA (ΔRBS) by introducing a glycosylation site in the sialic acid binding pocket at residue 190 (Supplementary Fig. 7)23. Ferret immune sera were absorbed with ΔRBS HA-expressing cells to remove abs to HA outside of this region and tested for binding against WT or ΔRBS HA. RBS-directed abs were detected with titers of >2,000 in all HA-nanoparticle-immunized ferrets, but only in 1 of 6 ferrets that received TIV (Fig. 4b, middle).
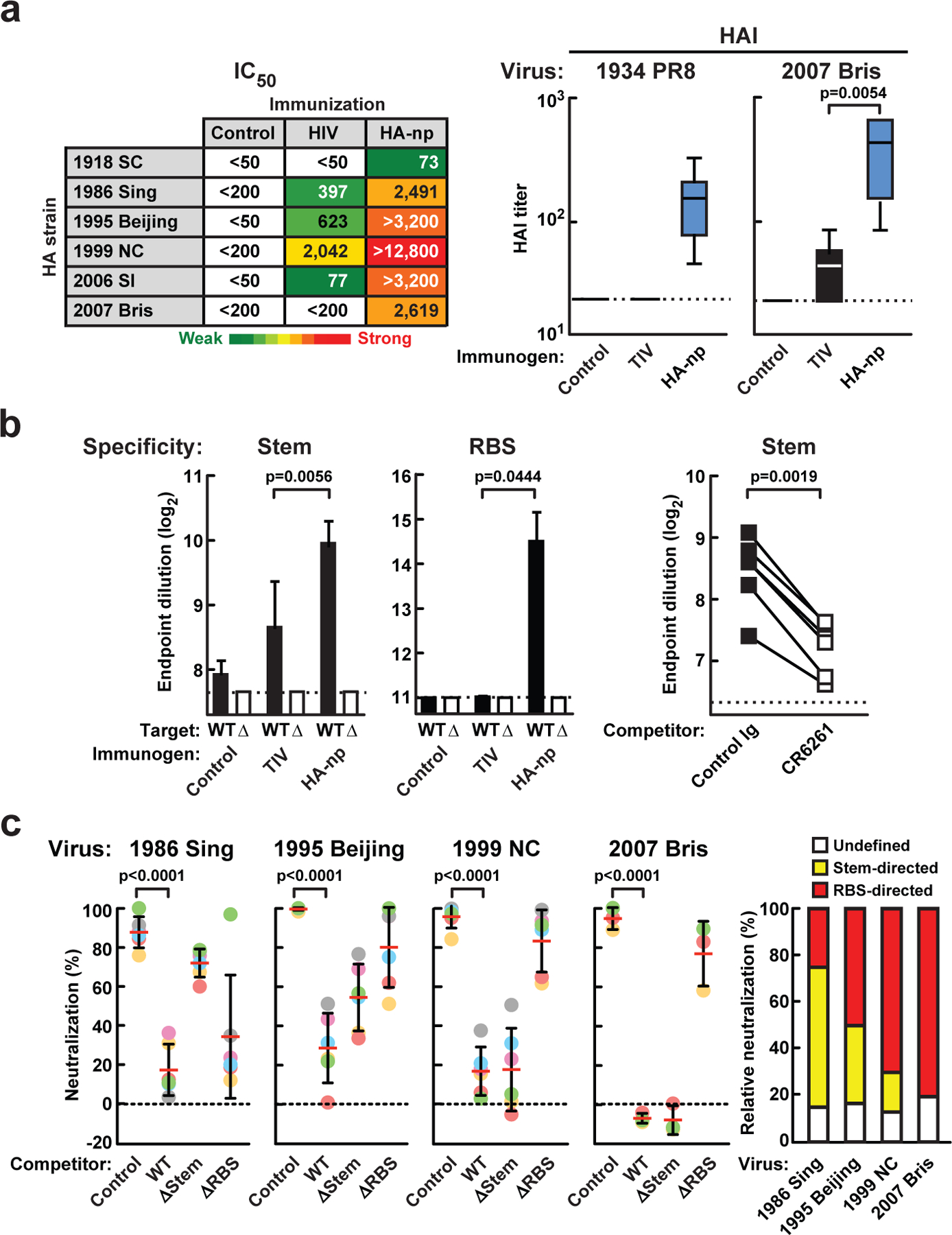
a, Breadth of serum neutralization in immune ferrets. IC50 titers against a panel of H1N1 pseudotyped viruses (left) and HAI titers against 1934 PR8 and 2007 Bris H1N1 viruses (right) were determined. Heat map is colored as in Figure 2. HAI titers are presented as box-and-whiskers plots with lines at the mean (n=6). b, Stem- and RBS-directed abs elicited by HA-nanoparticle immunization. Immune sera were pre-absorbed with ΔStem (left) and ΔRBS (middle) HA-expressing cells and analyzed for their binding to WT and a respective mutant (Δ) HA. The mean endpoint titers were plotted with s.d. (n=6). Binding of ΔStem HA pre-absorbed immune sera to HA pre-incubated with a control or CR6261 mAbs (right). Each symbol represents the titer of an individual ferret (n=6). c, Neutralization competition with WT, ΔStem or ΔRBS HA (left). The neutralization of HA-nanoparticle-immune sera was measured in the presence of indicated competitor proteins. Percent neutralizations at serum dilution 1/200 (1986 Sing and 2007 Bris), 1/800 (1995 Beijing) or 1/3,200 (1999 NC) were plotted. Each symbol represents an individual ferret and mean is indicated as a red line with s.d. (n=6 except for 2007 Bris (n=3)). The relative contributions of stem- and RBS-directed neutralization were calculated and plotted as mean percent (n=6).
To define the relative contributions of stem- and RBS-directed abs to the breadth of neutralization, we performed neutralization assays in the presence of competitor proteins: WT, ΔStem or ΔRBS HA. In the presence of excess ΔStem HA, only stem-directed abs can neutralize viruses; similarly, ΔRBS HA interferes with all abs except those targeting the RBS. Four H1N1 strains were tested in this assay and the pattern of neutralization inhibition varied by strain. Neutralization of 1999 NC or 2007 Bris was mediated predominantly by RBS-directed abs. However, neutralization of 1986 Sing was due mainly to stem-directed abs. Interestingly, both stem- and RBS-directed abs contributed to neutralize 1995 Beijing virus (Fig. 4c). While the neutralization specificities of mouse and ferret abs differ somewhat from previously described human abs, we have observed these differences previously8. This variation is most likely due to differences in the origins of the VH genes that give rise to them and affect their fine specificity23. In particular, anti-stem abs isolated from humans derive predominantly from the VH1-69 gene19, which is not present in other species. Even different human VH1-69 abs show differences in breadth and fine specificity among influenza subtypes19.
Based on the premise that highly ordered repetitive arrays induce yet stronger immune responses24, we have successfully designed an HA-nanoparticle to present trimeric HA spike in its native fold, rigidly and symmetrically, with sufficient spacing to ensure optimal access to potential bnAbs directed to the stem. These nanoparticles not only had the desired physical properties but also enhanced the potency and breadth of nAb responses compared to TIV, the current commercial vaccine, directed to two independent highly conserved epitopes. Although not yet a universal influenza vaccine, these nanoparticles provide a major increment in influenza protection by eliciting potent nAbs against a broad spectrum of H1N1 viruses. Moreover, the synthetic nanoparticles are fully recombinant, eliminating the need to produce potentially dangerous virus in eggs or in cell culture, and allowing for modifications that improve immunogenicity which would otherwise not be tolerated in replication-competent viruses currently used for vaccines. HA-nanoparticle technology therefore represents a foundation for a new generation of influenza vaccines and could be adapted to create analogous vaccines for a wide variety of pathogens.
METHODS SUMMARY
All genes used for recombinant proteins and pseudoviruses were synthesized using mammalian preferred codons. The HA-ferritin fusion gene was generated by fusing the ectodomain of HA (residues HA1 1-HA2 174, H3 numbering system) to H. pylori ferritin (residues 5–167) with a Ser-Gly-Gly linker. Recombinant proteins were produced by transient transfection of expression vectors in 293F cells (Invitrogen) and purified by chromatography techniques (see Methods for detail). The TIV used in this study were 2006–2007 and 2011–2012 Fluzone® (Sanofi Pasteur). Animal experiments were carried out in accordance with all federal regulations and NIH guidelines. Mice were immunized intramuscularly twice with 0.17 μg (Fig. 2a and andb)b) or 1.67 μg (Fig. 2d) of HA-nanoparticles (HA amount) or matched amount of TIV with or without Ribi adjuvant system (Sigma) or with MF59 (Novartis) at a 3-week interval. Ferrets were immunized intramuscularly with 2.5 μg of HA-nanoparticles or 7.5 μg of TIV with Ribi at weeks 0 and 4. H1N1 virus challenge was performed five weeks after the last immunization with 106.5 50% egg infectious dose (EID50) of 2007 Bris virus via intranasal inoculation. Statistical analyses were performed using Prism 5 (GraphPad Software).
Full Methods and any associated references are available in the online version of the paper.
METHODS
Vector construction.
The gene encoding Helicobacter pylori nonheme iron-containing ferritin (GenBank NP_223316) with a point mutation (N19Q) to abolish a potential N-linked glycosylation site was synthesized by PCR-based accurate synthesis25 using human-preferred codons. The human CD5 leader sequence and a serine-glycine-glycine (Ser-Gly-Gly) spacer were fused to the gene fragment encoding ferritin (residues 5–167) to generate a secreted protein. The plasmids encoding various influenza virus HAs, including A/South Carolina/1/1918 (1918 SC), A/Puerto Rico/8/1934 (1934 PR8), A/Singapore/6/1986 (1986 Sing), A/Beijing/262/1995 (1995 Beijing), A/New Caledonia/20/1999 (1999 NC), A/Solomon Islands/3/2006 (2006 SI), A/Brisbane/59/2007 (2007 Bris), A/California/04/2009 (2009 CA), A/Perth/16/2009 (H3 2009 Perth), B/Florida/04/2006 (B 2006 Florida), and their corresponding NAs with human preferred codons were synthesized as previously reported8. HA-ferritin fusion genes were generated by fusing the ectodomain of HAs (residues HA1 1-HA2 174, H3 numbering) from 1999 NC, 2009 CA, H3 2009 Perth and B 2006 Florida to H. pylori ferritin (residues 5–167) with a Ser-Gly-Gly linker. Soluble trimeric HA proteins were purified as previously described26. Transmembrane and soluble forms of 1999 NC ΔStem8 and ΔRBS23 HA mutants were generated by introducing an N-linked glycosylation site at residues HA2 45 (I45N/G47T) and HA1 190 (R192T), respectively. The soluble form of 2007 Bris ΔRBS HA mutant was generated by introducing an N-linked glycosylation site at the same site. All genes were then cloned into mammalian expression vectors for efficient expression27. Plasmids encoding the mAbs CR6261 (ref. 4), CH65 (ref. 6) and a single-chain variable fragment (ScFv) F10 (ref. 5) were synthesized as described previously8.
Protein biosynthesis and purification.
To produce ferritin nanoparticles, HA-nanoparticles and trimeric HA, the expression vectors were transfected into 293F cells (Invitrogen) using 293fectin (Invitrogen) according to the manufacturer’s instructions. Matched NAs were initially co-transfected at 20:1 HA:NA (wt:wt) to minimize the self-aggregation of HAs often observed with the soluble trimeric HA proteins, though further analysis showed that proper formation of the HA-nanoparticles did not require NA co-expression. The cells were grown in Freestyle 293 expression medium (Invitrogen) and the culture supernatants were collected 4 days post-transfection. The supernatants were concentrated and then buffer exchanged to a Tris buffer (20 mM Tris, 50 mM NaCl, pH 7.5 for ferritin nanoparticles; 20 mM Tris, 500 mM NaCl, pH 7.5 for HA-nanoparticles). The ferritin nanoparticles were purified by ion-exchange chromatography using a HiLoad 16/10 Q Sepharose HP column (GE Healthcare). The HA-nanoparticles were purified by affinity column chromatography using Erythrina cristagalli agglutinin (ECA, coral tree lectin; EY Laboratories, Inc.) specific for galactose β(1,4) N-acetylglucosamine. The ferritin nanoparticles and HA-nanoparticles were further purified by size exclusion chromatography with a Superose 6 PG XK 16/70 column (GE Healthcare) in PBS. The molecular weights of the ferritin nanoparticle and HA-nanoparticles were calculated based on two equations generated by least squares linear regression on a semi-log plot using gel filtration low and high molecular weight standards (Bio-Rad), respectively. The yield of the HA-nanoparticles was typically 2–10 mg l−1 depending on the HA strains. The trimeric HA proteins were purified as described previously26. Protein purity and size were verified by SDS-PAGE and dynamic light scattering using a DynaPro system (Wyatt Technology). MAbs and F10 scFv were produced in 293F cells and purified as described previously8,28. MAbs against 1999 NC HA were purified from hybridoma supernatants as previously described8.
Electron microscopic analysis.
Purified ferritin nanoparticles and HA-nanoparticles were negatively stained with phosphotungstic acid and ammonium molybdate, respectively, and images were recorded on a Tecnai T12 microscope (FEI) at 80 kV with a CCD camera (AMT Corp.).
Hemagglutinin inhibition assay (HAI).
Seed stocks of the influenza viruses were obtained from the CDC (Atlanta, GA) and the viruses were expanded in embryonated chicken eggs or in Madin-Darby canine kidney (MDCK) cells. Immune sera were pretreated with receptor-destroying enzyme (RDE II; Denka Seiken Co., Ltd.) and HAI assays were performed using four hemagglutinating units well−1 and 0.5% turkey or chicken red blood cells.
Pseudotyped virus neutralization and protein competition assays.
The pseudotype neutralization assay was performed as previously described and has been widely accepted for defining the specificity of nAbs targeting influenza virus HAs8,26–29. For the protein competition assay, neutralizing activity of the F10, CR6261 or immune sera was measured in the presence of competitor proteins, trimeric HA (WT, ΔStem or ΔRBS), HA-nanoparticles, ferritin nanoparticles or irrelevant protein (HIV-1 gp120) at final concentration of 20 and 25 μg ml−1 for mAbs and immune sera, respectively.
ELISA.
Purified trimeric HA, HA-nanoparticles, and TIV (2 μg of H1 HA ml−1), ferritin nanoparticles (0.68 μg ml−1 for Supplementary Fig. 2 or 2 μg ml−1 for the rest), mouse liver ferritin (2 μg ml−1, Alpha Diagnostic International, Inc.), ΔStem and ΔRBS HA trimer (2 μg ml−1) were coated (100 μl well−1) onto MaxiSorp™ plates (Nunc). For the ELISA-based competition assay, HA trimer (2 μg ml−1) was coated onto the plates and the plates were incubated with CR6261 or an isotype control (VRC01) at 8 μg ml−1 (refs. 30,31) before adding serially diluted pre-absorbed ferret immune sera.
Intracellular cytokine staining (ICS) assay.
CD4+ and CD8+ T-cell responses were evaluated by ICS for interferon-γ (IFN-γ), tumor necrosis factor α (TNFα), and interleukin-2 (IL-2) as described previously32. We used individual peptide pools (15-mer overlapping by 11 residues, 2.5 μg ml−1 for each peptide) covering H. pylori ferritin or mouse ferritin light and heavy chains to stimulate cells.
Immunization.
The TIV used in this study was 2006–2007 Fluzone® (Sanofi Pasteur) containing HAs from A/New Caledonia/20/1999 (H1N1), A/Wisconsin/67/2005 (H3N2) and B/Malaysia/2504/04 (influenza B), or 2011–2012 Fluzone® with HAs from A/California/07/09-like (H1N1), A/Perth/16/09 (H3N2) and B/Brisbane/60/08 (influenza B) (Supplementary Fig. 6). The TIV split vaccines are treated with a detergent (octoxinol-9) to solubilize membranes on influenza viruses and form rosettes that contain full-length HAs and NAs. Female BALB/c mice (6–8 weeks old; Charles River Laboratories) were immunized (5 mice/group) with 0.5 μg (0.17 μg of H1 HA) or 0.22 μg (0.17 μg of HA) of TIV or HA-nanoparticles, respectively (Fig. 2a, ,b),b), or 5 μg (1.67 μg of H1 HA), 2.24 μg (1.67 μg of HA) or 0.57 μg (equimolar to HA-nanoparticles) of TIV, HA-nanoparticles or ferritin nanoparticles, respectively (Fig. 2d). All immunizations were given intramuscularly in 100 μl of PBS or in 100 μl of 50% (v/v) mixture of Ribi adjuvant system (Sigma) in PBS at weeks 0 and 3. In a separate experiment, MF59 (Novartis) was used as the adjuvant in place of Ribi. A group of BALB/c mice (n=4) was immunized with 20 μg of trimeric HA (thrombin cleaved) with Ribi adjuvant at weeks 0 and 4. For the experiment in Supplementary Fig. 6b, mice were immunized (n=5) with 5 μg (1.67 μg of each HA component) of TIV, 2.24 μg (1.67 μg of HA) of monospecific HA-nanoparticles or 6.72 μg (1.67 μg of each HA component) of multispecific HA-nanoparticles with Ribi adjuvant at weeks 0 and 3. Blood samples were collected prior to the first dose, and at 2 weeks after each immunization. For ferret studies, male Fitch ferrets (6 months old; Triple F Farms), seronegative for H1N1, H3N2 and type B influenza viruses, were housed and cared for at BIOQUAL, Inc. (Rockville, MD). Ferrets were immunized (6 ferrets/group) intramuscularly with 500 μl of PBS, 7.5 μg (2.5 μg of H1 HA) of TIV or 3.35 μg (2.5 μg of HA) of HA-nanoparticles in 500 μl of 50% (v/v) mixture of Ribi adjuvant in PBS at weeks 0 and 4. Blood was collected prior to the first dose and 3 and 2 weeks after the first and the second immunizations, respectively.
Virus challenge.
Five weeks after the last immunization, the ferrets were challenged with 106.5 EID50 of 2007 Bris virus. The virus was expanded in embryonated chicken eggs from a seed stock obtained from CDC (Atlanta, GA) and has a titer of 106.5 EID50 ml−1. The ferrets were observed for clinical signs twice daily and weight and temperature measurements recorded daily. Nasal washes were obtained on days 1, 3 and 5 and infectious viral titers were determined by a 50% tissue culture infectious dose (TCID50) assay using MDCK cells as described previously8.
Serum absorption.
Ferret immune sera taken 2 weeks after the second immunization were subjected to the assay. One ml of the immune sera diluted at 1:100 and 1:1,000 was incubated with 100 μl of pre-washed ΔStem and ΔRBS HA-expressing 293F cell pellets, respectively. After incubating for 1 hour at 4°C, supernatants were harvested by centrifugation and binding to WT and mutant HAs was examined by ELISA. The ΔStem HA-pre-absorbed sera were also used for competition ELISA.
Statistical analysis.
All data plotted with error bars are expressed as means with s.d. unless otherwise mentioned. The p values were generated by analyzing data with a two-tail unpaired t test using the Prism 5 program (GraphPad Software). In Fig. 4b, right panel, the data were analyzed by two-way ANOVA using Prism 5.
Molecular representations.
All structural renderings of proteins were generated using the UCSF Chimera package33, Version 1.7.0 (http://www.cgl.ucsf.edu/chimera/) or The PyMOL Molecular Graphics System, Version 1.5.0.4 (Schrödinger, LLC; http://www.pymol.org/). UCSF Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
Supplementary Material
Supplementary Figures and Legends [1]
SI Guide [1]
Acknowledgements
We thank H. Andersen (BIOQUAL), A. Taylor, A. Zajac and C. Chiedi (VRC) for help with the animal studies; U. Baxa, K. Nagashima and A. Harned (NCI SAIC Frederick) for EM studies; X. Chen (VRC) for technical support; A. Panet, B. Graham, R. Schwartz (VRC) and members of the Nabel lab for discussions; S. Sun and M. Rossmann (Purdue University) for technical and conceptual advice; A. Tislerics, B. Hartman and J. Farrar for manuscript preparation. The MF59 adjuvant was kindly provided by Novartis. This work was supported by the Intramural Research Program of the Vaccine Research Center, NIAID, National Institutes of Health.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
The authors declare that an intellectual property application has been filed by NIH based on data presented in this paper.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1038/nature12202
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8312026
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Advanced technologies for the development of infectious disease vaccines.
Nat Rev Drug Discov, 21 Oct 2024
Cited by: 0 articles | PMID: 39433939
Review
The self-assembled nanoparticle-based multi-epitope influenza mRNA vaccine elicits protective immunity against H1N1 and B influenza viruses in mice.
Front Immunol, 15:1483720, 08 Oct 2024
Cited by: 0 articles | PMID: 39445022 | PMCID: PMC11497263
Tyrosinase-Mediated Conjugation for Antigen Display on Ferritin Nanoparticles.
Bioconjug Chem, 27 Sep 2024
Cited by: 0 articles | PMID: 39332819 | PMCID: PMC11487507
Ferritin Vaccine Platform for Animal and Zoonotic Viruses.
Vaccines (Basel), 12(10):1112, 27 Sep 2024
Cited by: 0 articles | PMID: 39460279 | PMCID: PMC11511493
Review Free full text in Europe PMC
Self-assembling nanoparticle engineered from the ferritinophagy complex as a rabies virus vaccine candidate.
Nat Commun, 15(1):8601, 04 Oct 2024
Cited by: 0 articles | PMID: 39366932 | PMCID: PMC11452399
Go to all (457) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Development of a Pan-H1 Influenza Vaccine.
J Virol, 92(22):e01349-18, 29 Oct 2018
Cited by: 28 articles | PMID: 30185594 | PMCID: PMC6206494
Elicitation of broadly neutralizing influenza antibodies in animals with previous influenza exposure.
Sci Transl Med, 4(147):147ra114, 01 Aug 2012
Cited by: 38 articles | PMID: 22896678
Elicitation of Protective Antibodies against a Broad Panel of H1N1 Viruses in Ferrets Preimmune to Historical H1N1 Influenza Viruses.
J Virol, 91(24):e01283-17, 30 Nov 2017
Cited by: 39 articles | PMID: 28978709 | PMCID: PMC5709581
Implications of broadly neutralizing antibodies in the development of a universal influenza vaccine.
Curr Opin Virol, 17:110-115, 28 Mar 2016
Cited by: 27 articles | PMID: 27031684 | PMCID: PMC4940123
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (3)
Grant ID: Z99 AI999999
Grant ID: Z01 AI005003
Grant ID: ZIA AI005003





