Abstract
Objective
To evaluate progressive cerebral degeneration in amyotrophic lateral sclerosis (ALS) by assessing alterations in N-acetylaspartate (NAA) ratios in the motor and prefrontal cortex within clinical subgroups of ALS.Methods
Seventy-six patients with ALS and 59 healthy controls were enrolled in a prospective, longitudinal, multicenter study in the Canadian ALS Neuroimaging Consortium. Participants underwent serial clinical evaluations and magnetic resonance spectroscopy at baseline and 4 and 8 months using a harmonized protocol across 5 centers. NAA ratios were quantified in the motor cortex and prefrontal cortex. Patients were stratified into subgroups based on disease progression rate, upper motor neuron (UMN) signs, and cognitive status. Linear mixed models were used for baseline and longitudinal comparisons of NAA metabolite ratios.Results
Patients with ALS had reduced NAA ratios in the motor cortex at baseline (p < 0.001). Ratios were lower in those with more rapid disease progression and greater UMN signs (p < 0.05). A longitudinal decline in NAA ratios was observed in the motor cortex in the rapidly progressing (p < 0.01) and high UMN burden (p < 0.01) cohorts. The severity of UMN signs did not change significantly over time. NAA ratios were reduced in the prefrontal cortex only in cognitively impaired patients (p < 0.05); prefrontal cortex metabolites did not change over time.Conclusions
Progressive degeneration of the motor cortex in ALS is associated with more aggressive clinical presentations. These findings provide biological evidence of variable spatial and temporal cerebral degeneration linked to the disease heterogeneity of ALS. The use of standardized imaging protocols may have a role in clinical trials for patient selection or subgrouping.Classification of evidence
This study provides Class II evidence that MRS NAA metabolite ratios of the motor cortex are associated with more rapid disease progression and greater UMN signs in patients with ALS.Trial registration information
ClinicalTrials.gov Identifier: NCT02405182.Free full text

Progressive Neurochemical Abnormalities in Cognitive and Motor Subgroups of Amyotrophic Lateral Sclerosis
Associated Data
Abstract
Objective
To evaluate progressive cerebral degeneration in amyotrophic lateral sclerosis (ALS) by assessing alterations in N-acetylaspartate (NAA) ratios in the motor and prefrontal cortex within clinical subgroups of ALS.
Methods
Seventy-six patients with ALS and 59 healthy controls were enrolled in a prospective, longitudinal, multicenter study in the Canadian ALS Neuroimaging Consortium. Participants underwent serial clinical evaluations and magnetic resonance spectroscopy at baseline and 4 and 8 months using a harmonized protocol across 5 centers. NAA ratios were quantified in the motor cortex and prefrontal cortex. Patients were stratified into subgroups based on disease progression rate, upper motor neuron (UMN) signs, and cognitive status. Linear mixed models were used for baseline and longitudinal comparisons of NAA metabolite ratios.
Results
Patients with ALS had reduced NAA ratios in the motor cortex at baseline (p < 0.001). Ratios were lower in those with more rapid disease progression and greater UMN signs (p < 0.05). A longitudinal decline in NAA ratios was observed in the motor cortex in the rapidly progressing (p < 0.01) and high UMN burden (p < 0.01) cohorts. The severity of UMN signs did not change significantly over time. NAA ratios were reduced in the prefrontal cortex only in cognitively impaired patients (p < 0.05); prefrontal cortex metabolites did not change over time.
Conclusions
Progressive degeneration of the motor cortex in ALS is associated with more aggressive clinical presentations. These findings provide biological evidence of variable spatial and temporal cerebral degeneration linked to the disease heterogeneity of ALS. The use of standardized imaging protocols may have a role in clinical trials for patient selection or subgrouping.
Classification of Evidence
This study provides Class II evidence that MRS NAA metabolite ratios of the motor cortex are associated with more rapid disease progression and greater UMN signs in patients with ALS.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT02405182.
The clinical heterogeneity of amyotrophic lateral sclerosis (ALS) contributes to challenges in developing targeted therapeutics and evaluating outcomes for disease-modifying treatments.1 Clinical trials would benefit from phenotypically homogeneous cohorts (a method of clinical trial enrichment) and from a noninvasive biomarker that can accurately and objectively monitor disease progression.
Magnetic resonance spectroscopy (MRS) is able to evaluate cerebral neurochemistry in vivo, providing biomarkers of cerebral degeneration with diagnostic, prognostic, and disease monitoring potential.2 N-acetylaspartate (NAA), a marker of neuronal integrity, is reduced when expressed in absolute terms and as a ratio to other metabolites, including creatine (Cr; a marker of energy metabolism), choline (Cho; a marker of membrane turnover), and myo-inositol (Ino; a putative marker of gliosis). This has been observed in motor3-11 and extramotor regions, including the frontal lobe.7,12,13 Longitudinal MRS studies are few in number, report inconsistent findings, and are limited by small sample sizes.3,4,8,14,15 There is a critical need for data assessing spectroscopy performance in multicenter studies toward the potential of MRS-derived biomarkers facilitating clinical trials.
The objective of this study was to evaluate progressive cerebral degeneration in ALS in a multicenter fashion. We hypothesized that (1) there is a progressive decline in NAA ratios (decrease in neuronal integrity) in motor and prefrontal regions and (2) ALS phenotypes with faster clinical progression, greater upper motor neuron (UMN) burden, and greater cognitive impairment have faster decline in NAA. The study was conducted as part of the Canadian ALS Neuroimaging Consortium (CALSNIC) using an imaging and clinical protocol harmonized across 5 centers.16
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Informed written consent was obtained from all participants. The study was approved by the Institutional Review Board at each research site and is registered with ClinicalTrials.gov (NCT02405182).
Data Availability
Anonymized data will be made available on reasonable request from qualified investigators to the corresponding author.
Participant Recruitment
Clinical and MRI data were collected from 5 research sites across Canada as part of CALSNIC. Patients with ALS who met the criteria for possible, probable, or definite ALS as defined by the El Escorial criteria were recruited from specialized ALS clinics.17 All patients had concurrent signs of clinical UMN and lower motor neuron impairment in at least 1 region detected on neurologic examination and presented with limb or bulbar symptoms at onset. Furthermore, patients with ALS must have had sufficient cognitive capacity to provide informed consent for the study and were excluded if they had a comorbid diagnosis of frontotemporal dementia. Healthy participants from the adult general population were also recruited to the study. Participants were excluded if they had history of any other neurologic or psychiatric disorders or were unable to tolerate lying flat for the hour-long duration of the MRI scan.
All participants underwent comprehensive clinical evaluations and multimodal MRI. Imaging and clinical testing were done at baseline (time point 1) and repeated at 4 months (time point 2) and 8 months (time point 3) after the baseline visit.
For the purpose of assessing the test-retest and multicenter reliability, 6 additional healthy participants were recruited. Each participant in this cohort traveled to and were imaged twice (scans 1 and 2) at each participating research site using identical protocols, for a total of 60 imaging sessions. Between the scans, each participant exited the MRI system for a minimum of 2 hours.
Clinical Evaluations
Disease severity was assessed with the ALS Functional Rating Scale–Revised (ALSFRS-R), a 12-item questionnaire assessing speech, swallowing, limb, and respiratory function.18 The scale ranges from 0 to 48, with lower scores indicating greater functional impairment. The baseline ALSFRS-R scores were used to determine the disease progression rate (DPR) using the following formula: (48 − ALSFRS-R score)/symptom duration in months. One patient did not have an ALSFRS-R score recorded and was excluded from analyses related to DPR.
Neurologic examinations were performed for each patient by neurologists specializing in ALS from each research site. A clinical scale was used to evaluate the degree of UMN burden. This scale used lateralized subscores of tone and reflexes, as well as presence of the Babinski sign and pseudobulbar affect (further information available from Dryad, table e-1: doi.org/10.5061/dryad.73n5tb2wx). Six patients were excluded from the baseline analysis and 2 from the longitudinal analysis due to missing or incomplete neurologic examinations.
The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) is a comprehensive screening tool for cognitive changes in ALS. The tool evaluates cognitive domains of executive functioning, verbal fluency, language, memory, and visuospatial abilities.19 Fifteen patients were excluded from the baseline analysis due to incomplete cognitive assessments.
Clinical subtypes of ALS were created by dichotomizing each feature at baseline into 3 groups:
Faster or slower DPR (ALS-FP and ALS-SP): on the basis of the median of the ALS cohort, a cutoff score of DPR = 0.29 was used to stratify patients into 2 groups.
Higher or lower UMN burden from neurologic examination (ALS-UMNH and ALS-UMNL): on the basis of the median of the ALS cohort, a cutoff score of 6 was used to stratify patients into 2 groups.
Presence or absence of cognitive impairment (ALS–cognitively impaired [ALS-CI] and ALS–cognitively normal [ALS-CN]): patients were classified as ALS-CI if they scored 2 SDs below the control mean in ECAS verbal fluency score (of 24) or executive function score (of 48). The cutoffs used were 13.1 and 33.3, respectively.
Data Acquisition
Previous studies have consistently demonstrated reductions in neurometabolite ratios in the motor cortex3-11 and prefrontal cortex7,12,13 in single-center studies at baseline. As a result, the initial reason for selection of the motor and prefrontal cortex was to replicate and validate earlier findings in a large-scale multicenter study.20 Subsequently, these initial baseline findings were extended to investigate progressive cerebral degeneration in the same regions.
The CALSNIC MRS data acquisition parameters and procedures for voxel placements have been described previously.20 In brief, the data were acquired at the participating centers using research MRI systems operating at 3T using General Electric (Fairfield, CT) systems (University of Calgary, University of Toronto), Siemens (Malvern, PA) systems (University of Alberta, McGill University), and a Philips (Best, the Netherlands) system (University of British Columbia). Three-dimensional T1-weighted images were acquired at 1 mm3 for MRS voxel localization. Single-voxel proton MRS was performed with a stimulated echo acquisition mode sequence (repetition time 3 seconds, echo time 160 milliseconds, mixing time 40 milliseconds) to acquire water-suppressed spectroscopic data from the motor cortex and mesial prefrontal cortex (mPFC) (figure 1). The voxel sizes for the motor and mPFC were 20 × 50 × 20 and 25 × 32 × 25 mm, respectively.

(A) Representative magnetic resonance (MR) spectrum from the (B) motor cortex and (C) an MR spectrum from the (D) mesial prefrontal cortex (mPFC). Cho = choline; Cr = creatine; Ino = myo-inositol; NAA = N-acetylaspartate.
Data Postprocessing
Postprocessing and quality inspection of all acquired data were performed at a single center, as previously reported.20 Cerebral metabolite quantification of in vivo MRS was performed with LCModel (version 6.1) to obtain peak area estimates for the following metabolites: NAA, N-acetylaspartylglutamate (NAAG), Ino, Cr, and Cho. The total amount of N-acetylaspartyl moieties was quantified by combining the metabolite levels of NAA and NAAG. Subsequently in this article, NAA refers to the total levels of NAA and NAAG. Three ratios were used for analysis: NAA/Cho, NAA/Cr, and NAA/Ino.
The variable mix of gray matter (GM) relative to the white matter (WM) and CSF within the voxel may be a confounding factor in MRS analysis. Because CSF has negligible contributions to MRS metabolite signal, the percentage of GM in each voxel was determined by taking the concentration of GM and dividing by the sum of the estimated subvolumes of GM and WM. To calculate the GM and WM subvolume, the MRS voxels of interest were coregistered to T1-weighted images, and SPM-12 segmentation was used to extract the tissue subvolumes.
Statistical Analysis
The demographics and clinical features were compared for participants at baseline both between patients with ALS and healthy controls (HCs) and between ALS subgroups. Two-tailed t tests and Mann-Whitney U tests were conducted to test for differences in continuous variables, and χ2 tests were used for categorical features. The Shapiro-Wilk test of normality was performed for each parameter to determine the distribution of the data. The Tukey test was used to detect outliers in the metabolite ratios. Any detected outliers were excluded from further statistical analysis. Statistical analyses were performed with MedCalc (version 17.6, MedCalc Software, Ostend, Belgium).
Intraclass correlation coefficients (ICCs) were calculated to assess test-retest reliability. ICCs evaluate the degree of agreement and extent of correlation between 2 measures. Ranging between 0 and 1, ICCs are classified as poor (<0.40), fair (0.41–0.60), good (0.61–0.75), and excellent (>0.75) according to standard convention.21 A 2-way mixed-effects model was used to compute the ICCs using neuronal metabolite data acquired from the traveling healthy participants. Test-retest (intrasite) reliability was assessed with data from scans 1 and 2 from individuals, and multicenter (intersite) reliability was estimated with scan 1 from each site. Reliability assessments were then used to determine the neurometabolite ratios selected for further analysis.
Metabolite ratios were compared at baseline with linear mixed models, controlled for age, site, and percentage GM in the voxel. The threshold for statistical significance was p < 0.05. Linear mixed models were used to estimate linear slopes over time for metabolite ratios for the ALS group, HC group, and ALS subgroups to account for missing data due to participant attrition. The models accounted for clustering within repeated time measures and were controlled for age, site, and percentage GM in the voxel. Only participants who completed at least 1 follow-up visit were included. Mean annual rate of change was estimated for each cohort. Linear trends were considered significant at p < 0.05.
Similarly, linear mixed models were used to evaluate the progression of clinical scores over time such as the ALSFRS-R and the UMN burden score for ALS patient groups, controlling for age and site of acquisition. However, with only a single version of the ECAS, it was administered only at baseline for all participants to avoid memory effects. Thus, ECAS scores were not included in the longitudinal analysis. Linear trends were considered significant at p < 0.05.
Effect sizes for significant longitudinal linear mixed model slopes were estimated with a previously described method.22 With this method, the Cohen ƒ2 was calculated to measure the local effect size of the repeated-measures data; ƒ2 values ≥0.02, ≥0.15, and ≥0.35 indicate small, medium, and large effect sizes, respectively. Subsequently, power analyses were performed using the linear rates from the mixed models to determine the minimum sample sizes needed to detect longitudinal significant changes in a hypothetical analysis.
All linear mixed models, reliability models, effect sizes, and power analyses were conducted with SPSS (released 2016, IBM SPSS Statistics for Windows, version 20.0, IBM Corp, Armonk, NY).
The primary objective of this study was to evaluate patterns of progressive cerebral degeneration within subgroups of ALS stratified by clinical characteristics. The methods of this study meet Class II criteria for classification of evidence.
Results
Study Demographics
A total of 135 participants were recruited for this study (76 patients with ALS and 59 HCs). After stratification at baseline, 38 and 37 patients were included in the ALS-FP and ALS-SP cohorts, respectively. Furthermore, 32 and 38 patients were stratified into ALS-UMNH and ALS-UMNL, respectively. Twenty-six ALS-CI and 35 ALS-CN patients were present in the analyses at baseline. Further information on the number of participants in each of the cohorts at each time point is summarized in table 1. Patients with ALS and HCs were significantly different in age (p = 0.045). There were no significant differences in age between the patient groups. There were no significant differences in sex between patients with ALS and HCs, ALS-FP and ALS-SP, ALS-UMNH and ALS-UMNL, or ALS-CI and ALS-CN. Between the ALS-FP and ALS-SP subgroups, significant differences were present in site of onset (p = 0.01), ALSFRS-R score (p = 0.003), symptom duration (p < 0.001), DPR (p < 0.001), and UMN burden (p < 0.001). The ALS-UMNH and ALS-UMNL subgroups had different ALSFRS-R scores (p = 0.005), symptom duration (p < 0.001), DPR (p < 0.001), and UMN burden (p < 0.001). Between the ALS-CI and ALS-CN subgroups, significant differences were present only in ECAS Verbal Fluency (p < 0.001) and ECAS Executive Function (p < 0.001) subscores. Table 1 summarizes the demographics for each cohort in the study. A number of participants did not complete all 3 visits due to deteriorating health or other logistical reasons. The number of participants at each time point is illustrated in figure 2.
Table 1
Baseline Demographics of ALS, ALS Subtypes, and HCs

Data Quality Assurance
Metabolite ratios were flagged for exclusion with the use of quality assurance protocols during postprocessing. At baseline, 5.2% (n = 21 of 405) of motor and 5.4% (n = 22 of 405) of mPFC metabolite were excluded. At time point 2, 4.8% (n = 14 of 294) of motor and 6.1% (n = 18 of 294) of mPFC were excluded. At time point 3, 4.8% (n = 11 of 231) of motor and 1.7% (n = 4 of 231) of mPFC were excluded.
Reliability Analysis
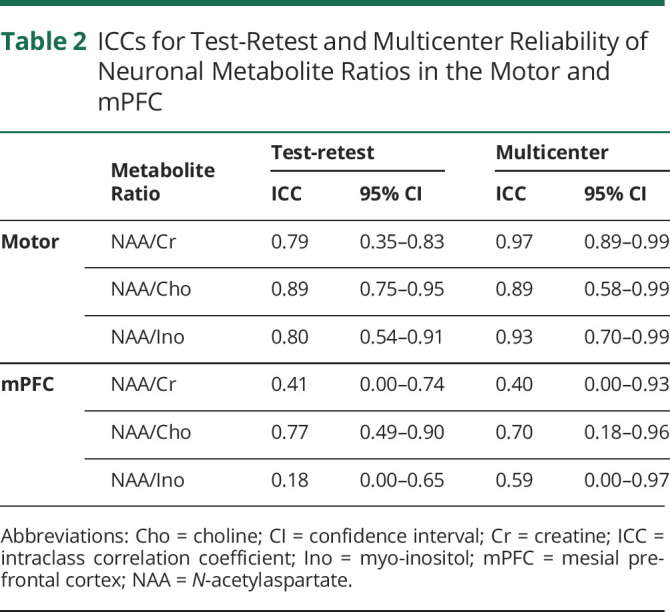
Motor NAA/Cr, NAA/Cho and NAA/Ino, and mPFC NAA/Cho had good test-retest reliability and intersite reliability and were retained for further analyses. mPFC NAA/Cr and NAA/Ino had poor reliability and were excluded from subsequent analyses (table 2).
Table 2
ICCs for Test-Retest and Multicenter Reliability of Neuronal Metabolite Ratios in the Motor and mPFC
Cross-Sectional Results
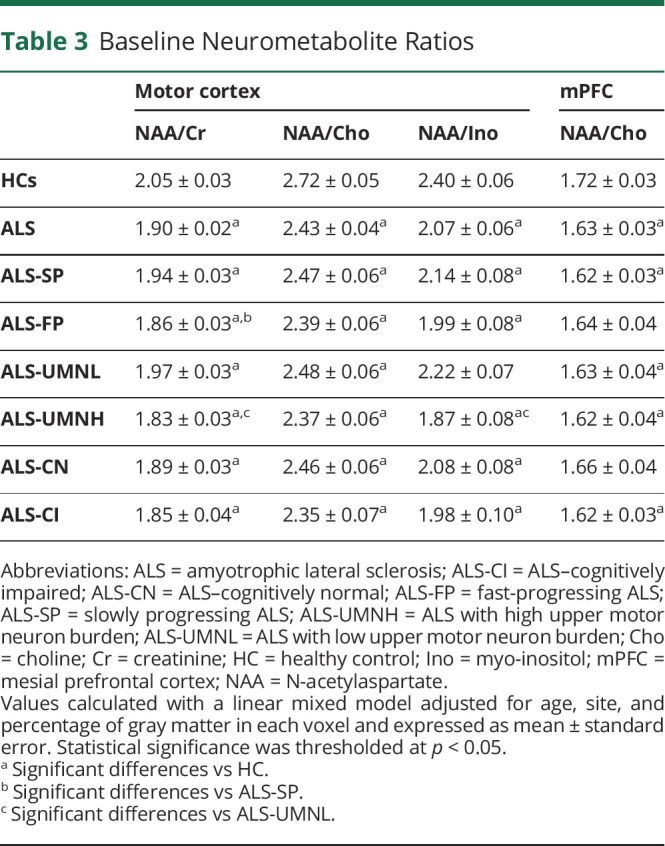
At baseline, motor cortex NAA/Cr (p < 0.001), NAA/Cho (p < 0.001), NAA/Ino (p < 0.001), and mPFC NAA/Cho (p = 0.019) were reduced in patients with ALS compared to HCs. Motor cortex NAA/Cr was lower in ALS-FP compared to ALS-SP (p = 0.04). Furthermore, motor cortex NAA/Cr (p = 0.002) and NAA/Ino (p = 0.002) were lower in the ALS-UMNH group compared to the ALS-UMNL group. Figure 3 illustrates the boxplots for the above analyses. Compared to HCs, NAA/Cho was reduced in the mPFC in ALS-CI but was not significantly different in ALS-CN. No significant differences were found for NAA/Cho in the mPFC between any ALS subgroups. Table 3 summarizes the metabolite levels from the baseline analyses.
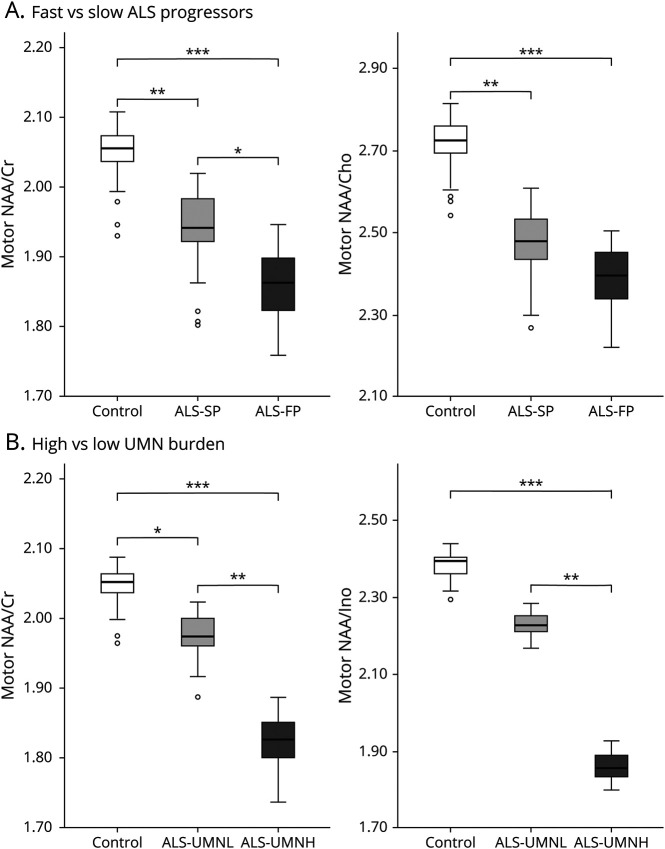
Metabolite ratios are adjusted for age, site of acquisition, and percentage of voxel gray matter. Patients are subgrouped according to baseline characteristics of (A) disease progression rate and (B) degree of upper motor neuron (UMN) burden on clinical examination. Statistical analyses were conducted with linear mixed regression to compare groupwise differences. ALS = amyotrophic lateral sclerosis; ALS-FP = fast-progressing ALS; ALS-SP = slowly progressing ALS; ALS-UMNH = ALS with high UMN burden; ALS-UMNL = ALS with low UMN burden; Cho = choline; Cr = creatine; Ino = myo-inositol; NAA = N-acetylaspartate.
Table 3
Baseline Neurometabolite Ratios
Longitudinal Results
Longitudinal metabolite changes were observed in the motor cortex. NAA/Cr (−0.078 ± 0.04/y [SE] or 4.34%/y, p = 0.037, ƒ2 = 0.17) and NAA/Cho (−0.151 ± 0.05/y or 6.40%/y, p = 0.009, ƒ2 = 0.14) declined over time in the ALS-FP group. Similarly, NAA/Cr (−0.132 ± 0.03/y or 7.24%/y, p = 0.001, ƒ2 = 0.34) and NAA/Cho (−0.130 ± 0.06/y or 5.51%/y, p = 0.04, ƒ2 = 0.14) declined over time in the ALS-UMNH group. Figure 4 demonstrates the longitudinal trajectories in the ALS subgroups. No significant longitudinal changes in metabolites were found in the total ALS group, HC group, or ALS-CI, ALS-CN, ALS-SP, or ALS-UMNL groups. Longitudinal metabolite changes were not observed in the mPFC.

Slopes were derived from linear mixed models, accounting for age, site of acquisition, and percentage of voxel grey matter. Trajectories of motor N-acetylaspartate (NAA)/creatine (Cr) and NAA/choline (Cho) decline for (A) patients with amyotrophic lateral sclerosis (ALS) that is fast progressing (ALS-FP) and (B) patients with ALS with high upper motor neuron (UMN) burden (ALS-UMNH) were significantly faster (p < 0.05). Dashed lines = 95% confidence interval. ALS-SP = slowly progressing ALS; ALS-UMNL = ALS with low UMN burden; ns = nonsignificant.
In the total ALS group, ALSFRS-R score declined significantly over time (−7.4 ± 1.19/y or 19.05%/y, p < 0.001), while UMN burden did not. ALSFRS-R scores declined in both ALS-FP (−10.7 ± 1.76/y or 28.45%/y, p < 0.001) and ALS-SP (−2.7 ± 0.77/y or 6.68%/y, p = 0.001) over time. Clinical UMN burden did not decline over time in either ALS-UMNH or ALS-UMNL.
Power Analyses
Power analyses were performed for the neurometabolites NAA/Cr and NAA/Cho in the total ALS, ALS-FP, and ALS-UMNH cohorts. To detect a 10%/y change, it was estimated that 73 and 51 ALS-FP patients and 21 and 95 ALS-UMNH patients would be required to detect longitudinal changes in NAA/Cr and NAA/Cho, respectively. If all patients with ALS were included, the sample sizes required would be 363 patients for NAA/Cr, 292 patients for NAA/Cho, and 18 patients for ALSFRS-R score. All power calculations were performed at 80% power with an α level of 0.05.
Discussion
In this multicenter study, progressive cerebral degeneration in different clinical subtypes of ALS was evaluated with MRS. The major findings of the study are as follows. First, progressive cerebral degeneration occurred in the motor cortex, with faster rates observed in patients with clinical characteristics suggesting more aggressive disease, namely faster DPR and greater UMN burden. Second, progressive motor cortex degeneration was observed even in the absence of clinical UMN progression. Third, patients with cognitive impairment have greater cerebral degeneration in the mPFC.
MRS studies in ALS have consistently revealed abnormalities in the motor cortex, demonstrating reductions in NAA/Cr,6,23-27 NAA/Cho,6,23,24,26,27 and NAA/Ino.2,23 Previously, neurochemical correlates were reported with DPR for both NAA/Cr28 and NAA/Cho3,26 in the motor region, which corroborate with the present study findings. This has also been reported for both motor and extramotor areas, particularly in frontotemporal regions with respect to the latter.29-31 Moreover, reductions in fractional anisotropy have previously been observed in fast ALS progressors compared to slow ALS progressors along the corticospinal tract and upper frontal lobe.32 Taken together, there is evidence for neuroanatomic correlates of clinical disease progression in ALS.
For the purpose of clinical trials as measures of therapeutic effects, potential biomarkers must demonstrate sensitivity to the biological progression of disease.33 Previous studies have shown inconsistent results regarding longitudinal change in neuronal metabolites in ALS. Several studies reported a significant decline in NAA moieties in the motor cortex over time3,6,8,34; others did not.4,15 Inconsistencies between studies may be due to numerous factors such as variations in technical methodologies (imaging acquisition protocols, analysis software, interscan time interval) and differences in patient cohorts (symptom duration, DPR, degree of cerebral involvement). Earlier studies have limited analyses to undifferentiated ALS cohorts; only 1 study distinguished patients by clinical phenotype, showing a decrease in NAA/Ino of patients with ALS with upper limb dysfunction.34 In the present study, longitudinal alterations were observed in patients with seemingly more aggressive disease at presentation, as evident by greater clinical UMN dysfunction, faster DPRs, and lower NAA indices. Thus, spectroscopy has potential as a tool for monitoring biological disease progression in clinical trials, specifically when targeting specific phenotypes of ALS.
Furthermore, the results of our longitudinal analysis suggest that varied trajectories of biological disease progression are present between clinical ALS subgroups. The ALS-FP cohort declined significantly in neuronal metabolites over time, suggesting that more aggressive cerebral pathology underlies faster clinical progression. This is further corroborated by a previous tensor-based morphometry study in which cerebral atrophy occurred faster in the motor region in patients with clinically faster-progressing ALS-FP.35 In contrast, ALS-SP patients demonstrated metabolite reductions at baseline without longitudinal changes. It is presumed that cerebral degeneration occurred in these patients before the baseline session, but further deterioration was of insufficient magnitude to be detected within the time frame of the observations.
In patients with greater clinical UMN dysfunction, larger reductions of neurometabolites were observed in the motor region but not the frontal region. Conversely, cognitively impaired patients had lower NAA in the frontal lobe, but their NAA ratios were unchanged in the motor region. Furthermore, DPR was not associated with frontal metabolites. These results suggest that focal neurodegeneration in specific regions of the brain contributes to clinical presentation, providing evidence of neuroanatomic substrates for decline in cognitive and motor function.
Previous correlations between clinical UMN burden and metabolite levels of the motor cortex have been established in the ALS literature.5,10,11,36 Taken together, these studies provide evidence that degeneration in the motor region is a pathologic process associated with UMN dysfunction and may serve as a surrogate marker. Moreover, ongoing decline of metabolite ratios was observed in patients with high UMN burden at baseline in the present study, while clinical scores remained stable over time. This suggests that MRS is more sensitive to subclinical UMN degeneration, giving confidence in its potential as a marker for underlying cerebral disease progression. Although baseline reductions were observed, patients with lower UMN burden at baseline had unchanging neurometabolites over time. Therefore, it can be speculated that cerebral involvement in the motor cortex is initially limited and may reach a maximal ceiling earlier in the disease course for certain patients, leading to less severe clinical UMN dysfunction. The findings of a previous study showing that progressive GM atrophy in the motor and frontotemporal regions occurred primarily within 13 months of initial symptom onset support this concept.37
This study also sought to evaluate progressing cerebral degeneration in the frontal lobe. Previous single-center studies have shown reductions in NAA/Ino13 and NAA/Cr.38 A recent study showed NAA/Ino in the mPFC as predictive of 2-year survival in patients with ALS.39 In the present study, mPFC NAA/Cho was reduced in ALS-CI but not in ALS-CN, presenting an in vivo cerebral correlate to cognitive impairment. In support, associations between verbal fluency and NAA/Cr in the dorsolateral prefrontal cortex have been shown in patients with ALS.12 This study furthers the idea that degeneration in extramotor regions, particularly in the prefrontal area, is associated with cognitive impairment within ALS populations.
Identifying markers of disease burden and progression is crucial for evaluating disease-modifying interventions in clinical trials. Currently, the ALSFRS-R is widely used as a primary outcome measure in ALS drug trials.40 However, the ALSFRS-R does not reflect specific pathologic changes but rather overall clinical progression as inferred from functional decline. Therefore, it is not biologically specific, for example, to a specific motor neuron pool and could be affected by cognitive function or motivation. MRS is advantageous in that it is able to capture biological involvement and to quantify specifically cerebral degeneration. Although MRS-based biomarkers are specific for cerebral degeneration, we found that relatively large sample sizes would be required in a nonstratified ALS cohort in clinical trials to detect a significant longitudinal change. However, our power calculations showed that significantly smaller, more reasonable sample sizes are sufficient when patients are selected on the basis of certain clinical phenotypes. Such means of trial enrichment may be useful to address the confounding effects of disease heterogeneity in ALS. Therefore, MRS may have a role in monitoring the effects of interventions and disease progression in future studies targeting specific clinical phenotypes.
Excellent test-retest reliability of MRS has previously been shown in various cerebral regions for NAA/Cr, with ICCs ranging from 0.84 to 0.90.41 However, NAA/Cr and NAA/Ino in the mPFC were poor in this study. This was likely due to technical factors that decrease the accuracy of metabolite quantification in the region. These issues include shimming performance and higher sensitivity to head motion during scanning. Compared to the motor region, there is also decreased signal-to-noise ratios due to a larger frontal region voxel. This study showed that NAA moieties in the motor cortex had excellent multicenter reliability. This provides overall confidence in MRS as a potential biomarker for cerebral degeneration in the motor region, although frontal metabolites may require more technical improvement.
This study is not without limitations. The median disease progression was 0.29/y, which is relatively slow compared to natural history studies in ALS.42 Another limitation of the study is the median symptom duration in the ALS-SP group (47.0 months), which was comparatively longer than the ALS-FP group (18.9 months). This may contribute to the lack of longitudinal progression in the ALS-SP group because of cases progressing very slowly. Furthermore, participant attrition was present due to disease-related or logistical reasons. The incorporation of MRI into clinical trials with therapeutic agents (rather than just observational studies) is recommended to obtain real-world data with larger sample sizes and to permit more refined estimates of attrition. Another limitation is the lack of genetic data included in the study because such data were rarely available for most patients. Previous studies have identified variable patterns of MRI findings in patients with ALS with the C9ORF expansion mutation.37,43 It would be beneficial to closely examine the relationship between genotype and the progression of disease as detected by MRS or other MRI metrics. This will be possible for future CALSNIC studies because complete collection of genetic data has been prioritized in the second phase of the consortium. Last, neurometabolites were sampled from the motor and mesial prefrontal cortices due to previously identified alterations in these regions. Subsequent MRS studies could seek to include other pathologically relevant regions of interest or to use whole-brain 3-dimensional MRS to investigate progressive changes in ALS and ALS subgroups.
Overall, this study shows that progressive cerebral degeneration in the motor cortex can be detected in vivo in patients with ALS with more aggressive clinical presentations. These findings underscore the heterogeneity of the disease, providing further biological evidence of variable spatial and temporal cerebral degeneration in patients with ALS. This has relevance for clinical trials to assist with classification of patients into more homogeneous cohorts to evaluate disease-modifying interventions.
Acknowledgment
The authors thank all the participants and caregivers for their time and energy for the purpose of this study. They also acknowledge and thank the research staff, including research coordinators and MRI technologists, at each research site for their efforts. Data management and quality control was facilitated by the Canadian Neuromuscular Disease Registry.
Glossary
| ALS | amyotrophic lateral sclerosis |
| ALSFRS-R | ALS Functional Rating Scale–Revised |
| ALS-CI | ALS–cognitively impaired |
| ALS-CN | ALS–cognitively normal |
| ALS-FP | fast-progressing ALS |
| ALS-SP | slowly progressing ALS |
| ALS-UMNH | ALS with high UMN |
| ALS-UMNL | ALS with low UMN |
| CALSNIC | Canadian ALS Neuroimaging Consortium |
| Cho | choline |
| Cr | creatine |
| DPR | disease progression rate |
| ECAS | Edinburgh Cognitive and Behavioural ALS Screen |
| GM | gray matter |
| HC | healthy control |
| ICC | intraclass correlation coefficient |
| Ino | myo-inositol |
| mPFC | mesial prefrontal cortex |
| MRS | magnetic resonance spectroscopy |
| NAA | N-acetylaspartate |
| NAAG | N-acetylaspartylglutamate |
| UMN | upper motor neuron |
| WM | white matter |
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
Funding provide by the Canadian Institutes of Health Research, ALS Society of Canada, Brain Canada Foundation, and Shelly Mrkonjic Research Fund.
Disclosure
D. Ta, A. Ishaque, O. Srivastava, C. Hanstock, P. Seres, D. T. Eurich, C. Luk, H. Briemberg, R. Frayne, A. Genge, S. J. Graham, L. Korngut, L. Zinman, and S. Kalra report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0000000000012367
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8397589
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/107683679
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1212/wnl.0000000000012367
Article citations
Mild cognitive impairment in amyotrophic lateral sclerosis: current view.
J Neural Transm (Vienna), 29 Oct 2024
Cited by: 0 articles | PMID: 39470847
Review
Temporal and spatial progression of microstructural cerebral degeneration in ALS: A multicentre longitudinal diffusion tensor imaging study.
Neuroimage Clin, 43:103633, 14 Jun 2024
Cited by: 0 articles | PMID: 38889523 | PMCID: PMC11231599
Mismatch between clinically defined classification of ALS stage and the burden of cerebral pathology.
J Neurol, 271(5):2547-2559, 28 Jan 2024
Cited by: 0 articles | PMID: 38282082
Potential of neuroimaging as a biomarker in amyotrophic lateral sclerosis: from structure to metabolism.
J Neurol, 271(5):2238-2257, 17 Feb 2024
Cited by: 0 articles | PMID: 38367047
Review
The Spectrum of Cognitive Dysfunction in Amyotrophic Lateral Sclerosis: An Update.
Int J Mol Sci, 24(19):14647, 27 Sep 2023
Cited by: 6 articles | PMID: 37834094 | PMCID: PMC10572320
Review Free full text in Europe PMC
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Data Citations
- (1 citation) DOI - 10.5061/dryad.73n5tb2wx
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT02405182
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study.
Amyotroph Lateral Scler Other Motor Neuron Disord, 5(3):141-149, 01 Sep 2004
Cited by: 59 articles | PMID: 15512902 | PMCID: PMC2744639
Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis.
Arch Neurol, 58(5):729-735, 01 May 2001
Cited by: 60 articles | PMID: 11346367
Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: subgroup analysis and follow-up measurements.
Arch Neurol, 55(7):931-936, 01 Jul 1998
Cited by: 74 articles | PMID: 9678310
[Proton MR spectroscopy studies of the brain in ALS patients].
Neurol Neurochir Pol, 35(1 suppl):61-70, 01 Jan 2001
Cited by: 0 articles | PMID: 11732281
Review
Funding
Funders who supported this work.






