Abstract
Free full text

Cholangiopathy as part of post-COVID syndrome
Abstract
Liver compromise in critically ill patients with coronavirus disease 2019 (COVID-19) is common but usually transient and self-limited. However, liver tests on some patients continue to show abnormal results. Herein, a 29-year-old patient with clinical and histological features of cholangiopathy is presented. Despite treatment with ursodeoxycholic acid and cholestyramine, bilirubin and transaminase levels remained elevated. This case report raises awareness of the difficulty of managing this condition in patients with COVID-19.
1. Introduction
Acute liver compromise in patients with coronavirus disease 2019 (COVID-19) is common. The overall frequency of acute liver injury in COVID-19 is about 26.5%, and up to 41.1% of patients present transaminitis [1]. In addition, COVID-19 patients presenting acute liver injury are more likely to exhibit the poorest outcomes (OR 1.68; 95% CI: 1.04 to 2.70) [1]. Furthermore, levels of glutamic-oxaloacetic transaminase (GOT) or glutamic pyruvic transaminase (GPT) are associated with mortality [1]. Elevated total bilirubin is also considered a biomarker of deleterious outcomes [2].
The mechanism of liver injury remains unknown. Initially, a direct assault of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on hepatocytes that led to abnormal liver enzyme levels was suggested. However, hepatocytes do not express high numbers of receptors for angiotensin converting enzyme 2 (ACE-2) [2], and direct infection of hepatocytes was not found [3]. Some studies, in turn, have suggested that over expression of ACE-2 receptors in cholangiocytes could be associated with direct viral damage in the liver and thus lead to cholangiopathy [4,5]. However, this hypothesis remains to be confirmed.
In a recent case series, three patients with clinical and histologic features resembling secondary sclerosing cholangitis were published [5]. These patients presented with a severe cholangiocyte injury and intrahepatic microangiopathy suggestive of direct injury from SARS-CoV-2 [5]. Herein, a patient with hyperbilirubinemia, transaminitis, and histologic findings suggestive of post-COVID cholangiopathy is presented.
2. Case presentation
A 29-year-old woman came to the emergency room in January 2021 with a 3-day history of dyspnea, dry cough, and fever. She had a history of obesity and was not on any regular medications. Family history was unremarkable. On examination, the patient had tachypnea, and rales were heard in both lungs. Oxygen saturation was below 80%, requiring high-flow nasal cannula. Cardiac, abdominal, and neurological examinations were normal.
Chest x-ray showed multilobar pneumonia, and SARS-CoV-2 infection was confirmed by antigen test. Blood count reported leukocytosis and lymphopenia. Ferritin, D-Dimer, and C reactive protein were elevated. In addition, the patient presented elevated troponin as well as creatinine and BUN (Fig. 1A and B). Myocarditis/Type 2 infarction were suspected, and an acute kidney injury was diagnosed (Fig. 1A and B). Transthoracic echocardiogram was normal. Patient was started on antibiotics because of suspected bacterial infection, colchicine, enoxaparin, aspirin, dexamethasone, furosemide, and intravenous fluids (Fig. 1A).
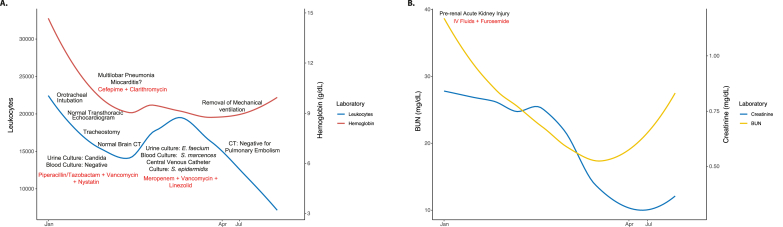
Longitudinal assessment of hematological and renal function. A. Total leukocytes and hemoglobin. B. Creatinine and BUN. In both panels, the line represents the locally estimated scatterplot smoothing. Relevant clinical features and interventions are shown chronologically. CT: computed tomography; IV: intravenous; BUN: blood urea nitrogen.
Nine days after admission, the patient deteriorated, and mechanical ventilation was initiated. Although blood cultures were negative, urine culture reported Candida spp. and nystatin was initiated (Fig. 1A). In February, given the long-lasting orotracheal intubation, a tracheostomy was done. During this period, she presented fasciculations in face, arm, and leg muscles. However, the brain computed tomography (CT) scan was normal. She remained in the intensive care unit (ICU) with slight clinical improvement.
At the beginning of March, urine, blood, and central venous catheter cultures were positive for E. faecium, S. marcences, and S. epidermidis respectively. Thus, antibiotics were modified (Fig. 1A). At the same time, the patient presented jaundice with mixed hyperbilirubinemia, elevated transaminases, and alkaline phosphatase (Fig. 2A).
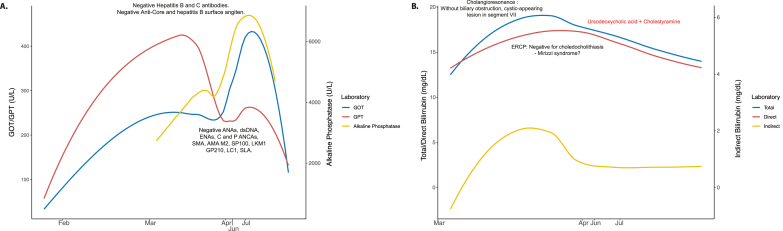
Longitudinal assessment of liver function. A. Transaminases and alkaline phosphatase. B. Total, direct, and indirect bilirubin. In both panels, the line represents the locally estimated scatterplot smoothing. Relevant clinical features and interventions are shown chronologically. GOT: glutamic-oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; ANAs: anti-nuclear antibodies; ENAs: extractable nuclear antigens antibodies; dsDNA: anti-double stranded DNA antibodies; ANCAs: anti-neutrophil cytoplasmic antibodies; SMA: smooth muscle antibodies; AMA-M2: anti-mitochondrial antibodies type 2; LKM1: liver kidney microsome type 1 antibodies; LC1: Anti-liver cytosolic antigen type 1; SLA: soluble liver antigen antibodies; ECRP: endoscopic retrograde cholangiopancreatography.
An obstructive biliary disease was suspected. However, cholangioresonance showed no biliary obstruction and reported a cystic-appearing lesion in segment VII of the liver. The endoscopic retrograde cholangiopancreatography (ERCP) was negative for choledocholithiasis, and Mirizzi syndrome was suspected. However, the latter was not confirmed by the cholangioresonance results, and a SARS-CoV-2 cholangiopathy was suspected (Fig. 2B). The liver biopsy exhibited a low periportal inflammatory infiltrate without necrosis but with a severe obstructive cholestatic pattern (Fig. 3). Standard therapy was continued, the tracheostomy was removed, and she was released from ICU.
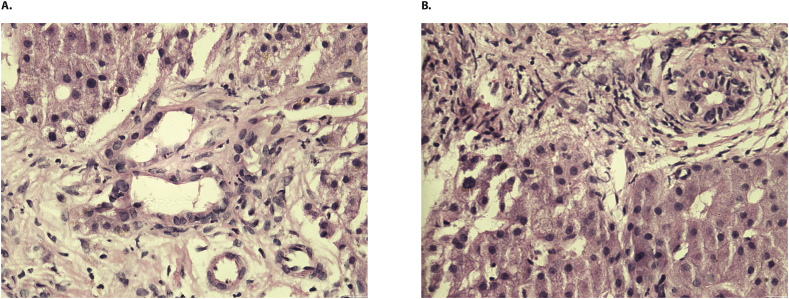
Histology of liver biopsy. A-B. Low periportal inflammatory infiltrate without necrosis but with a severe obstructive cholestatic pattern. H&E; × 40.
In the middle of June, the patient was transferred to our center due to the persistence of jaundice. Laboratory results for hepatotropic viruses were negative, and an autoimmune condition was suspected. However, the autoimmune profile was negative (by ELISA, INOVA Diagnostics, San Diego, CA, USA, and IMTEC-ANA-LIA Maxx and LIVER-LIA - Human Diagnostics Magdeburg, Germany) (Fig. 2A). Ursodeoxycholic acid and cholestyramine were prescribed to treat the mixed hyperbilirubinemia with a slight improvement in clinical manifestations. At present, jaundice persists, and patient presents post-COVID syndrome (PCS) including memory loss, attention disorders, headache, dizziness, and chest pain.
3. Discussion
A patient who recovered from critical illness but presented PCS mainly characterized by cholangiopathy was described. Cholangiopathy appeared during critical illness, and clinical manifestations persisted after ICU discharge. Four months later, the condition persists with no response to standard therapy. This case illustrates the troublesome management of patients with PCS.
Roth et al. [5], described three similar patients with cholangiopathy associated with COVID-19 in whom the liver biopsy showed bile duct paucity, periportal hepatocytes metaplasia (characteristic of obstructive cholestasis), and evidence of bridging fibrosis all of which indicated a risk of progression to a secondary biliary cirrhosis. This contrasts with our case, in which a low periportal inflammatory infiltrate without fibrosis but with a severe cholestatic pattern was observed. This may suggest that post-COVID-19 cholangiopathy presents with different pathways for development.
Another disorder named “secondary sclerosing cholangitis of critically ill patients” (SSC–CIP) has been described recently. This is a cholestatic liver disease of unknown etiology and represents the most prevalent form of secondary sclerosing cholangitis. It presents a rapid progression to liver cirrhosis and hepatic failure [6]. Mechanical ventilation with high positive end-expiratory pressure, prone positioning, and a higher volume of intraperitoneal fat have been suggested as risk factors for developing this condition. The ERCP and liver biopsy are the gold standard for diagnosis [6].
Although our case could resemble SSC-CIP, the ERCP was normal and liver biopsy did not show the classical SSC-CIP features (i.e., ischemic-like lesions, portal inflammation, duct loss, ductopenia, lymph follicles, granulomas, lymphocytic interface activity, or portal fibrosis). This suggests that our patient's condition was not associated with long lasting ICU management, and other mechanisms for injury should be considered.
Autoimmune mediated conditions such as primary biliary cholangitis have been associated with post–COVID cholangiopathy [7]. Patients presented mild hyperbilirubinemia, lymphocyte infiltration in a liver biopsy as well as positivity for antinuclear (ANAs), and anti-mitochondrial antibodies (AMA) [7]. In our case, the liver biopsy did not show periportal lymphocyte infiltrates, and the panel of autoantibodies was negative. Thus, this suggests a non-autoimmune phenomenon for liver injury.
The patient's hyperbilirubinemia could have biased the autoantibody testing. Bilirubin strongly binds to proteins and may have interfered with autoantibody detection. Indirect evidence from patients with SLE showed that high levels of bilirubin were associated with lower levels of anti-dsDNA antibodies [8]. The panel of autoantibodies should be repeated after resolution of hyperbilirubinemia.
4. Conclusions
Critically ill patients with COVID-19 exhibit a higher risk of post-COVID cholangiopathy. Liver injury could be mediated by a SARS-CoV-2 predilection for cholangiocytes. As a rule of thumb, autoimmune conditions should be ruled out in all cases of COVID-19-associated hyperbilirubinemia. Early diagnosis and management should be implemented to avoid long-lasting adverse outcomes. Cholangiopathy must be consider as a clinical manifestation of PCS [9].
Funding
This work was supported by the Universidad del Rosario, grant number (ABN-011).
Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all the members of the CREA for their contributions and fruitful discussions during the preparation of the manuscript.
References
Articles from Journal of Translational Autoimmunity are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/112759721
Article citations
Impact of prior SARS-CoV-2 infection on postoperative recovery in patients with hepatocellular carcinoma resection.
BMC Gastroenterol, 24(1):317, 17 Sep 2024
Cited by: 0 articles | PMID: 39289600 | PMCID: PMC11409749
Clinical and Histopathological Discoveries in Patients with Hepatic Injury and Cholangiopathy Who Have Died of COVID-19: Insights and Opportunities for Intervention.
Hepat Med, 15:151-164, 04 Oct 2023
Cited by: 1 article | PMID: 37814605 | PMCID: PMC10560482
Review Free full text in Europe PMC
Post-COVID-19 cholangiopathy: Systematic review.
World J Methodol, 13(4):296-322, 20 Sep 2023
Cited by: 3 articles | PMID: 37771872 | PMCID: PMC10523251
Secondary Sclerosing Cholangitis After SARS-CoV2: ICU Ketamine Use or Virus-Specific Biliary Tropism and Injury in the Context of Biliary Ischemia in Critically Ill Patients?
Hepat Med, 15:93-112, 01 Aug 2023
Cited by: 3 articles | PMID: 37547355 | PMCID: PMC10404108
Post-COVID-19 Cholangiopathy: A Recent Indication for Liver Transplantation.
J Clin Med Res, 15(4):250-254, 28 Apr 2023
Cited by: 0 articles | PMID: 37187714 | PMCID: PMC10181353
Go to all (16) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Post-COVID-19 cholangiopathy: Current understanding and management options.
World J Gastrointest Surg, 15(5):788-798, 01 May 2023
Cited by: 5 articles | PMID: 37342848 | PMCID: PMC10277943
Review Free full text in Europe PMC
Post-COVID-19 Cholangiopathy: A Systematic Review.
J Clin Exp Hepatol, 13(3):489-499, 30 Oct 2022
Cited by: 11 articles | PMID: 36337085 | PMCID: PMC9618303
Review Free full text in Europe PMC
COVID-Associated Cast-Forming Cholangiopathy: A Commentary on Disease Mechanism, Treatment, and Prognosis.
Hepat Med, 15:27-32, 28 Mar 2023
Cited by: 1 article | PMID: 37013139 | PMCID: PMC10066716
Multiorgan Failure and Omicron: A Suspected Case of Post-COVID-19 Cholangiopathy.
Cureus, 15(2):e35010, 15 Feb 2023
Cited by: 1 article | PMID: 36938182 | PMCID: PMC10021348
Funding
Funders who supported this work.
Universidad Del Rosario (1)
Grant ID: ABN-011




