Abstract
Free full text

Neurodevelopmental origins of substance use disorders: Evidence from animal models of early-life adversity and addiction
Abstract
Addiction is a chronic relapsing disorder with devastating personal, societal, and economic consequences. In humans, early-life adversity (ELA) such as trauma, neglect, and resource scarcity are linked with increased risk of later-life addiction, but the brain mechanisms underlying this link are still poorly understood. Here, we focus on data from rodent models of ELA and addiction, in which causal effects of ELA on later-life responses to drugs and the neurodevelopmental mechanisms by which ELA increases vulnerability to addiction can be determined. We first summarize evidence for a link between ELA and addiction in humans, then describe how ELA is commonly modeled in rodents. Since addiction is a heterogeneous disease with many individually varying behavioral aspects that may be impacted by ELA, we next discuss common rodent assays of addiction-like behaviors. We then summarize the specific addiction-relevant behavioral phenotypes caused by ELA in male and female rodents and discuss some of the underlying changes in brain reward and stress circuits that are likely responsible. By better understanding the behavioral and neural mechanisms by which ELA promotes addiction vulnerability, we hope to facilitate development of new approaches for preventing or treating addiction in those with a history of ELA.
Introduction
Substance use disorder (SUD) is characterized by loss of control over increasingly harmful substance use, often leading to physical dependence, as well as by persistent drug cravings and risk of relapse, which can last for years (Hasin et al., 2013). Addictive drugs are thought to hijack brain reward circuits that normally mediate seeking of and pleasure from natural rewards (Nesse & Berridge, 1997), thereby eliciting subjectively pleasurable experiences and continued recreational use (i.e. positive reinforcement) in some individuals. In others, initial drug use may instead result in relief of an underlying negative affective state (i.e. negative reinforcement), a process that is further exacerbated by subsequent escalating drug use and the affective dysregulation it causes (Koob & Le Moal, 1997). Moreover, continued chronic drug use may also promote excessive learning or inflexible drug habits (Berke & Hyman, 2000; Everitt & Wolf, 2002). The relative roles of positive or negative reinforcement to an individual’s drug use likely differ based on the abused drug of choice, specific drug availability, as well as one’s sex and heritable or environmental risk or resilience factors. In other words, there is more than one way to be at risk for addiction, and more than one manifestation of the disorder once it emerges (Fig. 1). Understanding how these complex factors interact to put individuals at risk of SUD and the contribution of ELA to these factors and mechanisms is essential for developing new ways to treat and prevent SUD.
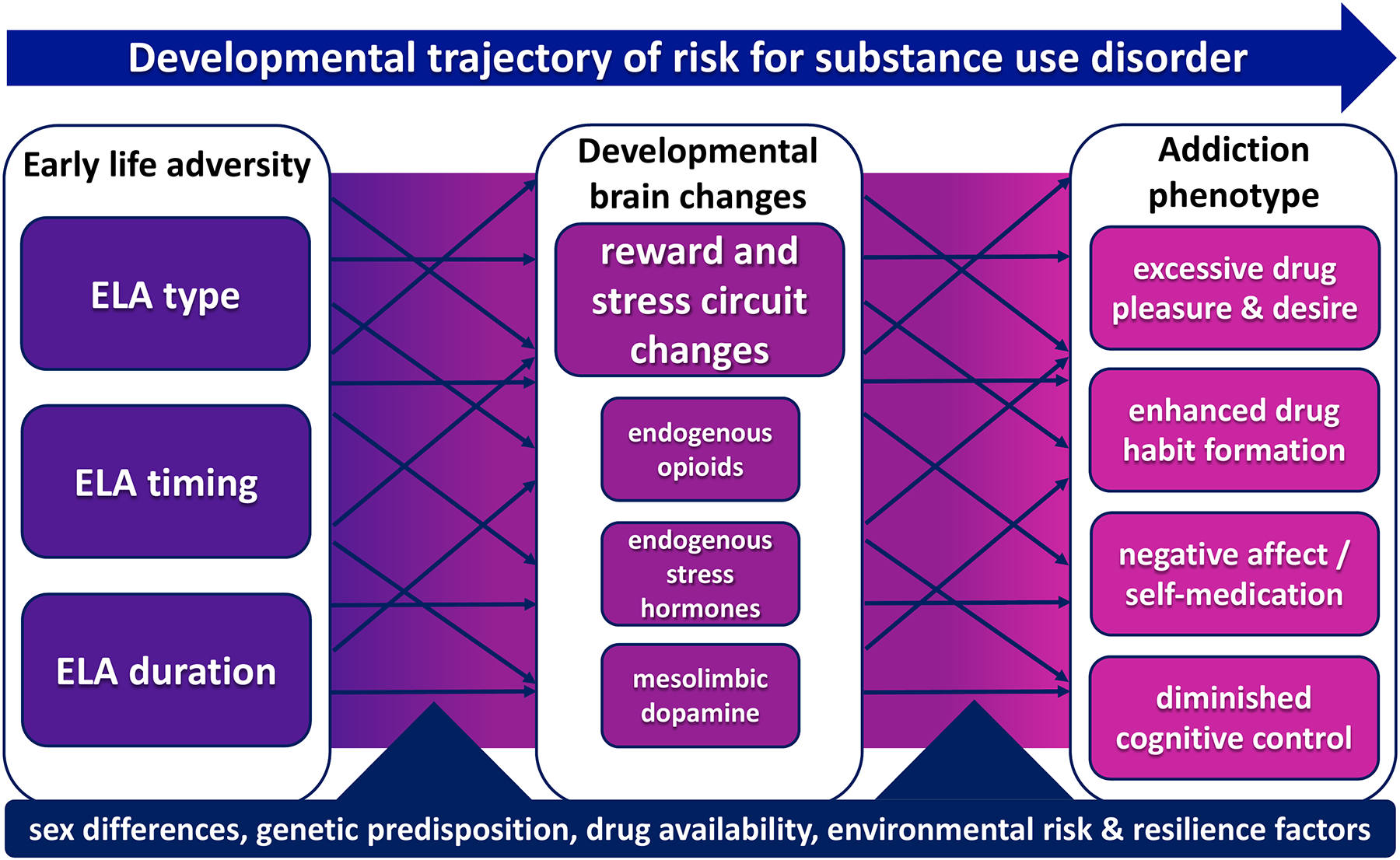
ELA perturbs multiple neurodevelopmental processes, including the development and maturation of reward and stress circuits. These alterations may lead to a variety of reward-related behaviors associated with addiction. Importantly, the developmental trajectory from ELA to substance use is mediated by a complex multitude of interacting features, ultimately manifesting as a heterogeneous constellation of neurobiological and behavioral outcomes that increase risk for substance use disorder.
Many factors contribute to the risk for developing SUD, including developmental experiences such as stress or insecure social relationships, drug availability, genetic predisposition and biological sex differences (Schuckit, 2002; Dube et al., 2003; Sinha, 2008; Volkow et al., 2011; Kreek et al., 2012; Wright et al., 2014; Becker & Chartoff, 2019; Crist et al., 2019; Jiang et al., 2019). Moreover, these factors may also interact in important ways. Here we will focus on ELA, an important environmental risk factor for SUD. We will describe the association of ELA with addiction in humans, then concentrate on preclinical research showing long-lasting, causal effects of ELA on addiction-related behaviors and aim to elucidate the brain mechanisms that may be involved.
Association of ELA with Addiction in Humans
ELA related to poverty, trauma and chaotic environment affects over 30% of children in the U.S. (American Psychological Association, 2018). When adversity occurs during critical neurodevelopmental stages, it can impact cognitive and emotional processing long into adulthood (Callaghan & Tottenham, 2016; Chen & Baram, 2016; Short & Baram, 2019). A classic psychological mechanism by which this occurs involves potential disruption of the attachment of infants to their primary caregivers (Ainsworth, 1969; Bowlby, 1974; 2008); such disruption may have long-lasting effects on social and emotional development. From a neurobiological perspective, ELA perturbs numerous neurodevelopmental processes, including the development and maturation of brain circuits involved in cognition and emotion. In this review, we consider how adverse sensory signals from the environment (i.e., early life experiences), especially during critical developmental periods, can disturb synaptic strengthening or pruning in reward and stress circuits (Korosi et al., 2010; Singh-Taylor et al., 2017; Bolton et al., 2018a; Granger et al., 2020). By impacting the maturation of brain circuits, adverse early-life experiences lead to long-lasting changes in the function of these circuits, potentially impacting vulnerability to the addictive effects of drugs.
Indeed, adverse early life experiences are robustly associated with later-life substance addiction in humans (Nurco et al., 1996; Simpson & Miller, 2002; Dube et al., 2003; Widom et al., 2006; Gershon et al., 2008; Sinha, 2008; Enoch, 2011; Shand et al., 2011; Stein et al., 2017; Marsh et al., 2018; Levis et al., 2021). The landmark Adverse Childhood Experiences study found that in addition to increasing the likelihood of early initiation of drug use (Dube et al., 2003), a risk factor for addiction in itself (Grant & Dawson, 1997; McCabe et al., 2007; Chen et al., 2009), ELA increases risk for smoking up to 2-fold, alcoholism up to 7-fold, injected drug use up to 11-fold, and other illicit drug use up to 4-fold (Anda et al., 2006). Yet, ELA does not inevitably lead to substance use disorder in all individuals. Reasons for this may be that specific long-term outcomes of ELA vary based on type of adversity experienced (Sheridan & McLaughlin, 2014; Dennison et al., 2019), the age of exposure to adversity (Luby et al., 2020), individual variability in traits associated with resilience to stress (Fergusson & Horwood, 2003; Hartmann & Schmidt, 2020; Zinn et al., 2020; Méndez Leal & Silvers, 2021), and societal factors such as the availability of specific drugs (Wright et al., 2014) or access to supportive interpersonal relationships and community resources (Daskalakis et al., 2013; Gartland et al., 2019; Liu et al., 2020). Additionally, sex and gender differences may play a role in these outcomes. For example, in women, a history of neglect predicts a higher probability of opioid dependence, while dependence in men is instead better predicted by acute traumatic experiences and concomitant post-traumatic stress symptoms (Shand et al., 2011). In fact, women with a history of ELA appear to be particularly predisposed to substance use disorders relative to men and to individuals with no history of ELA (Widom et al., 1995; Najavits et al., 1997; Hyman et al., 2006; Gershon et al., 2008; Hyman et al., 2008; Lansford et al., 2010; Shand et al., 2011; Marsh et al., 2018; Peltier et al., 2019; Capusan et al., 2021). Notably, this sex-dependent relationship may be partially explained by the fact that girls and boys tend to be exposed to different types of adversities (Short & Baram, 2019; Haahr-Pedersen et al., 2020).
Although clinical studies strongly support an association of ELA with later-life SUD, it is challenging to establish causality in human studies. Therefore, animal models are essential for parsing the mechanisms by which ELA impacts neurodevelopment and characterizing the resulting differences in behavioral responses to drugs of abuse. In the following sections, we 1) describe two of the most commonly used rodent models of ELA, 2) overview common rodent tests used to model addiction-relevant behavioral processes, 3) describe how rodent ELA models impact addiction-relevant behaviors, and 4) discuss known effects of ELA upon brain reward and stress circuit development in rodents which may underlie this association.
Animal Models of Early Life Adversity
Several animal models have been developed to study the effects of ELA on brain development and behavior in rodents as well as nonhuman primates [For comprehensive reviews of these models see (Molet et al., 2014; Nishi et al., 2014; Doherty et al., 2017; Walker et al., 2017; Wakeford et al., 2018; Brenhouse & Bath, 2019)]. Here, we focus on two of the most commonly used rodent models; maternal separation (MS) and limited bedding and nesting (LBN). Notably, as in humans (Shand et al., 2011; Sheridan & McLaughlin, 2014; Strathearn et al., 2020), the outcomes of ELA in rodents varies based on such factors as the type and timing of adversity, as well as on the animal species and strain used, the outcome measures assayed, sex, and other factors (van Oers et al., 1998; Pryce & Feldon, 2003; Moffett et al., 2007; Schmidt et al., 2011; Kundakovic et al., 2013; Andersen, 2015; Di Segni et al., 2018; Bonapersona et al., 2019; Brenhouse & Bath, 2019; Walters & Kosten, 2019; Bath, 2020; Demaestri et al., 2020; Lundberg et al., 2020). It is important to be aware of this diversity and embrace the notion that different rodent models of ELA may lead to different neurodevelopmental changes and ultimately to distinct addiction-relevant phenotypes.
A number of studies examining the effects of ELA on reward-seeking behavior employ a version of the maternal separation (MS) procedure, first introduced by Seymore Levine (1957). Pups are separated from their mother daily during the first 1–2 weeks of life, for a period of time ranging between 15 minutes and 24 hours. This causes an acute, predictable daily stressor accompanied by transient corticosterone elevations during the period of separation (McCormick et al., 1998). Notably, the duration of separation period itself (minutes vs. hours) and resulting impact on maternal behavior is a crucial variable that determines the nature of long-term outcomes (Fenoglio et al., 2006; Korosi et al., 2010; Tractenberg et al., 2016; Orso et al., 2019). For example, following 15 minutes of separation, pups typically receive augmented care when returned to their mother (Pryce et al., 2001; Orso et al., 2019). Some have found that this augmented care following 15-minute separations (“handling”) promotes resilience and improve long-term outcomes (Levine, 1957; Korosi & Baram, 2009), whereas daily MS of three hours, the most common approach, tends to lead to more detrimental outcomes (Tractenberg et al., 2016; Bonapersona et al., 2019). However, some apparent contradictions in the literature exist, and others have observed that brief (minutes) or long (hours) maternal separation can in some cases result in reward-related outcomes that are similar to non-handled controls (Meaney et al., 2002; Schmidt et al., 2011; Nylander & Roman, 2013; Bian et al., 2015). These apparent inconsistencies highlight the complexities of the model, as well as the need for appropriate experimental controls (e.g., handled vs. non-handled conditions). In addition, the age of separation critically influences the outcomes of MS (van Oers et al., 1998; Peña et al., 2019). Relatedly, sex mediates MS effects on neurodevelopment in a manner that is still incompletely understood, but which may involve sex differences in neurodevelopmental sensitive periods, hormonal interactions, and other factors (Flagel et al., 2003; Bath, 2020).
A more recently developed model of ELA involves simulating chronic resource poverty by limiting bedding and nesting materials (LBN) from a postpartum rodent dam and her litter. This procedure has been adopted widely in rats and mice (Gilles et al., 1996; Wang et al., 2012; Molet et al., 2014; Bath et al., 2017; Walker et al., 2017) in original or modified formats (Walker et al., 2017; Opendak et al., 2019). In this model, most of the bedding and nesting materials are removed from the home cage environment, causing in the mother a mild, chronic stress that leads to unpredictable and fragmented maternal care (Ivy et al., 2008; Rice et al., 2008; Molet et al., 2014) and a transient increase in basal corticosterone levels in both dam and pups (Brunson et al., 2005; Ivy et al., 2008). Importantly, the total quantity of care received by pups is unaffected by LBN (Molet et al., 2016a). Instead, the quality of care is disrupted by LBN, such that dams more frequently and unpredictably switch between care elements (licking, feeding, etc). This chaotic patterning of care leads to pronounced long-term cognitive and affective deficits in rodents (Ivy et al., 2010; Molet et al., 2016a; Bolton et al., 2017; Short & Baram, 2019). Notably, unpredictable parental care is also a strong predictor of negative cognitive and emotional outcomes in humans and nonhuman primates (Rosenblum & Paully, 1987; Coplan et al., 1996; Davis et al., 2017; Wakeford et al., 2018; Davis et al., 2019; Glynn & Baram, 2019).
In both MS and LBN models of ELA, the developmental stage(s) at which adversity occurs is important for determining the long-term behavioral and neural outcomes. In rodents, early postnatal life, a period roughly analogous to the first year of life in humans (Avishai-Eliner et al., 2002; Birnie et al., 2020), seems to be a sensitive period for long-term negative outcomes of ELA. This may be because this period is especially important for organizing brain reward and stress circuits (Molet et al., 2014; Birnie et al., 2020; Luby et al., 2020), leaving them susceptible to perturbation by ELA. When adverse events or chaotic parental care occur during this sensitive period, circuits are impacted in a manner that may be irreversible once the sensitive window is closed, analogous to how sensory systems are shaped by appropriate environmental stimuli occurring at the necessary developmental stage (Hubel et al., 1977; Zhang et al., 2001; Hensch, 2004; Li et al., 2006; Espinosa & Stryker, 2012). Just as sensory systems require regular inputs at specific times during development to mature properly, these reward and stress circuits may be similarly “tuned” by factors like acute stressors or the predictability of parental care patterning (Hane & Fox, 2016; Davis et al., 2017; Andersen, 2018; Glynn & Baram, 2019), thereby permanently impacting reward and stress circuit function (Baram et al., 2012; Glynn & Baram, 2019; Birnie et al., 2020; Luby et al., 2020). Of note, the ability of brains to postnatally “tune” development of survival-critical reward and stress circuits may be an adaptive feature. By responding to environmental signals conveying information about the safety or predictability of the world in which one is born, circuits may develop in a manner that could promote survival and evolutionary fitness (Schmidt, 2011). Yet in our modern world, circuits developing under adversity seem too often to lead to unwanted adverse outcomes, such as vulnerability to SUD.
Modeling Behavioral Aspects of Addiction in Rodents
In order to understand how ELA may cause susceptibility to addiction-like outcomes later in life, it is essential to be precise about what is meant by “addiction-relevant behavior.” Drug addiction is a chronic, relapsing disorder characterized a heterogeneous set of maladaptive drug-seeking behaviors. It has been conceptualized as a “downward spiral” beginning with cycles of binging and intoxication motivated by positive reinforcement from pleasurable drug effects or by negative reinforcement due to drug-induced relief of negative affective states. Subsequently, drug abuse can transition into uncontrolled use, when discontinuation of use results in highly aversive withdrawal symptoms, drug cravings, persistent and invasive thoughts about drugs, and impaired cognitive control that can lead to relapse (Koob & Le Moal, 1997). However, trajectories through these stages are not uniform; individuals with SUD may present with different combinations of symptoms or reasons for seeking treatment, and relapse may be triggered by a variety of emotional, physiological, and environmental factors. Consideration of these potential individual differences will be important for understanding the neural mechanisms underlying the various factors associated with risk for SUD, as well as for developing effective prevention and treatment strategies. Accordingly, when using animal models to investigate the brain mechanisms by which ELA leads to SUD vulnerability, it is essential to consider the specific addiction-related behavioral processes being modeled.
The initial phases of SUD typically involve acute, repeated, pleasurable intoxication that is liable to be repeated (i.e. it is positively reinforcing). These reinforcing drug effects, as well as their potential alterations by ELA, can be assessed through several different behavioral measures. For example, conditioned place preference (CPP) models measure an animal’s ability to associate the pleasurable effects of a drug with a specific place, which can be recalled later in a drug-free state, causing the animal to return to that place. The reinforcing (or rewarding) effects of addictive drugs might also be inferred by measuring effects of acute drug exposure on intracranial self-stimulation (ICSS), or operant responding by an animal to receive increasingly intense patterns of rewarding electrical brain stimulation (Olds & Milner, 1954; Carlezon & Chartoff, 2007). Abused drugs tend to reduce ICSS threshold, which has been interpreted to result from the pleasurable effects of the drug substituting for pleasure derived from intracranial stimulation (Negus & Miller, 2014), though this interpretation has been questioned (Smith et al., 2010). Another behavioral model that may measure the addiction-promoting effects of drugs is locomotor sensitization, or an increase in the locomotor-activating effects of abused drugs after repeated experimenter administration. In addiction, desire for drugs increases markedly with repeated use (sometimes called incentive sensitization). Therefore, locomotor sensitization has been interpreted as a proxy for incentive motivational processes that fuel addiction-like drug seeking behaviors (Robinson & Berridge, 2008). In this manner, locomotor sensitization may model the excessive motivation to take drugs that characterizes addiction. ELA may impact any or all of these behavioral responses to experimenter-administered drugs, each of which may rely on distinct underlying brain circuits.
The aforementioned models involve non-voluntary administration of drug to experimental animals; yet, drug effects on the brain and behavior in both humans and rodents differ markedly based on whether they are experimenter- or self-administered (Robinson et al., 2002; Jacobs et al., 2003; Steketee & Kalivas, 2011). Researchers have therefore created models in rodents which measure voluntary drug use, for example via oral ingestion or intravenous self-administration. Voluntary consumption approaches can be used to model recreational drug-taking over short periods of time or escalating and compulsive use over more extended access periods (Markou et al., 1993; Ahmed et al., 2000; Ward et al., 2006; Rogers et al., 2008). ELA may therefore impact initial acquisition of drug taking, short-term “recreational” drug use, or escalation of use over extended periods, each implying impact on distinct neural substrates.
Drug self-administration models can also be adapted to measure several distinct types of drug intake, such as highly motivated, effortful drug seeking as opposed to drug taking under free access conditions. This is important, because these types of drug taking behaviors have different underlying neural mechanisms (Berridge & Robinson, 2003; Di Ciano & Everitt, 2005; Baldo & Kelley, 2007; Bentzley et al., 2013; Salamone et al., 2016; Volkow et al., 2017) that may be differentially affected by ELA. Analyses of high- versus low-effort drug seeking can capitalize on behavioral economic theory, which stipulates that consumption of any commodity is sensitive to increasing price, and that some commodities are more sensitive to price than others. This concept is referred to as “demand elasticity” (Hursh, 1980). Inelastic demand, or relative insensitivity to price, is a feature of SUD, in that addiction can be characterized as an excessively inelastic demand for a drug (Bickel et al., 2014). In other words, addicted individuals will pay higher prices (financial or in life consequences) for drugs than will non-addicted individuals. Importantly, demand elasticity for drugs is distinct from preferred drug intake when the drug is free or cheap, in which case consumption is governed instead by “hedonic setpoint” (Hursh & Silberberg, 2008; Bickel et al., 2014; Strickland et al., 2019). In rodents, demand elasticity and hedonic setpoint for abused drugs can be modeled by examining intake at different “prices,” operationalized as the amount of effort required to receive a unit of drug (Hursh & Silberberg, 2008; Oleson & Roberts, 2009). Recently, a version of this protocol was developed in which both demand elasticity and hedonic setpoint can be determined in a single ~2hr test session (Oleson & Roberts, 2008; Bentzley et al., 2013; Bentzley et al., 2014; Levis et al., 2019; Newman & Ferrario, 2020). Notably, the neural substrates of demand elasticity and hedonic setpoint for abused drugs including cocaine and opioids are distinct (Bentzley & Aston-Jones, 2015; Bolton et al., 2018b; Mahler et al., 2018; Salamone et al., 2018; Levis et al., 2019), meaning that ELA could alter one or both of these processes, leading to distinct addiction-relevant behavioral phenotypes.
Negative reinforcement, or reinforcement motivated by elimination of an unpleasant stimulus, is often endorsed by individuals with SUD, and likely contributes to addiction in several ways. One of these serves as the basis for “self-medication” theories of substance use, in which drugs are used to relieve pre-existing negative affective states. Self-medication likely plays a major role in addiction for some individuals (Khantzian, 1987; Markou et al., 1998), especially for pain-relieving or anxiolytic drugs like opioids or alcohol. Use of drugs to relieve negative states can be measured in animals, for example by examining how pain impacts seeking of analgesic opioid drugs (Martin & Ewan, 2008; Evans & Cahill, 2016). When drug use becomes chronic and escalating, negative reinforcement also underlies continued use in order to reverse withdrawal-induced sickness and negative affect. In rodents, somatic symptoms of acutely aversive withdrawal such as piloerection, “wet dog” shakes, and rapid weight loss can be measured (Gellert & Holtzman, 1978; Hildebrand et al., 1997; Becker, 2000), as can acute or persistent affective dysregulation occurring after cessation of drug exposure (Malin et al., 2000; Malin & Goyarzu, 2009; Rothwell et al., 2012). It is possible that ELA impacts one or more of these negative reinforcement processes, for example by inducing negative affective states that are relieved by initial drug use, by impacting physiological dependence upon drug with chronic use, or by influencing the severity of acute and/or protracted withdrawal symptoms.
Another important aspect of addiction is its chronic, relapsing nature. Indeed, risk for relapse often continues to be significant even after years of abstinence. Relapse is often precipitated by specific environmental triggers, such as experiencing drug-associated cues, acute stressors, or ingestion of small, “priming” doses of drug. Each of these factors can be modeled in rodents by imposing abstinence following a period of drug self-administration, then introducing one or more relapse triggers, causing animals to reinstate their drug seeking (Stewart & de Wit, 1987; Shaham et al., 2003). Adaptations of these models have also been recently developed in which animals voluntarily abstain from drug, a behavior that is characteristic of humans attempting to cease or curtail their drug use. This can be achieved in rodents by imposing punishments (e.g. shocks) along with drugs, or by forcing a choice between drugs and highly salient rewards such as palatable foods or social interactions (Panlilio et al., 2003; Ahmed, 2018; Farrell et al., 2018; Marchant et al., 2019; Venniro et al., 2019). Individual differences in these choice behaviors potentially represent one aspect of an individual’s risk for addiction that might be influenced by ELA, though choice behaviors have not been thoroughly examined in the context of ELA. Indeed, it is estimated that only a subset of outbred rats exhibit “compulsive” drug seeking (Belin et al., 2008; Flagel et al., 2009; Belin et al., 2011; George & Koob, 2017; Farrell et al., 2018), and it is possible that developmental environment manipulations such as ELA could alter this ratio. Given the variability in behavioral traits, ELA might therefore affect the manifestation of addiction-like drug-seeking behaviors by influencing reactivity to potential relapse triggers, the sensitivity to factors that suppress drug intake, or both.
It is also important to recognize that addiction-related behaviors in the models discussed above and their underlying neural substrates may vary based on the studied drug of abuse (Schuster & Thompson, 1969; Thompson & Pickens, 1970; Shalev et al., 2002; Meyer et al., 2016). Likewise, humans may have specific vulnerabilities only to certain drug classes (e.g. stimulants vs. depressants), and the mechanisms driving specific drug choices (beyond immediate drug availability) are not well understood. Furthermore, although some addiction-related drug effects are common to all major abused drugs (Wise & Rompré, 1989; Saal et al., 2003; Nestler, 2004; Scofield et al., 2016), there are also major differences in the neural mechanisms by which different classes of drugs act. Therefore, it is possible that the neurodevelopmental changes in brain reward and stress circuits caused by ELA will lead to susceptibility to addiction to specific classes of drug, and more work is required to test this possibility.
In sum, addiction is a heterogeneous disorder. Its multiple and interacting features and components can be impacted by ELA in complex ways. These facts necessitate sophisticated and precise modeling in rodents. Understanding exactly which addiction-relevant behaviors are affected by ELA will be essential for understanding the nature of the risk ELA imposes on individuals with SUD. In the next sections we review evidence that ELA in rodents leads to a variety of changes in addiction-relevant behaviors (summarized in Table 1), and discuss salient modulating factors including the specific ELA model, sex, and abused drug which contribute to these relationships.
Table 1.
Summary of findings on the effects of ELA on stimulant, alcohol, and opioid-seeking behaviors and relevant stress and reward circuit correlates.
| Reference | Drug Class | ELA Procedure | Species | ELA Effect on Addiction-like Behavior ND = not done; Ø = no effect | Reward / Stress Circuit Correlate | Procedural Notes | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Levis et al., 2019 | opioid | LBN P2-9 | rat | ND | ↑ | N/A | |
| Ordoñes Sanchez et al., 2021 | opioid | LBN P2-9 | rat | ↑ | Ø | transcription changes in NAc (M) | |
| Kalinichev et al., 2002 | opioid | MS15/180 P2-14 | rat | ↑ (MS180 > MS15) | ND | N/A | |
| Matthews & Robbins, 2003 | opioid | REMS360* P5-20 | rat | ND | ↓ | N/A | * separations occurred on 10 randomly spaced occasions |
| Vazquez et al., 2005 | opioid | MS180 P1-14 | rat | ↑ | ND | ↓ striatal endogenous opioid mRNA | |
| Michaels & Holtzman, 2008 | opioid | 24h MS P2; MS180-360* P4-12 | rat | ↑ | Ø | N/A | * alternating 3h and 6h separations |
| Abad et al., 2016 | opioid | MS180 P2-14 | rat | ↑ | ↑ | N/A | |
| Mohammadian et al., 2019 | opioid | MS180 P2-14 | rat | ↑ | ↑ | N/A | |
| Bolton et al., 2018b | psychostimulant | LBN P2-9 | rat | ↓ | ND | ↑ cocaine-induced c-Fos in NAc, lateral habenula, central amygdala | |
| Campbell & Spear, 1999 | psychostimulant | MS15 P1-12 | rat | ↓ | ↓ | N/A | |
| Matthews et al., 1999 | psychostimulant | REMS360* P5-20 | rat | ↓ | ↑ | N/A | * separations occurred on 10 randomly spaced occasions |
| Kosten et al., 2000, 2004 | psychostimulant | MS60 P2-9 | rat | ↑ | ↑ | N/A | |
| Li et al., 2003 | psychostimulant | MS15/180 P1-21 | rat | ND | ↓ (MS15/180) | N/A | |
| Brake et al., 2004 | psychostimulant | MS0/15/180 P1-14 | rat | ↑ (MS180) ↓ (MS15) | ND | ↓ striatal DAT (MS180) | |
| Marquardt et al., 2004 | psychostimulant | MS+ P1-10 | rat | ↑ | ND | N/A | + additional aversive stimulus during separation |
| Zhang et al., 2005 | psychostimulant | MS60 P2-9 | rat | ↑ | ND | N/A | |
| Moffett et al., 2006 | psychostimulant | MS15/180 P2-15 | rat | ↑ (MS180) | ND | N/A | |
| Vazquez et al., 2006 | psychostimulant alcohol opioid | MS180 P1-14 | rat | slight ↑ no effect ↑ | ND | no effect of MS on VTA or striatal DAT | |
| Der-Avakiann & Markou, 2010 | psychostimulant | MS180 P1-14 | rat | ↑ | ND | N/A | |
| Lewis et al., 2013, 2016 | psychostimulant | MS15/180 P2-14 | rat | ↑ (MS180) | ND | ↑ protective MeCP2 expression in NAc core (MS15) | |
| Hensleigh & Pritchard, 2014, 2015 | psychostimulant | MS180 P2-8 | rat | ↑ | Ø | MS potentiates methamphetamine-induced decrease in striatal DAT and TH expression (M) | |
| O’Connor et al., 2015 | psychostimulant | MS180 P2-12 | rat | ↓ | ND | N/A | |
| Ganguly et al., 2019 | psychostimulant | MS240 P2-20 | rat | ↑ | Ø | ↓ GluA2 expression in PFC, NAc (M) | |
| Kikusui et al., 2005 | psychostimulant | MS60 P1-13 | mouse | ↑ (M > F) | ↑ | N/A | |
| Gracia-Rubio et al., 2016 | psychostimulant | MS240 P2-5, MS480 P6-16, weaning at P17 | mouse | ↓ | ND | ↓ striatal D2R expression | |
| Mitchell et al., 2018 | psychostimulant | LBN P2-9 | mouse | ↓ | ND | ↓ NAc α2 subunit of GABA-A receptor mRNA | |
| Castro-Zavala et al., 2020a,b | psychostimulant | MS240 P2-5, MS480 P6-16, weaning at P17 | mouse | ↑ | Ø | sex and drug experience-dependent changes of GluA1, GluA2, CREB, and pCREB expression in NAc and VTA | |
| Okhuarobo et al., 2020 | alcohol | LBN P2-9 | mouse | ↑ | Ø | N/A | |
| Huot et al., 2001 | alcohol | MS15/180 P2-14 | rat | ↑ (MS180) | ND | ↑ HPA axis reactivity (MS180) | |
| Ploj et al., 2003a | alcohol | MS15/360 P1-21 | rat | ↑ (MS360) ↓ (MS15) | ND | MS duration and alcohol-experience dependent changes in mesocorticolimbic dopamine and opioid receptor expression | |
| Roman et al., 2004 | alcohol | MS15/360 P1-21 | rat | ND | Ø | N/A | |
| Romano-López et al., 2012 | alcohol | MS360* P2-15 | rat | ↑ | ND | MS-induced changes in PFC, NAc, and hippocampal glutamate and GABA expression | * two daily 180-min separations |
| Gondré-Lewis et al., 2016 | alcohol | MS180 P2-21 | rat | ↑ | ↑ | ↓ VTA dopamine-like neurons, ↑ amygdala neuron number & density | |
| Bassey & Gondré-Lewis, 2019 | alcohol | MS180 P2-21 | rat | ↑ | ↑ | ↓ VTA, ↑ amygdala neuron number & density | |
| Amancio-Belmont et al., 2020 | alcohol | MS180 P2-15 | rat | ↑ | ND | ↑ NAc D2R and D3R expression | |
| Portero-Tresserra et al., 2018 | alcohol | MS240 P2-5, MS480 P6-16, weaning at P17 | mouse | ↑ | ND | ↓ PFC and striatal endocannabinoid expression | |
| Kawakami et al., 2007 | alcohol | MS15/180 P2-14 | mouse | Ø | ↑ (MS180 > MS15) | ↑ basal CORT (F; MS180) ↑ EtOH CORT response (M; MS15/180) | |
| Cruz et al., 2008 | alcohol | MS180 P1-14 | mouse | ↑ | ND | N/A | |
| García-Gutiérrez et al., 2016 | alcohol | 12h MS P8 & P12 | mouse | ↑ | ND | ↑ NAc dopamine, opioid peptide & receptor, and CRH expression | |
Table abbreviations: CORT, corticosterone; CREB, cAMP-response element binding protein; pCREB, phosphorylated CREB; CRH, corticotropin releasing hormone; D2R, dopamine receptor type 2; D3R, dopamine receptor type 3; DAT, dopamine transporter; GluA1, AMPA glutamate receptor subunit A1; GluA2, AMPA glutamate receptor subunit A2; HPA axis, hypothalamic-pituitary-adrenal axis; LBN, limited bedding and nesting; MeCP2, methyl CpG binding protein 2; MS, maternal separation; NAc, nucleus accumbens; PFC, prefrontal cortex; TH, tyrosine hydroxylase; VTA, ventral tegmental area
Early Life Adversity Effects on Responses to Addictive Drugs
Effects of Maternal Separation ELA
Numerous studies have shown that MS impacts later-life responses to addictive drugs, and these effects may differ by drug class as well as sex.
While work on effects of ELA on opioid-seeking is still limited, evidence suggests that MS may augment opioid drug addiction-relevant behaviors. In male rats and mice, MS enhances morphine reward across multiple behavioral tests, including CPP, locomotor sensitization, and voluntary oral consumption (Kalinichev et al., 2002; Vazquez et al., 2005; Vazquez et al., 2006; Michaels & Holtzman, 2008). In females, effects of MS depend on the opioid response being measured. MS yields similar pro-opioid outcomes in females as in males on morphine CPP and oral intake tasks (Abad et al., 2016; Mohammadian et al., 2019), yet MS led to a heroin-induced increase in ICSS threshold in females at a dose of heroin that reduces ICSS threshold in controls, suggesting a potential MS-induced blunting of heroin’s hedonic effects in that sex (Matthews & Robbins, 2003).
Effects of MS on psychostimulant responses have been consistently reported, and these also appear to differ in males and females. In male rats and mice, MS increases oral and intravenous self-administration of the psychostimulants cocaine and methamphetamine, and both the locomotor sensitizing and place preference-inducing effects of stimulants are stronger in MS males than in females (Kosten et al., 2000; Brake et al., 2004; Marquardt et al., 2004; Kikusui et al., 2005; Zhang et al., 2005; Moffett et al., 2006; Lewis et al., 2013; Lewis et al., 2016; Castro-Zavala et al., 2020a; Castro-Zavala et al., 2020b). Indeed, some evidence suggests that MS females may in fact have blunted cocaine sensitization compared to control-reared females (Li et al., 2003). MS also enhances the “pro-hedonic” properties of amphetamine, as indicated by a larger reduction in ICSS threshold in male rats relative to control-reared males (Der-Avakian & Markou, 2010). However, the degree to which this psychostimulant-prone MS effect is specific to males is still unclear. Though some studies show a male-specific enhancement of psychostimulant responses by MS (Hensleigh & Pritchard, 2014; Ganguly et al., 2019; Castro-Zavala et al., 2020b), other reports suggest that MS also has similar effects in females (Matthews et al., 1999; Kosten et al., 2004), and others still show instead a blunting of psychostimulant reward in MS males relative to controls (Matthews et al., 1999; Matthews & Robbins, 2003; O’Connor et al., 2015), an effect also seen after short (15-minute) periods of MS (Campbell & Spear, 1999). The reason for these apparently conflicting findings is unclear, but could depend upon differences in the precise protocol used, timing of MS, species/strain differences, or other experimental differences. For example, Hensleigh and Pritchard (2014) and Ganguly et al (2019) separated pups individually, Castro-Zavala et al (2020b) included early weaning, whereas Matthews et al (1996; 1999; 2003) and O’Connor et al (2015) all separated pups in a group by litter. Investigation into whether these or other procedural differences might be causally related to the variability observed drug-related outcomes is needed.
MS also affects responses to alcohol in a persistent, and potentially sex-dependent manner. MS-reared male but not female rats, show greater voluntary oral alcohol consumption than their control counterparts (Ploj et al., 2003a; Roman et al., 2004), and MS increases preference for alcohol over water in male mice and rats (Huot et al., 2001; Cruz et al., 2008; Romano-López et al., 2012; Amancio-Belmont et al., 2020). Male MS mice also consume more alcohol when it is intermittently available in a “drinking in the dark” protocol (Portero-Tresserra et al., 2018). Although these effects were shown in male animals, other studies have shown that MS increases operant self-administration of alcohol in both male and female rats (Gondré-Lewis et al., 2016; Bassey & Gondré-Lewis, 2019), and MS increases the locomotor sensitizing effects of alcohol only in females (Kawakami et al., 2007).
In sum, MS clearly impacts behavioral effects of several classes of addictive drugs, potentially in a sex-dependent manner. Most likely, methodological differences such as duration and timing of the MS protocol, potential species and strain differences, drug of abuse tested, and the aspect of addiction-like behavior measured explains the complex pattern of findings using the MS ELA manipulation (Jaworski et al., 2005; van der Veen et al., 2008; Orso et al., 2019). More work is also required to understand how factors like sex, hormonal influences, and others affect how MS alters responses to addictive drugs.
Effects of Limited Bedding and Nesting ELA
Several groups have examined how chronic ELA in the limited bedding and nesting model affects later-life responses and addiction vulnerability to opioids, cocaine, and alcohol.
Our group has recently begun to examine how LBN affects opioid addiction-related behaviors. In female LBN rats, we found a striking increase in addiction-like behaviors in pursuit of opioid drugs (Levis et al., 2019). LBN-reared females had stronger reinstatement of heroin seeking triggered by either heroin priming injections or heroin cues than female controls. In addition, when we examined demand elasticity for the short-acting fentanyl-derivative opioid drug remifentanil, LBN females showed relatively inelastic, addiction-like demand, without measurable changes in hedonic setpoint. A similar decreased sensitivity to price of a highly palatable food reward was observed in LBN females, though no such effect was seen for a less palatable chow reward. Our recent unpublished observations indicate that these pro-opioid effects of LBN in females may not occur to the same extent in males. Notably, Ordoñes Sanchez et al. (2021) also observed sex differences in the effects of ELA on opioid addiction-like behaviors. In this study, male LBN rats self-administered less morphine and were less impulsive than their control counterparts, whereas females did not show LBN-induced changes in these behaviors. LBN also induced sex-specific changes in NAc gene expression. The differences in opioid reward-related effects of LBN between studies might involve procedural differences such as rat strain (Sprague-Dawley vs. Long-Evans), opioid drugs tested (heroin/remifentanil vs. morphine), or differences in prenatal handling procedures between the studies (shipping timed-pregnant dams vs. in-house breeding) (Levis et al., 2019; Ordoñes Sanchez et al., 2021). Regardless, the clear sex differences in ELA effects on susceptibility to OUD-related behaviors seem likely to have important implications for understanding human opioid use and addiction. Indeed, it is notable that women addicted to heroin have a much greater prevalence of adverse experiences during development than heroin-addicted men, and the association between ELA and substance abuse appears also to be stronger in women than in men (Hyman et al., 2006; Hyman et al., 2008; Shand et al., 2011).
In contrast to our opioid-related findings, our group recently found that LBN facilitates acquisition of cocaine self-administration in male rats, though stable intake of the drug was equivalent in LBN and controls. However, when we subsequently measured cocaine behavioral economic demand elasticity, we found no change in sensitivity of cocaine intake to price (elasticity), though there was a decrease in cocaine hedonic setpoint, or intake when price was very low (Bolton et al., 2018b). We interpreted this result as LBN-induced “anhedonia” for cocaine, similar to the reduced engagement with natural rewards like sucrose solution or social play observed in LBN males (Molet et al., 2016a; Yan et al., 2017; Bolton et al., 2018a). In male mice, LBN also leads to blunted cocaine locomotor sensitization (Mitchell et al., 2018), suggesting reduced cocaine reward. While LBN did not seem to increase addiction-like cocaine seeking in our study of male rats, this more general anhedonic phenotype could still impact addiction susceptibility, perhaps especially for other classes of drugs (like opioids) that could more effectively “self-medicate” this underlying affective dysregulation.
In a model of alcohol dependence in adult mice, males that have experienced LBN develop excessive alcohol drinking more rapidly than control-reared mice (as measured by escalation of voluntary alcohol consumption), an effect not seen in LBN females (Okhuarobo et al., 2020), suggesting that LBN may confer a specific vulnerability to alcohol reward in males. Further exploring sex differences in the effects of LBN on addiction and determining how LBN females respond to other classes of addictive drugs are important questions that remain open.
In summary, clinical and pre-clinical evidence, including some congruent findings from nonhuman primate models suggesting that ELA enhances drug abuse in adolescents (Wakeford et al., 2018), suggest that ELA can increase vulnerability to addiction to a wide range of drugs. This may occur by enhancing the rewarding or motivating effects of drugs themselves, by impacting factors like susceptibility to relapse triggers, or perhaps by inducing a state of affective dysregulation that may be self-medicated with certain drugs. Understanding the specific addiction-relevant behaviors which are most impacted by ELA may help elucidate causal mechanisms, such as changes in neural circuit structure and function caused by developmental adversity. We review some of these circuit and substrate-level ELA-induced changes in the following section.
Does ELA “Rewire” Brain Reward and Stress Circuits?
Considerable evidence links dysfunction of brain reward and stress circuits with addiction vulnerability and severity. These circuits undergo substantial maturation in the first weeks (rodents) or year (humans) of life, and mounting evidence supports the notion that ELA induces long-lasting developmental changes, leading to addiction-relevant neuroadaptations in brain reward and stress circuits and increased vulnerability to SUD (Koob, 2008; Sinha, 2008; Koob & Volkow, 2016; Ironside et al., 2018).
Here, we will focus specifically on the roles of specific brain systems for which a large body of evidence exists on the effects of ELA in mediating their function. We will first provide an overview of the behavioral functions of key reward-related systems, namely dopamine and opioid signaling molecules and receptors in mesolimbic circuits, as well as stress-related systems, specifically corticotropin releasing hormone (CRH) and dynorphin/kappa opioid receptors in extended amygdala. We then summarize findings about the specific changes in these molecules and circuits which may underlie ELA effects on addiction susceptibility. Notably, the effects of ELA are not limited to classical stress and reward circuits, as pronounced effects on memory-linked regions like hippocampus are also seen (Ivy et al., 2010; Chen & Baram, 2016; Molet et al., 2016b), which may lead to cognitive deficits or other psychiatric symptoms that may indirectly affect drug seeking. Likewise, the neural substrates altered by ELA that might mediate reward seeking are not limited to the dopamine and opioid systems (Forster et al., 2018).
Roles for Mesolimbic Opioids and Dopamine in “Reward Circuits”
Addictive drugs are thought to “hijack” neural circuits of reward, pharmacologically engaging the neural mechanisms responsible for registering pleasurable experiences, and generating motivation to pursue these rewards again in the future. These mechanisms normally operate in service of learning about and pursuing natural rewards like food, water, and sex, but repeated drug use may cause them to be specifically, and excessively, centered on drugs instead. The neural mechanisms by which drugs cause pleasurable states and/or states of compulsive seeking and desire are the subject of much study, and involve complex circuit, synaptic, and molecular mechanisms. Here we concentrate on two such mechanisms that are particularly strongly linked to drug reward: dopamine and opioids within “mesolimbic reward circuit” nodes like ventral tegmental area (VTA), prefrontal cortex (PFC), and nucleus accumbens (NAc).
Similar to other rewards, drugs of abuse are thought to generate pleasurable subjective states via actions in mesolimbic circuits. Reward-induced pleasure is complex, but a role for endogenous opioid signaling in nucleus accumbens seems to be particularly important. Endogenous opioid systems involve at least 3 opioid peptides (endorphin, enkephalin, and dynorphin), acting via three primary g-protein coupled receptors (mu, delta, and kappa opioid receptors) to modulate neural activity (Kieffer et al., 1992; Chen et al., 1993; Minami et al., 1993). Endogenous and exogenous ligands engage inhibitory intracellular signaling cascades, inhibiting neural firing postsynaptically, and suppressing neurotransmitter release from axon terminals (Mansour et al., 1995; Valentino & Volkow, 2018). Opioid receptors are densely expressed in NAc, where they are localized both pre- and post-synaptically (Mansour et al., 1994). Of particular relevance, opioid receptors in an anatomically segregated “hedonic hotspot” within the nucleus accumbens dorsomedial shell subregion play a major role in registering affective pleasure from food reward, in a manner suggesting that this restricted anatomical zone is of special importance for registering the pleasurable aspects of food or other types of rewards (Peciña & Berridge, 2005; Thompson & Swanson, 2010; Zahm et al., 2013; Castro & Berridge, 2014). Given this link between NAc opioids and pleasure, it is not surprising that addictive opioid drugs generate highly euphoric states. However, other major drugs of abuse also engage accumbens opioidergic signaling (Kreek, 1996; Olive et al., 2001; Gerrits et al., 2003; Yoo et al., 2012), which may likewise contribute to euphoric and pleasurable responses to these drugs.
Dopamine signaling within mesolimbic circuits, and especially in NAc, are another crucial mechanism by which drugs engage reward circuits to promote addiction. The mesolimbic dopamine circuit entails projections from VTA to NAc, PFC, and other forebrain limbic sites, which are thought to mediate multiple addiction-related behavioral processes (Kalivas & Volkow, 2005; Salamone et al., 2007; Kalivas, 2008). Addictive drugs, regardless of class and mechanism of action, engage the mesolimbic dopamine system, as do natural rewards of various types (Wise & Bozarth, 1987). The precise roles played by dopamine neurons is still debated, but it likely involves addiction-relevant psychological processes such as reward prediction (Schultz, 1998), inflexible habitual aspects of drug taking (Everitt & Robbins, 2005), and highly effortful drug seeking, especially when triggered by drug-associated cues (Shaham et al., 2003; Robinson & Berridge, 2008; Mahler et al., 2018).
In sum, mesolimbic opioids and dopamine play critical and nuanced roles within brain circuits that mediate pleasure, motivation, learning, and habits. Depending on precisely how ELA impacts these circuits, we may therefore see consequences on a range of SUD-relevant behaviors, any of which could lead to an addiction-vulnerable phenotype via distinct neural mechanisms.
“Stress Circuits” in Addiction
Stress also plays a key role in addiction, and ELA effects on stress circuits is likely to contribute to ELA-induced addiction risk. Physiologically, stress can be defined as activation of the hypothalamic-pituitary-adrenal (HPA) axis leading to release of CRH from the hypothalamus into the bloodstream, as well as directly into brain emotional systems via neural projections (Joëls & Baram, 2009; Koob & Zorrilla, 2010; McEwen & Gianaros, 2011). Brain circuits in which CRH is synthesized locally and acts to promote stressful and aversive states include extended amygdala regions such as central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), as well as the dorsal raphe, the paraventricular and lateral hypothalamic nuclei.
Stress may impact addiction risk via the ability of some drugs to counteract negative emotional or affective states. Many users of anxiolytic drugs such as opioids, benzodiazepines, and alcohol report that when they began using these drugs, their underlying anxieties and negative emotions suddenly lifted. In this way, drug use may provide relief to an already suffering person, resulting in strong negative reinforcement, or “self-medication.” This may occur via direct or indirect recruitment by drugs of endogenous opioids such as enkephalin and beta-endorphin, which counteract neural responses to stress and help promote recovery from stressful events (Cohen et al., 1983; Curtis et al., 2001; Bowers et al., 2012; Valentino & Van Bockstaele, 2015; Valentino & Volkow, 2018). CRH and opioid receptors co-localize in regions related to stress and reward (Van Bockstaele et al., 2010; Williams & Milner, 2011; Reyes et al., 2017; Castro & Bruchas, 2019), and neuroadaptations induced by chronic opioid exposure in stress and reward regions appear to be modulated by glucocorticoids as well (García-Pérez et al., 2012), further supporting a link between opioid transmission and self-medication of negative affect with abused drugs.
Stress also plays an important role in maintaining compulsive substance use, particularly of drugs that cause physiological dependence and severe withdrawal symptoms, such as opioids and alcohol (Bruchas et al., 2010; George & Koob, 2017). Stress circuits and molecules play a key role in mediating these highly aversive acute withdrawal symptoms (Koob, 2008; Logrip et al., 2011; Gilpin & Roberto, 2012; Chartoff & Carlezon, 2014). For example, CRH is released in the extended amygdala structures CeA and BNST during alcohol withdrawal (Olive et al., 2002), and blockade of CeA CRH receptors prevents acute withdrawal-enhanced consumption of ethanol in dependent rats (Funk et al., 2006). Some have also described affective dysregulation that persists for extended periods after cessation of drug use, which may help promote relapse (Kenny & Markou, 2001; Aston-Jones & Harris, 2004). Moreover, protracted abstinence can also enhance the incentive salience of drug-associated cues upon drug re-exposure (Smith & Aston-Jones, 2014). Multiple brain circuits are involved in this excessive drug seeking seen even after persistent abstinence. For example, protracted withdrawal is associated with altered glutamate-dependent plasticity in the VTA and its afferent inputs such as the amygdala, BNST, lateral hypothalamus, VTA, and NAc (Aston-Jones & Harris, 2004), as well as altered function of the PFC that may be mediated by CRH (Zorrilla et al., 2014; Quadros et al., 2016; Blaine & Sinha, 2017). In addition, opioid withdrawal memories appear to promote opioid seeking via interactions between these stress and reward-related circuits (Frenois et al., 2005). Withdrawal-associated dysphoria and stress-induced reinstatement of drug-seeking is also thought to be mediated in part by the dynorphin/kappa opioid receptor system, likely by acting in concert with CRH (Land et al., 2008; Redila & Chavkin, 2008; Bruchas et al., 2010; Nygard et al., 2016).
In addition to withdrawal, acute stress in any form is thought be a major trigger for relapse in humans, and stress also potently induces reinstatement of drug seeking in rodents (Shalev et al., 2000; Sinha, 2001; Shaham et al., 2003; See & Waters, 2010; McReynolds et al., 2014; Mantsch et al., 2016). Specifically, activation of stress circuit nodes such as the BNST, CeA, BLA, and medial septum play a key role in stress-induced relapse, as do stress-linked transmitters like CRH and norepinephrine in these structures and elsewhere in the brain (Shaham et al., 2000; Koob & Zorrilla, 2010; Logrip et al., 2011). Notably, stressors may activate drug seeking via their recruitment of motivation circuits (Sarnyai et al., 2001; Yap & Miczek, 2008; Shalev et al., 2010; George et al., 2012; Lemos et al., 2019). For example, physical or psychological stressors elicit the release of CRH in the VTA, causing dopamine release in the NAc and leading to reinstatement of drug-seeking behaviors (Wang et al., 2005; Wang et al., 2007; Shalev et al., 2010; Ungless et al., 2010). CRH signaling within NAc itself may also play stress-independent roles in reward seeking, for example by increasing the incentive salience of reward-paired cues (Peciña et al., 2006; Baumgartner et al., 2021).
Clearly, stress is an important and multifaceted factor influencing SUD, and ELA-induced changes in stress circuits may impact initiation of drug use, maintenance of use/avoidance of withdrawal, and relapse risk in response to life stressors. In the next section, we review what is known about how ELA affects development of brain reward and stress circuits, and how this may influence the development of, and recovery from addiction.
Effects of ELA on Reward and Stress Circuits
Mounting evidence suggests that ELA causes profound, likely permanent changes in brain reward and stress systems, including mesolimbic and extended amygdala circuits, dopamine, endogenous opioids, and CRH. Given the importance of these systems to addiction, it is likely that these disruptions contribute to the ability of ELA to enhance addiction susceptibility in vulnerable individuals.
Adult function of stress-related circuits and molecules are profoundly impacted by ELA, and this may impact addiction propensity or severity. For example, ELA provoked enduring changes in the expression levels of several stress modulators. CRH expression is augmented in CeA (Dubé et al., 2015) and hippocampus (Ivy et al., 2010) of ELA rodents, leading to major changes in circuit functions (Brunson et al., 2005; Ivy et al., 2010). In the context of addiction, these changes in circuit function are evident from studies examining circuit activation in adult rodents that have experienced ELA. Thus, palatable food, social play, and acute cocaine rewards induce a stronger Fos response in CeA of LBN males than of control males, an effect accompanied by anhedonia-like behavioral responses to those same rewards (Bolton et al., 2018a; Bolton et al., 2018b). This may indicate a stress-like response to these normally rewarding stimuli following ELA. ELA also alters functional connectivity and microstructure of stress- and reward-related brain regions. For example, LBN males have increased adulthood amygdala-PFC structural connectivity relative to controls (Bolton et al., 2018a). Pre-weaning LBN males, but not females, have reduced BLA-PFC, and altered PFC-striatum resting state functional connectivity (Guadagno et al., 2018a; Guadagno et al., 2018b), a finding that persists into adulthood, accompanied by reduced sucrose preference and social interaction (Yan et al., 2017). Likewise, both MS and LBN disrupt early maturation of BLA-PFC connections (Brenhouse et al., 2013; Honeycutt et al., 2020; Manzano Nieves et al., 2020), further implicating this circuit in the effects of ELA. Notably, human studies suggest that ELA’s impact on amygdala development is essential for the resulting depression and anxiety (Callaghan & Tottenham, 2016; Fareri & Tottenham, 2016). The latter could set the stage for “self-medicating” drug use in vulnerable individuals (Kessler, 2004).
Mesolimbic dopamine system development is strongly impacted by ELA (Rodrigues et al., 2011; Ventura et al., 2013; Peña et al., 2014; Bonapersona et al., 2018), thereby potentially facilitating dopamine-dependent incentive motivational, learning, or habitual aspects of addiction. While it is clear that ELA affects the mesolimbic dopamine circuit, the precise changes are somewhat inconsistent across studies, and appear to be partially sex dependent. For example, MS females have more dopamine cells in the VTA than controls, and also show enhanced excitability of VTA dopamine neurons, whereas males appear instead to have more non-dopamine cells in VTA relative to control males, but no change in the number of dopamine cells there (Chocyk et al., 2011; Chocyk et al., 2015; Majcher-Maślanka et al., 2017; Spyrka et al., 2020). MS females, but not males, have increased dopamine turnover in prefrontal cortex, and turnover of other monoamines in the striatum also differ between sexes after ELA (González-Pardo et al., 2020). However, MS does appear to affect dopamine signaling in males in some cases. For example, in males, MS-enhanced sensitivity to amphetamine and cocaine are associated with decreased dopamine transporter expression in the NAc (Meaney et al., 2002; Brake et al., 2004). Others have found that alcohol self-administration in MS but not control male rats is negatively correlated with the number of dopamine neurons in VTA, a phenomenon that is also seen in genetically alcohol-preferring rats (Gondré-Lewis et al., 2016; Bassey & Gondré-Lewis, 2019). MS combined with limited nesting materials during the second week of life has also recently been found to alter histone modification and gene transcription in dopamine receptor type 2 (D2R) containing NAc medium spiny neurons more robustly in male mice than females, suggesting a sex-dependent change in function of those cells (Kronman et al., 2021). As mentioned above, differences in ELA protocols, age, and models used for addiction or quantification approaches may be responsible for this range of outcomes.
ELA-induced changes in dopamine receptor protein and mRNA expression are also observed across the mesolimbic circuit in both sexes following ELA. Consistent decreases in dopamine receptor expression in striatum are induced by MS in both males (Zhu et al., 2010; Romano-López et al., 2016) and females (Majcher-Maślanka et al., 2017), and striatal reductions of D2R correlate with the magnitude of MS-suppressed cocaine locomotor sensitization (Gracia-Rubio et al., 2016). However, others have observed that D2R and D3R in NAc are instead increased by MS in male rats, an effect associated with increased alcohol intake in male MS rats (Amancio-Belmont et al., 2020). Similarly, MS male mice have increased prefrontal cortex dopamine receptor gene expression relative to controls (Tractenberg et al., 2020).
Brain endogenous opioid systems are enduringly altered by ELA, a fact that may impact drug-induced pleasure or other addiction-relevant processes. MS persistently alters endogenous opioid peptides, as well as opioid and dopamine receptor expression in reward and stress related areas, including striatum, midbrain, hippocampus, and hypothalamus in both a sex- and ELA timing-dependent manner (Ploj et al., 1999; Ploj et al., 2001; Ploj & Nylander, 2003; Ploj et al., 2003a; Ploj et al., 2003b; Gustafsson et al., 2008). Specifically, long bouts of daily separation (360 minutes) lead to higher ethanol consumption in adulthood, whereas brief bouts (15 minutes) may be protective against chronic escalating ethanol consumption, even though both protocols lead to elevated expression of opioid peptides in the hypothalamus and pituitary (Ploj & Nylander, 2003; Ploj et al., 2003a; Ploj et al., 2003b), PFC, and VTA (Gustafsson et al., 2008). Additionally, 15-minute daily MS leads to higher delta opioid receptor density in amygdala, and enhanced dynorphin-mediated HPA-axis inhibition in males but not females, whereas females but not males have reduced dynorphin expression in PFC and amygdala (Ploj et al., 1999; Ploj et al., 2001). In a 12-hr MS model that led to enhanced ethanol consumption in male mice, mu opioid receptor gene expression was elevated in NAc (García-Gutiérrez et al., 2016). MS also alters the ability of addictive drugs to induce plasticity in opioidergic circuits. For example, MS male rats do not show the typical chronic ethanol-induced downregulation of delta, mu, and kappa opioid receptor gene expression in striatum (Granholm et al., 2017), potentially contributing to the excessive alcohol consumption seen in these animals. Clearly, MS leads to persistent changes in endogenous opioids, though more work is needed to link these changes to addiction-like behaviors.
Perhaps as a result of the above molecular circuit-development changes, ELA persistently alters neural responses to drugs of abuse themselves in a manner that may facilitate their rewarding effects via actions in limbic reward circuits. For example, in male rats, MS increases the sensitivity of limbic and striatal regions to ethanol-induced gene expression and DNA methylation changes (Vrettou et al., 2017) and alcohol exposure leads to MS-specific alterations in mesocorticolimbic dopamine and opioid receptor expression (Ploj et al., 2003a). In response to psychostimulants, MS increases cocaine-induced striatal c-Fos expression in both male and female rats after chronic cocaine exposure (Kohut et al., 2009) and potentiates methamphetamine-induced depletion of striatal dopamine transporter and tyrosine hydroxylase, but only in males (Hensleigh & Pritchard, 2015). Additionally, microdialysis experiments reveal enhanced dopamine release in NAc in response to d-amphetamine in MS males (Hall et al., 1999). LBN also increases cocaine-induced c-Fos in NAc core, lateral habenula, and CeA of male rats, which may be related to quicker acquisition of cocaine self-administration, but an eventual reduction of hedonic setpoint for the drug (Bolton et al., 2018b).
Finally, it is possible that ELA enhances addiction vulnerability by simultaneously altering both stress- and reward-related processes. For example, relative to controls, MS increases dopamine, endogenous opioid, and CRH expression simultaneously in NAc in male mice that also show increased ethanol consumption relative to controls (García-Gutiérrez et al., 2016). In male rats, the magnitude of MS-enhanced alcohol consumption is strongly correlated with HPA axis responses to startle stress (Huot et al., 2001). In female rats, MS also increases VTA neuron excitability, an effect that is accompanied by elevated peripheral stress hormones (Spyrka et al., 2020). These findings support the notion that an imbalance of stress and reward processes may play a mediating role in the effects of ELA on SUD vulnerability (Koob, 2008; Valentino & Van Bockstaele, 2015).
Conclusions
Both ELA and addiction are complex processes, yet it is clear that ELA is a predisposing factor to SUD. However, the link between ELA and addiction is intricate and remains poorly understood. ELA effects on brain reward and stress circuit development are likely contributors to this link, though other mechanisms certainly also contribute (Kim et al., 2017; Baracz et al., 2020). The effects of ELA may also differ based on the type of ELA, its timing, sex of the individual involved, and many other factors. In addition, the behavioral phenotypes caused by ELA are nuanced and can differ based on the drug of abuse and stage of the addiction process that is tested. Therefore, additional investigation is required to determine exactly how ELA impacts brain development, and how these resulting changes put individuals at risk for specific addiction-related behaviors that could all lead to SUD, possibly through a range of neural mechanisms. We propose that understanding the precise links between ELA and addiction-like outcomes opens the possibility of developing better strategies for preventing and reversing addiction in those predisposed by their history of ELA.
Acknowledgements:
This work is funded by the National Institutes of Health grants R01 MH073136 (TZB), P50 MH096889 (TZB), P50 DA044118 (SVM), F30 DA051137 (SCL), T32 GM 008620 (SCL), and the Tobacco Related Disease Research Program Grant T31IR1767 (SVM). The authors thank Joshua Nykamp for his contribution to the figure concept and design.
Abbreviations:
| BNST | bed nucleus of the stria terminalis |
| CeA | central amygdala |
| CORT | corticosterone |
| CREB | cAMP-response element binding protein |
| pCREB | phosphorylated CREB |
| CPP | conditioned place preference |
| CRH | corticotropin releasing hormone |
| D2R | dopamine receptor type 2 |
| D3R | dopamine receptor type 3 |
| DAT | dopamine transporter |
| ELA | early life adversity |
| GluA1 | AMPA glutamate receptor subunit A1 |
| GluA2 | AMPA glutamate receptor subunit A2 |
| HPA axis | hypothalamic-pituitary-adrenal axis |
| ICSS | intracranial self-stimulation |
| LBN | limited bedding and nesting |
| MeCP2 | methyl CpG binding protein 2 |
| MS | maternal separation |
| NAc | nucleus accumbens |
| PFC | prefrontal cortex |
| SUD | substance use disorder |
| TH | tyrosine hydroxylase |
| VTA | ventral tegmental area |
Footnotes
Conflict of Interest Statement:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Statement:
No human or animal subjects were involved in this study (literature review).
Data Sharing Statement:
Data sharing is not applicable to this review article as no new data were created or analyzed in this study.
REFERENCES
- Abad AT, Miladi-Gorji H & Bigdeli I (2016) Effects of swimming exercise on morphine-induced reward and behavioral sensitization in maternally-separated rat pups in the conditioned place preference procedure. Neurosci Lett, 631, 79–84. [Abstract] [Google Scholar]
- Ahmed SH (2018) Trying to make sense of rodents’ drug choice behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87, 3–10. [Abstract] [Google Scholar]
- Ahmed SH, Walker JR & Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology, 22, 413–421. [Abstract] [Google Scholar]
- Ainsworth MDS (1969) Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child development, 969–1025. [Abstract] [Google Scholar]
- Amancio-Belmont O, Becerril Meléndez AL, Ruiz-Contreras AE, Méndez-Díaz M & Prospéro-García O (2020) Maternal separation plus social isolation during adolescence reprogram brain dopamine and endocannabinoid systems and facilitate alcohol intake in rats. Brain Res Bull, 164, 21–28. [Abstract] [Google Scholar]
- American Psychological Association (2018) Stress in America: Generation Z Stress in America(TM) Survey. [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR & Giles WH (2006) The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci, 256, 174–186. [Europe PMC free article] [Abstract] [Google Scholar]
- Andersen SL (2015) Exposure to early adversity: Points of cross-species translation that can lead to improved understanding of depression. Development and psychopathology, 27, 477–491. [Europe PMC free article] [Abstract] [Google Scholar]
- Andersen SL (2018) Stress, sensitive periods, and substance abuse. Neurobiology of Stress, 10, 100140. [Europe PMC free article] [Abstract] [Google Scholar]
- Aston-Jones G & Harris GC (2004) Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology, 47, 167–179. [Abstract] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA & Baram TZ (2002) Stressed-out, or in (utero)? Trends in Neurosciences, 25, 518–524. [Europe PMC free article] [Abstract] [Google Scholar]
- Baldo BA & Kelley AE (2007) Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology, 191, 439–459. [Abstract] [Google Scholar]
- Baracz SJ, Everett NA & Cornish JL (2020) The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci Biobehav Rev, 110, 114–132. [Abstract] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A & Stern H (2012) Fragmentation and Unpredictability of Early-Life Experience in Mental Disorders. American Journal of Psychiatry, 169, 907–915. [Europe PMC free article] [Abstract] [Google Scholar]
- Bassey RB & Gondré-Lewis MC (2019) Combined early life stressors: Prenatal nicotine and maternal deprivation interact to influence affective and drug seeking behavioral phenotypes in rats. Behav Brain Res, 359, 814–822. [Europe PMC free article] [Abstract] [Google Scholar]
- Bath KG (2020) Synthesizing Views to Understand Sex Differences in Response to Early Life Adversity. Trends in Neurosciences, 43, 300–310. [Europe PMC free article] [Abstract] [Google Scholar]
- Bath KG, Nitenson A, Lichtman E, Lopez C, Chen W, Gallo M, Goodwill H & Manzano-Nieves G (2017) Early life stress leads to developmental and sex selective effects on performance in a novel object placement task. Neurobiology of Stress, 7, 57–67. [Europe PMC free article] [Abstract] [Google Scholar]
- Baumgartner HM, Schulkin J & Berridge KC (2021) Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation? Biological Psychiatry. [Europe PMC free article] [Abstract] [Google Scholar]
- Becker HC (2000) Animal models of alcohol withdrawal. Alcohol Research & Health, 24, 105. [Europe PMC free article] [Abstract] [Google Scholar]
- Becker JB & Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology, 44, 166–183. [Europe PMC free article] [Abstract] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV & Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology, 36, 569–579. [Europe PMC free article] [Abstract] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW & Everitt BJ (2008) High Impulsivity Predicts the Switch to Compulsive Cocaine-Taking. Science, 320, 1352–1355. [Europe PMC free article] [Abstract] [Google Scholar]
- Bentzley BS & Aston-Jones G (2015) Orexin-1 receptor signaling increases motivation for cocaine-associated cues. European Journal of Neuroscience, 41, 1149–1156. [Europe PMC free article] [Abstract] [Google Scholar]
- Bentzley BS, Fender KM & Aston-Jones G (2013) The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology, 226, 113–125. [Europe PMC free article] [Abstract] [Google Scholar]
- Bentzley BS, Jhou TC & Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences, 111, 11822–11827. [Europe PMC free article] [Abstract] [Google Scholar]
- Berke JD & Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron, 25, 515–532. [Abstract] [Google Scholar]
- Berridge KC & Robinson TE (2003) Parsing reward. Trends in Neurosciences, 26, 507–513. [Abstract] [Google Scholar]
- Bian Y, Yang L, Wang Z, Wang Q, Zeng L & Xu G (2015) Repeated Three-Hour Maternal Separation Induces Depression-Like Behavior and Affects the Expression of Hippocampal Plasticity-Related Proteins in C57BL/6N Mice. Neural plasticity, 2015, 627837–627837. [Europe PMC free article] [Abstract] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J & Murphy JG (2014) The Behavioral Economics of Substance Use Disorders: Reinforcement Pathologies and Their Repair. Annual review of clinical psychology, 10, 641–677. [Europe PMC free article] [Abstract] [Google Scholar]
- Birnie MT, Kooiker CL, Short AK, Bolton JL, Chen Y & Baram TZ (2020) Plasticity of the Reward Circuitry After Early-Life Adversity: Mechanisms and Significance. Biol Psychiatry, 87, 875–884. [Europe PMC free article] [Abstract] [Google Scholar]
- Blaine SK & Sinha R (2017) Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. [Europe PMC free article] [Abstract] [Google Scholar]
- Bolton JL, Molet J, Ivy A & Baram TZ (2017) New insights into early-life stress and behavioral outcomes. Current Opinion in Behavioral Sciences, 14, 133–139. [Europe PMC free article] [Abstract] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A & Baram TZ (2018a) Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biological Psychiatry, 83, 137–147. [Europe PMC free article] [Abstract] [Google Scholar]
- Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, Cross C, Baram TZ & Mahler SV (2018b) Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiology of Stress, 8, 57–67. [Europe PMC free article] [Abstract] [Google Scholar]
- Bonapersona V, Joëls M & Sarabdjitsingh RA (2018) Effects of early life stress on biochemical indicators of the dopaminergic system: A 3 level meta-analysis of rodent studies. Neuroscience & Biobehavioral Reviews, 95, 1–16. [Abstract] [Google Scholar]
- Bonapersona V, Kentrop J, Van Lissa CJ, van der Veen R, Joëls M & Sarabdjitsingh RA (2019) The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neuroscience & Biobehavioral Reviews, 102, 299–307. [Abstract] [Google Scholar]
- Bowers ME, Choi DC & Ressler KJ (2012) Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiology & behavior, 107, 699–710. [Europe PMC free article] [Abstract] [Google Scholar]
- Bowlby J (1974) Attachment and loss. volume I. Attachment Attachment and loss. volume I. Attachment, pp. 401 p.–401 p. [Google Scholar]
- Bowlby J (2008) Attachment. Basic books. [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ & Gratton A (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience, 19, 1863–1874. [Abstract] [Google Scholar]
- Brenhouse HC & Bath KG (2019) Bundling the haystack to find the needle: Challenges and opportunities in modeling risk and resilience following early life stress. Front Neuroendocrinol, 54, 100768. [Europe PMC free article] [Abstract] [Google Scholar]
- Brenhouse HC, Lukkes JL & Andersen SL (2013) Early Life Adversity Alters the Developmental Profiles of Addiction-Related Prefrontal Cortex Circuitry. Brain Sciences, 3, 143–158. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruchas MR, Land BB & Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain research, 1314, 44–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G & Baram TZ (2005) Mechanisms of late-onset cognitive decline after early-life stress. The Journal of neuroscience : the official journal of the Society for Neuroscience, 25, 9328–9338. [Europe PMC free article] [Abstract] [Google Scholar]
- Callaghan BL & Tottenham N (2016) The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology, 41, 163. [Europe PMC free article] [Abstract] [Google Scholar]
- Campbell J & Spear LP (1999) Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl), 143, 183–189. [Abstract] [Google Scholar]
- Capusan AJ, Gustafsson PA, Kuja-Halkola R, Igelström K, Mayo LM & Heilig M (2021) Correction: Re-examining the link between childhood maltreatment and substance use disorder: a prospective, genetically informative study. Molecular Psychiatry. [Abstract] [Google Scholar]
- Carlezon WA Jr. & Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc, 2, 2987–2995. [Abstract] [Google Scholar]
- Castro DC & Berridge KC (2014) Opioid Hedonic Hotspot in Nucleus Accumbens Shell: Mu, Delta, and Kappa Maps for Enhancement of Sweetness “Liking” and “Wanting”. The Journal of Neuroscience, 34, 4239–4250. [Europe PMC free article] [Abstract] [Google Scholar]
- Castro DC & Bruchas MR (2019) A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron, 102, 529–552. [Europe PMC free article] [Abstract] [Google Scholar]
- Castro-Zavala A, Martin-Sanchez A, Lujan MA & Valverde O (2020a) Maternal separation increases cocaine intake through a mechanism involving plasticity in glutamate signalling. Addict Biol, n/a, e12911. [Abstract] [Google Scholar]
- Castro-Zavala A, Martín-Sánchez A & Valverde O (2020b) Sex differences in the vulnerability to cocaine’s addictive effects after early-life stress in mice. Eur Neuropsychopharmacol, 32, 12–24. [Abstract] [Google Scholar]
- Chartoff EH & Carlezon WA Jr. (2014) Drug withdrawal conceptualized as a stressor. Behav Pharmacol, 25, 473–492. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen C-Y, Storr CL & Anthony JC (2009) Early-onset drug use and risk for drug dependence problems. Addictive Behaviors, 34, 319–322. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen Y & Baram TZ (2016) Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology, 41, 197. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA & Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Molecular pharmacology, 44, 8–12. [Abstract] [Google Scholar]
- Chocyk A, Majcher-Maślanka I, Przyborowska A, Maćkowiak M & Wędzony K (2015) Early-life stress increases the survival of midbrain neurons during postnatal development and enhances reward-related and anxiolytic-like behaviors in a sex-dependent fashion. Int J Dev Neurosci, 44, 33–47. [Abstract] [Google Scholar]
- Chocyk A, Przyborowska A, Dudys D, Majcher I, Maćkowiak M & Wędzony K (2011) The impact of maternal separation on the number of tyrosine hydroxylase-expressing midbrain neurons during different stages of ontogenesis. Neuroscience, 182, 43–61. [Abstract] [Google Scholar]
- Cohen MR, Pickar D & Dubois M (1983) The Role of the Endogenous Opioid System in the Human Stress Response. Psychiatric Clinics of North America, 6, 457–471. [Abstract] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM & Nemeroff CB (1996) Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences, 93, 1619–1623. [Europe PMC free article] [Abstract] [Google Scholar]
- Crist RC, Reiner BC & Berrettini WH (2019) A review of opioid addiction genetics. Current Opinion in Psychology, 27, 31–35. [Europe PMC free article] [Abstract] [Google Scholar]
- Cruz FC, Quadros IM, da S Planeta C & Miczek KA (2008) Maternal separation stress in male mice: Long-term increases in alcohol intake. Psychopharmacology, 201, 459–468. [Europe PMC free article] [Abstract] [Google Scholar]
- Curtis A, Bello N & Valentino R (2001) Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J Neurosci, 21. [Europe PMC free article] [Abstract] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH & de Kloet ER (2013) The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38, 1858–1873. [Europe PMC free article] [Abstract] [Google Scholar]
- Davis EP, Korja R, Karlsson L, Glynn LM, Sandman CA, Vegetabile B, Kataja E-L, Nolvi S, Sinervä E, Pelto J, Karlsson H, Stern HS & Baram TZ (2019) Across continents and demographics, unpredictable maternal signals are associated with children’s cognitive function. EBioMedicine, 46, 256–263. [Europe PMC free article] [Abstract] [Google Scholar]
- Davis EP, Stout SA, Molet J, Vegetabile B, Glynn LM, Sandman CA, Heins K, Stern H & Baram TZ (2017) Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc Natl Acad Sci U S A, 114, 10390–10395. [Europe PMC free article] [Abstract] [Google Scholar]
- Demaestri C, Pan T, Critz M, Ofray D, Gallo M & Bath KG (2020) Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm Behav, 124, 104763. [Europe PMC free article] [Abstract] [Google Scholar]
- Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA & McLaughlin KA (2019) Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Dev, 90, e96–e113. [Europe PMC free article] [Abstract] [Google Scholar]
- Der-Avakian A & Markou A (2010) Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience, 170, 1189–1198. [Europe PMC free article] [Abstract] [Google Scholar]
- Di Ciano P & Everitt BJ (2005) Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. European Journal of Pharmacology, 526, 186–198. [Abstract] [Google Scholar]
- Di Segni M, Andolina D & Ventura R (2018) Long-term effects of early environment on the brain: Lesson from rodent models. Seminars in Cell & Developmental Biology, 77, 81–92. [Abstract] [Google Scholar]
- Doherty TS, Blaze J, Keller SM & Roth TL (2017) Phenotypic outcomes in adolescence and adulthood in the scarcity-adversity model of low nesting resources outside the home cage. Dev Psychobiol, 59, 703–714. [Europe PMC free article] [Abstract] [Google Scholar]
- Dubé CM, Molet J, Singh-Taylor A, Ivy A, Maras PM & Baram TZ (2015) Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiology of Stress, 2, 10–19. [Europe PMC free article] [Abstract] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH & Anda RF (2003) Childhood Abuse, Neglect, and Household Dysfunction and the Risk of Illicit Drug Use: The Adverse Childhood Experiences Study. Pediatrics, 111, 564–572. [Abstract] [Google Scholar]
- Enoch M-A (2011) The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology, 214, 17–31. [Europe PMC free article] [Abstract] [Google Scholar]
- Espinosa JS & Stryker MP (2012) Development and plasticity of the primary visual cortex. Neuron, 75, 230–249. [Europe PMC free article] [Abstract] [Google Scholar]
- Evans CJ & Cahill CM (2016) Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research, 5, 1748. [Europe PMC free article] [Abstract] [Google Scholar]
- Everitt BJ & Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci, 8, 1481–1489. [Abstract] [Google Scholar]
- Everitt BJ & Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci, 22, 3312–3320. [Europe PMC free article] [Abstract] [Google Scholar]
- Fareri DS & Tottenham N (2016) Effects of early life stress on amygdala and striatal development. Developmental Cognitive Neuroscience, 19, 233–247. [Europe PMC free article] [Abstract] [Google Scholar]
- Farrell M, Schoch H & Mahler S (2018) Modeling cocaine relapse in rodents: Behavioral considerations and circuit mechanisms. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87. [Europe PMC free article] [Abstract] [Google Scholar]
- Fenoglio KA, Chen Y & Baram TZ (2006) Neuroplasticity of the Hypothalamic–Pituitary–Adrenal Axis Early in Life Requires Recurrent Recruitment of Stress-Regulating Brain Regions. The Journal of Neuroscience, 26, 2434–2442. [Europe PMC free article] [Abstract] [Google Scholar]
- Fergusson DM & Horwood LJ (2003) Resilience to childhood adversity: Results of a 21-year study. Resilience and vulnerability: Adaptation in the context of childhood adversities, 130–155. [Google Scholar]
- Flagel SB, Akil H & Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology, 56, 139–148. [Europe PMC free article] [Abstract] [Google Scholar]
- Flagel SB, Vázquez DM & Robinson TE (2003) Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology, 28, 1741–1751. [Abstract] [Google Scholar]
- Forster GL, Anderson EM, Scholl JL, Lukkes JL & Watt MJ (2018) Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiology of stress, 9, 29–39. [Europe PMC free article] [Abstract] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M & Le Moine C (2005) A Specific Limbic Circuit Underlies Opiate Withdrawal Memories. The Journal of Neuroscience, 25, 1366–1374. [Europe PMC free article] [Abstract] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF & Koob GF (2006) Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. Journal of Neuroscience, 26, 11324–11332. [Europe PMC free article] [Abstract] [Google Scholar]
- Ganguly P, Honeycutt JA, Rowe JR, Demaestri C & Brenhouse HC (2019) Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF. Brain, Behavior, and Immunity, 78, 41–51. [Europe PMC free article] [Abstract] [Google Scholar]
- García-Gutiérrez MS, Navarrete F, Aracil A, Bartoll A, Martínez-Gras I, Lanciego JL, Rubio G & Manzanares J (2016) Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addict Biol, 21, 847–858. [Abstract] [Google Scholar]
- García-Pérez D, Laorden ML, Milanés MV & Núñez C (2012) Glucocorticoids regulation of FosB/ΔFosB expression induced by chronic opiate exposure in the brain stress system. PLoS One, 7, e50264. [Europe PMC free article] [Abstract] [Google Scholar]
- Gartland D, Riggs E, Muyeen S, Giallo R, Afifi TO, MacMillan H, Herrman H, Bulford E & Brown SJ (2019) What factors are associated with resilient outcomes in children exposed to social adversity? A systematic review. BMJ Open, 9, e024870–e024870. [Europe PMC free article] [Abstract] [Google Scholar]
- Gellert VF & Holtzman SG (1978) Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. Journal of Pharmacology and Experimental Therapeutics, 205, 536–546. [Abstract] [Google Scholar]
- George O & Koob GF (2017) Individual differences in the neuropsychopathology of addiction. Dialogues Clin Neurosci, 19, 217–229. [Europe PMC free article] [Abstract] [Google Scholar]
- George O, Le Moal M & Koob GF (2012) Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiology & Behavior, 106, 58–64. [Europe PMC free article] [Abstract] [Google Scholar]
- Gerrits MAFM, Lesscher HBM & van Ree JM (2003) Drug dependence and the endogenous opioid system. European Neuropsychopharmacology, 13, 424–434. [Abstract] [Google Scholar]
- Gershon A, Minor K & Hayward C (2008) Gender, victimization, and psychiatric outcomes. Psychol Med, 38, 1377–1391. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilles EE, Schultz L & Baram TZ (1996) Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol, 15, 114–119. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilpin NW & Roberto M (2012) Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev, 36, 873–888. [Europe PMC free article] [Abstract] [Google Scholar]
- Glynn LM & Baram TZ (2019) The Influence of Unpredictable, Fragmented Parental Signals on the Developing Brain. Frontiers in neuroendocrinology. [Europe PMC free article] [Abstract] [Google Scholar]
- Gondré-Lewis MC, Darius PJ, Wang H & Allard JS (2016) Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats. J Chem Neuroanat, 76, 122–132. [Europe PMC free article] [Abstract] [Google Scholar]
- González-Pardo H, Arias JL, Gómez-Lázaro E, López Taboada I & Conejo NM (2020) Sex-Specific Effects of Early Life Stress on Brain Mitochondrial Function, Monoamine Levels and Neuroinflammation. Brain Sciences, 10, 447. [Europe PMC free article] [Abstract] [Google Scholar]
- Gracia-Rubio I, Martinez-Laorden E, Moscoso-Castro M, Milanés MV, Laorden ML & Valverde O (2016) Maternal Separation Impairs Cocaine-Induced Behavioural Sensitization in Adolescent Mice. PLOS ONE, 11, e0167483. [Europe PMC free article] [Abstract] [Google Scholar]
- Granger SJ, Glynn LM, Sandman CA, Small SL, Obenaus A, Keator DB, Baram TZ, Stern H, Yassa MA & Davis EP (2020) Aberrant Maturation of the Uncinate Fasciculus Follows Exposure to Unpredictable Patterns of Maternal Signals. The Journal of Neuroscience, JN-RM-0374–0320. [Europe PMC free article] [Abstract] [Google Scholar]
- Granholm L, Todkar A, Bergman S, Nilsson K, Comasco E & Nylander I (2017) The expression of opioid genes in non-classical reward areas depends on early life conditions and ethanol intake. Brain Research, 1668, 36–45. [Abstract] [Google Scholar]
- Grant BF & Dawson DA (1997) Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse, 9, 103–110. [Abstract] [Google Scholar]
- Guadagno A, Kang MS, Devenyi GA, Mathieu AP, Rosa-Neto P, Chakravarty M & Walker C-D (2018a) Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Structure and Function, 223, 3711–3729. [Abstract] [Google Scholar]
- Guadagno A, Wong TP & Walker C-D (2018b) Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 25–37. [Abstract] [Google Scholar]
- Gustafsson L, Oreland S, Hoffmann P & Nylander I (2008) The impact of postnatal environment on opioid peptides in young and adult male Wistar rats. Neuropeptides, 42, 177–191. [Abstract] [Google Scholar]
- Haahr-Pedersen I, Perera C, Hyland P, Vallières F, Murphy D, Hansen M, Spitz P, Hansen P & Cloitre M (2020) Females have more complex patterns of childhood adversity: implications for mental, social, and emotional outcomes in adulthood. European Journal of Psychotraumatology, 11, 1708618. [Europe PMC free article] [Abstract] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T & Robbins TW (1999) Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse, 32, 37–43. [Abstract] [Google Scholar]
- Hane AA & Fox NA (2016) Early caregiving and human biobehavioral development: a comparative physiology approach. Current Opinion in Behavioral Sciences, 7, 82–90. [Europe PMC free article] [Abstract] [Google Scholar]
- Hartmann J & Schmidt MV (2020) Chapter 11 - Stress resilience as a consequence of early-life adversity. In Chen A (ed) Stress Resilience. Academic Press, pp. 149–164. [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M & Grant BF (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry, 170, 834–851. [Europe PMC free article] [Abstract] [Google Scholar]
- Hensch TK (2004) Critical period regulation. Annu Rev Neurosci, 27, 549–579. [Abstract] [Google Scholar]
- Hensleigh E & Pritchard LM (2014) The effect of early environmental manipulation on locomotor sensitivity and methamphetamine conditioned place preference reward. Behavioural Brain Research, 268, 66–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Hensleigh E & Pritchard LM (2015) Maternal separation increases methamphetamine-induced damage in the striatum in male, but not female rats. Behavioural Brain Research, 295, 3–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Hildebrand BE, Nomikos GG, Bondjers C, Nisell M & Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology (Berl), 129, 348–356. [Abstract] [Google Scholar]
- Honeycutt JA, Demaestri C, Peterzell S, Silveri MM, Cai X, Kulkarni P, Cunningham MG, Ferris CF & Brenhouse HC (2020) Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife, 9, e52651. [Europe PMC free article] [Abstract] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S, Barlow HB & Gaze RM (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 278, 377–409. [Abstract] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ & Plotsky PM (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl), 158, 366–373. [Abstract] [Google Scholar]
- Hursh SR (1980) ECONOMIC CONCEPTS FOR THE ANALYSIS OF BEHAVIOR. Journal of the Experimental Analysis of Behavior, 34, 219–238. [Europe PMC free article] [Abstract] [Google Scholar]
- Hursh SR & Silberberg A (2008) Economic demand and essential value. Psychological Review, 115, 186. [Abstract] [Google Scholar]
- Hyman SM, Garcia M & Sinha R (2006) Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Drug Alcohol Abuse, 32, 655–664. [Europe PMC free article] [Abstract] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ & Sinha R (2008) Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug and Alcohol Dependence, 92, 208–216. [Europe PMC free article] [Abstract] [Google Scholar]
- Ironside M, Kumar P, Kang MS & Pizzagalli DA (2018) Brain mechanisms mediating effects of stress on reward sensitivity. Curr Opin Behav Sci, 22, 106–113. [Europe PMC free article] [Abstract] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C & Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience, 154, 1132–1142. [Europe PMC free article] [Abstract] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G & Baram TZ (2010) Hippocampal Dysfunction and Cognitive Impairments Provoked by Chronic Early-Life Stress Involve Excessive Activation of CRH Receptors. The Journal of Neuroscience, 30, 13005–13015. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ & Schoffelmeer AN (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci, 24, 566–573. [Abstract] [Google Scholar]
- Jaworski J, Francis D, Brommer C, Morgan E & Kuhar M (2005) Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology, 181, 8–15. [Abstract] [Google Scholar]
- Jiang S, Kamei N, Bolton JL, Ma X, Stern HS, Baram TZ & Mortazavi A (2019) Intra-individual methylomics detects the impact of early-life adversity. Life Sci Alliance, 2. [Europe PMC free article] [Abstract] [Google Scholar]
- Joëls M & Baram TZ (2009) The neuro-symphony of stress. Nat Rev Neurosci, 10, 459–466. [Europe PMC free article] [Abstract] [Google Scholar]
- Kalinichev M, Easterling KW & Holtzman SG (2002) Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology, 27, 518–533. [Abstract] [Google Scholar]
- Kalivas PW (2008) Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotoxicity research, 14, 185–189. [Abstract] [Google Scholar]
- Kalivas PW & Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry, 162, 1403–1413. [Abstract] [Google Scholar]
- Kawakami SE, Quadros IM, Takahashi S & Suchecki D (2007) Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice. Behav Brain Res, 184, 109–116. [Abstract] [Google Scholar]
- Kenny PJ & Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav, 70, 531–549. [Abstract] [Google Scholar]
- Kessler RC (2004) The epidemiology of dual diagnosis. Biological Psychiatry, 56, 730–737. [Abstract] [Google Scholar]
- Khantzian EJ (1987) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. The cocaine crisis, 65–74. [Abstract] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C & HiRTH CG (1992) The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proceedings of the National Academy of Sciences, 89, 12048–12052. [Europe PMC free article] [Abstract] [Google Scholar]
- Kikusui T, Faccidomo S & Miczek KA (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology, 178, 202–210. [Abstract] [Google Scholar]
- Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJV & Strathearn L (2017) Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Ann N Y Acad Sci, 1394, 74–91. [Europe PMC free article] [Abstract] [Google Scholar]
- Kohut SJ, Roma PG, Davis CM, Zernig G, Saria A, Dominguez JM, Rice KC & Riley AL (2009) The impact of early environmental rearing condition on the discriminative stimulus effects and Fos expression induced by cocaine in adult male and female rats. Psychopharmacology, 203, 383–397. [Europe PMC free article] [Abstract] [Google Scholar]
- Koob GF (2008) A Role for Brain Stress Systems in Addiction. Neuron, 59, 11–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Koob GF & Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science, 278, 52–58. [Abstract] [Google Scholar]
- Koob GF & Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry, 3, 760–773. [Europe PMC free article] [Abstract] [Google Scholar]
- Koob GF & Zorrilla EP (2010) Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs, 11, 63–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Korosi A & Baram TZ (2009) The pathways from mother’s love to baby’s future. Frontiers in behavioral neuroscience, 3, 27–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu Z-W, Borok E, Gao X-B, Horvath TL & Baram TZ (2010) Early-Life Experience Reduces Excitation to Stress-Responsive Hypothalamic Neurons and Reprograms the Expression of Corticotropin-Releasing Hormone. The Journal of Neuroscience, 30, 703–713. [Europe PMC free article] [Abstract] [Google Scholar]
- Kosten TA, Miserendino MJ & Kehoe P (2000) Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res, 875, 44–50. [Abstract] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY & Kehoe P (2004) Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behavioural brain research, 151, 137–149. [Abstract] [Google Scholar]
- Kreek M, Levran O, Reed B, Schlussman SD, Zhou Y & Butelman ER (2012) Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. Journal of Clinical Investigation, 122, 3387–3393. [Europe PMC free article] [Abstract] [Google Scholar]
- Kreek MJ (1996) Cocaine, dopamine and the endogenous opioid system. Journal of addictive diseases, 15, 73–96. [Abstract] [Google Scholar]
- Kronman H, Torres-Berrío A, Sidoli S, Issler O, Godino A, Ramakrishnan A, Mews P, Lardner CK, Parise EM, Walker DM, van der Zee YY, Browne CJ, Boyce BF, Neve R, Garcia BA, Shen L, Peña CJ & Nestler EJ (2021) Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nature Neuroscience. [Europe PMC free article] [Abstract] [Google Scholar]
- Kundakovic M, Lim S, Gudsnuk K & Champagne FA (2013) Sex-Specific and Strain-Dependent Effects of Early Life Adversity on Behavioral and Epigenetic Outcomes. Frontiers in Psychiatry, 4, 78. [Europe PMC free article] [Abstract] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ & Chavkin C (2008) The Dysphoric Component of Stress Is Encoded by Activation of the Dynorphin κ-Opioid System. The Journal of Neuroscience, 28, 407–414. [Europe PMC free article] [Abstract] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS & Bates JE (2010) Does physical abuse in early childhood predict substance use in adolescence and early adulthood? Child Maltreat, 15, 190–194. [Europe PMC free article] [Abstract] [Google Scholar]
- Lemos JC, Shin JH & Alvarez VA (2019) Striatal Cholinergic Interneurons Are a Novel Target of Corticotropin Releasing Factor. The Journal of Neuroscience, 39, 5647–5661. [Europe PMC free article] [Abstract] [Google Scholar]
- Levine S (1957) Infantile experience and resistance to physiological stress. Science, 126, 405. [Abstract] [Google Scholar]
- Levis SC, Bentzley BS, Molet J, Bolton JL, Perrone CR, Baram TZ & Mahler SV (2019) On the early life origins of vulnerability to opioid addiction. Molecular Psychiatry. [Europe PMC free article] [Abstract] [Google Scholar]
- Levis SC, Mahler SV & Baram TZ (2021) The Developmental Origins of Opioid Use Disorder and Its Comorbidities. Frontiers in Human Neuroscience, 15. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis CR, Bastle RM, Manning TB, Himes SM, Fennig P, Conrad PR, Colwell J, Pagni BA, Hess LA, Matekel CG, Newbern JM & Olive MF (2016) Interactions between Early Life Stress, Nucleus Accumbens MeCP2 Expression, and Methamphetamine Self-Administration in Male Rats. Neuropsychopharmacology, 41, 2851–2861. [Europe PMC free article] [Abstract] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L & Olive MF (2013) The Effects of Maternal Separation on Adult Methamphetamine Self-Administration, Extinction, Reinstatement, and MeCP2 Immunoreactivity in the Nucleus Accumbens. Front Psychiatry, 4, 55. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Fitzpatrick D & White LE (2006) The development of direction selectivity in ferret visual cortex requires early visual experience. Nature Neuroscience, 9, 676–681. [Abstract] [Google Scholar]
- Li Y, Robinson TE & Bhatnagar S (2003) Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Research, 960, 42–47. [Abstract] [Google Scholar]
- Liu SR, Kia-Keating M, Nylund-Gibson K & Barnett ML (2020) Co-Occurring Youth Profiles of Adverse Childhood Experiences and Protective Factors: Associations with Health, Resilience, and Racial Disparities. Am J Community Psychol, 65, 173–186. [Abstract] [Google Scholar]
- Logrip ML, Koob GF & Zorrilla EP (2011) Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs, 25, 271–287. [Europe PMC free article] [Abstract] [Google Scholar]
- Luby JL, Baram TZ, Rogers CE & Barch DM (2020) Neurodevelopmental Optimization after Early-Life Adversity: Cross-Species Studies to Elucidate Sensitive Periods and Brain Mechanisms to Inform Early Intervention. Trends Neurosci, 43, 744–751. [Europe PMC free article] [Abstract] [Google Scholar]
- Lundberg S, Nylander I & Roman E (2020) Behavioral Profiling in Early Adolescence and Early Adulthood of Male Wistar Rats After Short and Prolonged Maternal Separation. Front Behav Neurosci, 14, 37. [Europe PMC free article] [Abstract] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, Cope ZA, Lin EC, Riedy MD, Scofield MD, Messinger J, Ruiz CM, Riegel AC, España RA & Aston-Jones G (2018) Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse. Journal of Neuroscience, 39, 537–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Majcher-Maślanka I, Solarz A, Wędzony K & Chocyk A (2017) The effects of early-life stress on dopamine system function in adolescent female rats. International Journal of Developmental Neuroscience, 57, 24–33. [Abstract] [Google Scholar]
- Malin DH & Goyarzu P (2009) Rodent models of nicotine withdrawal syndrome Nicotine Psychopharmacology. Springer, pp. 401–434. [Abstract] [Google Scholar]
- Malin DH, Moon WD, Moy ET, Jennings RE, Moy DM, Warner RL & Wilson OB (2000) A rodent model of cocaine abstinence syndrome. Pharmacology Biochemistry and Behavior, 66, 323–328. [Abstract] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H & Watson SJ (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. Journal of Comparative Neurology, 350, 412–438. [Abstract] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ & Akil H (1995) The cloned μ, δ and κ receptors and their endogenous ligands: Evidence for two opioid peptide recognition cores. Brain Research, 700, 89–98. [Abstract] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD & Shaham Y (2016) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology, 41, 335. [Europe PMC free article] [Abstract] [Google Scholar]
- Manzano Nieves G, Bravo M, Baskoylu S & Bath KG (2020) Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. eLife, 9, e55263. [Europe PMC free article] [Abstract] [Google Scholar]
- Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM & Shaham Y (2019) Context-induced relapse after extinction versus punishment: similarities and differences. Psychopharmacology, 236, 439–448. [Europe PMC free article] [Abstract] [Google Scholar]
- Markou A, Kosten TR & Koob GF (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology, 18, 135–174. [Abstract] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G & Koob GF (1993) Animal models of drug craving. Psychopharmacology, 112, 163–182. [Abstract] [Google Scholar]
- Marquardt AR, Ortiz-Lemos L, Lucion AB & Barros HM (2004) Influence of handling or aversive stimulation during rats’ neonatal or adolescence periods on oral cocaine self-administration and cocaine withdrawal. Behav Pharmacol, 15, 403–412. [Abstract] [Google Scholar]
- Marsh JC, Park K, Lin YA & Bersamira C (2018) Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. J Subst Abuse Treat, 87, 79–85. [Europe PMC free article] [Abstract] [Google Scholar]
- Martin TJ & Ewan E (2008) Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Experimental and clinical psychopharmacology, 16, 357–366. [Europe PMC free article] [Abstract] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS & Robbins TW (1996) Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding,d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology, 126, 75–84. [Abstract] [Google Scholar]
- Matthews K & Robbins TW (2003) Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev, 27, 45–55. [Abstract] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ & Caine SB (1999) Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology, 141, 123–134. [Abstract] [Google Scholar]
- McCabe SE, West BT, Morales M, Cranford JA & Boyd CJ (2007) Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction, 102, 1920–1930. [Europe PMC free article] [Abstract] [Google Scholar]
- McCormick CM, Kehoe P & Kovacs S (1998) Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. Int J Dev Neurosci, 16, 175–185. [Abstract] [Google Scholar]
- McEwen BS & Gianaros PJ (2011) Stress- and allostasis-induced brain plasticity. Annu Rev Med, 62, 431–445. [Europe PMC free article] [Abstract] [Google Scholar]
- McReynolds JR, Peña DF, Blacktop JM & Mantsch JR (2014) Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress, 17, 22–38. [Abstract] [Google Scholar]
- Meaney MJ, Brake W & Gratton A (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology, 27, 127–138. [Abstract] [Google Scholar]
- Méndez Leal AS & Silvers JA (2021) Neurobiological Markers of Resilience to Early-Life Adversity During Adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6, 238–247. [Abstract] [Google Scholar]
- Meyer PJ, King CP & Ferrario CR (2016) Motivational Processes Underlying Substance Abuse Disorder. Curr Top Behav Neurosci, 27, 473–506. [Europe PMC free article] [Abstract] [Google Scholar]
- Michaels CC & Holtzman SG (2008) Early Postnatal Stress Alters Place Conditioning to Both μ- and κ-Opioid Agonists. Journal of Pharmacology and Experimental Therapeutics, 325, 313–318. [Abstract] [Google Scholar]
- Minami M, Toya T, Katao Y, Maekawa K, Nakamura S, Onogi T, Kaneko S & Satoh M (1993) Cloning and expression of a cDNA for the rat k-opioid receptor. FEBS letters, 329, 291–295. [Abstract] [Google Scholar]
- Mitchell SJ, Maguire EP, Cunningham L, Gunn BG, Linke M, Zechner U, Dixon CI, King SL, Stephens DN, Swinny JD, Belelli D & Lambert JJ (2018) Early-life adversity selectively impairs α2-GABAA receptor expression in the mouse nucleus accumbens and influences the behavioral effects of cocaine. Neuropharmacology, 141, 98–112. [Europe PMC free article] [Abstract] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI & Kuhar MJ (2006) Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther, 317, 1210–1218. [Abstract] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD & Kuhar MJ (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochemical Pharmacology, 73, 321–330. [Europe PMC free article] [Abstract] [Google Scholar]
- Mohammadian J, Najafi M & Miladi-Gorji H (2019) Effect of enriched environment during adolescence on spatial learning and memory, and voluntary consumption of morphine in maternally separated rats in adulthood. Dev Psychobiol, 61, 615–625. [Abstract] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ & Stern H (2016a) Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry, 6. [Europe PMC free article] [Abstract] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S & Baram TZ (2014) Naturalistic rodent models of chronic early-life stress. Developmental Psychobiology, 56, 1675–1688. [Europe PMC free article] [Abstract] [Google Scholar]
- Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, Solodkin A, Obenaus A & Baram TZ (2016b) MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus, 26, 1618–1632. [Europe PMC free article] [Abstract] [Google Scholar]
- Najavits LM, Weiss RD & Shaw SR (1997) The Link Between Substance Abuse and Posttraumatic Stress Disorder in Women: A Research Review. American Journal on Addictions, 6, 273–283. [Abstract] [Google Scholar]
- Negus SS & Miller LL (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev, 66, 869–917. [Europe PMC free article] [Abstract] [Google Scholar]
- Nesse RM & Berridge KC (1997) Psychoactive drug use in evolutionary perspective. Science, 278, 63–66. [Abstract] [Google Scholar]
- Nestler EJ (2004) Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends in Pharmacological Sciences, 25, 210–218. [Abstract] [Google Scholar]
- Newman M & Ferrario CR (2020) An improved demand curve for analysis of food or drug consumption in behavioral experiments. Psychopharmacology (Berl), 237, 943–955. [Europe PMC free article] [Abstract] [Google Scholar]
- Nishi M, Horii-Hayashi N & Sasagawa T (2014) Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Frontiers in Neuroscience, 8, 166. [Europe PMC free article] [Abstract] [Google Scholar]
- Nurco DN, Kinlock TW, O’Grady KE & Hanlon TE (1996) Early family adversity as a precursor to narcotic addiction. Drug and Alcohol Dependence, 43, 103–113. [Abstract] [Google Scholar]
- Nygard SK, Hourguettes NJ, Sobczak GG, Carlezon WA & Bruchas MR (2016) Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. Journal of Neuroscience, 36, 9937–9948. [Europe PMC free article] [Abstract] [Google Scholar]
- Nylander I & Roman E (2013) Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology, 229, 555–569. [Europe PMC free article] [Abstract] [Google Scholar]
- O’Connor RM, Moloney RD, Glennon J, Vlachou S & Cryan JF (2015) Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology, 99, 168–176. [Abstract] [Google Scholar]
- Okhuarobo A, Bolton JL, Igbe I, Zorrilla EP, Baram TZ & Contet C (2020) A novel mouse model for vulnerability to alcohol dependence induced by early-life adversity. Neurobiol Stress, 13, 100269. [Europe PMC free article] [Abstract] [Google Scholar]
- Olds J & Milner P (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol, 47, 419–427. [Abstract] [Google Scholar]
- Oleson EB & Roberts D (2009) Parsing the addiction phenomenon: Self-administration procedures modeling enhanced motivation for drug and escalation of drug intake. Drug Discovery Today: Disease Models, 5, 217–226. [Europe PMC free article] [Abstract] [Google Scholar]
- Oleson EB & Roberts DCS (2008) Behavioral Economic Assessment of Price and Cocaine Consumption Following Self-Administration Histories that Produce Escalation of Either Final Ratios or Intake. Neuropsychopharmacology, 34. [Europe PMC free article] [Abstract] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA & Hodge CW (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. Journal of Neuroscience, 21, RC184–RC184. [Europe PMC free article] [Abstract] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA & Hodge CW (2002) Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology Biochemistry and Behavior, 72, 213–220. [Abstract] [Google Scholar]
- Opendak M, Robinson-Drummer P, Blomkvist A, Zanca RM, Wood K, Jacobs L, Chan S, Tan S, Woo J, Venkataraman G, Kirschner E, Lundström JN, Wilson DA, Serrano PA & Sullivan RM (2019) Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology, 44, 1247–1257. [Europe PMC free article] [Abstract] [Google Scholar]
- Ordoñes Sanchez E, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, Flowers J, Rogers CM, Langrehr MG, Ardekani CS, Famularo ST, Bongiovanni AR, Knouse MC, Floresco SB, Briand LA, Wimmer ME & Bangasser DA (2021) Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proceedings of the National Academy of Sciences, 118, e2020173118. [Europe PMC free article] [Abstract] [Google Scholar]
- Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Tractenberg SG, Benetti F & Grassi-Oliveira R (2019) How Early Life Stress Impact Maternal Care: A Systematic Review of Rodent Studies. Frontiers in Behavioral Neuroscience, 13. [Europe PMC free article] [Abstract] [Google Scholar]
- Panlilio LV, Thorndike EB & Schindler CW (2003) Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology, 168, 229–235. [Abstract] [Google Scholar]
- Peciña S & Berridge KC (2005) Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do μ-Opioids Cause Increased Hedonic Impact of Sweetness? The Journal of Neuroscience, 25, 11777–11786. [Europe PMC free article] [Abstract] [Google Scholar]
- Peciña S, Schulkin J & Berridge KC (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC biology, 4, 1–16. [Europe PMC free article] [Abstract] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR & McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiol Stress, 10, 100149. [Europe PMC free article] [Abstract] [Google Scholar]
- Peña C, Nestler EJ & Bagot RC (2019) Environmental Programming of Susceptibility and Resilience to Stress in Adulthood in Male Mice. Frontiers in Behavioral Neuroscience, 13, 40. [Europe PMC free article] [Abstract] [Google Scholar]
- Peña CJ, Neugut YD, Calarco CA & Champagne FA (2014) Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. European Journal of Neuroscience, 39, 946–956. [Abstract] [Google Scholar]
- Ploj K & Nylander I (2003) Long-term effects on brain opioid and opioid receptor like-1 receptors after short periods of maternal separation in rats. Neurosci Lett, 345, 195–197. [Abstract] [Google Scholar]
- Ploj K, Pham TM, Bergström L, Mohammed AH, Henriksson BG & Nylander I (1999) Neonatal handling in rats induces long-term effects on dynorphin peptides. Neuropeptides, 33, 468–474. [Abstract] [Google Scholar]
- Ploj K, Roman E, Bergström L & Nylander I (2001) Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacology Biochemistry and Behavior, 69, 173–179. [Abstract] [Google Scholar]
- Ploj K, Roman E & Nylander I (2003a) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male wistar rats. Neuroscience, 121, 787–799. [Abstract] [Google Scholar]
- Ploj K, Roman E & Nylander I (2003b) Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides, 37, 149–156. [Abstract] [Google Scholar]
- Portero-Tresserra M, Gracia-Rubio I, Cantacorps L, Pozo OJ, Gómez-Gómez A, Pastor A, López-Arnau R, de la Torre R & Valverde O (2018) Maternal separation increases alcohol-drinking behaviour and reduces endocannabinoid levels in the mouse striatum and prefrontal cortex. European Neuropsychopharmacology, 28, 499–512. [Abstract] [Google Scholar]
- Pryce CR, Bettschen D & Feldon J (2001) Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol, 38, 239–251. [Abstract] [Google Scholar]
- Pryce CR & Feldon J (2003) Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience & Biobehavioral Reviews, 27, 57–71. [Abstract] [Google Scholar]
- Quadros IM, Macedo GC, Domingues LP & Favoretto CA (2016) An Update on CRF Mechanisms Underlying Alcohol Use Disorders and Dependence. Front Endocrinol (Lausanne), 7, 134. [Europe PMC free article] [Abstract] [Google Scholar]
- Redila VA & Chavkin C (2008) Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology, 200, 59–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Reyes BAS, Kravets JL, Connelly KL, Unterwald EM & Van Bockstaele EJ (2017) Localization of the delta opioid receptor and corticotropin-releasing factor in the amygdalar complex: role in anxiety. Brain Struct Funct, 222, 1007–1026. [Europe PMC free article] [Abstract] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR & Baram TZ (2008) A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology, 149, 4892–4900. [Europe PMC free article] [Abstract] [Google Scholar]
- Robinson TE & Berridge KC (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci, 363, 3137–3146. [Europe PMC free article] [Abstract] [Google Scholar]
- Robinson TE, Gorny G, Savage VR & Kolb B (2002) Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse, 46, 271–279. [Abstract] [Google Scholar]
- Rodrigues AJ, Leão P, Carvalho M, Almeida OF & Sousa N (2011) Potential programming of dopaminergic circuits by early life stress. Psychopharmacology (Berl), 214, 107–120. [Abstract] [Google Scholar]
- Rogers JL, De Santis S & See RE (2008) Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl), 199, 615–624. [Europe PMC free article] [Abstract] [Google Scholar]
- Roman E, Ploj K & Nylander I (2004) Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol, 33, 31–39. [Abstract] [Google Scholar]
- Romano-López A, Mendez-Diaz M, Ruiz-Contreras A, Carrisoza R & Prospero-Garcia O (2012) Maternal separation and proclivity for ethanol intake: a potential role of the endocannabinoid system in rats. Neuroscience, 223, 296–304. [Abstract] [Google Scholar]
- Romano-López A, Méndez-Díaz M, García FG, Regalado-Santiago C, Ruiz-Contreras AE & Prospéro-García O (2016) Maternal separation and early stress cause long-lasting effects on dopaminergic and endocannabinergic systems and alters dendritic morphology in the nucleus accumbens and frontal cortex in rats. Developmental neurobiology, 76, 819–831. [Abstract] [Google Scholar]
- Rosenblum LA & Paully GS (1987) Primate models of separation-induced depression. Psychiatr Clin North Am, 10, 437–447. [Abstract] [Google Scholar]
- Rothwell PE, Thomas MJ & Gewirtz JC (2012) Protracted manifestations of acute dependence after a single morphine exposure. Psychopharmacology (Berl), 219, 991–998. [Europe PMC free article] [Abstract] [Google Scholar]
- Saal D, Dong Y, Bonci A & Malenka RC (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron, 37, 577–582. [Abstract] [Google Scholar]
- Salamone JD, Correa M, Farrar A & Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl), 191, 461–482. [Abstract] [Google Scholar]
- Salamone JD, Correa M, Yang J-H, Rotolo R & Presby R (2018) Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Frontiers in Behavioral Neuroscience, 12. [Europe PMC free article] [Abstract] [Google Scholar]
- Salamone JD, Correa M, Yohn S, Lopez Cruz L, San Miguel N & Alatorre L (2016) The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behav Processes, 127, 3–17. [Abstract] [Google Scholar]
- Sarnyai Z, Shaham Y & Heinrichs SC (2001) The role of corticotropin-releasing factor in drug addiction. Pharmacological reviews, 53, 209–244. [Abstract] [Google Scholar]
- Schmidt MV (2011) Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology, 36, 330–338. [Abstract] [Google Scholar]
- Schmidt MV, Wang XD & Meijer OC (2011) Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl), 214, 131–140. [Abstract] [Google Scholar]
- Schuckit MA (2002) Vulnerability factors for alcoholism. Neuropsychopharmacology: The fifth generation of progress, 1399–1411. [Google Scholar]
- Schultz W (1998) Predictive reward signal of dopamine neurons. Journal of neurophysiology, 80, 1–27. [Abstract] [Google Scholar]
- Schuster CR & Thompson T (1969) Self Administration of and Behavioral Dependence on Drugs. Annual Review of Pharmacology, 9, 483–502. [Abstract] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith ACW, Roberts-Wolfe D & Kalivas PW (2016) The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacological reviews, 68, 816–871. [Europe PMC free article] [Abstract] [Google Scholar]
- See RE & Waters RP (2010) Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res, 3, 81–89. [Europe PMC free article] [Abstract] [Google Scholar]
- Shaham Y, Erb S & Stewart J (2000) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews, 33, 13–33. [Abstract] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H & Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl), 168, 3–20. [Abstract] [Google Scholar]
- Shalev U, Erb S & Shaham Y (2010) Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain research, 1314, 15–28. [Europe PMC free article] [Abstract] [Google Scholar]
- Shalev U, Grimm JW & Shaham Y (2002) Neurobiology of Relapse to Heroin and Cocaine Seeking: A Review. Pharmacological Reviews, 54, 1–42. [Abstract] [Google Scholar]
- Shalev U, Highfield D, Yap J & Shaham Y (2000) Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl), 150, 337–346. [Abstract] [Google Scholar]
- Shand FL, Degenhardt L, Slade T & Nelson EC (2011) Sex differences amongst dependent heroin users: Histories, clinical characteristics and predictors of other substance dependence. Addictive Behaviors, 36, 27–36. [Europe PMC free article] [Abstract] [Google Scholar]
- Sheridan MA & McLaughlin KA (2014) Dimensions of early experience and neural development: deprivation and threat. Trends in cognitive sciences, 18, 580–585. [Europe PMC free article] [Abstract] [Google Scholar]
- Short AK & Baram TZ (2019) Early-life adversity and neurological disease: age-old questions and novel answers. Nat Rev Neurol, 15, 657–669. [Europe PMC free article] [Abstract] [Google Scholar]
- Simpson TL & Miller WR (2002) Concomitance between childhood sexual and physical abuse and substance use problems A review. Clinical Psychology Review, 22, 27–77. [Abstract] [Google Scholar]
- Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, Simeone K, Cope J, Chen Y, Mortazavi A & Baram TZ (2017) NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Molecular Psychiatry, 23, 648. [Europe PMC free article] [Abstract] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158, 343–359. [Abstract] [Google Scholar]
- Sinha R (2008) Chronic Stress, Drug Use, and Vulnerability to Addiction. Annals of the New York Academy of Sciences, 1141, 105–130. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith KS, Mahler SV, Peciña S & Berridge KC (2010) Hedonic hotspots: generating sensory pleasure in the brain. Pleasures of the Brain. [Google Scholar]
- Smith RJ & Aston-Jones G (2014) Incentive learning for morphine-associated stimuli during protracted abstinence increases conditioned drug preference. Neuropsychopharmacology, 39, 373–379. [Europe PMC free article] [Abstract] [Google Scholar]
- Spyrka J, Gugula A, Rak A, Tylko G, Hess G & Blasiak A (2020) Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. Neurobiology of Stress, 13, 100250. [Europe PMC free article] [Abstract] [Google Scholar]
- Stein MD, Conti MT, Kenney S, Anderson BJ, Flori JN, Risi MM & Bailey GL (2017) Adverse childhood experience effects on opioid use initiation, injection drug use, and overdose among persons with opioid use disorder. Drug and alcohol dependence, 179, 325–329. [Europe PMC free article] [Abstract] [Google Scholar]
- Steketee JD & Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev, 63, 348–365. [Europe PMC free article] [Abstract] [Google Scholar]
- Stewart J & de Wit H (1987) Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs Methods of assessing the reinforcing properties of abused drugs. Springer, pp. 211–227. [Google Scholar]
- Strathearn L, Giannotti M, Mills R, Kisely S, Najman J & Abajobir A (2020) Long-term Cognitive, Psychological, and Health Outcomes Associated With Child Abuse and Neglect. Pediatrics, 146. [Europe PMC free article] [Abstract] [Google Scholar]
- Strickland JC, Lile JA & Stoops WW (2019) Evaluating non-medical prescription opioid demand using commodity purchase tasks: test-retest reliability and incremental validity. Psychopharmacology (Berl), 236, 2641–2652. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson RH & Swanson LW (2010) Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A, 107, 15235–15239. [Europe PMC free article] [Abstract] [Google Scholar]
- Thompson T & Pickens R (1970) Stimulant self-administration by animals: some comparisons with opiate self-administration. Fed Proc, 29, 6–12. [Abstract] [Google Scholar]
- Tractenberg SG, Levandowski ML, de Azeredo LA, Orso R, Roithmann LG, Hoffmann ES, Brenhouse H & Grassi-Oliveira R (2016) An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neuroscience & Biobehavioral Reviews, 68, 489–503. [Abstract] [Google Scholar]
- Tractenberg SG, Orso R, Creutzberg KC, Malcon LMC, Lumertz FS, Wearick-Silva LE, Viola TW, Riva MA & Grassi-Oliveira R (2020) Vulnerable and resilient cognitive performance related to early life stress: The potential mediating role of dopaminergic receptors in the medial prefrontal cortex of adult mice. International Journal of Developmental Neuroscience, 80, 13–27. [Abstract] [Google Scholar]
- Ungless MA, Argilli E & Bonci A (2010) Effects of stress and aversion on dopamine neurons: implications for addiction. Neuroscience & Biobehavioral Reviews, 35, 151–156. [Abstract] [Google Scholar]
- Valentino RJ & Van Bockstaele E (2015) Endogenous Opioids: The Downside of Opposing Stress. Neurobiology of stress, 1, 23–32. [Europe PMC free article] [Abstract] [Google Scholar]
- Valentino RJ & Volkow ND (2018) Untangling the complexity of opioid receptor function. Neuropsychopharmacology, 43, 2514–2520. [Europe PMC free article] [Abstract] [Google Scholar]
- Van Bockstaele EJ, Reyes BA & Valentino RJ (2010) The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res, 1314, 162–174. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza P-V & Deroche-Gamonet V (2008) Maternal Environment Influences Cocaine Intake in Adulthood in a Genotype-Dependent Manner. PLOS ONE, 3, e2245. [Europe PMC free article] [Abstract] [Google Scholar]
- van Oers HJ, de Kloet ER & Levine S (1998) Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res, 111, 245–252. [Abstract] [Google Scholar]
- Vazquez V, Giros B & Daugé V (2006) Maternal deprivation specifically enhances vulnerability to opiate dependence. Behavioural Pharmacology, 17, 715–724. [Abstract] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson M, Giros B & Daugé V (2005) Maternal Deprivation Increases Vulnerability to Morphine Dependence and Disturbs the Enkephalinergic System in Adulthood. The Journal of Neuroscience, 25, 4453–4462. [Europe PMC free article] [Abstract] [Google Scholar]
- Venniro M, Caprioli D & Shaham Y (2019) Novel models of drug relapse and craving after voluntary abstinence. Neuropsychopharmacology, 44, 234–235. [Europe PMC free article] [Abstract] [Google Scholar]
- Ventura R, Coccurello R, Andolina D, Latagliata EC, Zanettini C, Lampis V, Battaglia M, D’Amato FR & Moles A (2013) Postnatal aversive experience impairs sensitivity to natural rewards and increases susceptibility to negative events in adult life. Cereb Cortex, 23, 1606–1617. [Abstract] [Google Scholar]
- Volkow ND, McLellan TA, Cotto JH, Karithanom M & Weiss SRB (2011) Characteristics of Opioid Prescriptions in 2009. JAMA, 305, 1299–1301. [Europe PMC free article] [Abstract] [Google Scholar]
- Volkow ND, Wise RA & Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci, 18, 741–752. [Abstract] [Google Scholar]
- Vrettou M, Granholm L, Todkar A, Nilsson KW, Wallén-Mackenzie Å, Nylander I & Comasco E (2017) Ethanol affects limbic and striatal presynaptic glutamatergic and DNA methylation gene expression in outbred rats exposed to early-life stress. Addict Biol, 22, 369–380. [Abstract] [Google Scholar]
- Wakeford AGP, Morin EL, Bramlett SN, Howell LL & Sanchez MM (2018) A review of nonhuman primate models of early life stress and adolescent drug abuse. Neurobiol Stress, 9, 188–198. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y & Baram TZ (2017) Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20, 1–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Walters H & Kosten TA (2019) Early life stress and the propensity to develop addictive behaviors. Int J Dev Neurosci, 78, 156–169. [Abstract] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA & You Z-B (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. Journal of Neuroscience, 25, 5389–5396. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang B, You Z-B, Rice KC & Wise RA (2007) Stress-induced relapse to cocaine seeking: roles for the CRF 2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology, 193, 283–294. [Abstract] [Google Scholar]
- Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Müller MB & Schmidt MV (2012) Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci, 36, 2360–2367. [Abstract] [Google Scholar]
- Ward SJ, Läck C, Morgan D & Roberts DC (2006) Discrete-trials heroin self-administration produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl), 185, 150–159. [Abstract] [Google Scholar]
- Widom CS, Ireland T & Glynn PJ (1995) Alcohol abuse in abused and neglected children followed-up: are they at increased risk? J Stud Alcohol, 56, 207–217. [Abstract] [Google Scholar]
- Widom CS, Marmorstein NR & White HR (2006) Childhood victimization and illicit drug use in middle adulthood. Psychol Addict Behav, 20, 394–403. [Abstract] [Google Scholar]
- Williams TJ & Milner TA (2011) Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience, 179, 9–22. [Europe PMC free article] [Abstract] [Google Scholar]
- Wise RA & Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev, 94, 469–492. [Abstract] [Google Scholar]
- Wise RA & Rompré P-P (1989) Brain dopamine and reward. Annual Review of Psychology, 40, 191–225. [Abstract] [Google Scholar]
- Wright ER, Kooreman HE, Greene MS, Chambers RA, Banerjee A & Wilson J (2014) The iatrogenic epidemic of prescription drug abuse: County-level determinants of opioid availability and abuse. Drug and Alcohol Dependence, 138, 209–215. [Abstract] [Google Scholar]
- Yan CG, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, Yang Z, Gerum S, Biswal BB, Milham MP, Sullivan RM & Castellanos FX (2017) Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Translational Psychiatry, 7, e1005–e1005. [Europe PMC free article] [Abstract] [Google Scholar]
- Yap JJ & Miczek KA (2008) Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discov Today Dis Models, 5, 259–270. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoo JH, Kitchen I & Bailey A (2012) The endogenous opioid system in cocaine addiction: what lessons have opioid peptide and receptor knockout mice taught us? Br J Pharmacol, 166, 1993–2014. [Europe PMC free article] [Abstract] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM & Cheng AY (2013) On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Peciña and Berridge with commentary on the transitional nature of basal forebrain “boundaries”. J Comp Neurol, 521, 50–68. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang LI, Bao S & Merzenich MM (2001) Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci, 4, 1123–1130. [Abstract] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P & Kosten TA (2005) Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology, 177, 391–399. [Abstract] [Google Scholar]
- Zhu X, Li T, Peng S, Ma X, Chen X & Zhang X (2010) Maternal deprivation-caused behavioral abnormalities in adult rats relate to a non-methylation-regulated D2 receptor levels in the nucleus accumbens. Behavioural Brain Research, 209, 281–288. [Abstract] [Google Scholar]
- Zinn ME, Huntley ED & Keating DP (2020) Resilience in adolescence: Prospective Self moderates the association of early life adversity with externalizing problems. Journal of Adolescence, 81, 61–72. [Europe PMC free article] [Abstract] [Google Scholar]
- Zorrilla EP, Logrip ML & Koob GF (2014) Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol, 35, 234–244. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/ejn.15223
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8494863
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/103487087
Article citations
Neuroimaging of the effects of drug exposure or self-administration in rodents: A systematic review.
Neurosci Biobehav Rev, 164:105823, 01 Aug 2024
Cited by: 0 articles | PMID: 39094280 | PMCID: PMC11374361
Review Free full text in Europe PMC
Enduring Neurobiological Consequences of Early-Life Stress: Insights from Rodent Behavioral Paradigms.
Biomedicines, 12(9):1978, 02 Sep 2024
Cited by: 0 articles | PMID: 39335492 | PMCID: PMC11429222
Review Free full text in Europe PMC
Microglia: The Drunken Gardeners of Early Adversity.
Biomolecules, 14(8):964, 08 Aug 2024
Cited by: 1 article | PMID: 39199352 | PMCID: PMC11353196
Review Free full text in Europe PMC
Impact of childhood adversity on acute subjective effects of stimulant and opioid drugs: Evidence from placebo-controlled studies in healthy volunteers.
J Psychopharmacol, 38(11):986-997, 08 Aug 2024
Cited by: 0 articles | PMID: 39118370 | PMCID: PMC11528953
Individual longitudinal changes in DNA-methylome identify signatures of early-life adversity and correlate with later outcome.
Neurobiol Stress, 31:100652, 31 May 2024
Cited by: 3 articles | PMID: 38962694 | PMCID: PMC11219970
Go to all (24) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enduring disruption of reward and stress circuit activities by early-life adversity in male rats.
Transl Psychiatry, 12(1):251, 16 Jun 2022
Cited by: 16 articles | PMID: 35705547 | PMCID: PMC9200783
Neurobiological mechanisms of early life adversity, blunted stress reactivity and risk for addiction.
Neuropharmacology, 188:108519, 10 Mar 2021
Cited by: 22 articles | PMID: 33711348 | PMCID: PMC9195251
Review Free full text in Europe PMC
On the early life origins of vulnerability to opioid addiction.
Mol Psychiatry, 26(8):4409-4416, 10 Dec 2019
Cited by: 39 articles | PMID: 31822817 | PMCID: PMC7282971
Sex-specific behavioral outcomes of early-life adversity and emerging microglia-dependent mechanisms.
Front Behav Neurosci, 16:1013865, 04 Oct 2022
Cited by: 7 articles | PMID: 36268470 | PMCID: PMC9577368
Funding
Funders who supported this work.
NIDA NIH HHS (2)
Grant ID: P50 DA044118
Grant ID: F30 DA051137
NIGMS NIH HHS (1)
Grant ID: T32 GM008620
NIMH NIH HHS (3)
Grant ID: T32 MH119049
Grant ID: R01 MH073136
Grant ID: P50 MH096889
National Institutes of Health (5)
Grant ID: P50 MH096889
Grant ID: F30 DA051137
Grant ID: R01 MH073136
Grant ID: T32 GM008620
Grant ID: P50 DA044118
Tobacco-Related Disease Research Program (1)
Grant ID: T31IR1767




