Abstract
Importance
Infection with SARS-CoV-2 is associated with fatigue and sleep problems long after the acute phase of COVID-19. In addition, there are concerns of SARS-CoV-2 infection causing psychiatric illness; however, evidence of a direct effect is inconclusive.Objective
To assess risk of risk of incident or repeat psychiatric illness, fatigue, or sleep problems following SARS-CoV-2 infection and to analyze changes according to demographic subgroups.Design, setting, and participants
This cohort study assembled matched cohorts using the Clinical Practice Research Datalink Aurum, a UK primary care registry of 11 923 499 individuals aged 16 years or older. Patients were followed-up for up to 10 months, from February 1 to December 9, 2020. Individuals with less than 2 years of historical data or less than 1 week follow-up were excluded. Individuals with positive results on a SARS-CoV-2 test without prior mental illness or with anxiety or depression, psychosis, fatigue, or sleep problems were matched with up to 4 controls based on sex, general practice, and year of birth. Controls were individuals who had negative SARS-CoV-2 test results. Data were analyzed from January to July 2021.Exposure
SARS-CoV-2 infection, determined via polymerase chain reaction testing.Main outcomes and measures
Cox proportional hazard models estimated the association between a positive SARS-CoV-2 test result and subsequent psychiatric morbidity (depression, anxiety, psychosis, or self-harm), sleep problems, fatigue, or psychotropic prescribing. Models adjusted for comorbidities, ethnicity, smoking, and body mass index.Results
Of 11 923 105 eligible individuals (6 011 020 [50.4%] women and 5 912 085 [49.6%] men; median [IQR] age, 44 [30-61] years), 232 780 individuals (2.0%) had positive result on a SARS-CoV-2 test. After applying selection criteria, 86 922 individuals were in the matched cohort without prior mental illness, 19 020 individuals had prior anxiety or depression, 1036 individuals had psychosis, 4152 individuals had fatigue, and 4539 individuals had sleep problems. After adjusting for observed confounders, there was an association between positive SARS-CoV-2 test results and psychiatric morbidity (adjusted hazard ratio [aHR], 1.83; 95% CI, 1.66-2.02), fatigue (aHR, 5.98; 95% CI, 5.33-6.71), and sleep problems (aHR, 3.16; 95% CI, 2.64-3.78). However, there was a similar risk of incident psychiatric morbidity for those with a negative SARS-CoV-2 test results (aHR, 1.71; 95% CI, 1.65-1.77) and a larger increase associated with influenza (aHR, 2.98; 95% CI, 1.55-5.75).Conclusions and relevance
In this cohort study of individuals registered at an English primary care practice during the pandemic, there was consistent evidence that SARS-CoV-2 infection was associated with increased risk of fatigue and sleep problems. However, the results from the negative control analysis suggest that unobserved confounding may be responsible for at least some of the positive association between COVID-19 and psychiatric morbidity.Free full text

Association of SARS-CoV-2 Infection With Psychological Distress, Psychotropic Prescribing, Fatigue, and Sleep Problems Among UK Primary Care Patients
Key Points
Question
Is SARS-CoV-2 infection associated with risk of subsequent psychiatric morbidity, sleep problems, or fatigue?
Findings
In this cohort study of the health care records of 11 923
923 105 patients, including 226
105 patients, including 226 521 patients with SARS-CoV-2 infection, while infection was associated with increased risk of sleep problems and fatigue, associations with subsequent psychiatric morbidity were mixed.
521 patients with SARS-CoV-2 infection, while infection was associated with increased risk of sleep problems and fatigue, associations with subsequent psychiatric morbidity were mixed.
Meaning
These findings suggest that psychiatric morbidity associated with SARS-CoV-2 infection may be overstated in analyses of health care records that do not sufficiently control for confounding.
Abstract
Importance
Infection with SARS-CoV-2 is associated with fatigue and sleep problems long after the acute phase of COVID-19. In addition, there are concerns of SARS-CoV-2 infection causing psychiatric illness; however, evidence of a direct effect is inconclusive.
Objective
To assess risk of risk of incident or repeat psychiatric illness, fatigue, or sleep problems following SARS-CoV-2 infection and to analyze changes according to demographic subgroups.
Design, Setting, and Participants
This cohort study assembled matched cohorts using the Clinical Practice Research Datalink Aurum, a UK primary care registry of 11 923
923 499 individuals aged 16 years or older. Patients were followed-up for up to 10 months, from February 1 to December 9, 2020. Individuals with less than 2 years of historical data or less than 1 week follow-up were excluded. Individuals with positive results on a SARS-CoV-2 test without prior mental illness or with anxiety or depression, psychosis, fatigue, or sleep problems were matched with up to 4 controls based on sex, general practice, and year of birth. Controls were individuals who had negative SARS-CoV-2 test results. Data were analyzed from January to July 2021.
499 individuals aged 16 years or older. Patients were followed-up for up to 10 months, from February 1 to December 9, 2020. Individuals with less than 2 years of historical data or less than 1 week follow-up were excluded. Individuals with positive results on a SARS-CoV-2 test without prior mental illness or with anxiety or depression, psychosis, fatigue, or sleep problems were matched with up to 4 controls based on sex, general practice, and year of birth. Controls were individuals who had negative SARS-CoV-2 test results. Data were analyzed from January to July 2021.
Exposure
SARS-CoV-2 infection, determined via polymerase chain reaction testing.
Main Outcomes and Measures
Cox proportional hazard models estimated the association between a positive SARS-CoV-2 test result and subsequent psychiatric morbidity (depression, anxiety, psychosis, or self-harm), sleep problems, fatigue, or psychotropic prescribing. Models adjusted for comorbidities, ethnicity, smoking, and body mass index.
Results
Of 11 923
923 105 eligible individuals (6
105 eligible individuals (6 011
011 020 [50.4%] women and 5
020 [50.4%] women and 5 912
912 085 [49.6%] men; median [IQR] age, 44 [30-61] years), 232
085 [49.6%] men; median [IQR] age, 44 [30-61] years), 232 780 individuals (2.0%) had positive result on a SARS-CoV-2 test. After applying selection criteria, 86
780 individuals (2.0%) had positive result on a SARS-CoV-2 test. After applying selection criteria, 86 922 individuals were in the matched cohort without prior mental illness, 19
922 individuals were in the matched cohort without prior mental illness, 19 020 individuals had prior anxiety or depression, 1036 individuals had psychosis, 4152 individuals had fatigue, and 4539 individuals had sleep problems. After adjusting for observed confounders, there was an association between positive SARS-CoV-2 test results and psychiatric morbidity (adjusted hazard ratio [aHR], 1.83; 95% CI, 1.66-2.02), fatigue (aHR, 5.98; 95% CI, 5.33-6.71), and sleep problems (aHR, 3.16; 95% CI, 2.64-3.78). However, there was a similar risk of incident psychiatric morbidity for those with a negative SARS-CoV-2 test results (aHR, 1.71; 95% CI, 1.65-1.77) and a larger increase associated with influenza (aHR, 2.98; 95% CI, 1.55-5.75).
020 individuals had prior anxiety or depression, 1036 individuals had psychosis, 4152 individuals had fatigue, and 4539 individuals had sleep problems. After adjusting for observed confounders, there was an association between positive SARS-CoV-2 test results and psychiatric morbidity (adjusted hazard ratio [aHR], 1.83; 95% CI, 1.66-2.02), fatigue (aHR, 5.98; 95% CI, 5.33-6.71), and sleep problems (aHR, 3.16; 95% CI, 2.64-3.78). However, there was a similar risk of incident psychiatric morbidity for those with a negative SARS-CoV-2 test results (aHR, 1.71; 95% CI, 1.65-1.77) and a larger increase associated with influenza (aHR, 2.98; 95% CI, 1.55-5.75).
Conclusions and Relevance
In this cohort study of individuals registered at an English primary care practice during the pandemic, there was consistent evidence that SARS-CoV-2 infection was associated with increased risk of fatigue and sleep problems. However, the results from the negative control analysis suggest that unobserved confounding may be responsible for at least some of the positive association between COVID-19 and psychiatric morbidity.
Introduction
Many people infected with SARS-CoV-2 experience symptoms beyond the acute phase of COVID-19, particularly fatigue, brain fog, and sleep problems.1,2 Studies have also reported worsening mental health and an increased risk of psychiatric illness after COVID-19,3,4,5,6,7,8 and mechanisms linking the immune system, inflammation, and the brain have been proposed.9,10 Notably, not all studies have found an association of COVID-19 with anxiety or depression.11
To date, 37% of the US population has been infected with SARS-CoV-2 and only approximately 1 in 4 individuals present for testing.12,13 Observational studies investigating the outcomes associated with SARS-CoV-2 infection may be confounded by several sources affecting the likelihood that somebody is infected (eg, their occupation), the likelihood they present to services (eg, comorbidities), or the likelihood they seek a test (eg, health anxiety). When unobserved confounding is suspected, the extent that confounding bias is evident can be examined using a negative control.14 In a negative exposure control, a variable with no conceivable direct effect on the outcome, but with a similar confounding structure, is substituted for the exposure under investigation. If the result from the negative control is similar to that observed using the primary exposure, then unobserved confounding is implicated.
We examined the association between SARS-CoV-2 infection and psychiatric morbidity (ie, anxiety, depression, self-harm, psychosis, and prescription of psychotropic medication), sleep problems, and fatigue using UK primary care data. In the United Kingdom, most of health care takes place in primary care. Therefore, reexamining associations within this population might provide further detail as to the outcomes associated with COVID-19 in the population. To our knowledge, this is the first study to use a negative test result as a negative exposure control to detect unobserved confounding.
We consider both incident events as well as outcomes in people with preexisting mental illness, fatigue, or sleep problems. Our primary hypothesis was that SARS-CoV-2 infection would be associated with increased likelihood of new or repeat presentation of psychiatric morbidity, sleep problems, or fatigue independently of confounders. We also hypothesized that the increase in risk would be greatest for women and those living in more socioeconomically deprived areas. We investigated the specificity of infection with SARS-CoV-2 and psychological outcomes by repeating our analysis in individuals with an influenza diagnosis.
Methods
For this cohort study, data access was granted following approval from the Independent Scientific Advisory Committee for Medicines and Healthcare Products Regulatory Agency Database Research.15 All patient data were deidentified; thus, the requirement for patient consent was waived by CRPD. Individual patients can opt out of sharing their records with the Clinical Practice Research Datalink (CPRD) Aurum. This study is prepared in line with the Reporting of Studies Conducted Using Observational Routinely-Collected Data (RECORD) statement. The protocol was preregistered before any outcomes were analyzed and is available elsewhere.16
Data Source
Cohorts were assembled from the CPRD Aurum data set, a large UK primary care registry covering 19 million patients.17 It contains information on clinical events recorded by health care professionals, including diagnosis, symptoms, and therapies.
Eligible Cohort
Eligible individuals were those aged 16 years or older during 2020 and registered at a CPRD Aurum participating clinical practice from February 1 to December 8, 2020. Eligible follow-up began on the latest date of February 1, 2020, or their clinical practice registration, and ended at the earliest date of their death, the date they transferred out of a clinical practice, or the end of data collection (December 9, 2020). A total of 394 individuals were excluded if they had an indeterminate sex recorded. This resulted in 11 923
923 105 individuals for matching.
105 individuals for matching.
Exposure
Exposure to SARS-CoV-2 was defined as a positive result on a polymerase chain reaction (PCR) test using codes developed by the UK Medicines and Healthcare Products Regulatory Agency.18 In the United Kingdom, while most testing took place in the community, in primary care, individuals presenting with symptoms consistent with COVID-19 underwent PCR testing. In addition, since July 20, 2020, primary care physicians were notified of all PCR test results, regardless of outcome.19 In total, 232 780 of 11
780 of 11 923
923 105 eligible individuals (2.0%) had a record of a positive SARS-CoV-2 test result during the observation period.
105 eligible individuals (2.0%) had a record of a positive SARS-CoV-2 test result during the observation period.
Outcome
The primary outcomes were a diagnosis with or symptoms relating to depression, anxiety disorders, self-harm, affective or nonaffective psychosis, sleep problems, and fatigue or fatigue-like syndromes (eg, postviral fatigue syndrome). There were few cases of posttraumatic stress disorder (PTSD) so, in a departure from our protocol, these were combined with anxiety disorders. Secondary outcomes were prescriptions for psychotic medications (ie, antidepressants, anxiolytics, antipsychotics, mood stabilizers, benzodiazepines, and nonbenzodiazepines hypnotics). Outcome codes were identified in a prior analysis and are published elsewhere.20 Two senior clinical academics (C.A.C.-G. and N.K.) reviewed the clinical code list, and a senior academic pharmacist (D.M.A.) reviewed the medication code list.
Covariates
Covariates were identified from 10 years of patient data recorded before the start of each individual’s follow-up. Comorbidities were identified using individual diseases used for the Charlson comorbidity index21: cancer, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, connective tissue disease, dementia, diabetes, HIV/AIDS, hemiplegia, myocardial infarction, liver disease, renal disease, peripheral vascular disease, posterior vitreous detachment, and peptic ulcer disease. Additional data were extracted on sex, year of birth, and the practice’s Index of Multiple Deprivation score, an area-level ranking of socioeconomic deprivation divided into quintiles according to the national distribution. In the UK general practices, ethnicity is self-reported during registration or at clinical consultations. There are 296 different ethnicity codes in the CPRD, and these were categorized by the analysis team (M.P. and M.C.) as White, Asian, Black, Mixed and other (eg, Arab, Cook Island Māori, and Latin American) (eTable 1 in the Supplement).
Matched Cohorts
Five cohorts were constructed by matching individuals with a positive SARS-CoV-2 test result during follow-up with unexposed individuals (eFigure 1 in the Supplement). An individual’s earliest date of positive test result was defined as the index date. The first cohort (hereafter incident cohort) excluded individuals with recorded histories of psychiatric morbidity, fatigue, sleep problems, or psychotropic medications in the 5 years prior to their index date. The remaining 4 prevalent cohorts comprised individuals with common mental illness, psychosis, sleep problems, or fatigue in the last 6 months. Individuals in the common mental illness and psychosis cohorts must also have had a prescription for antidepressants, anxiolytics or antipsychotics, or mood stabilizers in the last 6 months. Individuals were not excluded from a prevalent cohort based on having a history of another mental illness, fatigue, or sleep problem. The prevalent cohorts were not mutually exclusive but were mutually exclusive with the incident cohort.
We excluded individuals with less than 1 week of eligible follow-up from their index date or less than 2 years registration prior to their index date to ensure adequate capture for prior mental illnesses. Patients were ineligible for being in the unexposed group if they had a record indicating suspected or confirmed COVID-19 in their history or in the week following the index date. Unexposed individuals may have had a positive test result at a later time, thus unexposed individuals could enter the cohort as an exposed individual at a later time. After these criteria were applied, up to 4 unexposed individuals were selected for each exposed individual, regardless of whether or not they had been selected previously (ie, matching with replacement), matched on sex, clinical practice, and year of birth. The psychosis cohort (in which there were fewer available unexposed individuals) was matched solely on sex and practice.
Additional Cohorts
Two further cohorts were constructed. The first substituted individuals with negative SARS-CoV-2 test results for those with a positive test result (560 495 negative test cases matched with 2
495 negative test cases matched with 2 232
232 733 comparators) and proceeded with the same analysis. The second repeated the analysis using those recorded by their general practitioner as having influenza or influenza-like symptoms and with a negative SARS-CoV-2 test result within 2 weeks. For the negative test cohort, we expected that there would be little (or no) association between receiving a negative test result (vs comparators) and any of the outcomes considered. Any divergence from a null finding would indicate potential bias from unobserved confounders. For the influenza cohort, we expected to see an increase in fatigue,22 but to a lesser extent than that seen for individuals with SARS-CoV-2 infection.3
733 comparators) and proceeded with the same analysis. The second repeated the analysis using those recorded by their general practitioner as having influenza or influenza-like symptoms and with a negative SARS-CoV-2 test result within 2 weeks. For the negative test cohort, we expected that there would be little (or no) association between receiving a negative test result (vs comparators) and any of the outcomes considered. Any divergence from a null finding would indicate potential bias from unobserved confounders. For the influenza cohort, we expected to see an increase in fatigue,22 but to a lesser extent than that seen for individuals with SARS-CoV-2 infection.3
Statistical Analysis
The index date for the exposed individuals became the start of follow-up for all in a matched set. Censoring occurred at death, when the practice ceased collecting data, or when the individual transferred to another practice. Hazard ratios (HRs) were estimated using Cox proportional hazard models, stratified on each matched set and with weights representing the number of times an individual was in a cohort. The proportional hazard assumption was investigated by graphically examining the cumulative hazard function and the scaled Schoenfeld residuals (eFigure 2 in the Supplement).
Adjusted models included variables for ethnicity, comorbidities (as binary indicators), smoking status, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared; linear and squared term) and a variable indicating how long the individual had been at a clinical practice. Effect modification was explored by including a multiplicative interaction between the exposure and (individually) the covariates: age (categorized as 16-24, 25-34, 35-49, 50-59, 60-69, 70-79, and ≥80 years), sex, Index of Multiple Deprivation quintile of the general practice, and follow-up time elapsed since index date (<1 month, 1 to <3 months, 3 to <6 months, or 6-10 months). The model using follow-up time was used to loosen the assumption of nonproportional hazards over the whole of follow-up.
There were missing data for ethnicity (2 327
327 219 individuals [19.5%]), smoking (881
219 individuals [19.5%]), smoking (881 268 individuals [7.4%]), and BMI (1
268 individuals [7.4%]), and BMI (1 757
757 001 individuals [14.7%]). To retain individuals, 10 imputed data sets were estimated after matching, using chained equations. Multiple imputation models included all covariates, a variable indicating the size of the general practice, and the patient’s height or weight (if recorded). Adjusted Cox models were fitted to each imputed data set, and estimates were combined using Rubin rules.
001 individuals [14.7%]). To retain individuals, 10 imputed data sets were estimated after matching, using chained equations. Multiple imputation models included all covariates, a variable indicating the size of the general practice, and the patient’s height or weight (if recorded). Adjusted Cox models were fitted to each imputed data set, and estimates were combined using Rubin rules.
We conducted 3 preplanned sensitivity analyses. For the first, we investigated the introduction of widespread testing by fitting an interaction between period (before vs after September 1, 2020) and the exposure. For the second sensitivity analysis, a propensity score was calculated and used as a covariate in the Cox model. For calculation of the propensity score, missing data items were given separate categories, and interactions between variables were included. The third sensitivity analysis repeated the analysis of the incident cohort but including only diagnosis codes in the outcome definition.
A post hoc sensitivity analysis was conducted after review to test robustness of findings to whether exposed and unexposed individuals were engaged with health care services. Thus, the incident matched cohort was constructed, again restricting to those who had routine clinical data (ie, BMI, ethnicity, or smoking status) recorded in the last 2 years, 1 year, and 6 months.
Stata statistical software version 16 (StataCorp) was used for all analyses and graphs were produced using R statistical software version 4.0.3 (R Project for Statistical Computing). P values were 2-sided, but we did not use a binary threshold to denote significance. Data were analyzed from January to July 2021.
Results
Of 11 923
923 105 individuals (6
105 individuals (6 011
011 020 [50.4%] women and 5
020 [50.4%] women and 5 912
912 085 [49.6%] men; median [IQR] age, 44 [30-61] years) in the eligible cohort, 232
085 [49.6%] men; median [IQR] age, 44 [30-61] years) in the eligible cohort, 232 780 (2.0%) were recorded as having a positive PCR test result for SARS-CoV-2 during their follow-up. Most positive test results occurred between April and May 2020 and October and November 2020 (eFigure 3 in the Supplement). Individuals with a positive test result were more likely than those without to be women (130
780 (2.0%) were recorded as having a positive PCR test result for SARS-CoV-2 during their follow-up. Most positive test results occurred between April and May 2020 and October and November 2020 (eFigure 3 in the Supplement). Individuals with a positive test result were more likely than those without to be women (130 775 [56.2%] women vs 5
775 [56.2%] women vs 5 880
880 245 [50.3%]) (Table 1), in the youngest or oldest age groups (45
245 [50.3%]) (Table 1), in the youngest or oldest age groups (45 456 individuals [19.5%] vs 1
456 individuals [19.5%] vs 1 665
665 762 [14.3%] aged 16-24 years; 16
762 [14.3%] aged 16-24 years; 16 083 individuals (6.9%) vs 683
083 individuals (6.9%) vs 683 993 individuals (5.9%) aged ≥80 years), and have higher BMI (median [IQR], 26.4 [22.9-30.8] vs 25.8 [22.6-29.7]) and more recorded comorbidities (51
993 individuals (5.9%) aged ≥80 years), and have higher BMI (median [IQR], 26.4 [22.9-30.8] vs 25.8 [22.6-29.7]) and more recorded comorbidities (51 500 individuals [22.1%] vs 2
500 individuals [22.1%] vs 2 303
303 458 individuals [19.7%] with ≥1 comorbid conditions). Individuals with positive SARS-CoV-2 test results were also more likely to have a clinical record in the preceding 5 years signifying psychiatric morbidity, fatigue, sleep problems, or a psychotropic medication prescription (Table 1).
458 individuals [19.7%] with ≥1 comorbid conditions). Individuals with positive SARS-CoV-2 test results were also more likely to have a clinical record in the preceding 5 years signifying psychiatric morbidity, fatigue, sleep problems, or a psychotropic medication prescription (Table 1).
Table 1.
| Characteristic | Positive SARS-CoV-2 test results, No. (%) | |
|---|---|---|
Yes (n = = 232 232 780) 780) | No (n = = 11 11 690 690 325) 325) | |
| Sex | ||
| Women | 130 775 (56.2) 775 (56.2) | 5 880 880 245 (50.3) 245 (50.3) |
| Men | 102 005 (43.8) 005 (43.8) | 5 810 810 080 (49.7) 080 (49.7) |
| Age group, y | ||
| 16-24 | 45 456 (19.5) 456 (19.5) | 1 665 665 762 (14.3) 762 (14.3) |
| 25-34 | 41 336 (17.8) 336 (17.8) | 2 208 208 954 (18.9) 954 (18.9) |
| 35-49 | 56 983 (24.5) 983 (24.5) | 2 881 881 606 (24.7) 606 (24.7) |
| 50-59 | 39 119 (16.8) 119 (16.8) | 1 827 827 489 (15.6) 489 (15.6) |
| 60-69 | 21 359 (9.2) 359 (9.2) | 1 362 362 308 (11.7) 308 (11.7) |
| 70-79 | 12 444 (5.4) 444 (5.4) | 1 060 060 213 (9.1) 213 (9.1) |
| ≥80 | 16 083 (6.9) 083 (6.9) | 683 993 (5.9) 993 (5.9) |
| Race and ethnicitya | ||
| White | 152 885 (79.5) 885 (79.5) | 7 545 545 915 (80.3) 915 (80.3) |
| Asian | 25 988 (13.5) 988 (13.5) | 996 746 (10.6) 746 (10.6) |
| Black | 7540 (3.9) | 499 926 (5.3) 926 (5.3) |
| Mixed | 3325 (1.7) | 185 221 (2.0) 221 (2.0) |
| Other | 2646 (1.4) | 175 694 (1.9) 694 (1.9) |
| Missing | 40 396b 396b | 2 286 286 823b 823b |
| BMI category | ||
| <18.5 | 7964 (3.9) | 386 194 (3.9) 194 (3.9) |
| ≥18.5 to <25 | 73 790 (36.1) 790 (36.1) | 3 975 975 991 (39.9) 991 (39.9) |
| ≥25 to <30 | 64 222 (31.4) 222 (31.4) | 3 217 217 104 (32.3) 104 (32.3) |
| ≥30 to <40 | 49 321 (24.1) 321 (24.1) | 2 058 058 755 (20.7) 755 (20.7) |
| ≥40 | 9106 (4.5) | 323 657 (3.3) 657 (3.3) |
| Missing | 28 377b 377b | 1 728 728 624b 624b |
| Time at clinical practice, median (IQR), y | 9.6 (2.3-21.0) | 9.4 (2.7-21.4) |
| Comorbidities, No.c | ||
| 0 | 181 280 (77.9) 280 (77.9) | 9 386 386 867 (80.3) 867 (80.3) |
| 1 | 26 143 (11.2) 143 (11.2) | 1 332 332 044 (11.4) 044 (11.4) |
| 2-4 | 23 262 (10.0) 262 (10.0) | 922 004 (7.9) 004 (7.9) |
| ≥5 | 2095 (0.9) | 49 410 (0.4) 410 (0.4) |
| Psychiatric morbidity in the last 5 y | ||
| Depression | 38 727 (16.6) 727 (16.6) | 1 601 601 431 (13.7) 431 (13.7) |
| Anxiety | 31 072 (13.4) 072 (13.4) | 1 271 271 468 (10.9) 468 (10.9) |
| Psychosis | 2941 (1.3) | 136 001 (1.2) 001 (1.2) |
| Eating disorder | 1344 (0.6) | 47 018 (0.4) 018 (0.4) |
| Personality disorder | 726 (0.3) | 42 531 (0.4) 531 (0.4) |
| Self-harm | 2975 (1.3) | 137 826 (1.2) 826 (1.2) |
| Fatigue | 18 396 (7.9) 396 (7.9) | 665 652 (5.7) 652 (5.7) |
| Sleep disorder | 16 759 (7.2) 759 (7.2) | 669 528 (5.7) 528 (5.7) |
| Medication in the last 5 y | ||
| Antidepressants | 65 364 (28.1) 364 (28.1) | 2 681 681 383 (22.9) 383 (22.9) |
| Benzodiazepines | 20 571 (8.8) 571 (8.8) | 826 794 (7.1) 794 (7.1) |
| Nonbenzodiazepine hypnotics | 12 596 (5.4) 596 (5.4) | 542 565 (4.6) 565 (4.6) |
| Antipsychotics | 5228 (2.3) | 209 463 (1.8) 463 (1.8) |
| Mood stabilizers | 15 306 (6.6) 306 (6.6) | 586 523 (5.0) 523 (5.0) |
Outcomes in Individuals Without Prior Mental Illness, Fatigue, or Sleep Problems (Incident Cohort)
The median (IQR) follow-up of the incident cohort was 6.3 (4.0-9.3) weeks. After matching on age, sex, and registered practice, and adjusting for ethnicity, smoking status, BMI, and comorbidities, having a positive result on a SARS-CoV-2 test was associated with an increase in risk of any psychiatric morbidity (adjusted HR [aHR], 1.83; 95% CI, 1.66-2.02) and of being prescribed psychotropic medication (aHR, 2.24; 95% CI, 2.09-2.40) (Table 2). The absolute risks were low: an estimated 1.4% of patients with a positive SARS-CoV-2 test result presented with psychiatric morbidity at 6 months, compared with 0.9% of individuals with a negative test result (eTable 2 in the Supplement).
Table 2.
| Outcome | Group | Events | Rate, per 1000 person-years | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Any psychiatric morbidity | SARS-CoV-2 | 447 | 30.24 | 1.80 (1.63-1.98) | 1.83 (1.66-2.02) |
| Unexposed | 1045 | 17.39 | 1 [Reference] | 1 [Reference] | |
| Depression | SARS-CoV-2 | 230 | 15.52 | 1.71 (1.50-1.95) | 1.74 (1.51-2.00) |
| Unexposed | 571 | 9.49 | 1 [Reference] | 1 [Reference] | |
| Anxiety | SARS-CoV-2 | 298 | 20.12 | 1.85 (1.65-2.08) | 1.93 (1.71-2.18) |
| Unexposed | 671 | 11.15 | 1 [Reference] | 1 [Reference] | |
| Psychosis | SARS-CoV-2 | 21 | 1.41 | 2.34 (1.48-3.70) | 1.84 (0.93-3.64) |
| Unexposed | 36 | 0.60 | 1 [Reference] | 1 [Reference] | |
| Self-harm | SARS-CoV-2 | 13 | 0.87 | 2.09 (1.20-3.64) | 2.21 (1.11-4.39) |
| Unexposed | 25 | 0.41 | 1 [Reference] | 1 [Reference] | |
| Sleep disorders | SARS-CoV-2 | 190 | 12.82 | 3.31 (2.82-3.89) | 3.16 (2.64-3.78) |
| Unexposed | 236 | 3.92 | 1 [Reference] | 1 [Reference] | |
| Fatigue | SARS-CoV-2 | 579 | 39.29 | 6.10 (5.47-6.80) | 5.98 (5.33-6.71) |
| Unexposed | 414 | 6.88 | 1 [Reference] | 1 [Reference] | |
| Any psychotropic medication | SARS-CoV-2 | 1055 | 72.05 | 2.41 (2.26-2.57) | 2.24 (2.09-2.40) |
| Unexposed | 1848 | 30.82 | 1 [Reference] | 1 [Reference] | |
| Antidepressants | SARS-CoV-2 | 583 | 39.54 | 1.79 (1.65-1.95) | 1.72 (1.57-1.88) |
| Unexposed | 1358 | 22.62 | 1 [Reference] | 1 [Reference] | |
| Benzodiazepines | SARS-CoV-2 | 266 | 17.95 | 3.84 (3.33-4.42) | 3.50 (2.95-4.15) |
| Unexposed | 305 | 5.07 | 1 [Reference] | 1 [Reference] | |
| Nonbenzodiazepine hypnotics | SARS-CoV-2 | 173 | 11.67 | 4.95 (4.10-5.97) | 4.90 (4.00-5.99) |
| Unexposed | 148 | 2.46 | 1 [Reference] | 1 [Reference] | |
| Antipsychotics | SARS-CoV-2 | 83 | 5.59 | 6.73 (4.99-9.08) | 7.61 (5.00-11.60) |
| Unexposed | 63 | 1.05 | 1 [Reference] | 1 [Reference] | |
| Mood stabilizers | SARS-CoV-2 | 105 | 7.08 | 3.80 (3.04-4.75) | 3.55 (2.74-4.61) |
| Unexposed | 115 | 1.91 | 1 [Reference] | 1 [Reference] | |
For almost all outcomes considered, positive SARS-CoV-2 test results were associated with increased risk. The largest increases were for receipt of antipsychotics (aHR, 7.61; 95% CI, 5.00-11.60), fatigue (aHR, 5.98; 95% CI, 5.33-6.71), receipt of nonbenzodiazepine hypnotics (aHR, 4.90; 95% CI, 4.00-5.99), receipt of mood stabilizers (a HR, 3.55; 95% CI, 2.74-4.61), and sleep problems (aHR, 3.16; 95% CI, 2.64-3.78).
There was effect modification by age, such that the association between SARS-CoV-2 infection and psychiatric morbidity was greater for older age groups (eg, ≥80 years: aHR, 4.17; 95% CI, 2.67-6.53 vs 16-24 years: aHR, 1.28; 95% CI, 1.06-1.55). For fatigue and sleep disorder, the association was greatest for those aged 60 to 69 years and remained elevated for all groups (Table 3). For all outcomes, women with positive SARS-CoV-2 test results had a higher incidence than men; however, the relative increase associated with a positive test result was larger for men. There was no association of effect modification by deprivation quintile. For sleep or psychiatric morbidity, the association was similar over follow-up.
Table 3.
| Group | Person-yearsa | Outcome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any psychiatric morbidity | Fatigue | Sleep | |||||||||||||
| SARS-CoV-2 | Unexposed | SARS-CoV-2, ratea | Unexposed, ratea | aHR (95% CI)b | P valuec | SARS-CoV-2, ratea | Unexposed, ratea | aHR (95% CI)b | P valuec | SARS-CoV-2, ratea | Unexposed, ratea | aHR (95% CI)b | P valuec | ||
| Sex | |||||||||||||||
| Women | 7.0 | 28.5 | 37.11 | 24.06 | 1.63 (1.43-1.85) | .003 | 43.01 | 8.68 | 5.25 (4.54-6.06) | .003 | 8.31 | 3.50 | 2.34 (1.78-3.06) | .003 | |
| Men | 7.8 | 31.5 | 24.14 | 11.53 | 2.20 (1.88-2.57) | 27.93 | 3.67 | 7.46 (6.19-9.01) | 12.45 | 3.07 | 4.06 (3.18-5.18) | ||||
| Age-group, y | |||||||||||||||
| 16-24 | 2.6 | 10.9 | 41.69 | 34.49 | 1.28 (1.06-1.55) | <.001 | 14.46 | 5.43 | 2.92 (2.14-4.00) | <.001 | 4.32 | 2.55 | 2.08 (1.25-3.46) | .002 | |
| 25-34 | 2.4 | 9.7 | 31.01 | 21.29 | 1.59 (1.26-2.02) | 20.37 | 5.17 | 4.24 (3.14-5.73) | 5.32 | 2.51 | 2.35 (1.48-3.75) | ||||
| 35-49 | 3.8 | 15.0 | 27.92 | 15.20 | 2.00 (1.64-2.45) | 39.67 | 5.79 | 6.49 (5.26-8.03) | 9.72 | 3.05 | 2.99 (2.16-4.13) | ||||
| 50-59 | 2.9 | 11.4 | 25.18 | 11.80 | 2.24 (1.75-2.88) | 42.38 | 4.80 | 8.74 (6.78-11.26) | 12.94 | 2.69 | 4.45 (3.00-6.59) | ||||
| 60-69 | 1.6 | 6.3 | 22.91 | 7.91 | 3.09 (2.16-4.43) | 38.94 | 3.95 | 9.15 (6.39-13.10) | 11.96 | 1.96 | 7.97 (4.56-13.94) | ||||
| 70-79 | 0.8 | 3.4 | 21.78 | 5.90 | 3.66 (2.00-6.68) | 37.80 | 4.74 | 7.23 (4.51-11.60) | 9.12 | 2.97 | 3.40 (1.41-8.17) | ||||
| ≥80 | 0.7 | 3.2 | 46.06 | 11.97 | 4.17 (2.67-6.53) | 32.17 | 9.76 | 5.07 (3.02-8.50) | 11.50 | 5.88 | 1.38 (0.60-3.18) | ||||
| IMD quintile | |||||||||||||||
| Highest | 1.8 | 7.2 | 21.93 | 15.21 | 1.54 (1.13-2.10) | .30 | 36.38 | 5.44 | 5.86 (4.34-7.91) | .06 | 5.78 | 2.16 | 3.11 (1.77-5.46) | .93 | |
| Second | 2.3 | 9.4 | 33.39 | 18.14 | 1.87 (1.47-2.38) | 43.24 | 4.51 | 8.66 (6.53-11.48) | 7.21 | 2.45 | 3.53 (2.13-5.84) | ||||
| Third | 2.2 | 9.1 | 32.91 | 16.96 | 2.01 (1.58-2.56) | 29.83 | 4.49 | 6.67 (4.89-9.12) | 9.65 | 3.09 | 3.43 (2.15-5.47) | ||||
| Fourth | 2.9 | 11.8 | 28.56 | 19.39 | 1.57 (1.25-1.96) | 28.98 | 6.80 | 5.04 (3.95-6.43) | 11.84 | 3.57 | 3.24 (2.24-4.70) | ||||
| Lowest | 3.6 | 14.9 | 33.02 | 16.61 | 2.06 (1.70-2.51) | 32.45 | 5.82 | 5.18 (4.13-6.51) | 8.82 | 3.31 | 2.76 (1.96-3.90) | ||||
| Follow-up time, mo | |||||||||||||||
| <1 | 6.1 | 24.5 | 22.02 | 15.78 | 1.57 (1.34-1.85) | .24 | 47.54 | 5.56 | 8.12 (6.71-9.82) | <.001 | 8.58 | 2.66 | 3.27 (2.33-4.61) | .12 | |
| 1-3 | 5.3 | 21.5 | 31.58 | 17.69 | 1.97 (1.70-2.29) | 24.66 | 5.64 | 4.47 (3.54-5.63) | 8.75 | 3.57 | 2.27 (1.59-3.24) | ||||
| 3-6 | 2.4 | 10.0 | 24.85 | 13.17 | 2.15 (1.68-2.75) | 25.76 | 6.33 | 4.66 (3.35-6.49) | 13.87 | 3.31 | 3.96 (2.49-6.31) | ||||
| ≥6 | 0.9 | 4.0 | 14.75 | 8.56 | 1.91 (1.20-3.03) | 17.49 | 5.61 | 3.03 (1.77-5.20) | 12.13 | 2.42 | 5.33 (2.40-11.82) | ||||
Abbreviation: aHR, adjusted hazard ratio.
Outcomes in Individuals With Prior History of Common Mental Illness, Psychosis, Fatigue, and Sleep Problems
For those with preexisting common mental illness (ie, depression or anxiety disorders), there was no association of a positive SARS-CoV-2 test result with increased risk of subsequent depression or anxiety events (Figure 1; eTable 3 in the Supplement). However, there was an increase in the risk of new prescriptions for antidepressants (aHR, 1.20; 95% CI, 1.13-1.26) and a larger increase for new prescriptions for benzodiazepines (aHR, 1.91; 95% CI, 1.68-2.17), and a positive SARS-CoV-2 test result was associated with more than 2-fold higher risk of subsequent fatigue (aHR, 2.24; 95% CI, 1.99-2.53).
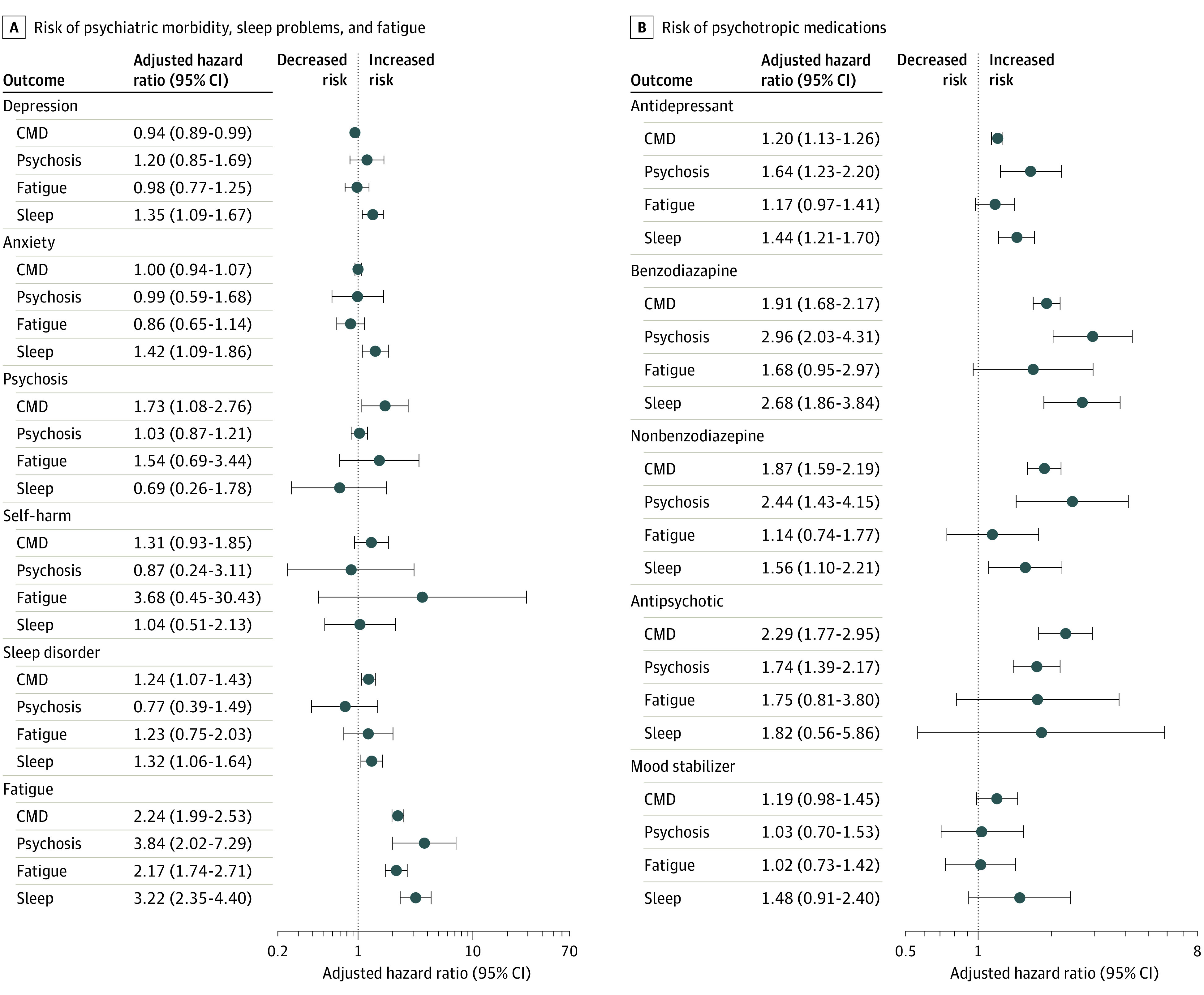
CMD indicates common mental disorders. Estimates are provided in eTable 3 in the Supplement.
There was no evidence of an increased risk of depression or anxiety associated with SARS-CoV-2 infection for those with preexisting psychosis or fatigue. There was an increased risk of depression and anxiety disorders associated with having a positive SARS-CoV-2 test result for those with preexisting sleep problems (depression: aHR, 1.35; 95% CI, 1.09-1.67; anxiety disorders: aHR, 1.42; 95% CI, 1.09-1.86) and an increased risk of subsequent fatigue associated with having a positive SARS-CoV-2 test result for all matched cohorts.
Negative Test Results and Influenza Cohorts
Among individuals with a negative SARS-CoV-2 test result, there was an increased risk of psychiatric morbidity compared with matched controls in the general population (Figure 2). There was a similar association to the main analysis between having a negative test result and the risk of all subcategories of psychiatric morbidity, fatigue, and sleep problems (eTable 4 in the Supplement). There was an association between having an influenza-like illness and either incident psychiatric morbidity, sleep, fatigue, or psychotropic prescribing. Prior to matching, individuals with a negative SARS-CoV-2 test result and those with influenza over follow-up had a higher rate of prior mental illness than those in the group with positive SARS-CoV-2 test results (eTable 5 in the Supplement).
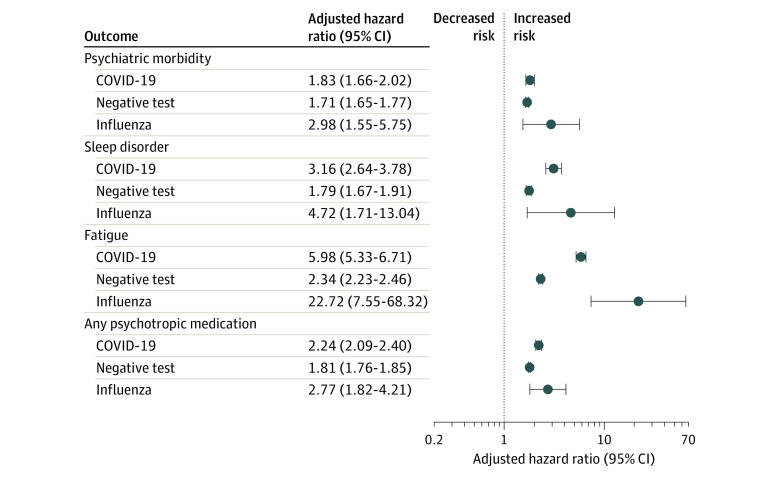
Psychotropic medications that were prescribed 6 months prior to index date were excluded. Estimates are provided in eTable 4 in the Supplement.
Sensitivity Analyses
There were some significant interactions between period of testing and the positive test result. For individuals who had positive test results prior to September 1, 2020, there was a stronger association between having a positive SARS-CoV-2 test result and psychiatric morbidity or psychotropic medication than those who had positive results on or after September 1, 2020 (eTable 6 in the Supplement). The estimates, after adjusting for a propensity score, were of a similar magnitude to that seen in the main analysis (eTable 7 in the Supplement). The estimates for anxiety and depression were also similar after including only diagnostic codes in the outcome; however, for psychosis, there was little evidence of an increase associated with SARS-CoV-2 infection (eTable 8 in the Supplement). When the cohort was restricted to those with recent clinical contact, there were similar associations to those seen in the main analysis (eTable 9 in the Supplement).
Discussion
In this cohort study, we found that SARS-CoV-2 infection confirmed with a positive PCR test result was associated with increased risk of incident psychiatric morbidity, sleep problems, and fatigue in the following months. However, sensitivity analyses provided doubt about whether some of these outcomes were directly associated with SARS-CoV-2 infection. Notably, using the same processes as the analysis of patients with a positive SARS-CoV-2 test result (eg, selection criteria, confounding control), individuals with a negative SARS-CoV-2 test result also experienced a substantial increase in risk of incident psychiatric morbidity. This association was of similar magnitude and with overlapping CIs to that observed in people with a positive SARS-CoV-2 test results. Having a negative test result for SARS-CoV-2, in and of itself, is unlikely to cause psychiatric morbidity through any direct mechanism; rather, this indicates there are unobserved confounders linking the likelihood of undergoing a test and the likelihood of having an incident psychiatric episode during the COVID-19 pandemic.
We believe the most likely unobserved confounding variables are occupation and health anxiety. While many individuals were placed on furlough or worked from home, many sectors of work (eg, health care workers) were required to continue as usual, raising the likelihood of exposure to SARS-CoV-2 and potentially leading to excess psychological strain.23 In addition, those who seek a test for COVID-19 symptoms could already be experiencing health anxieties that might indicate future mental illness. Those with a negative test result had a higher proportion of prior mental illness than those with a positive test, perhaps indicating that health anxiety might be more strongly associated with having a negative test result rather than a positive one. It has been estimated that approximately three-fourths of individuals infected with SARS-CoV-2 have not been tested,13 and the likelihood of seeking a test may be guided by similar behavioral factors regardless of whether the results would be positive or negative. The negative exposure control analysis showed these factors should not be ignored, and while this does not rule out a direct association of SARS-CoV-2 infection with subsequent psychiatric morbidity, it provides substantial doubt.
We included a separate investigation of individuals exposed to influenza during the same periods to examine the specificity of the association of incident psychiatric morbidity, fatigue, and sleep problems with SARS-CoV-2 infection as opposed to other respiratory diseases. For all outcomes, the increase in risk was considerably larger for individuals presenting for primary care with influenza than with SARS-CoV-2 infection. This is likely because these form a particularly selective group: overall there were fewer cases of influenza observed during the COVID-19 pandemic,24 and individuals who present with influenza may have been more likely to have severe infection, preexisting morbidity, and/or psychological risk factors.
Our finding contrasts with 2 recent studies using US administrative data3,4 that reported that individuals with positive test results for SARS-CoV-2 had approximately 2-fold higher risk of subsequent psychiatric illness compared with those with influenza infection. There are some key design differences that may explain this: the US analysis did not follow-up individuals with SARS-CoV-2 infection and the controls from the same date, nor did they adjust for geographical area. Because the pandemic has ecological effects on localized health care systems, as well as on population mental health, this could introduce substantive biases.25 Also, the US may have confounding that is less evident in the United Kingdom. For example, in the US, areas with high levels of socioeconomic deprivation and inequality had significantly higher incidence of COVID-19,26,27 these areas are also likely to have higher rates of mental illness.28,29
Our analysis is in agreement with a Danish registry study that did not find an association between having positive SARS-CoV-2 test results, vs negative test results, and subsequent mental illness.11 This analysis excluded patients hospitalized for COVID-19 (which we were unable to do); however, we note that the US study3 reported similar estimates for those who were hospitalized and those not.
Limitations
There are several limitations to our study, besides unobserved confounding. First, approximately 8.7% of the population of England had positive results in antibody testing for SARS-CoV-2 by December 2020,30 indicating they had previously been infected, compared with 2.0% of individuals in the study population who had positive results in SARS-CoV-2 PCR testing. Therefore, the comparison group will have contained many who had a SARS-CoV-2 infection but were asymptomatic or were symptomatic and did not report it. This misclassification will likely bias results toward the null. Second, because testing was relatively scarce during the early phases of the pandemic and there was no requirement automatically to notify general practices until July 20, 2020, most patients with SARS-CoV-2 infection were identified during the United Kingdom’s second wave of infections (ie, November-December 2020); therefore, the median follow-up was relatively short (6.3 weeks). Additionally, the cohort is based on administrative records, rather than active follow-up, and therefore will have contained some people who moved away. However, the results were sensitive to participants having recent clinical contact.
Conclusions
This cohort study of individuals with a positive SARS-CoV-2 PCR test result found an association of SARS-CoV-2 infection with fatigue and sleep problems; however there is some doubt about a direct association of SARS-CoV-2 with psychiatric outcomes. Other designs may be more appropriate to investigate the association of SARS-CoV-2 infection with mental health outcomes. For example, individuals, randomly selected, who took part in serological surveys might be more representative of the general population and, thus, less susceptible to bias.
Notes
Supplement.
eTable 1. Categorization of Race and Ethnicity From the Clinical Codes in CPRD
eFigure 1. Flowchart Showing Selection in to Matched Cohorts
eFigure 2. Cumulative Hazard and Scaled Schoenfeld Residual Plots
eFigure 3. Histogram of Frequency of Positive SARS-CoV-2 Test Results by Date During 2020
eTable 2. Proportion of Individuals With Positive Test Results and Controls With Outcomes After 6 Months Within Each Matched Cohort
eTable 3. Psychiatric Morbidity, Sleep Problems, Fatigue and Uniquely Prescribed Psychotropic Medications for Those With Preexisting Common Mental Illness, Psychosis, Fatigue, or Sleep Problems Matched on Year of Birth, Sex, and General Practice
eTable 4. Comparison of Adjusted Hazard Ratios From Matched Positive SARS-CoV-2 Test Results, Negative SARS-CoV-2 Test Results, and Influenza Cohorts
eTable 5. Description of Eligible Cohort According to SARS-CoV-2 Test and Influenza Status Over Follow-up
eTable 6. Estimates for Individuals With Positive SARS-CoV-2 Test Results in the First or Second Wave
eTable 7. Comparison of Estimates From Main Adjusted Analysis of the Incident Cohort With That Calculated Controlling for a Propensity Score
eTable 8. Estimates From the Main Analysis for Depression, Anxiety, and Psychosis and After Including Only Diagnosis Codes in the Outcome Definition
eTable 9. Repeating the Incident Matched Analysis for Individuals With Recent Clinical Contact, as Indicated by Recording of Routine Clinical Data
References
 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130-140. 10.1016/S2215-0366(20)30462-4 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130-140. 10.1016/S2215-0366(20)30462-4 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar] 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427. 10.1016/S2215-0366(21)00084-5 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427. 10.1016/S2215-0366(21)00084-5 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]Full text links
Read article at publisher's site: https://doi.org/10.1001/jamanetworkopen.2021.34803
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2786180/abel_2021_oi_210981_1646328482.89666.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/117010139
Article citations
COVID-19 and Mental Illnesses in Vaccinated and Unvaccinated People.
JAMA Psychiatry, 81(11):1071-1080, 01 Nov 2024
Cited by: 0 articles | PMID: 39167370 | PMCID: PMC11339697
Quality of sleep after COVID-19 infection: a cross-sectional study in the Southern Italy.
Front Psychiatry, 15:1428423, 24 Sep 2024
Cited by: 0 articles | PMID: 39386895 | PMCID: PMC11462549
Age-specific disparity in insomnia among COVID-19 patients in Fangcang shelter hospitals: a population-based study in Shanghai, China.
Front Neurol, 15:1420898, 26 Jul 2024
Cited by: 0 articles | PMID: 39131047 | PMCID: PMC11310121
Employment outcomes of people with Long Covid symptoms: community-based cohort study.
Eur J Public Health, 34(3):489-496, 01 Jun 2024
Cited by: 3 articles | PMID: 38423541 | PMCID: PMC11161149
Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea.
Mol Psychiatry, 29(11):3635-3643, 04 Jun 2024
Cited by: 2 articles | PMID: 38834668 | PMCID: PMC11541197
Go to all (29) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: A case-control study.
Cephalalgia, 41(13):1332-1341, 16 Jun 2021
Cited by: 33 articles | PMID: 34134526 | PMCID: PMC8212025
6-month consequences of COVID-19 in patients discharged from hospital: a cohort study.
Lancet, 397(10270):220-232, 08 Jan 2021
Cited by: 2428 articles | PMID: 33428867 | PMCID: PMC7833295
Mental burden and its risk and protective factors during the early phase of the SARS-CoV-2 pandemic: systematic review and meta-analyses.
Global Health, 17(1):34, 29 Mar 2021
Cited by: 122 articles | PMID: 33781283 | PMCID: PMC8006628
Review Free full text in Europe PMC
Are Sleep Problems Related to Psychological Distress in Healthy Aging during the COVID-19 Pandemic? A Review.
Int J Environ Res Public Health, 18(20):10676, 12 Oct 2021
Cited by: 8 articles | PMID: 34682423 | PMCID: PMC8536178
Review Free full text in Europe PMC
Funding
Funders who supported this work.
National Institute for Health Research (NIHR) (1)
Grant ID: NF-SI-0616-10114

 2
,
3
,
4
,
11
2
,
3
,
4
,
11



