Abstract
Background
The monoclonal-antibody combination AZD7442 is composed of tixagevimab and cilgavimab, two neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that have an extended half-life and have been shown to have prophylactic and therapeutic effects in animal models. Pharmacokinetic data in humans indicate that AZD7442 has an extended half-life of approximately 90 days.Methods
In an ongoing phase 3 trial, we enrolled adults (≥18 years of age) who had an increased risk of an inadequate response to vaccination against coronavirus disease 2019 (Covid-19), an increased risk of exposure to SARS-CoV-2, or both. Participants were randomly assigned in a 2:1 ratio to receive a single dose (two consecutive intramuscular injections, one containing tixagevimab and the other containing cilgavimab) of either 300 mg of AZD7442 or saline placebo, and they were followed for up to 183 days in the primary analysis. The primary safety end point was the incidence of adverse events after a single dose of AZD7442. The primary efficacy end point was symptomatic Covid-19 (SARS-CoV-2 infection confirmed by means of reverse-transcriptase-polymerase-chain-reaction assay) occurring after administration of AZD7442 or placebo and on or before day 183.Results
A total of 5197 participants underwent randomization and received one dose of AZD7442 or placebo (3460 in the AZD7442 group and 1737 in the placebo group). The primary analysis was conducted after 30% of the participants had become aware of their randomized assignment. In total, 1221 of 3461 participants (35.3%) in the AZD7442 group and 593 of 1736 participants (34.2%) in the placebo group reported having at least one adverse event, most of which were mild or moderate in severity. Symptomatic Covid-19 occurred in 8 of 3441 participants (0.2%) in the AZD7442 group and in 17 of 1731 participants (1.0%) in the placebo group (relative risk reduction, 76.7%; 95% confidence interval [CI], 46.0 to 90.0; P<0.001); extended follow-up at a median of 6 months showed a relative risk reduction of 82.8% (95% CI, 65.8 to 91.4). Five cases of severe or critical Covid-19 and two Covid-19-related deaths occurred, all in the placebo group.Conclusions
A single dose of AZD7442 had efficacy for the prevention of Covid-19, without evident safety concerns. (Funded by AstraZeneca and the U.S. government; PROVENT ClinicalTrials.gov number, NCT04625725.).Free full text

Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19
Associated Data
Abstract
Background
The monoclonal-antibody combination AZD7442 is composed of tixagevimab and cilgavimab, two neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that have an extended half-life and have been shown to have prophylactic and therapeutic effects in animal models. Pharmacokinetic data in humans indicate that AZD7442 has an extended half-life of approximately 90 days.
Methods
In an ongoing phase 3 trial, we enrolled adults (≥18 years of age) who had an increased risk of an inadequate response to vaccination against coronavirus disease 2019 (Covid-19), an increased risk of exposure to SARS-CoV-2, or both. Participants were randomly assigned in a 2:1 ratio to receive a single dose (two consecutive intramuscular injections, one containing tixagevimab and the other containing cilgavimab) of either 300 mg of AZD7442 or saline placebo, and they were followed for up to 183 days in the primary analysis. The primary safety end point was the incidence of adverse events after a single dose of AZD7442. The primary efficacy end point was symptomatic Covid-19 (SARS-CoV-2 infection confirmed by means of reverse-transcriptase–polymerase-chain-reaction assay) occurring after administration of AZD7442 or placebo and on or before day 183.
Results
A total of 5197 participants underwent randomization and received one dose of AZD7442 or placebo (3460 in the AZD7442 group and 1737 in the placebo group). The primary analysis was conducted after 30% of the participants had become aware of their randomized assignment. In total, 1221 of 3461 participants (35.3%) in the AZD7442 group and 593 of 1736 participants (34.2%) in the placebo group reported having at least one adverse event, most of which were mild or moderate in severity. Symptomatic Covid-19 occurred in 8 of 3441 participants (0.2%) in the AZD7442 group and in 17 of 1731 participants (1.0%) in the placebo group (relative risk reduction, 76.7%; 95% confidence interval [CI], 46.0 to 90.0; P<0.001); extended follow-up at a median of 6 months showed a relative risk reduction of 82.8% (95% CI, 65.8 to 91.4). Five cases of severe or critical Covid-19 and two Covid-19–related deaths occurred, all in the placebo group.
Conclusions
A single dose of AZD7442 had efficacy for the prevention of Covid-19, without evident safety concerns. (Funded by AstraZeneca and the U.S. government; PROVENT ClinicalTrials.gov number, NCT04625725.)
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has reduced the burden of coronavirus disease 2019 (Covid-19).1-4 However, some persons, including immunocompromised persons and those who cannot be vaccinated, remain at risk for severe Covid-19.5-13
Monoclonal antibodies, which protect against disease irrespective of immune system status and provide rapid protection,14,15 are potential options for Covid-19 immunoprophylaxis. Some combinations of monoclonal antibodies are already in use through emergency or temporary authorization for preexposure16 or postexposure17 prophylaxis against Covid-19 or treatment of mild-to-moderate disease.18,19
AZD7442 is a combination of two fully human, SARS-CoV-2–neutralizing monoclonal antibodies (tixagevimab and cilgavimab) that are derived from antibodies isolated from B cells obtained from persons infected with SARS-CoV-2. These antibodies contain the half-life–extending M252Y/S254T/T256E (YTE) modification20 and the L234F/L235E/P331S (TM) modification that decreases binding of the Fc receptor and complement component C1q.21,22 Tixagevimab and cilgavimab simultaneously bind to distinct, nonoverlapping epitopes of the SARS-CoV-2 spike-protein receptor-binding domain to potently neutralize the virus.22-25 AZD7442 has been shown to neutralize SARS-CoV-2 and its variants of concern in vitro and has prophylactic and therapeutic effects in nonhuman primates.22
In a phase 1 study, intramuscular administration of 300 mg of AZD7442 provided higher SARS-CoV-2 serum neutralizing titers than those associated with convalescent serum. SARS-CoV-2 serum neutralizing antibody titers remained three times as high as those associated with convalescent plasma after 9 months, and AZD7442 was also detected in the nasal mucosa.22 Here, we report results from the ongoing, phase 3 PROVENT trial, which evaluated AZD7442 for the prevention of symptomatic and severe Covid-19 in adults (≥18 years of age).
Methods
Trial Design and Oversight
In this ongoing, multicenter, double-blind, parallel-group, randomized, placebo-controlled trial, we assessed the safety and efficacy of a single dose of AZD7442 (two consecutive intramuscular injections; one each of tixagevimab and cilgavimab) for preexposure prophylaxis against Covid-19 in adults who had an increased risk of an inadequate response to Covid-19 vaccination, an increased risk of exposure to SARS-CoV-2, or both. Participants who were at increased risk for an inadequate response to Covid-19 vaccination were those who were classified as older (≥60 years of age), obese, immunocompromised, or unable to receive vaccines without adverse effects or as having congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, or chronic liver disease. Participants at increased risk for exposure to SARS-CoV-2 included, but were not limited to, health care workers (including staff working in long-term care facilities), workers in industrial settings such as meatpacking plants (who have been shown to be at high risk for SARS-CoV-2 transmission), military personnel, students living in dormitories, and others living together in close or high-density proximity. The trial is being conducted at 87 sites in Belgium, France, Spain, the United Kingdom, and the United States.
The primary analysis was planned after approximately 24 primary end-point events had been confirmed or 30% of the trial participants had become aware of their randomized assignment. The data cutoff for the primary analysis occurred on May 5, 2021. An additional extended follow-up data cutoff for the primary end point, key supportive analyses, and key secondary end points occurred on August 29, 2021. The estimated trial completion date is June 29, 2022.
The trial consisted of a screening period of up to 7 days, a 366-day safety and efficacy assessment period, and an optional additional safety assessment 91 days after the end of the 366-day safety and efficacy assessment period. Participants were randomly assigned in a 2:1 ratio to receive a single 300-mg dose of AZD7442 (one 1.5-ml intramuscular injection of each antibody administered consecutively) or saline placebo (two 1.5-ml intramuscular injections administered consecutively) on day 1. The participants were monitored for adverse events for 1 to 4 hours after the injections, and they were contacted weekly to monitor for Covid-19 symptoms. Full details of the trial conduct are provided in the protocol (which includes the statistical analysis plan), available with the full text of this article at NEJM.org.
The trial was conducted in accordance with the ethical principles derived from international guidelines, including the Declaration of Helsinki (7th revision, 2013), the international ethical guidelines of the Council for International Organizations of Medical Sciences, applicable Good Clinical Practice guidelines of the International Council for Harmonisation, and all applicable laws and regulations. The trial protocol and all other relevant documentation were reviewed and approved by a local or central institutional review board or ethics committee for each site. All the participants provided written informed consent (with assistance from a legally authorized representative if required) before enrollment. An independent, external adjudication committee, whose members were unaware of the randomized assignments, provided a systematic assessment of whether any deaths that occurred during the trial were associated with Covid-19. Details regarding the committee are provided in the Supplementary Appendix, available at NEJM.org.
Representatives of AstraZeneca designed the trial. Data were collected by the trial site investigators in collaboration with a contract research organization (IQVIA) and AstraZeneca and were analyzed by another contract research organization (ClinChoice) and AstraZeneca. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. All the authors contributed to the writing and editing of the manuscript and reviewed and approved the manuscript for submission, with legal approval from AstraZeneca. Agreements requiring authors to maintain data confidentiality were in place between AstraZeneca and the authors. AstraZeneca paid for medical writing and editorial support with an earlier version of the manuscript, which was developed in accordance with Good Publication Practice guidelines. Additional details regarding the trial design and methods are provided in the Supplementary Appendix.
Participants
Eligible participants were adults (≥18 years of age) who had an increased risk of an inadequate response to Covid-19 vaccination or exposure to SARS-CoV-2 owing to location or circumstance. All the participants were required to have a negative point-of-care SARS-CoV-2 serologic test result at screening. Participants were excluded if they had a history of SARS-CoV-2 infection, a positive SARS-CoV-2 result at screening, previous receipt of a vaccine or biologic agent indicated for the prevention of SARS-CoV-2 infection or Covid-19, or an allergy to any component of AZD7442 or the placebo.
End Points
The primary safety end point was the incidence of adverse events after intramuscular administration of a single dose of AZD7442, as compared with placebo. Adverse events, serious adverse events, medically attended adverse events, and adverse events of special interest were assessed. The primary efficacy end point was the first episode of symptomatic Covid-19, confirmed by positive results on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing, with an onset after the administration of AZD7442 or placebo and on or before day 183. Participants were considered to have had a primary end-point event if they presented with qualifying symptoms that were prespecified in the protocol (Table S1) and had a positive RT-PCR result between 5 days before and up to 10 days after the onset of symptoms. Data for participants who had become aware of their randomized assignment for any reason and data for participants who had received a Covid-19 vaccine were censored at the date of unblinding or vaccine administration, whichever was earlier.
Statistical Analysis
The safety analysis set consisted of all the participants who had undergone randomization and received at least one injection of AZD7442 or placebo. The primary and secondary efficacy analyses involved the full preexposure analysis set, which consisted of all the participants who had undergone randomization, received at least one injection of AZD7442 or placebo, and did not have RT-PCR–confirmed SARS-CoV-2 infection at baseline. The full analysis set consisted of all the participants who had undergone randomization and received at least one injection. For the primary efficacy end-point analysis, we calculated that a trial population of approximately 5150 participants who were randomly assigned in a 2:1 ratio, with a minimum of 18 observed events, would provide the trial with approximately 90% power to detect AZD7442 efficacy (a lower boundary of >0 for the two-sided 95% confidence interval). This calculation assumed 80% true efficacy and an annualized attack rate of 3% in the placebo group.
A Poisson regression with robust variance was used as the primary efficacy analysis model to estimate the relative risk of the incidence of symptomatic infection in the AZD7442 group as compared with the placebo group.26 The model included the trial group (AZD7442 or placebo) and age at informed consent (≥60 years or <60 years) as covariates, with the log of follow-up time used as an offset. AZD7442 efficacy was calculated as the relative risk reduction in the incidence of infection in the AZD7442 group as compared with that in the placebo group, or 100%×(1–the relative risk), with the result expressed as a percentage.
Missing events were not imputed for either trial group. A hierarchical approach was used to control for multiplicity of the primary, key supportive, and key secondary analyses on the basis of a two-sided alpha level of 0.05. A P value of less than 0.05 was considered to indicate statistical significance. No statistical testing was performed for the safety end points.
Results
Participants
Between November 21, 2020, and March 22, 2021, a total of 5973 participants underwent screening; in the full analysis set, 3460 were randomly assigned to receive AZD7442 and 1737 were randomly assigned to receive placebo. The last participant received an injection on March 29, 2021. In both groups, the median follow-up time from receipt of AZD7442 or placebo to the primary analysis was 83 days, and the median 6-month follow-up was 196 days. The primary analysis was conducted after 30% of the participants had elected to become aware of their randomized assignment (e.g., in order to consider Covid-19 vaccination).
The primary safety analysis included 3461 participants who had received AZD7442 and 1736 participants who had received placebo; 1 participant was assigned to receive placebo but incorrectly received AZD7442. The primary efficacy analysis included 3441 participants who had received AZD7442 and 1731 participants who had received placebo.
All the participants underwent SARS-CoV-2 RT-PCR testing at baseline; 25 of 5197 participants (0.5%; 19 in the AZD7442 group and 6 in the placebo group) tested positive after receiving AZD7442 or placebo and were excluded from the primary efficacy analysis. In the AZD7442 group, 1413 participants had become aware of their randomized assignment; 1406 participants (99.5%) had elected to become aware of their randomized assignment because they wanted to consider receiving a Covid-19 vaccine. In the AZD7442 group, 1161 received a Covid-19 vaccine (Fig. S1). In the placebo group, 749 participants had become aware of their randomized assignment; 742 participants (99.1%) had elected to become aware of their randomized assignment because they wanted to consider receiving a Covid-19 vaccine. In the placebo group, 853 received a Covid-19 vaccine. The percentages of participants with data that were censored because of loss to follow-up or discontinuation, unblinding, or vaccination were balanced between the AZD7442 and placebo groups (Tables S2 and S3).
The demographic and clinical characteristics at baseline were similar in the two groups (Table 1) and were consistent with those of the broader population of persons with SARS-CoV-2 infection (Table S4). The mean age was 53.5 years, 43.4% of the participants were 60 years of age or older, 46.1% were female, 14.5% identified as Hispanic or Latinx, 73.0% were White, and 17.3% were Black. At baseline, a large proportion of the participants were considered by the investigators to have an increased risk of an inadequate response to Covid-19 vaccination (73.3%) or exposure to SARS-CoV-2 (52.5%), and 77.5% had coexisting conditions that placed them at high risk for progression to severe Covid-19 disease.
Table 1
| Characteristic | AZD7442 (N=3460) | Placebo (N=1737) | Total (N=5197) |
|---|---|---|---|
| Age — yr | 53.6±15.0 | 53.3±14.9 | 53.5±15.0 |
| Age group — no. (%) | |||
| ≥60 yr | 1500 (43.4) | 757 (43.6) | 2257 (43.4) |
| ≥65 yr | 817 (23.6) | 409 (23.5) | 1226 (23.6) |
| ≥75 yr | 148 (4.3) | 70 (4.0) | 218 (4.2) |
| Female sex — no. (%) | 1595 (46.1) | 802 (46.2) | 2397 (46.1) |
| Race or ethnic group — no. (%)† | |||
| White race | 2545 (73.6) | 1249 (71.9) | 3794 (73.0) |
| Black race | 597 (17.3) | 302 (17.4) | 899 (17.3) |
| Asian race | 110 (3.2) | 60 (3.5) | 170 (3.3) |
| American Indian or Alaska Native ethnic group | 19 (0.5) | 10 (0.6) | 29 (0.6) |
| Native Hawaiian or other Pacific Islander ethnic group | 4 (0.1) | 4 (0.2) | 8 (0.2) |
| Other‡ | 185 (5.3) | 112 (6.4) | 297 (5.7) |
| Hispanic or Latinx ethnic group | |||
| No | 2731 (78.9) | 1412 (81.3) | 4143 (79.7) |
| Yes | 539 (15.6) | 215 (12.4) | 754 (14.5) |
| Unknown or not reported | 190 (5.5) | 110 (6.3) | 300 (5.8) |
| BMI§ | 29.6±6.9 | 29.6±7.0 | 29.6±6.9 |
| Resident in long-term care facility — no. (%) | 14 (0.4) | 12 (0.7) | 26 (0.5) |
| SARS-CoV-2 RT-PCR status — no. (%)¶ | |||
| Negative | 3334 (96.4) | 1672 (96.3) | 5006 (96.3) |
| Positive | 19 (0.5) | 6 (0.3) | 25 (0.5) |
| Missing data | 107 (3.1) | 59 (3.4) | 166 (3.2) |
| Increased risk of inadequate response to Covid-19 vaccination — no. (%)‖ | 2546 (73.6) | 1264 (72.8) | 3810 (73.3) |
| Increased risk of exposure to SARS-CoV-2 — no. (%)** | 1820 (52.6) | 909 (52.3) | 2729 (52.5) |
| High-risk factors for severe Covid-19 — no. (%) | |||
| Any high-risk factor | 2666 (77.1) | 1362 (78.4) | 4028 (77.5) |
| Obesity: BMI ≥30 | 1456 (42.1) | 712 (41.0) | 2168 (41.7) |
| Hypertension | 1229 (35.5) | 637 (36.7) | 1866 (35.9) |
| Smoking | 720 (20.8) | 370 (21.3) | 1090 (21.0) |
| Diabetes | 492 (14.2) | 242 (13.9) | 734 (14.1) |
| Asthma | 378 (10.9) | 198 (11.4) | 576 (11.1) |
| Cardiovascular disease | 272 (7.9) | 151 (8.7) | 423 (8.1) |
| Cancer | 250 (7.2) | 133 (7.7) | 383 (7.4) |
| COPD | 179 (5.2) | 95 (5.5) | 274 (5.3) |
| Chronic kidney disease | 184 (5.3) | 86 (5.0) | 270 (5.2) |
| Chronic liver disease | 149 (4.3) | 91 (5.2) | 240 (4.6) |
| Receipt of immunosuppressive therapy | 109 (3.2) | 63 (3.6) | 172 (3.3) |
| Immunosuppressive disease | 15 (0.4) | 9 (0.5) | 24 (0.5) |
| Sickle cell disease | 1 (<0.1) | 1 (0.1) | 2 (<0.1) |
Safety
At the data-cutoff date for the primary analysis, at least one adverse event was reported in 1221 of 3461 participants (35.3%) in the AZD7442 group and 593 of 1736 participants (34.2%) in the placebo group (Table 2). Most adverse events were mild or moderate in intensity. The most common adverse event of special interest was an injection-site reaction, which occurred in 2.4% of the participants in the AZD7442 group and in 2.1% of those in the placebo group. The incidence of serious adverse events was similar in the two groups (Table S5).
Table 2
| Adverse Event | AZD7442 (N=3461)† | Placebo (N=1736)† | Total (N=5197) |
|---|---|---|---|
| number of participants (percent) | |||
| Adverse events | |||
| Any adverse event | 1221 (35.3) | 593 (34.2) | 1814 (34.9) |
| Mild | 761 (22.0) | 369 (21.3) | 1130 (21.7) |
| Moderate | 387 (11.2) | 191 (11.0) | 578 (11.1) |
| Severe | 64 (1.8) | 27 (1.6) | 91 (1.8) |
| Serious adverse events | |||
| Any serious adverse event | 50 (1.4) | 23 (1.3) | 73 (1.4) |
| Related to AZD7442 or placebo‡ | 1 (<0.1)§ | 0 | 1 (<0.1) |
| Adverse events leading to trial discontinuation | 1 (<0.1)¶ | 0 | 1 (<0.1) |
| Medically attended adverse events | 360 (10.4) | 157 (9.0) | 517 (9.9) |
| Adverse events of special interest | |||
| Any adverse event of special interest | 93 (2.7) | 37 (2.1) | 130 (2.5) |
| Injection-site reaction | 82 (2.4) | 36 (2.1) | 118 (2.3) |
| Anaphylaxis‖ | 1 (<0.1) | 0 | 1 (<0.1) |
| Immune complex disease** | 1 (<0.1) | 0 | 1 (<0.1) |
| Other | 9 (0.3) | 2 (0.1) | 11 (0.2) |
| Related to AZD7442 or placebo‡ | 87 (2.5) | 36 (2.1) | 123 (2.4) |
| Adverse events leading to outcome of death†† | |||
| All adverse events | 4 (0.1) | 4 (0.2) | 8 (0.2) |
| Illicit-drug overdose | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Myocardial infarction | 1 (<0.1) | 0 | 1 (<0.1) |
| Renal failure | 1 (<0.1) | 0 | 1 (<0.1) |
| Covid-19‡‡ | 0 | 1 (0.1) | 1 (<0.1) |
| Covid-19–related ARDS‡‡ | 0 | 1 (0.1) | 1 (<0.1) |
Eight deaths occurred; two deaths (both in the placebo group) were assessed by the adjudication committee and were confirmed by testing as being related to Covid-19 (Table 2). The other deaths were the result of illicit-drug overdose (in 4 participants [0.1%], 2 each in the AZD7442 and placebo groups), myocardial infarction (in 1 participant [<0.1%] in the AZD7442 group), and renal failure (in 1 participant [<0.1%] in the AZD7442 group). All the deaths were assessed by the site investigators as being unrelated to AZD7442 or placebo.
The safety analysis at the median 6-month data-cutoff date revealed no additional adverse events of special interest (Table S6) or unexpected longer-term safety signals (Table S7). Nine deaths occurred in the AZD7442 group, and seven deaths occurred in the placebo group; none were considered by the investigator to be related to AZD7442 or placebo. No additional Covid-19–related deaths occurred.
Efficacy
At the data-cutoff date for the primary analysis, symptomatic SARS-CoV-2 RT-PCR–positive illness had occurred in 8 of 3441 participants (0.2%) in the AZD7442 group and in 17 of 1731 participants (1.0%) in the placebo group. The primary efficacy analysis met the statistical criterion for trial success; that is, it showed a significantly lower incidence of symptomatic SARS-CoV-2 RT-PCR–positive illness in the AZD7442 group than in the placebo group (relative risk reduction, 76.7%; 95% confidence interval [CI], 46.0 to 90.0; P<0.001) (Table 3). The unadjusted relative risk reduction was the same as the relative risk reduction after adjustment for age. The between-group differences in the key supportive analyses and the key secondary end point (Table S8) were statistically significant within the testing strategy.
Table 3
| First Case of SARS-CoV-2 RT-PCR–Positive Symptomatic Illness | Primary Analysis | Median 6-Mo Follow-up† | |||||
|---|---|---|---|---|---|---|---|
| AZD7442 (N=3441) | Placebo (N=1731) | Relative Risk Reduction % (95% CI) | P Value | AZD7442 (N=3441) | Placebo (N=1731) | Relative Risk Reduction % (95% CI) | |
| no. of participants (%) | no. of participants (%) | ||||||
| Primary end point: first case of illness, with data censored at unblinding or receipt of Covid-19 vaccine | 8 (0.2) | 17 (1.0) | 76.7 (46.0–90.0) | <0.001 | 11 (0.3) | 31 (1.8) | 82.8 (65.8–91.4) |
| Key supportive analyses | |||||||
| First case of illness, regardless of unblinding or receipt of Covid-19 vaccine | 10 (0.3) | 22 (1.3) | 77.3 (52.0–89.3) | <0.001 | 20 (0.6) | 44 (2.5) | 77.4 (61.7–86.7) |
| First case of illness, including all deaths, with data censored at unblinding or receipt of Covid-19 vaccine | 12 (0.3) | 19 (1.1) | 68.8 (35.6–84.9) | 0.002 | 18 (0.5) | 36 (2.1) | 75.8 (57.3–86.2) |
At the data-cutoff date for the primary analysis, SARS-CoV-2 RT-PCR–positive severe or critical illness (defined in Table S9) had occurred in none of the 3441 participants in the AZD7442 group and in 1 of the 1731 participants (0.1%) in the placebo group. In the median 6-month follow-up data analyses, an additional four cases of severe or critical Covid-19 were reported, for a total of five cases, all of which occurred in the placebo group. Six participants (0.2%) in the AZD7442 group and none of the participants in the placebo group had emergency department visits for symptoms that were consistent with Covid-19; the 6 participants were not hospitalized, and 3 of them subsequently tested positive for Covid-19. Results of the post hoc analysis of Covid-19–related hospitalizations are provided in the Supplementary Results section in the Supplementary Appendix.
As compared with the primary analysis of the primary end point, the median 6-month follow-up data analyses showed an even lower incidence of symptomatic Covid-19 (Table 3) in the AZD7442 group than in the placebo group, with a relative risk reduction of 82.8% (95% CI, 65.8 to 91.4). This increase in the efficacy estimate since the time of the primary analysis was driven by a greater percentage of events in the placebo group (1.2%, in 12 of 960 participants) than in the AZD7442 group (0.1%, in 3 of 2003 participants) during months 3 through 6, as compared with months 0 through 3 (Table S10).
The efficacy of AZD7442 was consistent across subgroups of participants with data that could be evaluated; all point estimates of the relative risk reduction in the incidence of symptomatic illness with AZD7442 as compared with placebo were greater than 44% (Figure 1). Among participants who were at increased risk for either an inadequate response to Covid-19 vaccination or exposure to SARS-CoV-2, the relative risk reductions (80.7% and 82.6%, respectively) were similar to the relative risk reduction in the overall population in the primary efficacy analysis (76.7%). The time until symptomatic illness was longer with AZD7442 than with placebo (hazard ratio, 0.17; 95% CI, 0.08 to 0.33) (Figure 2).
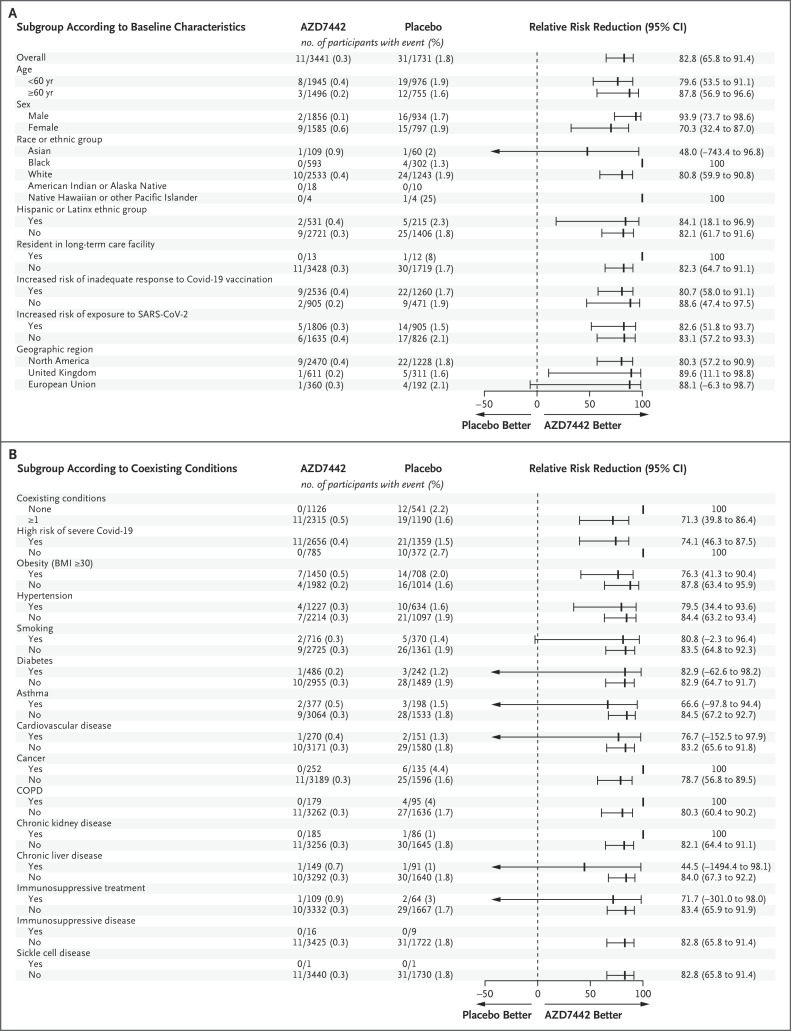
Estimates are based on Poisson regression with robust variance with the use of a full model or reduced model. An estimated relative risk reduction greater than 0 favored AZD7442. Panel A shows the relative risk reduction according to the baseline demographic and clinical characteristics of the participants. The relative risk reduction with AZD7442 could not be estimated for participants of American Indian or Alaskan Native heritage or those with immunosuppressive disease or sickle cell disease, because there were no instances of SARS-CoV-2 RT-PCR–positive symptomatic illness in participants in those subgroups. Panel B shows the relative risk reduction according to the participants’ coexisting conditions. The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. Immunosuppressive treatment is medication that suppresses the immune response, and immunosuppressive disease is a medical condition that could suppress the immune response, regardless of treatment. CI denotes confidence interval, COPD chronic obstructive pulmonary disease, Covid-19 coronavirus disease 2019, NE not estimable, RT-PCR reverse-transcriptase–polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.

Shown are Kaplan–Meier curves from a time-to-event analysis of the proportion of participants in the full preexposure analysis set who had a first SARS-CoV-2 RT-PCR–positive symptomatic illness, with a median follow-up of 6 months. The hazard ratio and corresponding 95% confidence interval were obtained from a Cox proportional-hazards model with the group as a covariate and with the stratification factor of age at informed consent (≥60 years or <60 years). Tick marks indicate censored data. The inset shows the same data on an enlarged y axis.
Pharmacokinetics
Serum levels of AZD7442 remained elevated for 6 months after administration (Fig. S2A). The geometric mean (±SD) serum level of AZD7442 was 18.9±2.1 μg per milliliter at day 8 and 24.0±1.8 μg per milliliter at day 29, which translated to a SARS-CoV-2 geometric mean neutralizing antibody titer of 493.1 (95% CI, 469.3 to 518.1) at day 8 and 677.3 (95% CI, 647.1 to 709.0) at day 29 in an 80% plaque-reduction neutralization test that used wild-type virus (Fig. S2B). These titers were 16 times and 22 times as high, respectively, as those from samples of convalescent plasma obtained from patients with Covid-19.22
SARS-CoV-2 Variants
Viral genotypic data collected at illness visits were available for 7 of 11 symptomatic participants in the AZD7442 group and 13 of 31 symptomatic participants in the placebo group. Eleven of these participants were infected with a SARS-CoV-2 variant of concern,27 including 1 participant with B.1.351 (beta) in the AZD7442 group and 10 participants in the placebo group (5 participants with B.1.1.7_1 [an alpha subvariant] and 5 participants with B.1.617.2 [delta]) (Table S11).
Discussion
The data reported here provide support for the use of AZD7442 as immunoprophylaxis to prevent Covid-19. The primary efficacy analysis and the key supportive analyses met the statistical criterion for trial success. The incidences of symptomatic RT-PCR–positive SARS-CoV-2 infection and severe disease among infected participants were lower in the AZD7442 group than in the placebo group, and there were no evident safety concerns. Pharmacokinetic data showed AZD7442 persistence in serum for 6 months after administration, which resulted in SARS-CoV-2–neutralizing antibody titers that remained higher at days 8 and 29 than those reported in convalescent serum.22
Although Covid-19 vaccines are highly effective, a need remains to protect persons who have an insufficient response to Covid-19 vaccination. Other monoclonal-antibody combinations that do not have extended half-lives can effectively prevent Covid-19,28,29 although those with half-lives of 18 to 32 days18,29 are administered monthly,16 and some are administered intravenously.28 Pharmacokinetic modeling data suggest that a single dose of AZD7442 could provide protection against Covid-19 for at least 6 months22; these data are supported by the pharmacokinetic data presented here. Data from a phase 1 study have shown that AZD7442 has an extended half-life of approximately 90 days, with levels detectable in serum for 9 months.22
The complementary binding of tixagevimab and cilgavimab to distinct regions of the viral spike protein receptor-binding domain presents a barrier to virus escape. In vitro studies have shown that AZD7442 and its parental antibodies (CoV-2130 plus CoV-2196) retain some neutralizing activity against the BA.1 subvariant of the B.1.1.259 (omicron) variant, with neutralizing activity reduced by a factor of 12 to 30 in live-virus assays30-32 and by a factor of 132 to 183 in pseudovirus30,33 assays. The potency of AZD7442 (half-maximal inhibitory concentration geometric mean titer of 51 to 277 ng per milliliter33,34) is higher than that of convalescent serum; therefore, AZD7442 is likely to be clinically active against the BA.1 subvariant. Emerging evidence suggests that the neutralizing activity of AZD7442 against the BA.2 subvariant is only minimally lower than that against the wild-type virus (lower by a factor of five in live-virus assays and by a factor of three in pseudovirus assays).30,35 Additional clinical studies are warranted to evaluate this issue further.
The current trial was designed early in the SARS-CoV-2 pandemic, when the effectiveness of Covid-19 vaccines in populations known to have an insufficient response to vaccines (e.g., adults ≥60 years of age) was uncertain. Although Covid-19 vaccination has since been shown to be effective in such populations, some immunocompromised persons still have a poor response to vaccination.36 As such, AZD7442 was authorized by the Food and Drug Administration (FDA) for the prevention of Covid-19 in persons with moderate-to-severe immune compromise or those in whom Covid-19 vaccination is not recommended.30 In February 2022, the FDA recommended an increase in the dose of AZD7442 to 600 mg because of the emergence of the BA.1 subvariant; recommendations for repeat dosing intervals have yet to be confirmed, pending further data. Other countries have continued to use the 300-mg dose that was studied in the current trial.37,38 Additional studies, including real-world evidence studies, are under way to assess the effectiveness of AZD7442 in immunocompromised populations, given the limited subgroup analyses afforded by this trial.
The limitations of our trial include the low number of events in smaller but important subgroups, including immunocompromised persons, so that efficacy in these groups could not be estimated. The allowance of participants to become aware of their randomized assignment in order to consider Covid-19 vaccination decreased the number of participants who were available for longer-term, double-blind follow-up. Changes in the clinical landscape that were driven by the availability of vaccines resulted in a higher-than-anticipated proportion of participants who elected to become aware of their randomized assignment in order to consider Covid-19 vaccination. Guidance from the data and safety monitoring board and regulatory feedback from the FDA regarding the need for participants to become aware of their randomized assignment so that they could consider vaccination increased the incidence of unblinding in the latter part of the trial. Also, the introduction of vaccines in participating countries may have affected the incidence of SARS-CoV-2 infection during the trial.
The strengths of our trial include the diverse trial population that encompassed a high proportion of adults older than 60 years of age and persons with coexisting conditions who had an increased risk of severe Covid-19, although there were too few events to statistically assess the benefit of AZD7442 in preventing severe disease in these groups. Populations that are disproportionately affected by Covid-19 (e.g., Black and Hispanic or Latinx populations39,40) were well represented.
Clinical and pharmacokinetic assessments from this trial are expected to continue for at least 12 months, with studies under way to evaluate the effectiveness of AZD7442 in immunocompromised persons who receive this agent as immunoprophylaxis under emergency use authorization. The results of this trial support the use of a single dose of AZD7442 (two consecutive intramuscular injections) for the prevention of symptomatic and severe Covid-19.
Acknowledgments
We thank the trial participants, their families, and all investigators involved in this trial; the members of the adjudication committee, who assessed deaths that occurred during the trial: Mark A. Tidswell, M.D., of Tufts University School of Medicine, Toby M. Maher, M.D., of the University of Southern California, and Ashley Whittington, M.D., of the London North West University Healthcare NHS Trust; Yousef Fawadleh, B.Sc., and Elaine Harrop, M.Sc., of AstraZeneca, for leading the operational management of the trial; Yee-Man Ching, Ph.D., of AstraZeneca, for facilitating author discussions, coordinating manuscript development, and critically reviewing the manuscript; Lorna Forse, Ph.D., for medical writing support and preparing the first draft of the manuscript, and Joe Alling, B.Sc., Linda Brown, B.Sc., Cellan Ellis, B.Sc., Matthew Stone, M.Res., and India Wright, M.Sc., all of Core Medica, Knutsford, United Kingdom, for editorial support with an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
Notes
This article was published on April 20, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by AstraZeneca and the U.S. government. AZD7442 is being developed under a contract (W911QY-21-9-0001) with the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, in partnership with the Department of Defense, and with the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmoa2116620
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9069994
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/126995594
Article citations
Antiviral effect of Evusheld in COVID-19 hospitalized patients infected with pre-Omicron or Omicron variants: a modelling analysis of the randomized DisCoVeRy trial.
J Antimicrob Chemother, 79(11):2887-2895, 01 Nov 2024
Cited by: 0 articles | PMID: 39236218 | PMCID: PMC11531825
Ibrutinib as a Secondary Treatment for Steroid-Refractory or Steroid-Dependent Chronic Graft-Versus-Host Disease: A Case Series of 11 Patients During the COVID-19 Era.
Cureus, 16(10):e71474, 14 Oct 2024
Cited by: 0 articles | PMID: 39539855 | PMCID: PMC11560293
Real-world clinical outcomes of tixagevimab/cilgavimab in the Omicron outbreak in China: baseline characteristics and interim analysis of the CLEAR study.
Virol J, 21(1):262, 24 Oct 2024
Cited by: 0 articles | PMID: 39448986 | PMCID: PMC11515397
Advancements in the Development of Anti-SARS-CoV-2 Therapeutics.
Int J Mol Sci, 25(19):10820, 09 Oct 2024
Cited by: 0 articles | PMID: 39409149 | PMCID: PMC11477007
Review Free full text in Europe PMC
Tixagevimab-cilgavimab for preventing breakthrough COVID-19 in dialysis patients: a prospective study.
Clin Kidney J, 17(11):sfae309, 18 Oct 2024
Cited by: 0 articles | PMID: 39539359 | PMCID: PMC11558061
Go to all (350) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04625725
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Efficacy, Safety, and Pharmacokinetics of AZD7442 (Tixagevimab/Cilgavimab) for Prevention of Symptomatic COVID-19: 15-Month Final Analysis of the PROVENT and STORM CHASER Trials.
Infect Dis Ther, 13(6):1253-1268, 04 May 2024
Cited by: 0 articles | PMID: 38703336 | PMCID: PMC11128422
Safety, Tolerability and Pharmacokinetics of Half-Life Extended Severe Acute Respiratory Syndrome Coronavirus 2 Neutralizing Monoclonal Antibodies AZD7442 (Tixagevimab-Cilgavimab) in Healthy Adults.
J Infect Dis, 227(10):1153-1163, 01 May 2023
Cited by: 5 articles | PMID: 36683419 | PMCID: PMC10175065
Safety, Efficacy and Pharmacokinetics of AZD7442 (Tixagevimab/Cilgavimab) for Treatment of Mild-to-Moderate COVID-19: 15-Month Final Analysis of the TACKLE Trial.
Infect Dis Ther, 13(3):521-533, 25 Feb 2024
Cited by: 0 articles | PMID: 38403865 | PMCID: PMC10965850
A Critical Analysis of the Use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld™) for COVID-19 Prophylaxis and Treatment.
Viruses, 14(9):1999, 09 Sep 2022
Cited by: 27 articles | PMID: 36146805 | PMCID: PMC9505619
Review Free full text in Europe PMC
Funding
Funders who supported this work.
AstraZeneca
U.S. Department of Defense (1)
Grant ID: W911QY-21-9-0001
U.S. Department of Health and Human Services (1)
Grant ID: W911QY-21-9-0001

 , the PROVENT Study Group*
, the PROVENT Study Group*


