Abstract
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) provides natural immunity against reinfection. Recent studies have shown waning of the immunity provided by the BNT162b2 vaccine. The time course of natural and hybrid immunity is unknown.Methods
Using the Israeli Ministry of Health database, we extracted data for August and September 2021, when the B.1.617.2 (delta) variant was predominant, on all persons who had been previously infected with SARS-CoV-2 or who had received coronavirus 2019 vaccine. We used Poisson regression with adjustment for confounding factors to compare the rates of infection as a function of time since the last immunity-conferring event.Results
The number of cases of SARS-CoV-2 infection per 100,000 person-days at risk (adjusted rate) increased with the time that had elapsed since vaccination with BNT162b2 or since previous infection. Among unvaccinated persons who had recovered from infection, this rate increased from 10.5 among those who had been infected 4 to less than 6 months previously to 30.2 among those who had been infected 1 year or more previously. Among persons who had received a single dose of vaccine after previous infection, the adjusted rate was low (3.7) among those who had been vaccinated less than 2 months previously but increased to 11.6 among those who had been vaccinated at least 6 months previously. Among previously uninfected persons who had received two doses of vaccine, the adjusted rate increased from 21.1 among those who had been vaccinated less than 2 months previously to 88.9 among those who had been vaccinated at least 6 months previously.Conclusions
Among persons who had been previously infected with SARS-CoV-2 (regardless of whether they had received any dose of vaccine or whether they had received one dose before or after infection), protection against reinfection decreased as the time increased since the last immunity-conferring event; however, this protection was higher than that conferred after the same time had elapsed since receipt of a second dose of vaccine among previously uninfected persons. A single dose of vaccine after infection reinforced protection against reinfection.Free full text

Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2
Associated Data
Abstract
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) provides natural immunity against reinfection. Recent studies have shown waning of the immunity provided by the BNT162b2 vaccine. The time course of natural and hybrid immunity is unknown.
Methods
Using the Israeli Ministry of Health database, we extracted data for August and September 2021, when the B.1.617.2 (delta) variant was predominant, on all persons who had been previously infected with SARS-CoV-2 or who had received coronavirus 2019 vaccine. We used Poisson regression with adjustment for confounding factors to compare the rates of infection as a function of time since the last immunity-conferring event.
Results
The number of cases of SARS-CoV-2 infection per 100,000 person-days at risk (adjusted rate) increased with the time that had elapsed since vaccination with BNT162b2 or since previous infection. Among unvaccinated persons who had recovered from infection, this rate increased from 10.5 among those who had been infected 4 to less than 6 months previously to 30.2 among those who had been infected 1 year or more previously. Among persons who had received a single dose of vaccine after previous infection, the adjusted rate was low (3.7) among those who had been vaccinated less than 2 months previously but increased to 11.6 among those who had been vaccinated at least 6 months previously. Among previously uninfected persons who had received two doses of vaccine, the adjusted rate increased from 21.1 among those who had been vaccinated less than 2 months previously to 88.9 among those who had been vaccinated at least 6 months previously.
Conclusions
Among persons who had been previously infected with SARS-CoV-2 (regardless of whether they had received any dose of vaccine or whether they had received one dose before or after infection), protection against reinfection decreased as the time increased since the last immunity-conferring event; however, this protection was higher than that conferred after the same time had elapsed since receipt of a second dose of vaccine among previously uninfected persons. A single dose of vaccine after infection reinforced protection against reinfection.
Although a decline in protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after two doses of BNT162b2 vaccine (Pfizer–BioNTech) has been observed in several studies,1-3 the level of protection remains unclear, as does the presence or extent of waning of natural immunity. Several studies have shown that 6 or more months after infection, persons still have substantial natural immunity against SARS-CoV-2.4-8 However, one recent study showed that messenger RNA (mRNA)–based vaccines confer a level of protection against hospitalization that is five times as high as that provided by previous infection.9
Waning of the humoral response of the immune system is well documented in vaccinated persons and in those who have been infected with SARS-CoV-2.10,11 In addition, studies of seasonal coronaviruses have shown waning of natural immunity and the possibility of reinfection.12,13 It is also unclear how natural immunity interacts with immunity conferred by vaccination. Some laboratory studies have indicated that “hybrid immunity” (i.e., immunity conferred by the combination of previous infection and vaccination) offers greater broad-spectrum protection,14 elicits higher levels of neutralizing antibodies,15 and provides greater protection against infection16 than immunity conferred by vaccination or infection alone. The durability of immunity resulting from SARS-CoV-2 infection and how this immunity compares with that conferred by vaccination are essential questions both at the level of an individual person and at the national level.
In this study, we estimated the incidence of confirmed SARS-CoV-2 infection in the following cohorts: previously infected, unvaccinated persons; previously infected persons who had also received the BNT162b2 vaccine; and vaccinated persons who had not been previously infected. For each cohort, we quantified the association between the time that had passed since infection or vaccination and the rate of confirmed infection. By comparing the rates of infection among these groups, we were able to assess the level of protection afforded by hybrid immunity as compared with that afforded by natural immunity or immunity conferred by vaccination.
Methods
Study Population
Our analysis, which was based on data from the national database of the Israeli Ministry of Health, focused on infections that were confirmed during the study period, from August 1 to September 30, 2021. During this period, Israel was in the midst of a fourth pandemic wave that was dominated by the B.1.617.2 (delta) variant.17 Israel had already conducted a campaign offering two doses of the BNT162b2 vaccine and had initiated a campaign offering third and fourth booster doses (see the Supplementary Methods 1 section in the Supplementary Appendix, available with the full text of this article at NEJM.org). In addition, beginning in March 2021, unvaccinated persons who had recovered from coronavirus disease 2019 (Covid-19) at least 3 months previously were eligible to receive a single dose of BNT162b2 vaccine.
In this study, reinfection with SARS-CoV-2 was defined as a positive polymerase-chain-reaction (PCR) test in a person who had had a positive test of a sample obtained at least 90 days before the study day.18 The definition of severe Covid-19 was consistent with that of the National Institutes of Health19 — that is, a resting respiratory rate of more than 30 breaths per minute, an oxygen saturation of less than 94% while the person was breathing ambient air, or a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of less than 300. The Israeli Ministry of Health database includes, for all residents who have received a Covid-19 vaccine, been tested for Covid-19, or been previously infected with SARS-CoV-2, basic demographic information such as sex, age, place of residence, and population sector, as well as full records of vaccinations and confirmed infections.
Using these data at the individual resident level, we studied confirmed infections among persons 16 years of age or older who had tested positive for SARS-CoV-2 infection before July 1, 2021, or who had received at least two doses of BNT162b2 vaccine at least 7 days before the end of the study period. We excluded from the analysis the following persons: those whose data did not include information on age or sex; those who had tested positive for SARS-CoV-2 between July 1 and July 31, 2021; those who had recovered from a PCR-confirmed SARS-CoV-2 infection and then received more than one dose of BNT162b2 vaccine (a small group with limited follow-up data); those who had received more than one dose of BNT162b2 vaccine and then recovered from a PCR-confirmed SARS-CoV-2 infection (a small group); those who had spent the entire study period abroad; and those who had received a vaccine other than BNT162b2 before August 1, 2021 (Figure 1).
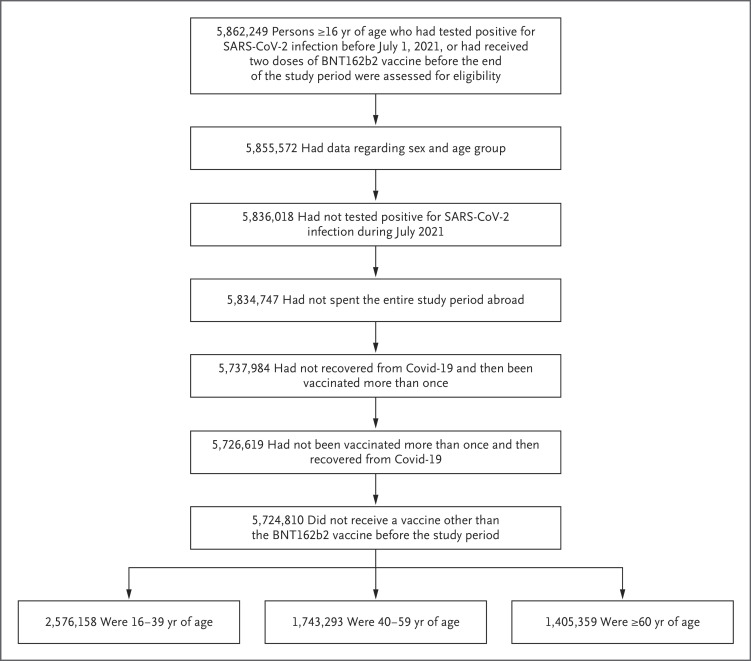
Eligible persons in the study did not have a documented positive polymerase-chain-reaction assay between July 1 and July 30, 2021, had received at most one vaccine dose before recovery or after recovery from coronavirus disease 2019 (Covid-19), and had not received a Covid-19 vaccine other than BNT162b2 before August 1, 2021. Age groups as of January 1, 2021, are shown. SARS-CoV-2 denotes severe acute respiratory syndrome coronavirus 2.
Study Design and Oversight
We compared the incidences of confirmed infection over the study period among cohorts of persons with various histories of immunity-conferring events (i.e., infection or vaccination). The recovered, unvaccinated cohort involved persons who had had a confirmed infection 90 or more days before the study day. There were two “hybrid” cohorts (i.e., cohorts with participants who had both natural immunity and immunity from vaccination); the recovered, one-dose cohort consisted of persons who had recovered from Covid-19 and had later received a single dose of vaccine at least 7 days before the study day, and the one-dose, recovered cohort involved those who had received a single dose of vaccine, followed by a confirmed infection at least 90 days before the study day. The two-dose cohort was composed of persons who had not been infected before the beginning of the study and who had received the second dose of vaccine at least 7 days before the study day, and the three-dose cohort was composed of those who had not been infected before the start of the study and who had received the third (booster) dose of vaccine at least 12 days before the study day.
These cohorts were divided into subcohorts according to the time that had elapsed since the last immunity-conferring event. We used 2 months as the basic time interval to define the subcohorts, but we combined months 12 to 18 for the recovered, unvaccinated cohort and omitted the period of 8 to less than 10 months for the vaccinated and hybrid cohorts because of the small number of persons in those cohorts.
A person could contribute follow-up days to different subcohorts and could also move from one cohort to another according to the following rules. A person who had recovered from Covid-19 and who received a first dose of BNT162b2 vaccine during the study period exited the recovered, unvaccinated cohort on the day of vaccination and entered the recovered, one-dose cohort 7 days later. A person who had recovered from Covid-19 and who had received a first vaccine dose but then received a second dose during the study period exited the recovered, one-dose cohort at the time of the second vaccination. A person in the two-dose cohort who received a third (booster) dose during the study period exited the two-dose cohort on the day of the booster dose and entered the three-dose cohort 12 days later.20 A person with a positive test for SARS-CoV-2 infection between May 1 and June 30, 2021, and who also received a single dose of BNT162b2 vaccine entered either the recovered, one-dose cohort or the one-dose, recovered cohort (according to whether or not confirmed infection predated vaccination) 90 days after the positive test. A person who received a vaccine other than BNT162b2 exited the study on the day of that vaccination.
Studies often compare infection rates among recovered or vaccinated persons with those among unvaccinated persons who have not been previously infected. However, owing to the high vaccination rate in Israel, the latter cohort is small and not representative of the overall population. Furthermore, the Israeli Ministry of Health database does not include complete information about such persons. Therefore, we did not include unvaccinated, previously uninfected persons in our analysis.
The study was approved by the institutional review board at the Sheba Medical Center. The Israeli Ministry of Health and Pfizer have a data-sharing agreement, but only the final results of this study were shared.
Statistical Analysis
To analyze the data, we used methods similar to those used in our previous studies.8,20,21 We assumed that the hazard of infection in each cohort would be independent of the sojourn time in previous cohorts (i.e., the time spent in the cohort before a confirmed infection), and we focused on the relationship between the proportional-hazards survival model and the Poisson regression model22 (see the Supplementary Methods 2 section). Specifically, the number of confirmed infections and the number of person-days at risk during the study period were counted for each subcohort.
A Poisson regression model was fitted, with adjustment for age group as of January 1, 2021 (16 to 39 years, 40 to 59 years, or ≥60 years), sex, population sector (general Jewish, Arab, or ultra-Orthodox Jewish), calendar week, and an exposure risk measure. The latter was calculated for each person on each follow-up day according to the rate of new confirmed infections during the previous 7 days in the person’s area of residence; this continuous measure was then categorized into 10 risk groups according to deciles.20 An average exposure risk was imputed to persons with missing data on residency. In order to ensure that the effect of missing data was small, a descriptive comparison of persons who had missing data with those who did not have missing data, as well as a multiple-imputation analysis, were performed (see the Supplementary Analysis 1 section). Goodness of fit of the model was checked by examining Pearson residuals across the categories.
In a supplementary analysis, we fitted a model with an interaction between age group and subcohort in order to estimate age-specific incidence rates in each subcohort. Each case of infection contributed an event to the respective subcohort. On the basis of the estimated parameters of the fitted regression model, the incidence rate in each subcohort, adjusted for the confounders, was estimated as the expected number of events per 100,000 days if all the person-days at risk were included in that subcohort (see the Supplementary Methods 3 section). The 95% confidence intervals were calculated with the use of a bootstraplike simulation approach23 without adjustment for multiplicity. We repeated the analysis of subcohorts with 1-month intervals (instead of 2-month intervals) to better distinguish between persons who chose to be vaccinated earlier and those who chose to be vaccinated later (or between those who were infected earlier and those who were infected later).
To examine the effect of misclassification of persons into cohorts owing to undocumented infections, we conducted a sensitivity analysis with the assumption that either 50% or 70% of true infections were undocumented. There were too few cases for an in-depth comparison of the incidences of severe disease within and between the cohorts with natural immunity and those with hybrid immunity; thus, only a descriptive analysis was performed. The results of a comparison of the incidences of severe Covid-19 between persons who had received two doses of BNT162b2 vaccine and those who had received a third (booster) dose are reported elsewhere.21
Results
Study Population and Descriptive Statistics
Our analysis was based on more than 5.7 million persons who contributed days in the five main cohorts (Figure 1). Figure 2 shows the dynamic inclusion of persons in the cohorts over time. Table 1 shows the number of events (confirmed SARS-CoV-2 infections and cases of severe Covid-19) according to the cohorts and demographic characteristics of the persons as well as the distribution of person-days at risk according to sex, age group, and population sector in the five cohorts. The sex distribution was similar in the five cohorts, with only slightly more person-days at risk for women than for men. There were clear differences among the cohorts in the distribution of the other covariates. Although persons who were 60 years of age or older contributed 53.4% of the person-days at risk in the three-dose cohort, persons of this age contributed only 8.3% of the person-days at risk in the recovered, unvaccinated cohort, 13.8% of the person-days at risk in the recovered, one-dose cohort, 14.3% of the person-days at risk in the one-dose, recovered cohort, and 12.6% of the person-days at risk in the two-dose cohort. The distributions of person-days at risk according to population sector also differed among the cohorts because the Arab and ultra-Orthodox Jewish groups have had a higher incidence of infection during the Covid-19 pandemic, resulting in higher percentages of these groups in the cohorts of recovered persons than in the cohorts of persons who were not previously infected. Figure S4 in the Supplementary Appendix shows the distribution of time between infection and vaccination in the hybrid cohorts.
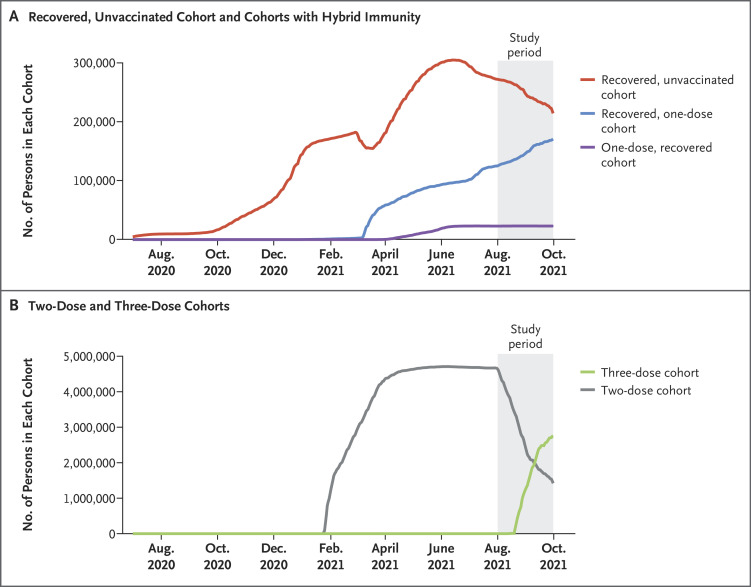
The numbers of persons in the cohorts increased and decreased as persons joined and exited the cohorts. In the study period, the area under the curves represents the number of person-days at risk in each cohort.
Table 1
| Cohort | Sex | Age Group | Population Sector | |||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | 16–39 yr | 40–59 yr | ≥60 yr | General Jewish | Arab | Ultra- Orthodox Jewish | |
| Recovered, unvaccinated cohort† | ||||||||
| Person-days at risk — % | 53.1 | 46.9 | 66.7 | 25.0 | 8.3 | 50.7 | 28.4 | 20.9 |
| SARS-CoV-2 infections/100,000 person-days at risk — no. | 2370 | 2021 | 3361 | 915 | 115 | 2522 | 1463 | 406 |
| Cases of severe Covid-19/100,000 person-days at risk — no. | 9 | 16 | 6 | 10 | 9 | 12 | 4 | 9 |
| Three-dose cohort‡ | ||||||||
| Person-days at risk — % | 51.0 | 49.0 | 16.4 | 30.1 | 53.4 | 89.2 | 4.1 | 6.7 |
| SARS-CoV-2 infections/100,000 person-days at risk — no. | 2835 | 3010 | 1156 | 2042 | 2647 | 4957 | 417 | 471 |
| Cases of severe Covid-19/100,000 person-days at risk — no. | 70 | 108 | 1 | 13 | 164 | 155 | 12 | 11 |
| Two-dose cohort§ | ||||||||
| Person-days at risk — % | 51.2 | 48.8 | 56.7 | 30.7 | 12.6 | 74.1 | 5.6 | 20.3 |
| SARS-CoV-2 infections/100,000 person-days at risk — no. | 77,475 | 63,003 | 84,471 | 42,825 | 13,182 | 111,174 | 12,328 | 16,976 |
| Cases of severe Covid-19/100,000 person-days at risk — no. | 579 | 793 | 44 | 264 | 1064 | 1071 | 74 | 227 |
| Recovered, one-dose cohort¶ | ||||||||
| Person-days at risk — % | 51.7 | 48.3 | 57.2 | 29.1 | 13.8 | 54.4 | 22.1 | 23.6 |
| SARS-CoV-2 infections/100,000 person-days at risk — no. | 441 | 381 | 543 | 209 | 70 | 539 | 170 | 113 |
| Cases of severe Covid-19/100,000 person-days at risk — no. | 8 | 5 | 1 | 5 | 7 | 8 | 2 | 3 |
| One-dose, recovered cohort‖ | ||||||||
| Person-days at risk — % | 52.2 | 47.8 | 52.6 | 33.1 | 14.3 | 60.8 | 15.3 | 23.9 |
| SARS-CoV-2 infections/100,000 person-days at risk — no. | 117 | 112 | 156 | 62 | 11 | 168 | 38 | 23 |
| Cases of severe Covid-19/100,000 person-days at risk — no. | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
Tables S1 through S4 provide a more detailed tabulation of the data, with each cohort divided into subcohorts according to the time that had elapsed since infection or vaccination. As expected, the differences in the distributions of covariates among the subcohorts within each cohort were smaller than those among the cohorts. The most prominent differences among subcohorts related to the tendency of older persons to receive vaccination earlier, according to the Israeli vaccination prioritization schedule. The numbers of person-days at risk in the subcohorts of persons who had recovered from Covid-19, regardless of whether they were vaccinated, were much smaller than those in the two-dose and three-dose subcohorts. The numbers of cases of severe Covid-19 among persons in each of the subcohorts of the recovered, unvaccinated cohort and in each of the subcohorts of the two hybrid cohorts were small (<10), so reliable quantification of the levels of protection against severe disease in each of these three cohorts was precluded. We therefore focused on comparing the incidences of confirmed infection among the subcohorts.
Waning Immunity against Reinfection
Table 2 and Figure 3 summarize the results of the Poisson regression analysis and show the estimated numbers of confirmed infections per 100,000 person-days at risk in each subcohort, with adjustment for age, sex, population sector, calendar week, and risk of exposure. Table 2 also provides two sets of rate ratios for each subcohort — one rate ratio that is relative to the reference subcohort of previously uninfected persons who had been vaccinated within the previous 2 months, and one rate ratio that is relative to the subcohort with the most recent immunity-conferring event within the cohort. The complete set of parameter estimates of the regression model is provided in Table S7. The adjusted incidence rates within age groups (16 to 39 years, 40 to 59 years, and ≥60 years) are provided in Table S8 and Figure S1. Figure S2 shows plots of the Pearson residuals indicating an overall satisfactory fit of the model to the data, with somewhat poorer fit in the cohorts vaccinated with two and three doses. Figure S3 shows the rates when the subcohorts were defined according to 1-month periods.
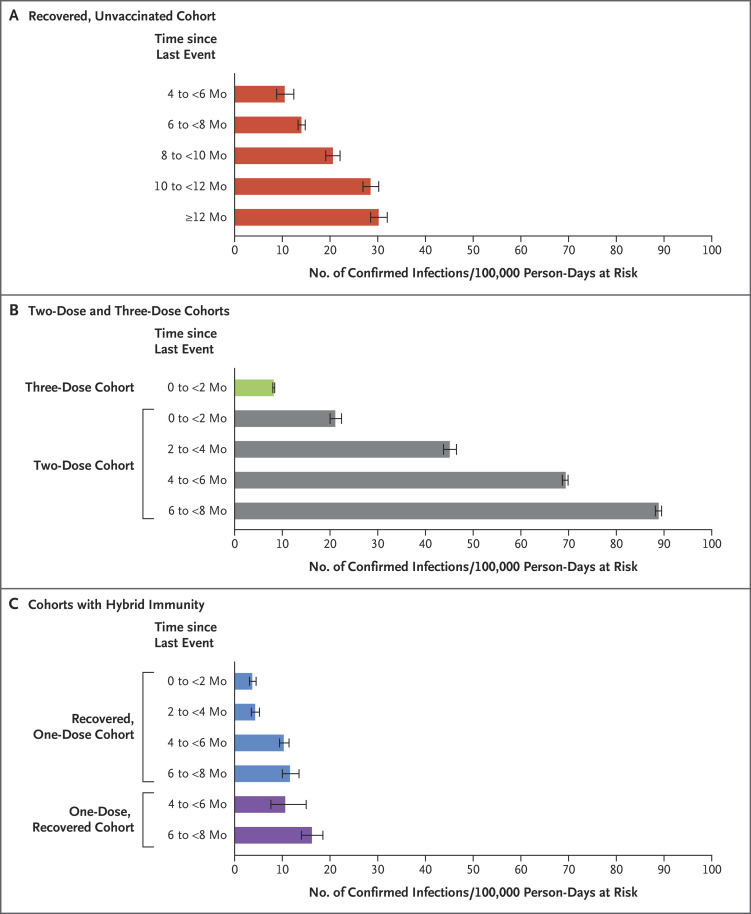
Data were obtained from the Poisson regression analysis for the study period, stratified according to subcohorts. Confidence intervals are not adjusted for multiplicity. The error bars denote 95% confidence intervals.
Table 2
| Cohort and Subcohort | Adjusted Rate (95% CI)† | Rate Ratio (95% CI) | Rate Ratio (95% CI) |
|---|---|---|---|
| Reference Subcohort vs. Other Subcohort | Subcohort with Most Recent Immunity-Conferring Event vs. Other Subcohort | ||
| no. of confirmed infections/ 100,000 person-days at risk | |||
| Recovered, unvaccinated cohort | |||
| 4 to <6 mo subcohort | 10.5 (8.8–12.4) | 2.0 (1.7–2.4) | Reference |
| 6 to <8 mo subcohort | 14.0 (13.3–14.8) | 1.5 (1.4–1.6) | 0.7 (0.6–0.9) |
| 8 to <10 mo subcohort | 20.6 (19.1–22.1) | 1.0 (0.9–1.1) | 0.5 (0.4–0.6) |
| 10 to <12 mo subcohort | 28.5 (26.9–30.2) | 0.7 (0.7–0.8) | 0.4 (0.3–0.4) |
| ≥12 mo subcohort | 30.2 (28.5–32.0) | 0.7 (0.6–0.8) | 0.3 (0.3–0.4) |
| Three-dose cohort | |||
| 0 to <2 mo subcohort | 8.2 (8.0–8.4) | 2.6 (2.4–2.7) | Reference |
| Two-dose cohort | |||
| 0 to <2 mo subcohort | 21.1 (20.0–22.4) | Reference | Reference |
| 2 to <4 mo subcohort | 45.1 (43.8–46.5) | 0.5 (0.4–0.5) | 0.5 (0.4–0.5) |
| 4 to <6 mo subcohort | 69.4 (68.7–69.9) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) |
| 6 to <8 mo subcohort | 88.9 (88.2–89.5) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) |
| Recovered, one-dose cohort | |||
| 0 to <2 mo subcohort | 3.7 (3.1–4.5) | 5.7 (4.6–6.9) | Reference |
| 2 to <4 mo subcohort | 4.3 (3.5–5.2) | 5.0 (4.0–6.1) | 0.9 (0.7–1.2) |
| 4 to <6 mo subcohort | 10.3 (9.4–11.4) | 2.0 (1.8–2.3) | 0.4 (0.3–0.4) |
| 6 to <8 mo subcohort | 11.6 (10.0–13.5) | 1.8 (1.5–2.2) | 0.3 (0.3–0.4) |
| One-dose, recovered cohort | |||
| 4 to <6 mo subcohort | 10.6 (7.6–15.0) | 2.0 (1.4–2.8) | Reference |
| 6 to <8 mo subcohort | 16.2 (14.0–18.5) | 1.3 (1.1–1.5) | 0.7 (0.5–0.9) |
We found evidence of waning immunity in all cohorts (Figure 3), with a steady decrease in protection over time. The adjusted rate of confirmed infections among recovered, unvaccinated persons 4 to less than 6 months after infection was 10.5 per 100,000 person-days at risk (95% confidence interval [CI], 8.8 to 12.4); this rate increased to 30.2 (95% CI, 28.5 to 32.0) among persons in this cohort 12 months or more after infection. In the two-dose cohort, the rate was 21.1 (95% CI, 20.0 to 22.4) among persons vaccinated within the previous 2 months, and this rate increased to 88.9 (95% CI, 88.2 to 89.5) among those vaccinated 6 to less than 8 months previously; in the recovered, one-dose cohort with the same times since vaccination, the rates were 3.7 (95% CI, 3.1 to 4.5) and 11.6 (95% CI, 10.0 to 13.5), respectively.
In the subcohorts of the recovered, unvaccinated cohort, the adjusted rates of confirmed infection were similar to those of the recovered, one-dose and one-dose, recovered subcohorts when the time elapsed since the last immunity-conferring event (either infection or vaccination) was the same (Figure 3). For example, at 4 to less than 6 months since the last immunity-conferring event, the rates per 100,000 person-days at risk were 10.5 (95% CI, 8.8 to 12.4) in the recovered, unvaccinated cohort, 10.3 (95% CI, 9.4 to 11.4) in the recovered, one-dose cohort, and 10.6 (95% CI, 7.6 to 15.0) in the one-dose, recovered cohort. At 6 to less than 8 months, the rates were 14.0 (95% CI, 13.3 to 14.8), 11.6 (95% CI, 10.0 to 13.5), and 16.2 (95% CI, 14.0 to 18.5), respectively. These rates were lower than those in the two-dose cohort 4 to less than 6 months after vaccination (69.4; 95% CI, 68.7 to 69.9) and 6 to less than 8 months after vaccination (88.9; 95% CI, 88.2 to 89.5). However, the protection conferred by two doses of vaccine was restored with the administration of a third dose; our study showed a rate of 8.2 (95% CI, 8.0 to 8.4) less than 2 months after booster vaccination (Table 2).
The sensitivity analysis for misclassification owing to unreported infections revealed that the rates of confirmed infection in the two-dose and three-dose cohorts as described above may have be underestimated by approximately 10% when the misclassification rate was 50% and by approximately 20% when the misclassification rate was 70%. However, such misclassification did not have a substantial effect on the estimates of waning protection (see the Supplementary Analysis 2 section).
Analysis of Cases of Severe Covid-19
The number of cases of severe Covid-19 was small in the cohorts of previously infected persons, with 25 in the recovered, unvaccinated cohort, 13 in the recovered, one-dose cohort, and 1 in the one-dose, recovered cohort. In the two-dose cohort, there were 1372 cases of severe Covid-19, and in the three-dose cohort, there were 178 cases (Table 1). The resulting crude rates of severe disease among persons 60 years of age or older, without consideration of the time since the last immunity-conferring event, were 0.6 per 100,000 person-days at risk in the recovered, unvaccinated cohort, 0.5 in the recovered, one-dose cohort, 0.5 in the one-dose, recovered cohort, 4.6 in the two-dose cohort, and 0.4 in the three-dose cohort.
Discussion
We evaluated the waning level of protection against confirmed infection with SARS-CoV-2 among persons who had recovered from previous infection and among previously uninfected persons who received the BNT162b2 vaccine. We compared protection in these groups with that in persons who had been vaccinated with a single dose and later infected with SARS-CoV-2 and with that in persons who had recovered from SARS-CoV-2 infection and later received a single vaccine dose. Previous studies showed higher protection in previously infected persons with or without an additional vaccine dose than in previously uninfected persons who had received two doses of mRNA vaccines.6,7 Our study quantifies the waning of natural and hybrid immunity at the national level in a real-world setting.
Waning immunity was evident in all the cohorts. This pattern of waning immunity was evident across all age groups. The adjusted rates of confirmed infection among the recovered, unvaccinated subcohorts were lower than those among the two-dose subcohorts when the time since the last immunity-conferring event was similar; nevertheless, the protection in the two-dose cohort could be restored by the administration of a booster shot.
In findings that were consistent with those of other studies,6,7,24 after several months, persons with hybrid immunity were better protected against reinfection than uninfected persons who had previously received two doses of vaccine (the two-dose cohort). Furthermore, we found that a single dose of the vaccine administered to a previously infected person or a booster dose administered to an uninfected person who had received two doses of vaccine restored the level of protection to the level in the early months after recovery or vaccination. The timing of vaccination after infection affects the protection.6 We did not have enough data to evaluate the level of protection as a function of time between infection and vaccination, while taking the waning effect into account.
The results reported here are in line with those of a study conducted by an Israeli health maintenance organization.7 That study showed that previously infected persons with or without one vaccine dose have better protection than uninfected persons who have received two doses of vaccine 3 to less than 8 months after the last immunity-conferring event. Our data on hospitalized patients who had severe Covid-19 did not contain enough cases for a definitive analysis but did not appear to support the findings in a recent report9 that suggested that vaccinated persons are more protected than previously infected persons 3 to less than 6 months after an immunity-conferring event.
In the recovered, unvaccinated cohort and the hybrid cohorts, the first infections were primarily infections with the original Wuhan-Hu-1 isolate and the B.1.1.7 (alpha) variant.17 If protection provided by previous infection depends on the variant, its effect is confounded with the effect of time since infection. Because a single variant was dominant in Israel during each of the pandemic waves,17 this study cannot disentangle the two effects. Moreover, during the study period, most infections were delta variant infections, and our analysis provides no information regarding protection against newer variants such as B.1.1.529 (omicron).
This was an observational study in which persons elected to receive a vaccine at different times, and there was no control for the probable differences in health care–seeking or risk-averse behavior of individual persons. Although the regression approach corrects for confounders for which data are available, including data on exposure risk, the possibility of residual bias remains. The residuals analysis revealed an overall reasonable fit, with a few large residuals in the cohorts vaccinated with two or three doses. These cohorts had large sample sizes, leading to substantial sensitivity to even a modest lack of fit.
Our results pertained to the rate of confirmed infection, so they were sensitive to detection bias due to different tendencies to perform PCR testing in the study cohorts. During the study period, the same official PCR testing policy applied to both previously infected persons and those who had received two doses of vaccine — namely, mandatory PCR testing on contact with an infected person. Although differences in testing rates among cohorts and among subcohorts within specified cohorts were observed, their overall magnitude was relatively small. The rate of PCR testing was typically lower in the recovered, unvaccinated cohort than in the other cohorts, so the level of protection in this cohort as compared with that in the two-dose cohort may have been overestimated. The data regarding severe disease were not affected by this bias.
Another source of potential bias was cohort misclassification. To be classified as a recovered person in our study, a PCR test must have been performed and found to have been positive. However, not all infected persons had received a diagnosis,25 and some of these persons had been vaccinated. Thus, some of the persons who were classified as being in the two-dose cohort or the three-dose cohort should have been considered to have had hybrid immunity. Under simple assumptions about the misclassification mechanism, we found that misclassification may have led to a 10% or even a 20% underestimation of the infection rate among vaccinated, uninfected persons, depending on the misclassification rate. Although the magnitude of the bias depends on our assumptions, the bias toward underestimation of the infection rate among vaccinated, uninfected persons is real if those who had recovered from Covid-19 and had been misclassified as belonging to the vaccinated cohorts were more protected from reinfection than their uninfected counterparts.
An understanding of the rates of waning immunity after immunity-conferring events is important for policy making regarding the need for and the timing of additional vaccine doses. We found that protection against the delta variant waned over time in both vaccinated and previously infected persons and that an additional vaccine dose restored protection.
Acknowledgments
We thank Ofra Amir for productive feedback on an earlier version of the manuscript.
Supplementary Appendix
Disclosure Forms
Notes
This article was published on May 25, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmoa2118946
Read article for free, from open access legal sources, via Unpaywall:
https://www.nejm.org/doi/pdf/10.1056/NEJMoa2118946?articleTools=true
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/128888808
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1056/nejmoa2118946
Article citations
Seroprevalence of SARS-CoV-2 antibodies among healthy blood donors: a systematic review and meta-analysis.
BMC Public Health, 24(1):2925, 22 Oct 2024
Cited by: 0 articles | PMID: 39438911 | PMCID: PMC11515703
Review Free full text in Europe PMC
Broad immunogenicity to prior SARS-CoV-2 strains and JN.1 variant elicited by XBB.1.5 vaccination in nursing home residents.
Geroscience, 12 Oct 2024
Cited by: 0 articles | PMID: 39395130
Humoral and cellular immune responses in vaccinated and unvaccinated children following SARS-CoV-2 Omicron infection.
Clin Transl Immunology, 13(10):e70008, 03 Oct 2024
Cited by: 0 articles | PMID: 39364394 | PMCID: PMC11447454
Did We Overreact? Insights on COVID-19 Disease and Vaccination in a Large Cohort of Immune-Mediated Inflammatory Disease Patients during Sequential Phases of the Pandemic (The BELCOMID Study).
Vaccines (Basel), 12(10):1157, 11 Oct 2024
Cited by: 0 articles | PMID: 39460324 | PMCID: PMC11510991
Navigating the Landscape of B Cell Mediated Immunity and Antibody Monitoring in SARS-CoV-2 Vaccine Efficacy: Tools, Strategies and Clinical Trial Insights.
Vaccines (Basel), 12(10):1089, 24 Sep 2024
Cited by: 0 articles | PMID: 39460256 | PMCID: PMC11511438
Review Free full text in Europe PMC
Go to all (219) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Incidence of SARS-CoV-2 Reinfection in Persons With Naturally Acquired Immunity With and Without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine : A Retrospective Cohort Study.
Ann Intern Med, 175(5):674-681, 15 Feb 2022
Cited by: 37 articles | PMID: 35157493 | PMCID: PMC8855786
Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection.
N Engl J Med, 386(13):1207-1220, 16 Feb 2022
Cited by: 368 articles | PMID: 35172051 | PMCID: PMC8908850
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study.
Clin Infect Dis, 75(1):e545-e551, 01 Aug 2022
Cited by: 129 articles | PMID: 35380632 | PMCID: PMC9047157
Breakthrough SARS-CoV-2 infections after vaccination: a critical review.
Hum Vaccin Immunother, 18(5):2051412, 18 Mar 2022
Cited by: 5 articles | PMID: 35302905 | PMCID: PMC9115792
Review Free full text in Europe PMC





