Abstract
Background
The coronavirus disease 2019 (COVID-19) epidemic, considered as the worst global public health event in nearly a century, has severely affected more than 200 countries and regions around the world. To effectively prevent and control the epidemic, researchers have widely employed dynamic models to predict and simulate the epidemic's development, understand the spread rule, evaluate the effects of intervention measures, inform vaccination strategies, and assist in the formulation of prevention and control measures. In this review, we aimed to sort out the compartmental structures used in COVID-19 dynamic models and provide reference for the dynamic modeling for COVID-19 and other infectious diseases in the future.Main text
A scoping review on the compartmental structures used in modeling COVID-19 was conducted. In this scoping review, 241 research articles published before May 14, 2021 were analyzed to better understand the model types and compartmental structures used in modeling COVID-19. Three types of dynamics models were analyzed: compartment models expanded based on susceptible-exposed-infected-recovered (SEIR) model, meta-population models, and agent-based models. The expanded compartments based on SEIR model are mainly according to the COVID-19 transmission characteristics, public health interventions, and age structure. The meta-population models and the agent-based models, as a trade-off for more complex model structures, basic susceptible-exposed-infected-recovered or simply expanded compartmental structures were generally adopted.Conclusion
There has been a great deal of models to understand the spread of COVID-19, and to help prevention and control strategies. Researchers build compartments according to actual situation, research objectives and complexity of models used. As the COVID-19 epidemic remains uncertain and poses a major challenge to humans, researchers still need dynamic models as the main tool to predict dynamics, evaluate intervention effects, and provide scientific evidence for the development of prevention and control strategies. The compartmental structures reviewed in this study provide guidance for future modeling for COVID-19, and also offer recommendations for the dynamic modeling of other infectious diseases.Free full text

Compartmental structures used in modeling COVID-19: a scoping review
Abstract
Background
The coronavirus disease 2019 (COVID-19) epidemic, considered as the worst global public health event in nearly a century, has severely affected more than 200 countries and regions around the world. To effectively prevent and control the epidemic, researchers have widely employed dynamic models to predict and simulate the epidemic’s development, understand the spread rule, evaluate the effects of intervention measures, inform vaccination strategies, and assist in the formulation of prevention and control measures. In this review, we aimed to sort out the compartmental structures used in COVID-19 dynamic models and provide reference for the dynamic modeling for COVID-19 and other infectious diseases in the future.
Main text
A scoping review on the compartmental structures used in modeling COVID-19 was conducted. In this scoping review, 241 research articles published before May 14, 2021 were analyzed to better understand the model types and compartmental structures used in modeling COVID-19. Three types of dynamics models were analyzed: compartment models expanded based on susceptible-exposed-infected-recovered (SEIR) model, meta-population models, and agent-based models. The expanded compartments based on SEIR model are mainly according to the COVID-19 transmission characteristics, public health interventions, and age structure. The meta-population models and the agent-based models, as a trade-off for more complex model structures, basic susceptible-exposed-infected-recovered or simply expanded compartmental structures were generally adopted.
Conclusion
There has been a great deal of models to understand the spread of COVID-19, and to help prevention and control strategies. Researchers build compartments according to actual situation, research objectives and complexity of models used. As the COVID-19 epidemic remains uncertain and poses a major challenge to humans, researchers still need dynamic models as the main tool to predict dynamics, evaluate intervention effects, and provide scientific evidence for the development of prevention and control strategies. The compartmental structures reviewed in this study provide guidance for future modeling for COVID-19, and also offer recommendations for the dynamic modeling of other infectious diseases.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40249-022-01001-y.
Background
After emerging in late 2019, the coronavirus disease 2019 (COVID-19) pandemic has affected more than 200 countries and territories, with more than 507.5 million confirmed cases and over 6.22 million deaths reported globally as of April 25, 2022 [1]. The speed, scope, and difficulty of prevention and control of the epidemic are unprecedented. It was declared a “global pandemic” by the World Health Organization (WHO) on March 11, 2020 [2]. The pandemic has not only posed a serious threat to human health but has also had profound consequences on society, economy, environment, public psychology, and so on [3]. In the early stages, as a newly emerging infectious disease, the epidemiological characteristics, transmission mechanisms, and clinical features of COVID-19 were not clear. At this time, due to the ability to combine expert advice and the limited data needed, dynamic models are being widely used to predict the dynamic trends, intensity, and temporal and spatial dynamic processes of the epidemic and to evaluate the potential impact and effectiveness of candidate prevention and control measures. Therefore, this has played an important role in allocating medical and health resources reasonably, determining effective prevention and control measures, and formulating strategies for the resumption of work and production in the early stages of the epidemic.
After almost two years of COVID-19, the scientific community has more in-depth research and a greater understanding of its epidemiology, characteristics, clinical manifestations, and other aspects. Due to the distinct incubation period of COVID-19, early studies using the susceptible-infected-recovered (SIR) model and its extension may be inaccurate, while the susceptible-exposed-infected-recovered (SEIR) model and its extension are more appropriate. As the characteristics of the COVID-19 epidemic have been revealed, and various non-pharmaceutical interventions (NPIs) have been applied, the compartmental structures of COVID-19 dynamic models have become increasingly rich and complex to reflect the true transmission dynamics of the epidemic to the greatest extent. A reasonable compartmental structure is critical for dynamic modeling; therefore, it is vital to review the compartmental structures of the COVID-19 dynamic models.
In this review, we analyzed the compartmental structures used in COVID-19 dynamic models, including the SEIR-based expanded models, meta-population models, and agent-based models, hoping to provide an important reference for the dynamic modeling for COVID-19 and other infectious diseases in the future.
Methods
Database searches
To conduct the search, we entered “COVID-19” in the title field, and “dynamics model” related fields in the abstract field. The search strategy was as follows: (COVID-19 +
+ Novel Coronavirus
Novel Coronavirus +
+ 2019-nCOV
2019-nCOV +
+ nCOV-19
nCOV-19 +
+ SARS-CoV-2) * (SIR
SARS-CoV-2) * (SIR +
+ SEIR
SEIR +
+ SIRQ
SIRQ +
+ SEIRQ
SEIRQ +
+ "the reproductive number"
"the reproductive number" +
+ "stochastic model"
"stochastic model" +
+ " deterministic model"
" deterministic model" +
+ "compartment model"
"compartment model" +
+ "dynamics model"
"dynamics model" +
+ "mathematical model"
"mathematical model" +
+ "mechanism model"
"mechanism model" +
+ "meta-population model"
"meta-population model" +
+ "agent-based model"
"agent-based model" +
+ "individual model"
"individual model" +
+ "epidemic model"
"epidemic model" +
+ "simulation model").
"simulation model").
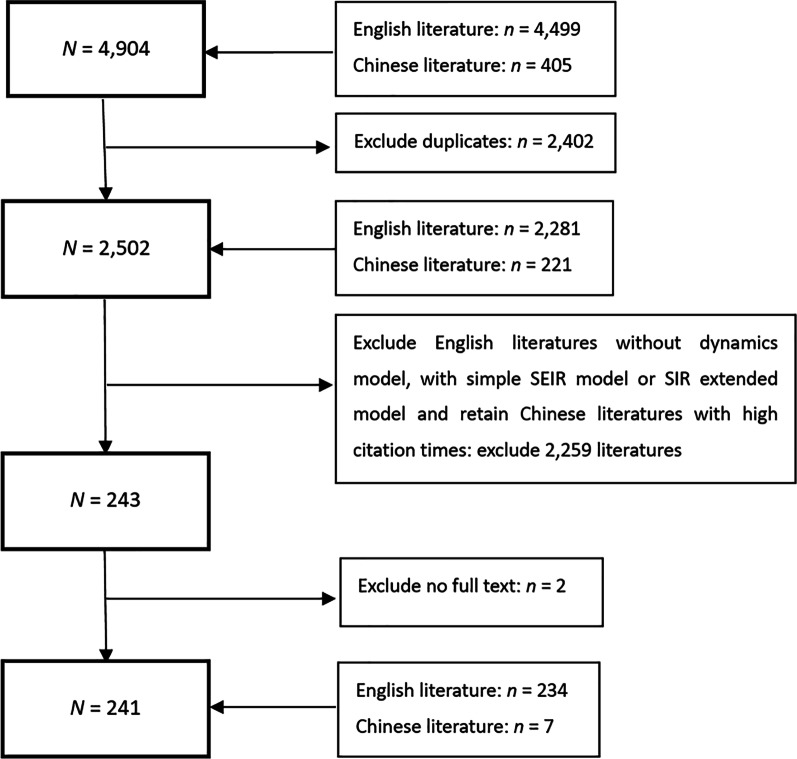
The searches, conducted on May 14, 2021, yielded 4499 records across PubMed, ScienceDirect, and Web of Science, and 405 records (in Chinese) across CNKI and Wanfang Database [4, 5].
Record screening
The inclusion criteria were as follows: (1) the literature language was either English or Chinese; (2) focus on COVID-19; (3) the compartmental structures of the dynamic model were described in detail; (4) literature with high reference value determined by internal expert discussion (mainly for papers in Chinese, based on the core journals of Peking University). The exclusion criteria were as follows: (1) repeated articles; (2) no dynamic model was established, SIR or its extension, or only a basic SEIR model was used; (3) full text unavailable. According to the above criteria, 234 English and 7 Chinese references were included in this review. The screening process is represented in Fig. 1.
Results
Due to incubation period of COVID-19, the SEIR model should theoretically be used as the basis to construct compartment models. Focusing on the compartmental structures used, this review analyzed the expanded compartment models based on the SEIR model, meta-population models, and agent-based models.
Expanded models based on SEIR
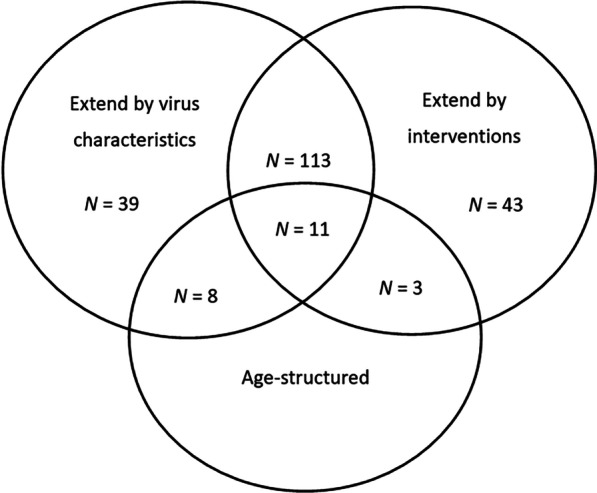
The main reasons to expand the compartments of the SEIR model include the following (Fig. 2):
the COVID-19 characteristics, such as asymptomatically infected, death, further subdividing the infected compartment by disease status, etc.
public health interventions, such as hospitalization, isolation, quarantine, etc.
age structure.
integrating the above reasons.
Expand compartments according to COVID-19 characteristics
Understanding the transmission mechanism and viral characteristics of COVID-19 is critical to control its spread. COVID-19 is primarily transmitted from person to person through respiratory droplets released when someone with COVID-19 sneezes, coughs, or talks. People can also be infected by touching objects contaminated with live viruses or by touching mucous membranes, such as the mouth, nose, and eyes after exposure to a contaminated environment [6]. Infected cases can be divided into symptomatic patients and asymptomatic patients according to the presence or absence of clinical symptoms; the symptomatic patients can be further divided into mild patients, severe patients, and critically ill patients (ICU patients), according to the severity of their symptoms. Therefore, to model the transmission dynamics, researchers expanded the compartments according to these divisions [7–9]. A proportion of those infected died, and the death rate of severe patients was higher than that of mild patients [10]. Thus, the death compartment (D) is included in some models. In addition, some studies added a compartment to account for the possible transmission to people via the live virus in contaminated environments [11–13]. A summary of the expanded compartments according to virus characteristics is presented in Table Table11 (complete compartmental structures and meaning are shown in Additional file 1).
Table 1
Summary of the expanded compartments according to virus characteristics of COVID-19
| Expanded compartments | Interpretation | References |
|---|---|---|
| A | Asymptomatic | [14] |
| Im, Is | Mild (Im)/ severe (Is) symptom | [7] |
| Ic | Critical | [9] |
| Ip, Ic, Is | Preclinical (Ip), clinical (Ic), subclinical infection (Is)a | [15] |
| P | Pre-symptomatic | [16] |
| D | Dead | [17–19] |
| B | Live virus in environment | [11] |
aSubclinical infected: infected persons with mild or no symptoms; clinical infected: infected persons with obvious symptoms.
Expand compartments according to public health interventions
Before the COVID-19 vaccination became available, countries worldwide adopted various NPIs, including border closure, active contact tracing, testing for isolating cases, quarantining suspected cases, wearing face masks, social distancing, school and/or workplace closure, travel restrictions, etc., in an effort to mitigate and contain the transmission. To simulate the true transmission dynamics of COVID-19 to the greatest extent and to assess the effects of different NPIs, researchers added compartments reflecting NPIs. These NPIs can be divided into the following two categories according to the target population of interventions:
Protect the susceptible population. The measures include restricting the activities of the susceptible population in medium- and high-risk areas [20, 21]; changing behaviors of the susceptible population through propaganda and education on epidemic prevention and control [22–24], requiring or encouraging people to wear face masks, maintain social distancing, wash hands frequently, reduce gatherings, and so on. These measures were meant to reduce the risk of exposure to COVID-19.
Track and isolate infected or suspected infected individuals and close contacts. The measures include isolating and hospitalizing infected individuals [25] and quarantining close contacts and suspected cases [26]. These measures can effectively reduce contact between the infectious and susceptible population, thereby reducing transmission rates. Considering these measures, researchers added corresponding isolation, quarantine, and hospitalization compartments based upon a basic SEIR model. The isolation compartments can be subdivided according to close contacts, suspected cases, and different types of infected persons. Hospitalized patients have been further subdivided according to their severity of symptoms, as indicated below.
In addition to the above two categories of NPIs, active nucleic acid testing and timely reporting of cases [27] were also important prevention and control measures. On the one hand, testing for close contacts, sub-close contacts (close contacts of close contacts), and suspected cases, testing for all people in high-risk areas, and testing for people out from high-risk areas can detect infected cases and take quarantine or other measures to stop the transmission; on the other hand, notification of confirmed cases and their travel paths can also improve the self-protective consciousness of the susceptible population and therefore reduce the risk of infection.
The expanded compartments reflecting public health interventions are presented in Table Table22 (see Additional file 2 for the complete structures and meaning of the compartments).
Table 2
Expanded compartments based on public health interventions
| Intervention | Expanded compartments | Interpretation | References |
|---|---|---|---|
| Categorize the susceptible | Sp | Protected susceptiblea | [20] |
| Sc | Confined susceptiblea | [21] | |
| Sr | Behavior changed susceptiblea | [24] | |
| M, U | Masked/unmasked humans | [23] | |
| Hospitalization/quarantine | H | Hospitalized infected | [25] |
| Q | Quarantine | [28] | |
| Sq, Eq | Quarantined susceptible (Sq), quarantined exposed (Eq) | [26] | |
| Active nucleic acid testing and notification of confirmed cases | M | Missed cases | [29] |
| Q1, Q2 | Suspected population under home quarantine (Q1), medical quarantine population of confirmed cases (Q2) | [30] | |
| Ir, Iu | Tested infected individuals (Ir), non-tested infected individuals (Iu) | [27] | |
| I1, I2 | Infectious people with timely diagnosis(I1), delayed diagnosis (I2) | [31] |
aProtected, confined, and behavior changed susceptible people are less likely to be infected than ordinary susceptible people
Expanded compartments based on age structure
Some studies considered the heterogeneity of the population, such as different contact rates among people, infection rates for different individuals, the protection awareness of people with different occupations and at different ages, and different development processes of the disease due to different physical fitness levels after infection. Additionally, the vaccination has certain regulations and priorities for different ages.
Table Table33 summarizes age groups and compartmental structures in the model in relation to age structure. Additional file 3 indicates the complete compartmental structures and age groups.
Table 3
Age groups and compartmental structures of dynamic models considering age structure
| Age group, years | Compartmental structures | Interpretation | References |
|---|---|---|---|
0–15, 15–29, 30–59, 59 + + | Si,Ei,Ai,Mi,Hi,Ci,Ri | Susceptible (Si), exposed (Ei), asymptomatic (Ai), mild (Mi), severe (Hi), critical (Ci), recovered (Ri) in age group i | [32] |
0–9, 10–19, …, 70–79, 80 + + | SiEiAiIiHiRiDi | Susceptible (Si), latently infected (Ei), asymptomatic infectious (Ai), infectious individuals with symptoms/clinically ill (Ii), hospitalized patients (Hi), recovered (Ri), death due to disease (Di) in group i | [33] |
0–14, 15–49, 50–69, 70–80, 80 + + | SiEiLiIiRiTpiAsiSsiSviCriRdiDi | Susceptible (Si), exposed (Ei), post latency (Li), infectious (Ii), undocumented recovered (Ri), tested positive (Tpi), asymptomatic (Asi), symptomatic (Ssi), severe (Svi), critical (Cri), dead (Di), documented recovered (Rdi) in age group i | [34] |
0–14, 15–49, 50–69, 70 + + | SijEijIijQijHijRijDij | Susceptible (Sij), exposed (Eij), presymptomatic (Ipij), mild to moderate (Imij), severe (Isij), quarantined and exposed (QEij), pre-symptomatic and isolated (QIpij), mild to moderate and isolated (QImij), severe and isolated (QIsij), isolated (Qij), admitted to hospital (Hij), pre-ICU (PICUij), ICU (HICUij), recovered (Rij), dead (Dij) in age group I and health status j | [35] |
0–10, 10–20, …, 60–70, 70 + + | SiViEiEviAiAviIiQiRiRviDi | Susceptible (Si), vaccinated (Vi), exposed (Ei), exposed and vaccinated (EVi), asymptomatic (Ai), asymptomatic and vaccinated (AVi), symptomatic (Ii), isolated (Qi), recovered (Ri), recovered and vaccinated (RVi), death (Di) in age group i | [36] |
Integrating virus characteristics and interventions
More studies have considered both virus characteristics and intervention measures when constructing dynamic models, including subdividing compartments into more detailed levels according to whether the individual has been inspected [37], discovered [38], and reported [39]. These compartmental structures reflect better the actual situation of intervention. For example, some studies have modeled intervention measures for patients with different infection status, which were common in reality: isolating asymptomatic infections at home, admitting symptomatic infections to hospital [40], and admitting severe patients to ICU for treatment [41], etc. The recovered individuals were also divided into different compartments according to whether they had been detected or not, symptomatic or asymptomatic [37]. With the successful development of COVID-19 vaccination, researchers added the vaccination compartment to evaluate its effectiveness and to develop immunization programs [42]. The expanded compartments are shown in Table Table44 (see Additional file 4 for the complete structures).
Table 4
Expanded compartments that considered both virus characteristics and interventions
| Interventions | Expansion compartments | Interpretation | References |
|---|---|---|---|
| Social distancing, wearing masks, washing hands, etc | U | Unsusceptible a(U) | [43] |
| Quarantined at home/hospitalization | ST | Susceptible persons removed from isolation b(ST) | [44] |
| I2 | Infectious after receiving ineffective treatment (I2) | [45] | |
| Q, D | Home quarantined individuals (Q), diagnosed individuals who are being treated and isolated (D) | [46] | |
| Ia, Is, Qa, Qs, Ru, Ra, Rs | Undetected asymptomatic infectious (Ia), undetected symptomatic infectious (Is), detected and quarantined asymptomatic (Qa), detected and quarantined symptomatic (Qs), undetected recovered asymptomatic (Ru), recovered detected asymptomatic (Ra), recovered detected symptomatic (Rs) | [37] | |
| W1, R1, D1, W2, R2, D2 | Hospitalized that never require an intensive care bed (W1), recovered from non-ICU (R1), deaths from non-ICU (D1), hospitalized that require an intensive care bed (W2), recovered from ICU (R2), deaths from ICU (D2) | [41] | |
| H1, H2 | Confirmed cases who are quarantined at home (H1), confirmed cases who are hospitalized (H2) | [40] | |
| Ip, Ic | Primarily infected (Ip), chronically infected (Ic)c | [47] | |
| Vaccination, testing or contact tracing | AC | Contact traced asymptomatic (AC) | [48] |
| Eu, Ed | Undetected exposed (Eu), detected exposed (Ed) | [38] | |
| Su, Sv | Unvaccinated susceptible (Su), vaccinated susceptible (Sv) | [42] |
aUnsusceptible: a susceptible person can become unsusceptible due to factors such as the use of facemasks, hand washing, and SD (social distance)
bSusceptible persons removed from isolation (ST): isolated susceptible individuals, after a period, are released from isolation and transferred to compartment ST
cPrimarily infected: individuals that remain infectious within the reported duration of the infectious period after the incubation period; chronically infected: individuals that are less infectious but remain infectious and may be diagnosed for a longer duration
Meta-population model
Meta-population models contain several subpopulations, each of them representing a spatial area from a country or a city, to a school or a family, to investigate interactions and movements among different subpopulations. A whole compartmental structure was conducted in each subpopulation to distinguish different populations and the movement of people among different subpopulations interacting with the whole population. Therefore, the meta-population models can be regarded as a combination of classic compartment models with network models. The compartments of the former have been analyzed above, and the latter is related to network analysis, with a relatively simple structure. The key to meta-population models lies in how to accurately describe the network to reflect reality and therefore to describe the movements of individuals among subpopulations, as well as their influence on the entire population.
By considering the contact heterogeneity and movements among subpopulations, meta-population models partially overcome the shortcomings of homogeneous mixing that traditional compartment models have typically assumed. They can also analyze the spatiotemporal dynamic process of infectious diseases on a relatively large spatial scale. Readers interested in this can refer to relevant studies [49].
Most of the compartmental structures of current meta-population models for COVID-19 were relatively simple. For example, Chang et al. established a SEIR meta-population model to identify high-risk areas of disease transmission and evaluated the potential influence of local travel restrictions in Taiwan, China with the population flow data [50]. Chan et al. employed a SEIR meta-population model combined with a dynamic mobile network to describe the prevalence of COVID-19 in 10 major cities of the United States [51]. Chinazzi et al. established a global SEIR meta-population model based on air flight networks to analyze the impact of travel restrictions on the spread of COVID-19 [52]. This type of model generally has a simple compartmental structure and can be found in previous summaries; we will not list them separately.
Agent-based model
Traditional compartment models typically assume that the population were homogeneously mixing, as do the subpopulations in meta-population models; therefore, they cannot describe the heterogeneity between individuals. Agent-based models (ABMs) are simulation models in which entities (referred to as agents) interact with each other, considering the individual’s demographics, social environment, and natural environment. ABMs consist of a series of interaction rules to make agents regularly move between different places; therefore, they can simulate the real spatiotemporal spread of infectious diseases optimally in small-scale spaces from a microscopic level, and they can provide scientific evidence for the implementation of precise prevention and control. Therefore, ABMs can be regarded as an integration of dynamic models and interaction rules. Considering the complex interactions between individuals in a heterogeneous population, Hoertel et al. established a stochastic agent-based microsimulation model to examine the potential impact of post-lockdown measures in France [53]; Aleta et al. used mobility and demographic data in the Boston metropolitan area to build a detailed agent-based model, demonstrating the importance of testing and tracing in the context of relaxed social distancing [54]. However, ABMs need detailed data and heavy computation, especially with a large number of agents and complex rules; the results were influenced greatly by initial values and interaction rules. Therefore, in the published ABM-related research, the compartmental structures were relatively simple and not as complicated as the expanded compartments summarized above (Some compartmental structures applied by ABM were shown in Additional file 5).
Discussion
Dynamic models are important tools in the study of infectious disease. By constructing compartments according to infection states and related interventions and simulating transformation among different compartments, they reflect the transmission process of diseases. The models can predict the development trends of the diseases and reflect the potential transmission process and evaluate the effectiveness of various intervention measures; therefore, they play a key role in creating prevention and control measures. Establishing appropriate compartmental structures is the basis and premise of making dynamic models work. We divided the dynamics models of COVID-19 into three categories, expanded compartment models based on SEIR model, meta-population models, and ABMs and review their compartmental structures accordingly, hoping to provide a reference for modeling COVID-19 in different scenarios and provide scientific guidance for modeling research on other diseases.
After reviewing current COVID-19 modeling studies, we found that the SEIR-based expanded models consider the latent period of the virus and expand the compartments according to the infection status and measures, such as tracking, diagnosis, and isolation. With the normalization of epidemic prevention and control, methods to reflect the intensity and time of various intervention measures in the model are still worthy to study. All the three types of models have their own characteristics and application scope, with different requirements for data. Traditional compartmental models generally assume homogeneous mixing of people, which is not realistic in the real world. However, they are simple in mathematical form, easy to analyze, low data requirements, and easy to apply. Meta-population models take population movements into account, therefore, are suitable for studying the spread of infectious diseases between different countries/regions. ABMs integrated heterogeneity of agents, make them be available for building more realistic models and provide accurate support for making decisions about the prevention and control of infectious diseases. These two latter types of models are more in line with real world, while need more parameters and complex rules of individual interaction, and require a greater demand on calculation resources. In practical application, appropriate models should be selected according to different research objectives, specific problems and data availability.
As time transpires, the COVID-19 epidemic, interventions, and people’s responses exhibit different characteristics: due to the effective implementation of early NPIs, subsequent successful development and mass vaccinations against COVID-19, the continued emergence of variant strains, and the overall acceleration of variation, people's productivity and lives are gradually returning to normal, and various sports events and public activities that had been cancelled or postponed due to the epidemic have been resuming. These characteristics render the development trend of the COVID-19 epidemic more complicated and unpredictable. In addition, adjusting the model according to changes in people’s awareness of prevention is also an issue that warrants close attention. At the same time, we also need to consider the possibility of reinfection among the vaccinated population, changes in the transmission capacity of mutant viruses, and the frequency and time points of implementation of various measures in the models, which can help us predict the epidemic, evaluate the effectiveness of prevention and control measures, and formulate prevention and control strategies more accurately, so as to ensure the safe and smooth development and recovery of public events and sports events as well as provide a scientific basis to meet the new demands in the pandemic.
Conclusions
We comprehensively reviewed the current COVID-19 dynamic models and mainly analyzed the expanded models based on the SEIR model, meta-population models, and ABMs. We found that the SEIR-based expanded models were created mainly according to the COVID-19 characteristics, NPIs, and the age structure of the population, which have been relatively mature and comprehensive, but further research is needed with the vaccination and the emergence of mutant strains. The meta-population models and the ABMs usually adopt a relatively basic or simple extended compartmental structure, which can be the focus of future research. Unsolved problems such as how and when to implement prevention and control measures accurately still require the help of dynamic models, for which the compartmental structures are of primary importance. This study can provide an important reference for the construction of compartments in future COVID-19 modeling. This may be applicable, for example, when constructing a reasonable dynamic model to simulate and evaluate the effects of different interventions (and their different implementation intensity and frequency) on the prevention and control of COVID-19 during large-scale sports events, or when lifting NPIs stepwise, etc. For the modeling of other respiratory infectious diseases, it also offers important guidance value.
Acknowledgements
Not applicable.
Abbreviations
| COVID-19 | The coronavirus disease 2019 |
| WHO | World Health Organization |
| SIR | Susceptible-infected-recovered |
| SEIR | Susceptible-exposed-infected-recovered |
| NPIs | Non-pharmaceutical interventions |
| ABMs | Agent-based models |
Author contributions
The authors ZJZ, LCK and ZRC conceived and designed this review. MWD, JS and JH conducted the literature search. MWD and LCK interpreted the findings and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Major Project of Scientific and Technical Winter Olympics from National Key Research and Development Program of China (2021YFF0306000), the National Natural Science Foundation of China (81973102), Public Health Talents Training Program of Shanghai Municipality (GWV-10.2-XD21), the Shanghai New Three-year Action Plan for Public Health (GWV-10.1-XK16), 13th Five-Year National Science and Technology Major Project for Infectious Diseases (2018ZX10725-509), Key projects of the PLA logistics Scientific research Program (BHJ17J013), the Natural Science Funds of Hebei (Grant No. D2019502010) and the Fundamental Research Funds for the Central Universities (No. 2021MS074).
Availability of data and materials
Not applicable.
Declarations
Not applicable.
Not applicable.
The authors declare no competing interests.
References
Articles from Infectious Diseases of Poverty are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s40249-022-01001-y
Read article for free, from open access legal sources, via Unpaywall:
https://idpjournal.biomedcentral.com/counter/pdf/10.1186/s40249-022-01001-y
Citations & impact
Impact metrics
Citations of article over time
Article citations
Genomic profiling and spatial SEIR modeling of COVID-19 transmission in Western New York.
Front Microbiol, 15:1416580, 27 Sep 2024
Cited by: 0 articles | PMID: 39397798 | PMCID: PMC11468862
A data-driven analysis on the mediation effect of compartment models between control measures and COVID-19 epidemics.
Heliyon, 10(13):e33850, 29 Jun 2024
Cited by: 0 articles | PMID: 39071698 | PMCID: PMC11283110
Autonomous and policy-induced behavior change during the COVID-19 pandemic: Towards understanding and modeling the interplay of behavioral adaptation.
PLoS One, 19(5):e0296145, 02 May 2024
Cited by: 0 articles | PMID: 38696526 | PMCID: PMC11065316
Prediction of cross-border spread of the COVID-19 pandemic: A predictive model for imported cases outside China.
PLoS One, 19(4):e0301420, 09 Apr 2024
Cited by: 0 articles | PMID: 38593140 | PMCID: PMC11003692
Pharmaceutical and non-pharmaceutical interventions for controlling the COVID-19 pandemic.
R Soc Open Sci, 10(12):230621, 20 Dec 2023
Cited by: 2 articles | PMID: 38126062 | PMCID: PMC10731327
Go to all (15) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Trade-Off between COVID-19 Pandemic Prevention and Control and Economic Stimulus.
Int J Environ Res Public Health, 19(21):13956, 27 Oct 2022
Cited by: 2 articles | PMID: 36360836 | PMCID: PMC9653931
Simulation and forecasting models of COVID-19 taking into account spatio-temporal dynamic characteristics: A review.
Front Public Health, 10:1033432, 18 Oct 2022
Cited by: 8 articles | PMID: 36330112 | PMCID: PMC9623320
Review Free full text in Europe PMC
Dynamics and Development of the COVID-19 Epidemic in the United States: A Compartmental Model Enhanced With Deep Learning Techniques.
J Med Internet Res, 22(8):e21173, 21 Aug 2020
Cited by: 11 articles | PMID: 32763892 | PMCID: PMC7451112
An SEIR Model with Time-Varying Coefficients for Analyzing the SARS-CoV-2 Epidemic.
Risk Anal, 43(1):144-155, 19 Nov 2021
Cited by: 4 articles | PMID: 34799850 | PMCID: PMC9011870
Funding
Funders who supported this work.
13th Five-Year National Science and Technology Major Project for Infectious Diseases (1)
Grant ID: 2018ZX10725-509
Fundamental Research Funds for the Central Universities (1)
Grant ID: 2021MS074
Key projects of the PLA logistics Scientific research Program (1)
Grant ID: BHJ17J013
Major Project of Scientific and Technical Winter Olympics from National Key Research and Development Program of China (1)
Grant ID: 2021YFF0306000
Natural Science Foundation of China (2)
Grant ID: 81773487
Grant ID: 81973102
Natural Science Funds of Hebei (1)
Grant ID: D2019502010
Public Health Talents Training Program of Shanghai Municipality (1)
Grant ID: GWV-10.2-XD21
Three-Side Innovation Projects for Aquaculture in Jiangsu Province (1)
Grant ID: GWV-10.1-XK16

 2
2