Abstract
Free full text

Real-world use of tisagenlecleucel in infant acute lymphoblastic leukemia
Abstract
Infants with B-cell acute lymphoblastic leukemia (B-ALL) have poor outcomes because of chemotherapy resistance leading to high relapse rates. Tisagenlecleucel, a CD19-directed chimeric antigen receptor T-cell (CART) therapy, is US Food and Drug Administration approved for relapsed or refractory B-ALL in patients ≤25 years; however, the safety and efficacy of this therapy in young patients is largely unknown because children <3 years of age were excluded from licensing studies. We retrospectively evaluated data from the Pediatric Real-World CAR Consortium to examine outcomes of patients with infant B-ALL who received tisagenlecleucel between 2017 and 2020 (n = 14). Sixty-four percent of patients (n = 9) achieved minimal residual disease-negative remission after CART and 50% of patients remain in remission at last follow-up. All patients with high disease burden at time of CART infusion (>M1 marrow) were refractory to this therapy (n = 5). Overall, tisagenlecleucel was tolerable in this population, with only 3 patients experiencing ≥grade 3 cytokine release syndrome. No neurotoxicity was reported. This is the largest report of tisagenlecleucel use in infant B-ALL and shows that this therapy is safe and can be effective in this population. Incorporating this novel immunotherapy into the treatment of infant B-ALL offers a promising therapy for a highly aggressive leukemia.
Introduction
Infant B-cell acute lymphoblastic leukemia (B-ALL) is an aggressive form of ALL diagnosed in patients younger than 12 months of age. Most have KMT2A gene rearrangement (KMT2Ar), which is a high-risk feature associated with chemotherapy resistance and relapse.1 Despite intensive chemotherapy regimens, estimated 5-year event-free survival (EFS) for infants with KMT2Ar B-ALL is <40% and overall survival (OS) after relapse remains dismal at 20%.2-6 Novel therapies such as chimeric antigen receptor T (CART) cells are needed. The apheresis and manufacturing of CART in infants with B-ALL can be challenging because of small patient size, high leukemia burden, and heavily pretreated disease resulting in impaired T-cell function and expansion.7,8
Tisagenlecleucel (KYMRIAH, Novartis), a CD19-directed CART, was US Food and Drug Administration approved in August 2017 for treatment of relapsed/refractory (R/R) B-ALL and is commercially available to patients up to age 25 years.9-12 The ELIANA trial excluded patients younger than age 3 years; therefore, the published CART experience in infant B-ALL is limited.7,13-15 Because of poor outcomes with chemotherapy and a US Food and Drug Administration indication including infants, experience is accumulating treating R/R infant B-ALL with tisagenlecleucel. We describe the real-world experience of R/R infant B-ALL treated with tisagenlecleucel using data from the Pediatric Real-World CAR Consortium (PRWCC).
Methods
The PRWCC, a multi-institutional consortium, has collected retrospective data on pediatric and young adult patients with B-ALL who received commercial tisagenlecleucel from August 2017 through March 6, 2020. Retrospective data were abstracted from the PRWCC database of patients with infant ALL, defined as <12 months of age at the time of original diagnosis. The primary outcome for this study was 1-month complete remission (CR) rate. Responders were defined as having minimal residual disease (MRD)-negative (<0.01%) CR by flow cytometry at day 28 after infusion. Additional outcomes included 6-month OS and EFS, toxicities including cytokine release syndrome (CRS) and neurotoxicity, and rationale for noninfusion. CRS was retrospectively graded according to American Society for Transplantation and Cellular Therapy guidelines, and neurotoxicity was graded per the American Society for Transplantation and Cellular Therapy, CART Related Encephalopathy Syndrome, or institutional standard.16,17 Each center obtained independent institutional review board approval. The study was conducted in accordance with the Declaration of Helsinki.
Results and discussion
Patient characteristics
Of 16 patients with infant B-ALL within the PRWCC database, 14 underwent infusion with tisagenlecleucel. Two patients with primary refractory disease were not infused: 1 because manufacturing failure and the other because of progression of leukemia and death. Table 1 includes baseline characteristics and response to therapy for infused patients.
Table 1.
Baseline characteristics and CART details
| All patients | Responders* | Nonresponders | |
|---|---|---|---|
| n = 14 | n = 9 | n = 5 | |
| Age at CART, median (range), y | 0 (0-9) | 2 (0-9) | 1 (0-2) |
| KMT2Ar status | |||
 Yes Yes | 12 | 8 | 4 |
 No No | 1 | 0 | 1 |
 Unknown Unknown | 1 | 1 | 0 |
| Frontline therapy EOI response | |||
 MRD-negative CR MRD-negative CR | 5 | 3 | 2 |
 MRD-positive CR MRD-positive CR | 7 | 5 | 2 |
 >M1 >M1 | 1 | 1 | 0 |
 Not assessed Not assessed | 1 | 0 | 1 |
| Prior immunotherapy and HSCT | |||
 Blinatumomab Blinatumomab | 3 | 3 | 0 |
 Inotuzumab Inotuzumab | 3 | 1 | 2 |
 HSCT HSCT | 4 | 2 | 2 |
| Disease category before CART | |||
 Primary refractory Primary refractory | 5 | 4 | 1 |
 First relapse† First relapse† | 5 | 2 | 3 |
 Second or greater relapse Second or greater relapse | 4 | 3 | 1 |
| MRD status before CAR | |||
 MRD-negative CR MRD-negative CR | 3 | 3 | 0 |
 MRD-positive CR MRD-positive CR | 6 | 6 | 0 |
 >M1 >M1 | 5 | 0 | 5 |
| ALC at time of T-cell collection, median (range), cells/μL | 1610 (240-3330) | 1564 (240-5120) | 1692 (300-3330) |
| CART cell dose infused, median (range), (×106 CAR T cells/kg) | 2.29 (1.3-4.6) | 2.65 (1.6-4.6) | 1.72 (1.3-2.4) |
| CRS | |||
 Any grade Any grade | 11 | 6 | 5 |
 ≥Grade 3 ≥Grade 3 | 3 | 1 | 2 |
| HSCT while in CART-mediated remission ‡ | |||
 Yes Yes | 1 | 1 | 0 |
 No No | 13 | 8 | 5 |
| Disease status after CART | |||
 Refractory to CART Refractory to CART | 4 | 0 | 4 |
 Remains in CR after CART Remains in CR after CART | 7 | 7 | 0 |
 Relapsed after CART Relapsed after CART | 3§ | 2 | 1 |
| Myeloid transformation | |||
 Yes Yes | 4 | 1 | 3 |
 No No | 3 | 1 | 2 |
| Death | 4 | 0 | 4 |
| Time after CART at last follow-up, median (range), d | 231 (44-856) | 290 (137-856) | 85 (44-389) |
ALC, absolute lymphocyte count; EOI, end of induction.
Of 14 infused patients, the majority (86%) had KMT2Ar. Of the 2 remaining patients, 1 had normal (KMT2A-germline) and 1 had unknown cytogenetics. Four patients (29%) had prior hematopoietic stem cell transplant (HSCT). Three patients (21%) had prior blinatumomab and 3 patients (21%) had prior inotuzumab. Indications for CART included primary refractory (n = 5), first relapse (n = 5), and second or greater relapse (n = 4). Before CART infusion, 5 patients (36%) had >M1 marrow (>5% blasts; range, 6%-90% blasts) and 9 patients (64%) were in morphologic CR, including 3 patients in MRD-negative CR. Before infusion, 9 patients were CNS1, 2 were CNS2, and 3 were not assessed. No patients had other active extramedullary disease at time of CART infusion. The median time from apheresis to infusion was 6 weeks (range, 5-15 weeks). All patients received lymphodepleting chemotherapy (fludarabine/cyclophosphamide) before infusion. Median CART dose was 2.2 × 106 cells/kg (range, 1.3-4.6 × 106 cells/kg).
Responders vs nonresponders
Nine patients (64%) achieved an MRD-negative CR at day 28 after CART infusion. Five patients (36%) failed to achieve MRD negativity (nonresponders), 1 of whom was in morphologic CR (flow MRD not assessed) and 4 of whom failed to achieve morphologic CR (≥5% disease). The median duration of follow-up was 231 days (range, 44-856 days).
Disease status before CART infusion affected outcomes for this population. All 5 patients with >M1 marrow before infusion were nonresponders, whereas all 9 responders were in morphologic CR at the last evaluation before infusion (MRD-positive, n = 6; MRD-negative, n = 3). The median CART dose for responders and nonresponders was 2.65 cells/kg (range, 1.6-4.6 × 106 cells/kg) vs 1.72 cells/kg (range, 1.3-2.4 × 106 cells/kg), respectively. There were equal numbers of responders and nonresponders with prior HSCT (each n = 2). All 3 patients with previous blinatumomab exposure achieved MRD-negative CR.
Seven of 9 responders (78%) had ongoing CR at last follow-up, with only 1 patient undergoing HSCT in remission after CART. This patient underwent HSCT preemptively per the discretion of the treating physician. Two responders relapsed after CART. One underwent HSCT following relapse, and both remain alive at last follow-up. The median duration of follow-up for responders was 290 days (range, 137-856 days). Of the nonresponders, all remained refractory and 4 died (refractory disease, n = 3; transplant-related mortality, n = 1) with a median time to death of 71 days (range, 44-132 days). The median duration of follow-up for nonresponders was 85 days (range, 44-389 days). The median time of the duration of B-cell aplasia was 171 days (28-414 days). The estimated post-CART 6-month EFS and OS in this cohort were 48% and 71%, respectively. Figure 1 illustrates each patient’s disease course from the time of CART infusion through last follow-up.
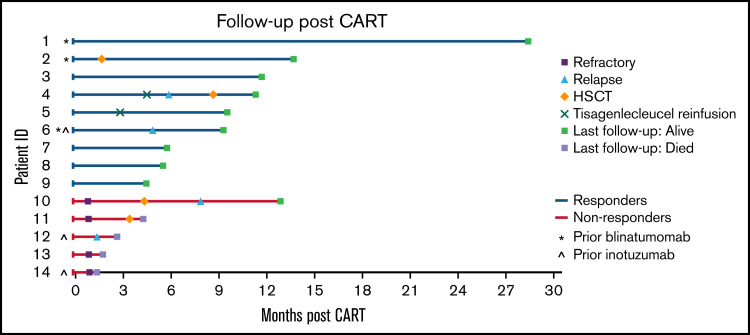
Swimmer plot illustrating the clinical course of patients with infant B-ALL following CART infusion.
Myeloid transformation has been described in KMT2Ar B-ALL following CD19-directed therapy.18-20 Lineage switch, including myeloid transformation, is reported in infant B-ALL, independent of CART.21 In this infant cohort, 4 patients (29%, 1 responder and 3 nonresponders) experienced myeloid transformation following CART, 3 of whom had KMT2Ar.
Safety
Overall, CART was tolerable in this cohort, with 11 patients (79%) experiencing CRS of any grade and only 3 patients (21%) experiencing ≥grade 3 CRS. One patient with grade 3 CRS received tocilizumab, steroids, and anakinra. One patient with grade 4 CRS received steroids and anakinra. No other patients with CRS required medications for immune suppression. There was no neurotoxicity reported.
This analysis demonstrates that CART is feasible, tolerable, and associated with clinical responses in R/R infant B-ALL. Of patients in morphologic remission (with or without MRD) at the time of infusion, all achieved, and the majority maintained, an MRD-negative remission after CART. Conversely, all patients with high disease burden at time of CART infusion (>M1 marrow) were refractory to this therapy. Although further validation is necessary, these data suggest infants with >M1 marrow may be at high risk of nonresponse to CART. Additionally, MRD detection at day 28 after CART emerged as a poor prognostic indicator, with 80% of MRD-positive patients experiencing death, in contrast to 78% of patients achieving MRD-negative CR who remain alive with sustained durable responses at time of last follow-up. This is a promising outcome for this chemotherapy refractory cohort of patients.
There are a number of challenges associated with leukapheresis and manufacturing of CART in small patients such as those with infant B-ALL. In this cohort, only 1 of the 16 patients who underwent leukapheresis was unable to be infused because of manufacturing failure. This is in line with other reports showing feasibility of collection and manufacturing in this cohort.22 Last, this report demonstrates the safety of tisagenlecleucel in infant B-ALL with manageable CRS and no neurotoxicity reported. Although these data are limited by the small number of patients, retrospective nature, and short duration of follow-up, in the absence of a clinical trial, it highlights that incorporation of CART into the treatment for this highly chemotherapy-resistant population offers promise for future improvements in outcomes for R/R infant B-ALL. Larger studies to better understand the long-term outcomes in responders and optimal timing of therapy are needed.
Acknowledgments
The authors appreciate the efforts of all those who have contributed data to the PRWCC. The authors also appreciate the support from Data, Regulatory, Administrative, and Contractual teams at Stanford University. The authors are thankful to our patients and families who allow the world to learn from them.
Authorship
Contribution: A.M., L.P., E.M.G., E.H.B., and L.M.S. designed the study, performed data analysis and interpretation, wrote the initial manuscript draft, and contributed to subsequent revisions of the manuscript; C.B. and S.P. assisted with data collection and analysis; and all authors provided assistance with data collection, interpretation of data, and reviewed the manuscript.
Conflict-of-interest disclosure: C.L.P. reports an advisory committee for Novartis. H.E.S. reports Novartis ad board and speakers bureau. S.P.M. reports ad boards for Novartis and Jazz. M.R.V. reports Novartis ad board, consulting and stocks for Fate Therapeutics and Bmogen, and consulting for Uptodate. G.D.M. reports Novartis ad board and speakers bureau and consulting for Eliana Trial and on Steering Committee. P.A.B. reports ad boards for Servier, Jazz, Novartis, and Janssen. M.Q. reports Novartis ad board and Mesoblast. M.L.H. reports Novartis ad board and Sobi ad board. P.S. reports Consulting or Advisory Role for Mesoblast and Takeda. K.J.C. reports research support and consulting for Novartis. C.L.M. reports consulting for Lyell Pharmaceuticals, Apricity, BMS, Medimmune Tech, and Nektar and equity from Allogene, Apricity, and Lyell Pharmaceuticals. T.W.L. reports consulting for Novartis, Cellectis, and Bayer and research funding from Novartis, Pfizer, and Bayer. E.M.G. reports consulting for Syndax Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Amy Moskop, Division of Hematology/Oncology/Blood and Marrow Transplantation, Department of Pediatrics, Medical College of Wisconsin and Children’s Wisconsin, 8701 W Watertown Plank Rd, MFRC 3018, Milwaukee, WI 53226; e-mail: ude.wcm@poksoma.
References
Articles from Blood Advances are provided here courtesy of The American Society of Hematology
Full text links
Read article at publisher's site: https://doi.org/10.1182/bloodadvances.2021006393
Read article for free, from open access legal sources, via Unpaywall:
https://ashpublications.org/bloodadvances/article-pdf/6/14/4251/1909076/advancesadv2021006393.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/128458517
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1182/bloodadvances.2021006393
Article citations
Treatment of Relapsed Acute Lymphocytic Leukemia in Adult Patients.
Curr Treat Options Oncol, 25(8):993-1010, 25 Jun 2024
Cited by: 0 articles | PMID: 38916714 | PMCID: PMC11329612
Review Free full text in Europe PMC
Allogeneic chimeric antigen receptor T cells for children with relapsed/refractory B-cell precursor acute lymphoblastic leukemia.
Haematologica, 109(6):1689-1699, 01 Jun 2024
Cited by: 0 articles | PMID: 38832424 | PMCID: PMC11141659
Review Free full text in Europe PMC
Chimeric antigen receptor T-cell therapy in childhood acute myeloid leukemia: how far are we from a clinical application?
Haematologica, 109(6):1656-1667, 01 Jun 2024
Cited by: 0 articles | PMID: 38832421 | PMCID: PMC11141645
Review Free full text in Europe PMC
Overcoming the challenges of primary resistance and relapse after CAR-T cell therapy.
Expert Rev Clin Immunol, 20(7):745-763, 14 May 2024
Cited by: 0 articles | PMID: 38739466
Review
Finding Your CAR: The Road Ahead for Engineered T Cells.
Am J Pathol, 194(8):1409-1423, 01 May 2024
Cited by: 0 articles | PMID: 38697513
Review
Go to all (20) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Impact of High Disease Burden on Survival in Pediatric Patients with B-ALL Treated with Tisagenlecleucel.
Transplant Cell Ther, 28(2):73.e1-73.e9, 04 Dec 2021
Cited by: 15 articles | PMID: 34875402 | PMCID: PMC8816862
Systematic Review and Meta-analysis of CD19-Specific CAR-T Cell Therapy in Relapsed/Refractory Acute Lymphoblastic Leukemia in the Pediatric and Young Adult Population: Safety and Efficacy Outcomes.
Clin Lymphoma Myeloma Leuk, 21(4):e334-e347, 17 Dec 2020
Cited by: 23 articles | PMID: 33573914
Review
CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials.
Lancet Haematol, 8(10):e711-e722, 01 Oct 2021
Cited by: 51 articles | PMID: 34560014 | PMCID: PMC9026766
Chimeric antigen receptor T cells for acute lymphoblastic leukemia.
Am J Hematol, 94(s1):S24-S27, 22 Mar 2019
Cited by: 22 articles | PMID: 30784101
Review

 1,*
1,* 


