Abstract
Free full text

Endothelial cell-specific loss of eNOS differentially affects endothelial function
Abstract
The endothelium maintains and regulates vascular homeostasis mainly by balancing interplay between vasorelaxation and vasoconstriction via regulating Nitric Oxide (NO) availability. Endothelial nitric oxide synthase (eNOS) is one of three NOS isoforms that catalyses the synthesis of NO to regulate endothelial function. However, eNOS’s role in the regulation of endothelial function, such as cell proliferation and migration remain unclear. To gain a better understanding, we genetically knocked down eNOS in cultured endothelial cells using sieNOS and evaluated cell proliferation, migration and also tube forming potential in vitro. To our surprise, loss of eNOS significantly induced endothelial cell proliferation, which was associated with significant downregulation of both cell cycle inhibitor p21 and cell proliferation antigen Ki-67. Knockdown of eNOS induced cell migration but inhibited formation of tube-like structures in vitro. Mechanistically, loss of eNOS was associated with activation of MAPK/ERK and inhibition of PI3-K/AKT signaling pathway. On the contrary, pharmacologic inhibition of eNOS by inhibitors L-NAME or L-NMMA, inhibited cell proliferation. Genetic and pharmacologic inhibition of eNOS, both promoted endothelial cell migration but inhibited tube-forming potential. Our findings confirm that eNOS regulate endothelial function by inversely controlling endothelial cell proliferation and migration, and by directly regulating its tube-forming potential. Differential results obtained following pharmacologic versus genetic inhibition of eNOS indicates a more complex mechanism behind eNOS regulation and activity in endothelial cells, warranting further investigation.
Introduction
The endothelium forms an innermost layer of every blood vessel and acts as a macromolecular barrier that regulates movement of small and large molecules, paracrine factors and gases between blood vessels and adjacent tissues [1]. The endothelial cells also modulate platelet adherence, leukocyte migration, smooth muscle cell proliferation and apoptosis [1]. In addition, they modulate blood fluidity by regulating platelet production and control inflammatory responses by producing cytokines and adhesion molecules [2]. One of the major function of endothelium is to regulate vascular tone by balancing the production of flow-sensitive nitric oxide (NO), which is an endothelium-dependent vasodilator of the underlying smooth muscle cells [3]. Under normal physiological conditions, NO has cardioprotective effects, where it diffuses to the surrounding tissues and results in muscle relaxation and prevents inflammation by inhibiting leukocyte adhesion and platelet aggregation [3]. Kubes et al., have shown that inhibition of NO in the vascular endothelium results in upregulation of CD11/CD18, protein essential for leukocyte adhesion [4]. Additionally, NO is also critical for the process of vascular remodelling, specifically VEGF-induced angiogenesis. It is reported that angiogenesis is proportional to the level of NO production in mouse vessels [5].
Inhibition of NO production occurs in the cardiovascular diseases such as atherosclerosis [6]. The shortage of NO is thought to occur either by decreased NO production or increased NO degradation [7]. NO is produced by an enzyme called endothelial nitric oxide synthases (eNOS). This enzyme catalyzes the conversion of L-Arginine into L-Citrulline, producing NO in the process [8]. Therefore, decreased NO production can be attributed to decreased availability of L-arginine or decreased availability and activity of eNOS [8]. ENOS, is expressed mainly in endothelium of the large arteries and impaired eNOS expression is implicated in development and progression of cardiovascular diseases [9]. ENOS and NO production constitutes a positive feed-back loop; upon changes in blood flow NO production increases and activates PI3-kinase dependent phosphorylation of protein kinase B leading to phosphorylation of eNOS and production of NO [10]. NO can also directly modulate expression of eNOS; such as inhibiting endogenous NO production using nitro-L-arginine-methyl ester (L-NAME) results in reduction of eNOS expression and increased exogenous NO causing increased eNOS expression [11].
In summary, eNOS is an essential regulator of endothelial function that constitute angiogenic potential, proliferation and migration. There are several reports investigating the role of eNOS and NO on endothelial angiogenic potential. Accordingly, Namba et al., reported that eNOS overexpression promotes angiogenesis and stimulates vascular endothelial growth factor (VEGF) production, and that L-NAME, a pharmacologic inhibitor of eNOS, inhibits eNOS-overexpression-associated blood flow [12]. There are other reports demonstrating inhibition of angiogenesis following knockdown of eNOS in vivo [5, 13]. There are also reports on cell proliferation following pharmacological inhibition of eNOS by its inhibitor L-NAME but this is not investigated following genetic loss of eNOS in endothelial cells. Therefore, to better delineate the role of eNOS in endothelial function and to understand related mechanisms, we genetically inhibited eNOS in cultured endothelial cells and investigated endothelial function and related regulators of endothelial function in vitro. We for the first time show that knockdown of eNOS significantly induces endothelial cell proliferation, downregulates both cell cycle inhibitor p21 and cell proliferation antigen Ki-67; induces migration and inhibits tube forming potential of endothelial cells. Loss of eNOS significantly activate MAPK/ERK, but inhibits PI3-K/AKT signaling pathway in endothelial cells. As previously reported, pharmacologic inhibition of eNOS inhibited cell proliferation and migration. Differential results obtained following pharmacologic versus genetic inhibition of eNOS indicates a more complex mechanism behind eNOS regulation and activity in endothelial cells, warranting further investigations.
Material and methods
Cell culture
Human umbilical vein or human pulmonary artery endothelial cells (HUVECs or HPAECs, pooled, Lonza; passage 4–6) were cultured in endothelial cell growth medium-2 (EGMTM-2 BulletkitTM; Lonza) supplemented with growth factors, serum and antibiotics at 37°C in humidified 5% CO2. siRNA-mediated eNOS gene knockdown was performed with sieNOS or scrambled control in accordance with the manufacture’s guidelines. A standard reverse transfection reagent (Lipofectamine ® 3000, Invitrogen), and 5 nm sieNOS [DharmaconTM: Cat # 106158 (sieNOS) or #106159 (sieNOS#)] or scrambled control (DharmaconTM: Cat # 2575450) were used. RNA or protein were collected using Trizol reagent (Invitrogen) or RIPA buffer, respectively, following 24, 48 or 72 hrs post-transfection.
Quantitative real time PCR
Total RNA was extracted, and complementary DNA (cDNA) was synthesized using 1μg RNA and the QuantiTech Reverse Transcription Kit (Qiagen). SYBR Select Master Mix (Applied Biosystems), forward and reverse primers for eNOS or GAPDH [14], and cDNA were mixed, and qPCR was performed using QuantStudio®3 Real-Time PCR instrument (Applied Biosystems). Comparative Delta Delta CT method was employed for data analysis.
Immunoblotting
Cell lysates from cultured endothelial cells were prepared in RIPA buffer (Sigma) 24, 48, and 72 hrs post-transfection with either sieNOS or its scrambled control. Equal amounts of protein were loaded on SDS-polyacrylamide gels and transferred to PVDF membrane (Thermo Fisher). Membranes were blocked for 1 hour and incubated with primary antibody specifically targeting eNOS, Ki67, p21, AKT, p-AKT, ERK, and p-ERK overnight at 4°C. After incubating with secondary antibody, bands were visualized with ECL substrate using chemiluminescence channel and 700 channel in Li-Cor Fc Odyssey imaging system, and quantified.
Proliferation assay
HUVECs were first cultured in endothelial cell growth medium-2 (EGMTM-2 BulletkitTM; Lonza) supplemented with growth factors, serum and antibiotics at 37°C in humidified 5% CO2, and seeded at a density of 1–2×104 cells/well in 96-well plates, transfected with sieNOS (5 nmol) or scrambled control (5 nmol), and then cell proliferation was evaluated 24, 48 and 72 hrs post-transfection using WST-8 Cell Proliferation Assay Kit (Cayman Chemicals) as described [15]. HUVECs were transfected in a 6-well plate (1.5–2×105 cells/well) with either sieNOS or scrambled control and cells were counted using CytoSmart cell counter 24, 48 and 72 hrs post-transfection. Cells were counted in triplicates for each biological replicate and average cell number was determined for each sample.
Scratch assay
HUVECs were cultured in endothelial cell growth medium-2 (EGMTM-2 BulletkitTM; Lonza) supplemented with growth factors, serum and antibiotics at 37°C in humidified 5% CO2 and transfected with sieNOS (5 nmol) or scrambled control (5 nmol), and seeded at a density of 2 x 105 cells/well in a 6-well plate and allowed to grow to 60–80% confluency. Each well was then administered a consistent straight scratch using p1000 pipette tip. Cells were then washed with 1X PBS for one time and incubated in DMEM supplemented with 1% FBS. Phase-contrast microscopy using an adapted camera (Optika) was employed to take pictures of cells in each well migrating into the scratch over 3 time points (0, 8 and 20 hrs) to evaluate for migrating capacity as described [16].
In vitro tube-formation assay
The In vitro Angiogenesis Kit (Millipore) was employed to evaluate endothelial angiogenic properties. HUVECs were transfected with sieNOS and seeded at a density of 2 x 105 cells/well in a 6-well plate and allowed to grow to ~75% confluency. The kit-provided matrix solution was added into designated wells of a 96-well plate. Transfected cells from the previous preparation were then harvested and seeded at a density of 1–1.5 x 104 cells/well onto the designated wells in EGM-2 medium. Phase-contrast microscopy was employed (Optika) to take pictures of cells under phase-contrast in each designated well over time to monitor formation of tube-like structures in vitro. Mesh area and tube-length was measured using angiogenesis analyzer software from Image J.
Statistical analysis
Data are expressed as the mean ± SD. Student’s t-test was applied when the means of two groups were compared using GraphPad-Prism software. A p-value <0.05 was considered statistically significant.
Results and discussion
In order to understand the role of eNOS in endothelial cell function in vitro, we first knocked down eNOS in cultured HUVECs and confirmed successful knockdown at transcript and protein levels in sieNOS-transfected endothelial cells (Fig 1A and 1B). We then evaluated cell proliferation in eNOS-knocked down and control endothelial cells using MTT assay. To our surprise, proliferation assay demonstrated significantly higher proliferation in sieNOS-transfected endothelial cells in comparison to control cells in a time-dependent manner at the studied 24, 48 and 72 hrs time-points (Fig 1C). We further validated our findings using automated Cytosmart cell-counter and another sieNOS# molecule, which also demonstrated significantly higher proliferation in the eNOS-knocked down endothelial cells (S1A–S1D Fig). Cell viability was also evaluated using Cytosmart cell-counter, but it was not affected by eNOS-knocked down at all three studied time points (S1B Fig). It is important to note that there are limited literatures investigating effect of knocking down eNOS on endothelial cells and there are almost no reports investigating the effect of eNOS-knock down on endothelial cell proliferation. However, the effect of eNOS-overexpression on cell proliferation has been investigated, which indirectly support our findings. Kader et al., reported that in bovine aortic endothelial cells eNOS overexpression inhibited endothelial cell proliferation [17]. Accordingly, we have previously also observed that breast cancer gene 2-deficiency in endothelial cells was associated with reduced eNOS expression and increased cell proliferation [14]. We then evaluated the effect of eNOS-knock down on p21 expression, which is a cyclin-dependent kinase inhibitor that promotes endothelial cell cycle arrest and inhibits proliferation [18]. Our transcript and protein data on p21 expression demonstrated reduced p21 expression in the eNOS-knocked down endothelial cells in comparison to control endothelial cells for all the studied (24, 48 and 72 hrs) time-points (Fig 1E and 1F). We also investigated the effect of eNOS-knockdown on the expression of endothelial Ki67, which is an endothelial cell proliferation marker [19]. Our Ki67-immunoblottng data demonstrated increased Ki67 expression following knockdown of eNOS in endothelial cells (Fig 1F). Knockdown of eNOS-associated reduced cell cycle inhibitor p21 expression and increased proliferation marker Ki67 expression, both support an increased endothelial cell proliferation following knockdown of eNOS in endothelial cells.
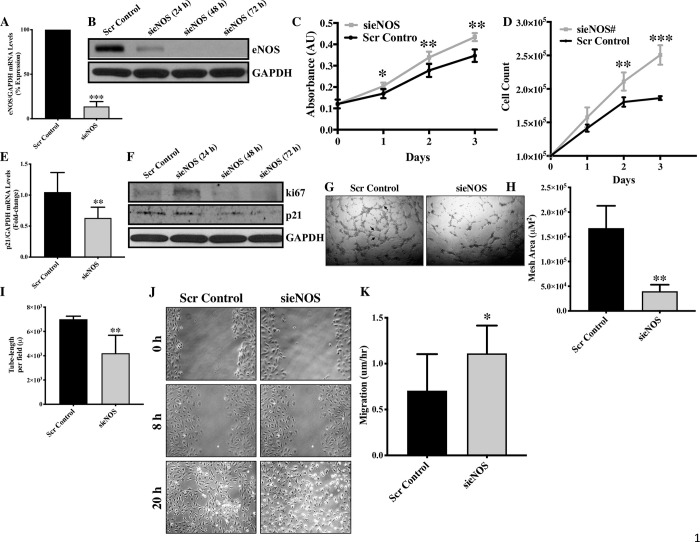
(A) HUVECs were transfected with sieNOS or scrambled control for 24, 48 and 72 hrs and the knockdown effect was confirmed by qPCR (B) western blot. (C) Endothelial cell proliferation was evaluated 24, 48 and 72 hrs post-transfection using proliferation kit and (D) by counting the cells using Cyto Smart cell counter. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate. (E) Later, RNA was extracted 24 hrs post-transfection and qPCR for p21 and (F) protein was extracted 24, 48 and 72 hrs post-transfection and immunoblot was performed for ki67, p21 and GAPDH. (G) ENOS-knockdown and control HUVECs were seeded on Matrigel, and pictures were taken 6 hrs post-seeding (tubes are marked by arrows) and (H) then quantification for mesh area and (I) tube-length were performed. (J) Scratch assay was performed in sieNOS and scrambled control-transfected HUVECs and pictures were taken at 0, 8 and 20 hrs and (K) the cell migration was quantified. *p<0.05, **p<0.01, ***p<0.001 vs. Scr Control. N = 3–5 in triplicates.
We also evaluated the other indices of endothelial function, such as cell migration and angiogenesis in the form of tube forming potential. Endothelial cell-specific knockdown of eNOS significantly inhibited tube forming potential in the form of reduced mesh area and tube-length in sieNOS-transfected endothelial cells (Fig 1G–1I). This finding is in line with previous finding, where inhibiting eNOS expression in endothelial cells is shown to inhibit angiogenesis [20, 21] However, knockdown of eNOS significantly enhanced the migratory ability of endothelial cells (Fig 1J and 1K).
HUVECs are very well characterized endothelial cells and have been used as representative endothelial cells to answer fundamental endothelial function-related questions [15, 16, 22], however, endothelial cells do display considerable functional and transcriptomic heterogeneity depending on their location [23]. To confirm that loss of eNOS-associated induction of proliferation and migration is not HUVEC-specific, we transfected Human Pulmonary Artery Endothelial Cells (HPAECSs) with sieNOS and confirmed successful eNOS silencing (Fig 2A). Similar to HUVECs, proliferation assay demonstrated significantly increased proliferation in eNOS-deficient in comparison to control HPAECs at all the three studied time-points (Fig 2B). Loss of eNOS-associated increased proliferation was again associated with reduced p21 expression in HPAECs (Fig 2C and 2D). Then, a scratch assay was performed on HPAECs and similar to HUVECs, an enhanced cell migration was observed in eNOS-deficient HPAECs (Fig 2E and 2F). This is contrasting to previous reports where eNOS pharmacologic inhibition by L-NAME is shown to inhibit endothelial cell migration[18, 19]. However, this contrasting finding may be attributed to the non-specificity of the inhibitor or the complex regulation of endothelial function by eNOS in a context-dependent manner. Interestingly, genetic transfer of eNOS in aortic smooth muscle cell is shown to inhibit migration [24], which indirectly support our data on knockdown of eNOS-associated induced cell migration. ENOS is an important regulator of VEGFa (vascular endothelial cell growth factor A), which further regulates angiogenesis and endothelial function by regulating VEGF/eNOS/AKT pathway [5]. We measured VEGFa transcript level in eNOS-knocked down endothelial cells, however VEGFa transcript levels were unaffected by eNOS-knock down (S1C Fig). To further investigate the underlying mechanism of how knockdown of eNOS led to an increased cell proliferation, we evaluated the expression and activation level of mechanistic regulators of endothelial cell proliferation PI3-K/AKT and MAPK/ERK signaling pathway [25, 26]. AKT is a downstream target of phosphatidylinositol 3-kinase (PI3K) and the direct binding of PI3K and phosphorylated AKT leads to AKT activation. Subsequently, AKT can phosphorylate its downstream target including eNOS [27], which eventually leads to an increased NO release.
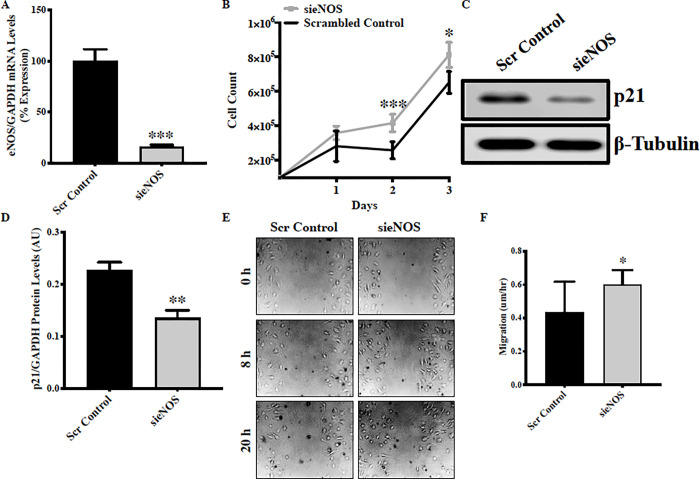
Human pulmonary artery endothelial cells (HPAECs) were transfected with sieNOS for 24, 48 and 72 hrs in HPAECs and (A) the knockdown effect was confirmed by qPCR. (B) Endothelial cell proliferation was evaluated 24, 48 and 72 hrs post-transfection using using Cyto Smart cell counter. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate. (C) Protein was extracted 48 hrs post-transfection and immunoblot was performed for p21 and β-tubulin. (D) RNA was extracted 24 hrs post-transfection and qPCR for p21 was performed. (E) Scratch assay was performed in sieNOS and scrambled control-transfected HPAECs and pictures were taken at 0, 8 and 20 hrs (F) and the cell migration was quantified. *p<0.05, **p<0.01, ***p<0.001 vs. Scr Control. N = 3–5 in triplicates.
There is no literature investigating activation of AKT following eNOS knockdown. However, our immunoblotting data demonstrated a significant reduction in the activation of AKT in eNOS-knocked down endothelial cells in comparison to control cells (Fig 3A–3C). Initially, following 24 hrs of transfection, pAKT appeared to be up-regulated but this time does not coincide with the complete eNOS protein-loss following sieNOS transfection, so we quantified AKT activation 48-hrs post-transfection, which is associated with eNOS-protein loss (Fig 1B). Interestingly, eNOS-protein loss was associated with significantly reduced AKT activation, however, the mechanism behind eNOS loss-associated reduced AKT activation is unknown. It is also reported that inhibition of MAPK/ERK signaling pathway; such that deletion of ERK in primary endothelial cells results in decreased cell proliferation indicating an important role of ERK in endothelial cell proliferation [28]. Mechanistically, eNOS contains a motif that can be recognized by ERK, causing eNOS activation and thereby increased NO production [29]. Accordingly, we also measured ERK activation in eNOS-deficient endothelial cells. Our data show an increased trend towards increased ERK activation in eNOS-deficient endothelial cells (Fig 3A, 3D and 3E). It remains to be elucidated that how increased ERK-activation promotes proliferation in eNOS-deficient endothelial cells. Our findings for the first time show that knockdown of eNOS activates MAPK/ERK signaling pathway, which may potentially mediate enhanced endothelial cell proliferation in eNOS-deficient endothelial cells.
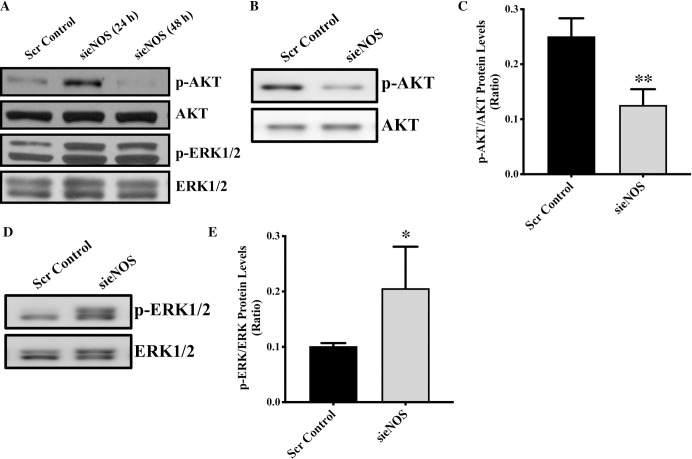
(A) HUVECs were transfected with sieNOS or scrambled control for 24 and 48 hrs. Total proteins were extracted 24 and 48 hrs post-transfection immunoblotting for p-AKT, AKT, p-ERK, ERK was performed. Immunoblots for (B, C) p-AKT, AKT, and (D, E) pERK1/2 and ERK1/2 were performed and quantified. *p<0.05, **p<0.01 vs. Scr Control. N = 3–5 in triplicates.
Given the unexpected nature of our findings about increased proliferation and migration but reduced angiogenesis in eNOS-deficient endothelial cells, we also evaluated proliferation, migration and angiogenesis following pharmacologic inhibition of eNOS using L-NAME. To our further surprise, we observed a dose-dependent inhibition of cell proliferation (Fig 4A) and tube forming potential (Fig 4B–4D) but increased cell migration (Fig 4E and 4F) in L-NAME-treated endothelial cells in comparison to vehicle-treated endothelial cells. Cell viability was not affected by L-NAME treatment (S1D Fig). However, this finding is in line with previous report, where L-NAME inhibited endothelial progenitor cells proliferation [20]. We next used another and more-direct eNOS inhibitor; L-NMMA, which is the active compound in L-NAME [30]. L-NMMA-treatment to the endothelial cells replicated our finding from L-NAME, where L-NMMA treatment also inhibited cell proliferation (Fig 5A) and promoted cell migration (Fig 5B and 5C). The exact mechanism of eNOS-associated inhibition of cell proliferation needs to be further investigated, however, it appears that this phenotype is not directly arising from the inhibition of eNOS activity leading to NO production as it is not affected by L-NAME or L-NMMA but mechanisms associated with processes up-stream to eNOS activation. Genetic and pharmacologic inhibition of eNOS-associated migration and angiogenic potential indicate that these mechanisms are directly associated with eNOS activation and related down-stream processes such as NO production.
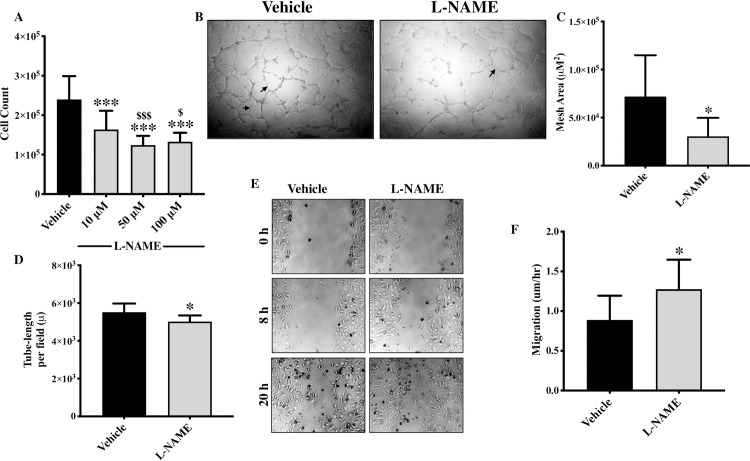
HUVECs were seeded until they reached 60–70% confluency and were treated with different dose of L-NAME (10, 50 and 100uM). (A) Cells were collected and counted at each dose using Cyto Smart cell counter. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate. (B) HUVECs were seeded on Matrigel, and pictures were taken 6 hrs post-seeding in the presence of L-NAME or vehicle (tubes are marked by arrows) and (C) then quantification for mesh area and (D) tube-length were performed. (E) HUVECs were seeded until they reached 70–80% confluency and were treated with L-NAME or vehicle. A scratch was made and pictures were taken at 0, 8 and 20 hrs and (F) the cell migration was quantified. *p<0.05, ***p<0.001 vs. vehicle. #p<0.05, ###p<0.001 vs. 10μM L-NAME. N = 3–5 in triplicate.
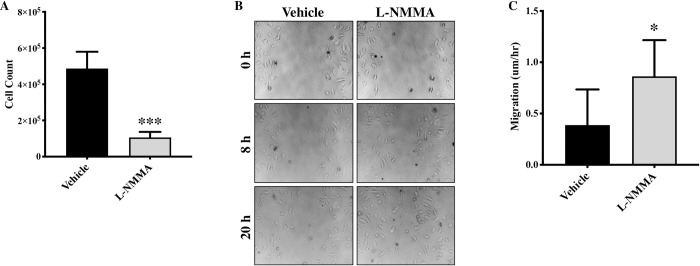
(A) HUVECs were seeded until they reached 60% confluency and were treated with L-NMMA (50uM) or vehicle and 24 hrs later cells were collected and counted using Cyto Smart cell counter. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate. (B) Cell migration assay was performed at 0, 8 and 20 hrs. HUVECs were seeded until they reached 70–80% confluency and were treated with L-NMMA or vehicle. A scratch was made and pictures were taken at 0, 8 and 20 hrs and (C) the cell migration was quantified. *p<0.05, ***p<0.001 vs. vehicle. N = 3–5 in triplicate.
Inhibition of eNOS and NO; both are associated with development of cardiovascular diseases, and accordingly there are therapeutics that are in use and/or being developed to enhance eNOS expression/activity and NO production to treat cardiovascular diseases, such as myocardial infarction, cardiac hypertrophy, diastolic heart failure, arteriosclerosis, and hypertension in humans and in animal models [31, 32]. However, both, beneficial and detrimental effects of eNOS activation have also been reported [33]. Our findings about knockdown of eNOS on endothelial cell proliferation, migration, p21, PI3-K/AKT and MAPK/ERK signaling pathway adds further complexity to the eNOS expression/activation-mediated treatment strategy and warrants a more detailed investigation particularly in the settings where these factors are associated with the progression of the disease.
Supporting information
S1 Fig
(A) HUVECs were transfected with either scrambled control or sieNOS# (5 nM each) for 24 hrs and RNA was extracted. The knockdown effect was confirmed by qPCR. ***p<0.0001 vs. Scr Control. (B) Cell viability was examined 24, 48 and 72 hrs post-transfection in HUVECs. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate. (C) HUVECs were transfected with either scrambled control or sieNOS for 48 hrs and RNA was extracted to perform qPCR for VEGFa. (D) Cell viability was evaluated following 24 hrs of vehicle or different dose of L-NAME treatment in HUVECs. Each triplicate was counted twice, and average of all triplicates was calculated for each biological replicate.
(TIF)
Funding Statement
Krishna Singh received funding from the Project Grant (FRN # 153216), Canadian Institutes of Health Research, Canada to KS. KS is also the recipient of the 2018/19 National New Investigator Award- Salary Support from the Heart and Stroke Foundation of Canada, Canada.
Data Availability
All relevant data are contained within the paper and its Supporting Information files.
References
Decision Letter 0
22 Feb 2022
PONE-D-22-01733Endothelial cell-specific Loss of eNOS Promotes ProliferationPLOS ONE
Dear Dr. Singh,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
The work is of interest since it proposes an original view of the potential role of eNOS on endothelial cells. However in the current form it seems yet preliminary and required many precisions and some experimental confirmations to be convincing. In particular it needs to take into account as underlined by one reviewer, in what context your hypothesis can take place and use the right tools for this aim (cell source, chemicals). Furthermore a point by point response to the concerns underlined by both reviewers is expected.
Please submit your revised manuscript by Apr 08 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Alain-Pierre Gadeau, Ph.D
Academic Editor
PLOS ONE
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
2. PLOS ONE now requires that authors provide the original uncropped and unadjusted images underlying all blot or gel results reported in a submission’s figures or Supporting Information files. This policy and the journal’s other requirements for blot/gel reporting and figure preparation are described in detail at https://journals.plos.org/plosone/s/figures#loc-blot-and-gel-reporting-requirements and https://journals.plos.org/plosone/s/figures#loc-preparing-figures-from-image-files. When you submit your revised manuscript, please ensure that your figures adhere fully to these guidelines and provide the original underlying images for all blot or gel data reported in your submission. See the following link for instructions on providing the original image data: https://journals.plos.org/plosone/s/figures#loc-original-images-for-blots-and-gels.
In your cover letter, please note whether your blot/gel image data are in Supporting Information or posted at a public data repository, provide the repository URL if relevant, and provide specific details as to which raw blot/gel images, if any, are not available. Email us at gro.solp@enosolp if you have any questions.
3. We note that you have included the phrase “data not shown” in your manuscript. Unfortunately, this does not meet our data sharing requirements. PLOS does not permit references to inaccessible data. We require that authors provide all relevant data within the paper, Supporting Information files, or in an acceptable, public repository. Please add a citation to support this phrase or upload the data that corresponds with these findings to a stable repository (such as Figshare or Dryad) and provide and URLs, DOIs, or accession numbers that may be used to access these data. Or, if the data are not a core part of the research being presented in your study, we ask that you remove the phrase that refers to these data.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Partly
Reviewer #2: No
**********
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: No
Reviewer #2: Yes
**********
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: No
**********
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: Title refer only to proliferation, but Fig 1K also migration.
The main question of the paper is quite unclear : Do the authors through proliferation and migration sought to modelize macrovascular endothelial cell healing, or do they sought to modelize angiogenesis ?
In addition, they refer to eNOS expression in SMC (ref 19) where the mechanisms are totaly different and which pathophysiological meaning is unclear.
FInally, they report rather preliminary results in nature, and acknowledge that at the end of the introduction « Our findings confirm that eNOS regulate endothelial function by directly controlling endothelial cell proliferation, and nonspecificity of L-NAME is responsible for inhibition of endothelial cell proliferation; both warranting further investigation.. ». Indeed, these results are very descriptive and the amount of limited to 2 Figures.
Thus, the apparent « discrepancy » between gene expression inhibition and eNOS actinity inhibition should be investigated more in deepth.
Major points :
1) INTRO :
a) « PKG increases extracellular concentration of calcium by facilitating the reuptake of cytosolic calcium into the sarcoplasmic reticulum and opening of calcium-activated potassium channels [5].
Ref 5 : Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium 1994. Is not appropriate as i) it demonstrate in SMC the opposite and ii) the roles of Calcium in SMC and in endothelial cells are very different and cannot be extrapolated.
2) METHODS :
a) Proliferation and Scratch Assay: the experimental conditions of cell culture should be better defined.
b) L-NAME is in vivo desestherified to L-NA, that is in fact the active coumpound. Thus,
Experiments should be done with L-NA and L-NMMA in order to minimize the pharmacological biais and to see if the results if these 3 complementary apporaches do concur. This would allow to attenuate the limitation indicated in the introduction : « non-specificity of eNOS pharmacologic inhibitor L-NAME « .
c) How many different sieNOS were used ? At least 3 should minimize the possible off-target effects.
Minor points :
Abstract : « On the contrary,.. » should be better explained : « at the level of the enzyme activity ».
Reviewer #2: The authors in their manuscript "Endothelial cell-specific Loss of eNOS Promotes Proliferation" attempted to describe function of eNOS as a negative regulator of cell proliferation. The study has a few major issues:
1. Authors should use correct term, authors claim that they are 'silencing' eNOS gene. Here, the word 'silencing' is used incorrectly, it should be replaced with 'siRNA mediated knockdown' or simply 'knockdown'. Genetic silencing, by definition, means traditional gene knockout using homologous recombination technology using the embryonic stem cells.
2. Can authors provide evidence that eNOS is a negative regulator of cell proliferation? For example, by over-expressing eNOS-cDNA in endothelial cells, which should increase the levels of cell cycle inhibitor such as p21, p27, p53 and down-regulation of Cyclin-D1. These can be done using cultured endothelial cells, at least two different primary endothelial cells must be used. The HUVECs at passage 4-6 are likely not quiescent, therefore, it would be more appropriate to use microvascular endothelial cells such as from lungs and heart. My fear is that the HUVECs may be displaying inappropriate adaptive response, in response to eNOS-knockdown.
3. Can authors normalize the activity of AKT (pAKT) in endothelial cells that received eNOS siRNA? Thereby rescue/restore the normal EC phenotype? Alternatively, can authors add back eNOS-cDNA to restore normal phenotype?
4. Figure 1L: Quantification of Western Blot is required.
5. The migration assay (wound healing) done over a period of 20 hours could include cell proliferation as well. Did author add BrdU during cell migration assay? This data is unclear.
6. In the Figure legend, can authors mention as to how many times experiments were repeated? Please explain N value clearly.
7. 'Tube formation' assay is misleading. Can authors indicate where the tubes are?
8. Methods are described inadequately.
Minor concerns:
1. 'matrigel' should be spelled Matrigel, where 'M' should be capitalized.
2. The manuscript could benefit from check on English grammar and clarity.
**********
6. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: Yes: JF ARNAL
Reviewer #2: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
22 Jul 2022
Reviewer 1
Comment #1 - Title refer only to proliferation, but Fig 1K also migration.
Response: Many thanks for your thoughtful comments. In cultured endothelial cells, loss of eNOS promoted cell proliferation and migration but inhibited tube-forming potential. Accordingly, to have more conclusive title, the revised title of the manuscript is that “Endothelial cell-specific Loss of eNOS Differentially Affects Endothelial Function”.
Comment #2: The main question of the paper is quite unclear: Do the authors through proliferation and migration sought to modelize macrovascular endothelial cell healing, or do they sought to modelize angiogenesis? In addition, they refer to eNOS expression in SMC (ref 19) where the mechanisms are totally different, and which pathophysiological meaning is unclear.?
Response: We mainly aimed to investigate the effect of loss of eNOS on in vitro measures of endothelial function such as proliferation, migration and tube formation, which contribute towards both; endothelial cell healing and angiogenesis. The statement regarding eNOS expression in SMC has been deleted from the revised manuscript.
Comment #3: Finally, they report rather preliminary results in nature, and acknowledge that at the end of the introduction “Our findings confirm that eNOS regulate endothelial function by directly controlling endothelial cell proliferation, and non-specificity of L-NAME is responsible for inhibition of endothelial cell proliferation; both warranting further investigation”. Indeed, these results are very descriptive and the amount of limited to 2 Figures. Thus, the apparent « discrepancy » between gene expression inhibition and eNOS activity inhibition should be investigated more in depth.
Response: We agree with the reviewer that to some extent the results are descriptive in nature; however, it confirms the effect of loss of eNOS on endothelial function in vitro, which has not been clearly reported so far, and that is the focus of the present study. We have plans to investigate the mechanisms behind observed effect, which will be part of our next manuscript. Explanation regarding non-specificity of L-NAME has been deleted from the revised manuscript. We believe that the differential effect of eNOS on endothelial function is observed due to mechanisms up-stream (before eNOS activation) and down-stream (after eNOS activation) to eNOS.
Comment #5: PKG increases extracellular concentration of calcium by facilitating the reuptake of cytosolic calcium into the sarcoplasmic reticulum and opening of calcium-activated potassium channels [5]. Ref 5 : Nitric oxide decreases [Ca2+] in vascular smooth muscle by inhibition of the calcium current. Cell Calcium 1994. Is not appropriate as i) it demonstrate in SMC the opposite and ii) the roles of Calcium in SMC and in endothelial cells are very different and cannot be extrapolated.
Response: We agree with your thoughtful comment and have deleted the original reference and added new references in Page 3 paragraph 1 of the revised manuscript.
Comment #6a: Proliferation and Scratch Assay: the experimental conditions of cell culture should be better defined.
Response: Thank you for your comment, the experimental conditions have now been further clarified in page 6, paragraph 1 and 2 of the revised manuscript. The migration assay was conducted as described in “An introduction to the wound healing assay using live-cell microscopy” (PMID: 25482647).
Comment #6b: L-NAME is in vivo desestherified to L-NA, that is in fact the active coumpound. Thus, Experiments should be done with L-NA and L-NMMA in order to minimize the pharmacological bias and to see if the results if these 3 complementary apporaches do concur. This would allow to attenuate the limitation indicated in the introduction : « non-specificity of eNOS pharmacologic inhibitor L-NAME.
Response: Many thanks for your suggestion. Now, we have included data on proliferation and migration following L-NMMA, which is similar to L-NAME data on HUVECS proliferation, migration and tube-formation (Please see Fig. 5 in the revised manuscript).
Comment #6c: How many different sieNOS were used? At least 3 should minimize the possible off-target effects.
Response: We used two sieNOS (sieNOS and sieNOS#) molecule and then measured proliferation following silencing. However, we observed the similar reduction in proliferation following transfection with both siRNA molecules (Please see supplementary figure 1 and figure 1D).
Comment #7: Abstract : “On the contrary,.. “ should be better explained : “at the level of the enzyme activity”.
Response: Abstract is revised as suggested.
Reviewer 2
Comment #1: Authors should use correct term, authors claim that they are 'silencing' eNOS gene. Here, the word 'silencing' is used incorrectly, it should be replaced with 'siRNA mediated knockdown' or simply 'knockdown'. Genetic silencing, by definition, means traditional gene knockout using homologous recombination technology using the embryonic stem cells.
Response: Thank you for your comment, it has now been corrected in the manuscript.
Comment #2: Can authors provide evidence that eNOS is a negative regulator of cell proliferation? For example, by over-expressing eNOS-cDNA in endothelial cells, which should increase the levels of cell cycle inhibitor such as p21, p27, p53 and down-regulation of Cyclin-D1. These can be done using cultured endothelial cells, at least two different primary endothelial cells must be used. The HUVECs at passage 4-6 are likely not quiescent, therefore, it would be more appropriate to use microvascular endothelial cells such as from lungs and heart. My fear is that the HUVECs may be displaying inappropriate adaptive response, in response to eNOS-knockdown.
Response: Thank you for the thoughtful comment. We have followed your suggestion and silenced eNOS in Human Pulmonary Artery Endothelial Cell (HPAECs) and confirmed downregulation of eNOS. Knockdown of eNOS in HPAECS was associated with increased proliferation, downregulation of p21 and enhanced migration as observed in eNOS-deficient HUVECs (Please see Fig. 2 in the revised manuscript). We agree that eNOS-overexpression will very well complement our findings. Accordingly, we have future plans but that will form the basis of another manuscript.
Comment #3: Can authors normalize the activity of AKT (pAKT) in endothelial cells that received eNOS siRNA? Thereby rescue/restore the normal EC phenotype? Alternatively, can authors add back eNOS-cDNA to restore normal phenotype?
Response: As suggested, we have normalized the AKT activity. Please see figure 3B and C.
Comment #4. Figure 1L: Quantification of Western Blot is required.
Response: As suggested, we have quantified AKT and ERK protein levels. Please see figure 3B-E.
Comment #5: The migration assay (wound healing) done over a period of 20 hours could include cell proliferation as well. Did author add BrdU during cell migration assay? This data is unclear.
Response: Thanks for your comment. We incubated cells in DMEM supplemented with 1%FBS to ensure there is only migration but not proliferation. Method is now clarified in page 6 paragraph 2.
Comment #6: In the Figure legend, can authors mention as to how many times experiments were repeated? Please explain N value clearly.
Response: Thank you for the comment. We have repeated the experiments at least 3 times in triplicates and the N values are now added in the figure legend.
Comment #7: 'Tube formation' assay is misleading. Can authors indicate where the tubes are?
Response: Thanks for pointing it out. As suggested, tubes are marked in the figure 1G of the revised manuscript.
Comment #8: Methods are described inadequately.
Response: Thank you for the comment, we have added more details to the methods section.
Comment #9: 'matrigel' should be spelled Matrigel, where 'M' should be capitalized.
Response: Thanks for the reminder, it has now been corrected.
Comment #10: The manuscript could benefit from check on English grammar and clarity.
Response: Thanks for the suggestion. We have now checked the manuscript for grammar and clarity.
Attachment
Submitted filename: Response to reviewers.docx
Decision Letter 1
15 Aug 2022
PONE-D-22-01733R1Endothelial Cell-Specific Loss of eNOS Differentially Affects Endothelial FunctionPLOS ONE
Dear Dr. Singh,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process. Please submit your revised manuscript by Sep 29 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Jeffrey S Isenberg, MD, MPH
Academic Editor
PLOS ONE
Journal Requirements:
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Additional Editor Comments (if provided):
The Reviewers felt that the revised manuscript was much improved, and the authors are thanked for this. Some concern still was found regarding terminology. See below and please address this. As well, a Reviewer requested an effort be made to improve the overall quality of the figures.
[However, the use of terminology remains confusing, e.g., tube formation. If authors claim that these are indeed tubes (Figure 4B, indicated by arrows), the Matrigel must be fixed with paraformaldehyde (4% PFA) and embedded in paraffin, make cross thin section, thereafter, stain with H&E and show lumen (vacuole/empty space). In other words, "lumen formation" is alternatively called "tube formation".]
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: All comments have been addressed
**********
2. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
Reviewer #2: Yes
**********
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: Yes
**********
4. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
6. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: Seems fine no additional comment Seems fine no additional comment Seems fine no additional comment Seems fine no additional comment
Reviewer #2: The authors have addressed most of my questions and concerns.
However, the use of terminology remains confusing, e.g., tube formation. If authors claim that these are indeed tubes (Figure 4B, indicated by arrows), the Matrigel must be fixed with paraformaldehyde (4% PFA) and embedded in paraffin, make cross thin section, thereafter stain with H&E and show lumen (vacuole/empty space). In other words, "lumen formation" is alternatively called "tube formation".
**********
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: Yes: Jean Francois ARNAL
Reviewer #2: No
**********
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 1
26 Aug 2022
Reviewer #1: Seems fine no additional comment Seems fine no additional comment Seems fine no additional comment Seems fine no additional comment
Response: Thank you very much.
Reviewer#2: The authors have addressed most of my questions and concerns.
However, the use of terminology remains confusing, e.g., tube formation. If authors claim that these are indeed tubes (Figure 4B, indicated by arrows), the Matrigel must be fixed with paraformaldehyde (4% PFA) and embedded in paraffin, make cross thin section, thereafter stain with H&E and show lumen (vacuole/empty space). In other words, "lumen formation" is alternatively called "tube formation".
Response: Thank you for your insightful comment. We agree that endothelial cells link to each other through formation of junctional complexes upon stimulation of angiogenic signals which lead to formation of lumen, and fixation will help visualize the lumen. We have now changed the “tube formation” to “formation of tube-like structures”.
Attachment
Submitted filename: Response to Reviewers August 25.docx
Decision Letter 2
30 Aug 2022
Endothelial Cell-Specific Loss of eNOS Differentially Affects Endothelial Function
PONE-D-22-01733R2
Dear Dr. Singh,
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua.
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno.
Kind regards,
Jeffrey S Isenberg, MD, MPH
Academic Editor
PLOS ONE
Additional Editor Comments (optional):
The authors are thanked for the minor adjustment in terminology. The manuscript is acceptable for publication.
Reviewers' comments:
Acceptance letter
15 Sep 2022
PONE-D-22-01733R2
Endothelial Cell-specific Loss of eNOS Differentially Affects Endothelial Function
Dear Dr. Singh:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno.
If we can help with anything else, please email us at gro.solp@enosolp.
Thank you for submitting your work to PLOS ONE and supporting open access.
Kind regards,
PLOS ONE Editorial Office Staff
on behalf of
Dr. Jeffrey S Isenberg
Academic Editor
PLOS ONE
Articles from PLOS ONE are provided here courtesy of PLOS
Full text links
Read article at publisher's site: https://doi.org/10.1371/journal.pone.0274487
Read article for free, from open access legal sources, via Unpaywall:
https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0274487&type=printable
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/136333782
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1371/journal.pone.0274487
Article citations
Screening of herbal extracts binding with vascular endothelial growth factor by applying HerboChip platform.
Chin Med, 19(1):122, 09 Sep 2024
Cited by: 0 articles | PMID: 39252102 | PMCID: PMC11382504
Complex Interplay between DNA Damage and Autophagy in Disease and Therapy.
Biomolecules, 14(8):922, 29 Jul 2024
Cited by: 0 articles | PMID: 39199310 | PMCID: PMC11352539
Review Free full text in Europe PMC
Trauma promotes heparan sulfate modifications and cleavage that disrupt homeostatic gene expression in microvascular endothelial cells.
Front Cell Dev Biol, 12:1390794, 24 Jul 2024
Cited by: 0 articles | PMID: 39114570 | PMCID: PMC11303185
Protein Disulfide Isomerase 4 Is an Essential Regulator of Endothelial Function and Survival.
Int J Mol Sci, 25(7):3913, 31 Mar 2024
Cited by: 2 articles | PMID: 38612722 | PMCID: PMC11011381
Neuronal nitric oxide synthase is required for erythropoietin stimulated erythropoiesis in mice.
Front Cell Dev Biol, 11:1144110, 21 Feb 2023
Cited by: 1 article | PMID: 36895793 | PMCID: PMC9988911
Go to all (6) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Berberine protects endothelial progenitor cell from damage of TNF-α via the PI3K/AKT/eNOS signaling pathway.
Eur J Pharmacol, 743:11-16, 23 Sep 2014
Cited by: 21 articles | PMID: 25257463
The role of eNOS in the migration and proliferation of bone-marrow derived endothelial progenitor cells and in vitro angiogenesis.
Cell Biol Int, 39(4):484-490, 05 Jan 2015
Cited by: 26 articles | PMID: 25492215
Propionyl-L-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases.
Vascul Pharmacol, 59(3-4):76-82, 12 Jul 2013
Cited by: 16 articles | PMID: 23850990
Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice.
J Allergy Clin Immunol, 132(5):1205-1214.e9, 08 Sep 2013
Cited by: 33 articles | PMID: 24021572
Funding
Funders who supported this work.
CIHR (2)
Grant ID: 153216
Grant ID: FRN 153216
Heart and Stroke Foundation of Canada (1)
Grant ID: 2018/19 National New Investigator Award- Salary Suppor

 1
,
2
,*
1
,
2
,*


