Abstract
Free full text

Immunostimulatory activity of fluoxetine in macrophages via regulation of the PI3K and P38 signaling pathways
Abstract
Fluoxetine is an antidepressant drug that is heavily preferred in the cure of depression, which is from the selective serotonin reuptake inhibitor (SSRI) group. There are many reports on the effect of fluoxetine on the immune system, and its effect on the macrophage cells has never been looked at before. We aimed to demonstrate the cytokine production potential of fluoxetine antidepressant, which is widely used in the clinic, in the J774.2 cell line and its effect on PI3K and P38 pathways. The use of fluoxetine alone in J774.2 macrophage cells showed immunostimulatory properties by inducing the production of tumor necrosis factor-α (TNF-α), interleukin (IL) IL-6, IL-12p40, and granulocyte–macrophage colony-stimulating factor (GM-CSF) cytokines. It showed anti-inflammatory properties by completely stopping the production of cytokines (IL-6, IL12p40, TNF-α, and GM-CSF) at all concentrations where LPS and fluoxetine were used together. While PI3K and P38 pathways were not effective in the immunostimulatory effect in the presence of the drug agent, we found that the PI3K and P38 pathways were influenced during their anti-inflammatory activity.
Introduction
Fluoxetine is in the selective serotonin reuptake inhibitor (SSRI) antidepressant drug group, which is frequently preferred in the treatment of depressive syndromes. SSRIs provide treatment by increasing the level of serotonin in the brain. Serotonin is a biogenic amine that is effective in regulating vital processes such as mood, food, sexual desire, sleep, and body temperature. Serotonin neurotransmitters are especially found in the immune-inflammatory axis and can regulate immune cell activity autocrine [1].
In the findings of recent studies, it has been emphasized that 5-hydroxytryptamine (5-HT) has an important regulatory role in the immune and nervous systems [2–4]. The main cells that produce enterochromaffin 5-HT, which is one of the gastrointestinal cells, release 5-HT through platelets. This event activates platelet-activating factors and immunoglobulin E (IgE), resulting in an inflammatory stimulus effect [2]. Lymphocytes and monocytes also synthesize 5-HT. Nerve fibers in contact with lymphoid tissues are also suggested to take up and release 5-HT to stimulate nerves [5, 6]. In studies on this subject, it has been reported that 5-HT has an immunomodulatory effect by increasing mitogen-activated lymphocyte proliferation, activating natural killer (NK) cells, and increasing macrophage and dendritic cell stimulation [7, 8].
Studies of fluoxetine’s effect on the immune system have been adhered to and are being done. In recent studies, it has been shown that fluoxetine reduces proinflammatory cytokine levels by affecting the inflammatory system in experimental animals. More recently, animal studies show that fluoxetine reduces proinflammatory cytokine levels by acting on the inflammatory process [9–11]. In a study conducted with patients treated with SSRI group antidepressants, they observed that the expression level of the gene encoding the NLRP3 gene, which has an inflammatory effect, decreased in mononuclear cells taken from patients. They also reported that IL-1β and IL-18 cytokine concentrations decreased in plasma [12]. In a meta-analysis including 22 studies, it was shown that IL-1β, 1IL-6, and TNF-α cytokine levels were decreased in the serum of patients using SSRIs [13]. In another study, it was reported that fluoxetine increased the expression of the serotonin transporter in the T lymphocyte surface region to improve serotonin reuptake, resulting in a decrease in the number of lymphocytes [14]. Studies are showing that fluoxetine is an immunomodulator. It was observed that T lymphocyte levels in the peripheral blood of mice treated with fluoxetine decreased, while the CD4 +
+ /CD8
/CD8 +
+ ratio remained stable [15]. It has been reported that patients treated with intravenous fluoxetine have a decrease in the level of T lymphocyte activity [14]. In another study conducted with 16 patients, a decrease in proinflammatory cytokine levels was observed, while an increase in Treg cell count was observed [16].
ratio remained stable [15]. It has been reported that patients treated with intravenous fluoxetine have a decrease in the level of T lymphocyte activity [14]. In another study conducted with 16 patients, a decrease in proinflammatory cytokine levels was observed, while an increase in Treg cell count was observed [16].
The P38 signaling pathway is a class of mitogen-activated protein kinases (MAPKs) that affects the production of cytokines by monocytes and macrophages [17]. The P38 pathway is activated as a result of ultraviolet, heat shock, stress, and activation of cytokines in the cell. While treating inflammatory diseases with intense cytokine production, drug production is made by targeting the P38 pathway [18]. In vitro-in vivo studies have stated that the P38 pathway is activated in the output of IL-6 and TNF-α with macrophages stimulated by lipopolysaccharide (LPS) [19, 20]. In a study, it was reported that paroxetine from the SSCI group induced apoptosis in breast malignant tumor cells concerning the Ca2 +
+ and P38 pathways [21].
and P38 pathways [21].
The PI3K signaling pathway is an oncogene-derived intracellular pathway that plays a crucial role in the setting of the cell events such as mitosis and apoptosis. PI3Ks expressing secondary messengers provide important mechanisms in intracellular signaling [22, 23]. It also has an significant role in inflammatory responses [24].
The inconsistency of the reports of studies investigating the effect of fluoxetine on immunity did not clarify these effects. In the current study, the amount of cytokine (IL-6, IL12p40, TNF-α, and GM-CSF) production of fluoxetine antidepressant alone and in combination with LPS on the mammalian macrophage cell line J774.2 was investigated. In addition, using salicylic acid combined with LPS, which has anti-inflammatory properties, its effects on the level of cytokines were compared with the group given fluoxetine. In addition, Al2O3, which is used as an adjuvant in vaccine formulations, has been used in combination with LPS to support proinflammatory activity [25]. There have not been comprehensive studies on the immune system cells specifically and their instruction with fluoxetine and how it affects intracellular pathways and their anti-inflammatory or pro-inflammatory behaviors. This study to our knowledge is the first one to focus on this aspect of fluoxetine’s effect on the immune system’s cells specifically on the macrophage.
Materials and methods
Fluoxetine was obtained from Sigma Aldrich (CAS number: 56296–78-7).
J774.2 macrophage cell culture
J774.2 mouse macrophage cell line had ATTC origin. Cells were seeded in Roswell Park Memorial Institute (RPMI) medium including 10% fetal bovine serum (FBS), 1% antibiotic (100 µg/mL for each penicillin–streptomycin), and sodium pyruvate for growth. Cells were left to incubate in the incubator (5% CO2, 37 °C). The cell culture medium was changed every 4 days and made ready for the experiments [26, 27].
Stimulating cells with lipopolysaccharide
J774.2 cells (106 cells/mL) were placed in 24-well plates and their effects were tested in combination with fluoxetine (1, 5, 10, and 20 µg/mL) and LPS. To increase the anti-inflammatory effect, LPS (1 µL, Enzo Life Sciences, Salmonella) was added to separate wells along with AL2O3 and salicylic acid, and the effects were compared with other results. One microliter of dimethyl sulfoxide (DMSO) was added to the control wells. The cells were incubated for 24 h (5% CO2, 37 °C) and then centrifuged (2000 RPM). Then they were put in Eppendorf tubes. Cells were placed at −
− 80 °C. For cytokine determination, cells emerging from
80 °C. For cytokine determination, cells emerging from −
− 80 were separated from the wells by gently pipetting. Statistical analyses were performed with 3 replications of each study. One hundred and twenty minutes before cytokine determination in macrophage cells, 5 mM ATP (Fisher Scientific) was added to each well [28].
80 were separated from the wells by gently pipetting. Statistical analyses were performed with 3 replications of each study. One hundred and twenty minutes before cytokine determination in macrophage cells, 5 mM ATP (Fisher Scientific) was added to each well [28].
ELISA test for cytokines
Enzyme-linked immunosorbent (ELISA) assay was performed to determine the number of cytokines IL-6 (cat number: 555183), IL12p40 (cat number: 555220), TNF-α (cat number: 555212), and GM-CSF (cat number: 555126). They were all used in 1:1000 dilution rate. The ELISA kit for the cytokine types to be administered was prepared according to the application procedure of the manufacturer of BD Biosciences, CA, USA. Ninety-six-well plates were coated (hamster anti-mouse cytokine pH =
= 9.5) and incubated overnight. Then the solutions were drained from the plates, washed three times with PBS (0.05% Tween), and allowed to rest at room temperature for 3 h. After the wells were cleaned, blocking buffer was added (200 µL—1% BSA PBS) followed by 3 washes and incubated at 4 °C overnight. Plates were washed 3 times with further biotin (100 µL) and human anti-mouse cytokine (0.5 µg/mL in 10). PBS was placed in each of the wells and left at room temperature for 120 min, and the solutions were removed and washed. After these procedures, TMB (100 µL—BD OptEIA) was added to each of the wells, and sulfuric acid (50 µL 1 M) was added to stop the reaction. Their absorbance was measured at 450 nm [29, 30]. Calculations were made for each sample (TNF-α, IL-6, IL12p40, and GM-CSF) using cytokine concentrations with known standards.
9.5) and incubated overnight. Then the solutions were drained from the plates, washed three times with PBS (0.05% Tween), and allowed to rest at room temperature for 3 h. After the wells were cleaned, blocking buffer was added (200 µL—1% BSA PBS) followed by 3 washes and incubated at 4 °C overnight. Plates were washed 3 times with further biotin (100 µL) and human anti-mouse cytokine (0.5 µg/mL in 10). PBS was placed in each of the wells and left at room temperature for 120 min, and the solutions were removed and washed. After these procedures, TMB (100 µL—BD OptEIA) was added to each of the wells, and sulfuric acid (50 µL 1 M) was added to stop the reaction. Their absorbance was measured at 450 nm [29, 30]. Calculations were made for each sample (TNF-α, IL-6, IL12p40, and GM-CSF) using cytokine concentrations with known standards.
Flow cytometry analysis for P38 and PI3K intracellular pathways
Cells were fixed and permeabilized according to the BD (BD Fix Buffer I -557,870) procedure. It was then stained with Anti PI3K (Invitrogen PE Mouse, p85/p55, cat no: MA5-36,954) and P38 MAPK (BD PE Mouse, pT180/pY182, cat no: 562065). Flow cytometry analysis of cells was performed by repeating each experiment data 3 times (BD FACS ARIA III) [31].
Statistical analysis
Statistical data were analyzed in GraphPad Prism Software version 5. The unpaired two-tailed t-test was used to determine statistical significance. Three replicates of all experimental conditions were made, p <
< 0.0001, N
0.0001, N =
= 3.
3.
Results
J774.2 macrophage cells incubated with fluoxetine for 24 h were observed to induce the production of IL-6 cytokines in direct proportion to the concentration used. It was observed that IL-6 production was completely stopped when compared with the positive control groups activated only by LPS and the concentrations using only fluoxetine (1, 5, 10, 20 µg/mL) from 1 μg/mL concentration to the highest concentration when LPS and fluoxetine were used together (Fig. 1). The use of anti-inflammatory salicylic acid with LPS blocked the production of IL-6 as expected. The combined use of AL2O3 and LPS stimulated the immune system, promoting cytokine production (Fig. 1).

Fluoxetine (F), the application of fluoxetine to macrophage cells stimulated IL-6 cytokine release and showed immunostimulatory properties. It showed an anti-inflammatory effect in the environment where it was LPS-positive. For IL-6 ELISAs (1 ×
× 10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (concentrations of 1, 5, 10, 20 µg/mL were used) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p
10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (concentrations of 1, 5, 10, 20 µg/mL were used) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p <
< 0.0001, N
0.0001, N =
= 3
3
J774.2 macrophage cells incubated with fluoxetine for 24 h exerted a pro-inflammatory effect by stimulating the production of IL-12p40 cytokines at each concentration (1, 5, 10, 20 µg/mL) used. In terms of IL-12p40 production, the drug molecule had immunostimulatory activity. When the immunomodulatory activity was measured, it was observed that IL12p40 cytokine production was completely knocked out in the presence of fluoxetine compared to the DMSive control groups activated only by LPS (Fig. 2). The use of anti-inflammatory salicylic acid with LPS reduced the IL-12p40 cytokine production by half compared to the LPS control well. The combined use of AL2O3 and LPS showed an immunostimulatory effect as expected and slightly increased IL-12p40 production compared to the single effect of LPS (Fig. 2).
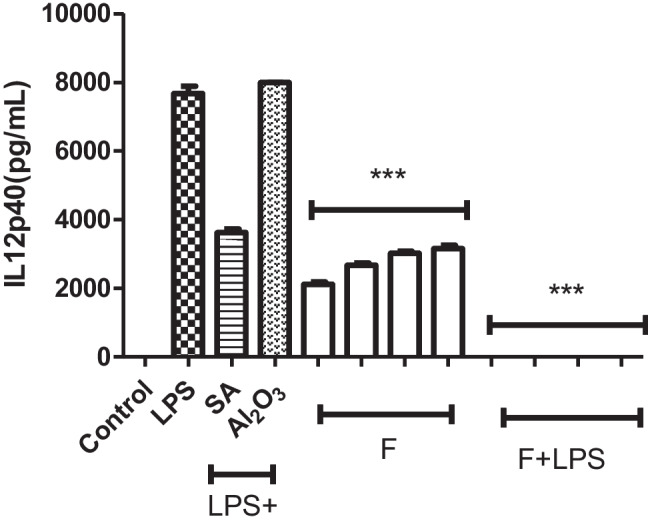
Fluoxetine (F), the application of fluoxetine to macrophage cells stimulated IL-12p40 cytokine release and showed immunostimulatory properties. It showed an anti-inflammatory effect in the environment where it was LPS-positive. For IL-12p1240 ELISAs (1 ×
× 10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (concentrations of 1, 5, 10, 20 µg/mL were used) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (1, 5, 10, 20 µg/mL) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p
10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (concentrations of 1, 5, 10, 20 µg/mL were used) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (1, 5, 10, 20 µg/mL) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p <
< 0.0001, N
0.0001, N =
= 3
3
Fluoxetine stimulated TNF-α cytokine production by showing immunostimulatory properties after incubation with J774.2 cells for 1 day. We found that when salicylic acid was applied in combination with LPS, it decreased the TNF-a grade compared to the LPS control well. The combined use of AL2O3 and LPS showed an immunostimulatory effect, as expected, and slightly increased IL-12p40 production equates to the effect of LPS alone. When fluoxetine was co-administered with LPS, it showed an anti-inflammatory effect and caused a decrease in TNF-α level (Fig. 3).

Fluoxetine (F), the application of fluoxetine to macrophage cells stimulated TNF-α cytokine release and showed immunostimulatory properties. In the environment with LPS, it showed an anti-inflammatory effect. For TNF-α ELISAs (1 ×
× 10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (concentrations of 1, 5, 10, 20 µg/mL were used) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p
10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (concentrations of 1, 5, 10, 20 µg/mL were used) and F added with 1 µg/mL LPS (1, 5, 10, 20 µg/mL) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p <
< 0.0001, N
0.0001, N =
= 3
3
J774.2 macrophage cells incubated with fluoxetine for 24 h exerted a pro-inflammatory effect by inducing the production of GM-CSF cytokines at each concentration used (especially 5, 10, 20 µg/mL). Therefore, fluoxetine has immunostimulatory activity by itself in the absence of LPS stimulation of the macrophages. While examining the immunomodulatory activities, it was observed that GM-CSF cytokine production was completely stopped when compared with the positive control groups activated only by LPS and the concentrations using only fluoxetine (1, 5, 10, 20 µg/mL) from the concentration of 1 μg/mL where LPS and fluoxetine were used together to the highest concentration (Fig. 4). The use of anti-inflammatory salicylic acid with LPS halted the IL-6 production as expected. The combined use of AL2O3 and LPS showed an immunostimulatory effect and slightly increased GMCSF production compared to the production levels achieved by the LPS alone (Fig. 4).
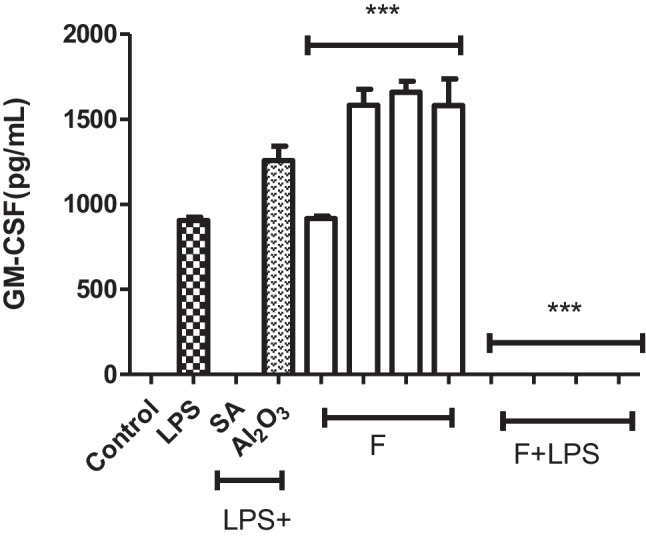
Fluoxetine (F), the application of fluoxetine to macrophage cells stimulated GM-CSF cytokine release and showed immunostimulatory properties. It showed an anti-inflammatory effect in the environment where it was LPS-positive. For GM-CSF ELISAs (1 ×
× 10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (1, 5, 10, 20 µg/mL) and F added with 1 µg/mL LPS (concentrations of 1, 5, 10, 20 µg/mL were used) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p
10.6 cells/mL cell concentration), J774.2 cells were stimulated with F (1, 5, 10, 20 µg/mL) for 24 h. In the environment with LPS, it showed an anti-inflammatory effect. DMSO was the negative control; F dissolved in DMSO (1, 5, 10, 20 µg/mL) and F added with 1 µg/mL LPS (concentrations of 1, 5, 10, 20 µg/mL were used) were positive controls. Each set of experiments was repeated 3 times and statistically calculated with Student’s t-test. ***p <
< 0.0001, N
0.0001, N =
= 3
3
Flow cytometry analysis was performed to define the effects of fluoxetine on PI3K and P38 intracellular changes. In this analysis, 20 µg/mL, which is the highest concentration of fluoxetine activity, which we determined as a result of our study, was used. J774.2 macrophage cells (106 cells/mL cell concentration) were stimulated for 24 h with LPS and LPS alone, salicylic acid (SA), 20 µg/mL fluoxetine alone, and LPS alone. Phosphorylated P38 cells were used in addition to DMSO for control groups, and LPS (1 µg/mL) was used for positive controls. When the effect of fluoxetine on the PI3K pathway was tested, the concentration of 20 µg/mL alone did not change the rate of PI3K positive cells relative to control data. Therefore, its immunostimulatory activity was not via activation of the PI3K pathway. Compared to the control group data, LPS +
+ 20 µg/mL cells decreased the % PI3K positive cell rate. While fluoxetine was not effective in this pathway due to its immunostimulating properties, we found that its anti-inflammatory effect was effective through this pathway (Fig. 5). Compared with the control group, 20 µg/mL fluoxetine did not increase the ratio of P38 cells. Compared with the control group, LPS
20 µg/mL cells decreased the % PI3K positive cell rate. While fluoxetine was not effective in this pathway due to its immunostimulating properties, we found that its anti-inflammatory effect was effective through this pathway (Fig. 5). Compared with the control group, 20 µg/mL fluoxetine did not increase the ratio of P38 cells. Compared with the control group, LPS +
+ 20 µg/mL cells increased their percentage of P38% cells. While fluoxetine was not effective on the p38 pathway in terms of its immunostimulatory properties, its anti-inflammatory effect was found to be effective through this pathway (Fig. 6). The PI3K and P38 pathways are inactive in the immunostimulatory effect in the presence of the drug agent. Meanwhile, fluoxetine acted through PI3K and P38 pathways for its anti-inflammatory properties.
20 µg/mL cells increased their percentage of P38% cells. While fluoxetine was not effective on the p38 pathway in terms of its immunostimulatory properties, its anti-inflammatory effect was found to be effective through this pathway (Fig. 6). The PI3K and P38 pathways are inactive in the immunostimulatory effect in the presence of the drug agent. Meanwhile, fluoxetine acted through PI3K and P38 pathways for its anti-inflammatory properties.
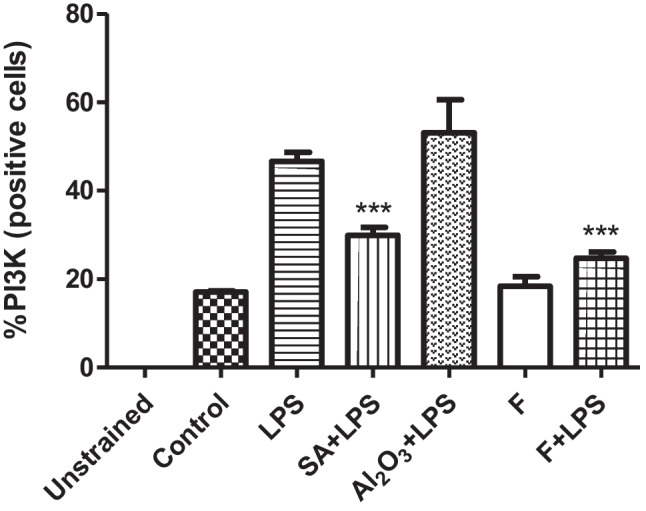
Rate of % PI3K (positive cells); macrophage cells (10.6 cells/mL) were stimulated for 1 day in combination with LPS and LPS alone, salicylic acid (SA), 20 μg/mL fluoxetine alone, and LPS in combination. Phosphorylated PI3K cells were used in addition to DMSO for control groups, and LPS (1 µg/mL) was used for positive controls. The reference point was accepted as 1 while making floor induction calculations. Three replicates were made for each data set; Student t-test was preferred for statistical analysis. ***p <
< 0.0001, N
0.0001, N =
= 3
3

Rate of % P38 (positive cells); macrophage cells (10.6 cells/mL) were stimulated for 1 day in combination with LPS and LPS alone, salicylic acid (SA), 20 μg/mL fluoxetine alone, and LPS in combination. Phosphorylated P38 cells were used in addition to DMSO for control groups, and LPS (1 µg/mL) was used for positive controls. The reference point was accepted as 1 while making floor induction calculations. Three replicates were made for each data set; Student t-test was preferred for statistical analysis.***p <
< 0.0001, N
0.0001, N =
= 3
3
Discussion
SSRI group antidepressants, which are frequently preferred in the cure of depression, regulate the level of serotonin. SSRIs cause an increase or decrease in the number of cytokines in circulation. There have been studies investigating the effect of fluoxetine, one of the SSRI group antidepressants, on the immune system, but there is no study on its effect on macrophage cells. In this study, IL-6, IL12p40, TNF-α, and GM-CSF cytokine production levels of J774.2 macrophage cells incubated with fluoxetine for 24 h were evaluated. In addition to fluoxetine, these effects were also examined in the presence of immunostimulating LPS. The use of fluoxetine alone in J774.2 macrophage cells showed immunostimulatory properties by inducing the production of IL-6, IL12p40, TNF-α, and GM-CSF cytokines. At all concentrations where LPS and fluoxetine were used together, it showed anti-inflammatory properties by completely stopping cytokine production (Figs. (Figs.1,1, ,2,2, ,3,3, and and44).
It is mentioned that proinflammatory cytokine production is increased in the pathophysiology of depressive disorders. Studies have found that depression is associated with changes in IL-6 and TNF-α cytokine levels [32, 33]. Kubera et al. showed that antidepressant drugs suppress the release of TNF-α and IL-6 induced by LPS [34].
A meta-analysis of 292 patients with major depressive disorder treated with fluoxetine revealed decreased levels of TNF-α or IL-6 cytokines. In the results of in vitro and in vivo studies with fluoxetine, it was stated that reducing IL-1 beta and IL-5 levels improved the symptoms of depression by regulating the neuroinflammatory system. It was found that fluoxetine regulates the neuroinflammatory system by suppressing proinflammatory cytokines, thus regulating the symptoms of depression [35, 36]. It is expected that most of the patients with depression will increase the level of proinflammatory cytokines, and the symptoms will be improved by the immunomodulatory effect of antidepressants on cytokines in the treatment. In some studies, severe decreases in C-reactive protein concentrations have been observed in patients with major depression treated with SSRIs. This decrease indicates that antidepressants initiate the anti-inflammatory response [37].
Liu et al. showed that fluoxetine inhibits IL-6 and TNF-α in the presence of LPS in LPS-stimulated microglial cells. The result of this study supports our study. In the use of fluoxetine, when there is a danger signal such as LPS in the environment, cytokine uremia is suppressed from macrophages and the inflammatory effect begins. Thus, the body remains our defense against external pathogens. They also showed that fluoxetine inhibited P38 MAPK phosphorylation [38]. When the anti-inflammatory effects of fluoxetine in mice with asthma were examined in peripheral studies with experimental animals, it was reported that it suppressed TNF and monocyte secretion [39, 40].
Ghosh et al. tested the anti-inflammatory property of fluoxetine in tumor-associated macrophages. They observed changes in some cytokines (IL-4, IL-6, IL-10, and IL-12) levels of macrophages retained in liquid tumor cells. They reported that after fluoxetine supplementation, CD3 +
+ T cells indicated a significant diminish in these cytokine levels like the control group, and this event triggered apoptosis. Fluoxetine may reduce the immunostimulatory properties of tumor cells by reprogramming macrophages in inflammatory conditions [41].
T cells indicated a significant diminish in these cytokine levels like the control group, and this event triggered apoptosis. Fluoxetine may reduce the immunostimulatory properties of tumor cells by reprogramming macrophages in inflammatory conditions [41].
Currently, TNF-α and IL are used in the treatment of autoimmune diseases and other immune-related diseases. For this reason, it is necessary to determine the cytokines that are effective in the pathogenesis of major depressive disorder (MDD) and explain how antidepressants used in the treatment of this disease change the number of cytokines. An abnormal immunological profile observed in the pathogenesis of the major depressive disorder, studies of pro-inflammatory (IL-1β, TNF, IFN-y, IL-12, IL-17, IL-18) the increase in the cytokines IL-12 and TGF-β anti-inflammatory cytokines have been shown to have low levels. [42, 43].
In autoimmune disorders such as rheumatoid arthritis and multiple sclerosis, differences in the number of cytokines have been observed with depressive symptoms [44, 45]. In studies, TNF-a and IL6 levels in the plasma and cerebrospinal fluid of patients with MDD were found to be significantly higher when compared to healthy individuals [46, 47]. In addition, TNF-α, IL-1β, IL-6, and IL-10 levels were found to be high in suicidal MDD patients [48]. MDD supports the importance of TNF-α in the pathogenesis of depression in developed rodent models. TNF-α levels have been shown to decrease when SSRI antidepressants are administered to rodent models of depression [49].
In in vitro and in vivo studies conducted with patients with major depressive disorder, it is known that IL-6 level is increased and its amount is high in the circulation. IL-6 contributes to the development of depression through the intestinal microbiota. Intravenously, IL-6 receptor antibody has been stated to perform antidepressant effects in the murine model of MDD [50].
Immunoinflammation and autoimmune went well with IL-12 but did not grow any further than that. In those with MDD, it is believed that pressor therapy reduces IL-12 and increases TGF-β1 [51]. Despite this study, in another study, proinflammatory levels (TNF-α IL-2, IL-12, and monocyte chemoattractant protein-1 (MCP-1)) were found to be very high [52]. In another study conducted on depressed patients, TNF-α, GM-CSF, IL-2, IL-5, IL-12, and IL-13 levels were found to be higher than in non-depressed groups. Compared to the non-obese and non-depressed groups, these cytokine levels were found to be higher [53, 54].
Immunoinflammatory disorders are common in the development of depression. There are existing preclinical and clinical studies in the literature suggesting that the proinflammatory cytokine macrophage migration inhibitory factor (MIF) and a member of the MIF family play a role in the pathogenesis [43]. In our study, fluoxetine stopped IL-6, IL12p40, TNF-a, and GM-CSF cytokine production in LPS-induced macrophage cells. However, future comprehensive studies are needed to evaluate the effects of other proinflammatory cytokines that play a role in major depressive disorder, such as fluoxetine MIF.
There are positive and negative results in studies on fluoxetine administration in autoimmune disease models. In a placebo-control group study investigating the effects of fluoxetine in patients with relapsing multiple sclerosis (MS), fluoxetine was shown to reduce the formation of lesions that strengthened in MS [55]. As a result of a study that tested the immunomodulatory effects of fluoxetine in a mouse multiple sclerosis model, it was reported that fluoxetine delayed the onset of autoimmune encephalomyelitis and reduced clinical paralysis. As a result, it has been stated that fluoxetine has immunomodulatory effects by suppressing the formation of T cells, antigen-presenting cells, and inflammatory cytokines [56]. In a recent study, it was stated that fluoxetine did not show any neuroprotective effect in the treatment of patients with progressive multiple sclerosis compared to the placebo group [57]. Fluoxetine and cytolamin from the SSCI group showed potent anti-inflammatory effects when applied to human and murine models of rheumatoid arthritis, greatly reducing disease progression [58]. Antidepressant drugs are preferred to reduce pain specific to rheumatoid arthritis. Fluoxetine antidepressant was administered to rats with rheumatoid arthritis for 26 days, and it was shown that there was no analgesic effect on rats with rheumatoid arthritis [59].
The P38 signaling pathway was discovered to regulate the proinflammatory cytokine production by macrophages [60]. It can activate the P38 pathway, which is one of the extracellular signals, in the activation of cytokines formed as a result of stress in the cell. The P38 pathway is targeted when treating inflammatory diseases with high cytokine production [18]. Studies have been conducted indicating that LPS-induced macrophages are included in the P38 signaling pathway and produce TNF-α and IL-6 [19, 20].
To see the effect of fluoxetine on macrophage intracellular pathways, cells were stained with PI3K and P38 in the presence of a drug agent alone or after incubation with LPS. Since fluoxetine observed its highest activity at a concentration of 20 µg/mL, this concentration was used to observe changes in PI3K and P38 intracellular levels in flow cytometry analysis. Compared with the control group, 20 µg/mL fluoxetine concentration did not increase the percentage of P38 and PI3K positive cells. LPS +
+ 20 µg/mL cells reduced the proportions of P38 and PI3K positive cells compared to LPS-containing control cells. While PI3K and P38 pathways were not effective in the immunostimulatory effect of the fluoxetine, we found that the PI3K and P38 pathways were part of the mechanism of its action during its anti-inflammatory activity.
20 µg/mL cells reduced the proportions of P38 and PI3K positive cells compared to LPS-containing control cells. While PI3K and P38 pathways were not effective in the immunostimulatory effect of the fluoxetine, we found that the PI3K and P38 pathways were part of the mechanism of its action during its anti-inflammatory activity.
Zhao et al. found that in rats treated with 10 mg/kg fluoxetine for 2 weeks, fluoxetine ameliorated depression-like behaviors by targeting P38 MAPK signaling, suppressing neuroinflammation and apoptosis [61]. Fluoxetine has been shown to inhibit P38 phosphorylation in LPS-stimulated microglial cells [38]. In another study, it was reported that fluoxetine and salicylic acid caused the inhibition of anti-inflammatory properties, NF-KB, and P38 MAPK pathways in BV2 microglia cells. In addition, it was stated that fluoxetine increased the anti-inflammatory effect in BV-2 cells induced by salicylic acid and LPS [60]. Consistent with this study, in our study, fluoxetine increased the anti-inflammatory effect through the P38 pathway.
It has been reported that the expression of TNF-α and IL-6 is decreased in the lungs of rats treated with fluoxetine, and it also provides protection against lung infection caused by methamphetamine by stopping oxidative stress via P38 [62]. The PI3K signaling pathway is an oncogene-derived intracellular pathway that has a critical role in the regulation of cell metabolisms such as proliferation and apoptosis. Secondary messengers expressed by PI3Ks provide important mechanisms for cell fate in intracellular signaling [22, 23]. Since the effects of fluoxetine on the PI3K signaling pathway have not been studied in macrophage cells before, there is no study in the literature on this subject. In a study with diabetic rats, fluoxetine was reported to regulate lipid metabolism via the PI3K/AKT signaling pathway [63]. Lei et al. reported that fluoxetine increased the proliferation of breast cancer cells by inducing low concentrations of PI3K/AKT signals in SKBR3 and MCF-7 breast cancer cells [64]. In our study, we also found that PI3K was another target of fluoxetine during its anti-inflammatory activity (Fig. 6). In addition to immunoinflammatory diseases, the PI3K/Akt/mTOR pathway includes infectious diseases such as HIV and SARS-CoV-2 and it also includes mTOR cancers as a multifunctional therapeutic target in HIV infection [65–67].
This study is the first to research the effect of fluoxetine, one of the SSRI group antidepressants, on the macrophage cells specifically. Fluoxetine exhibited immunostimulatory properties by promoting the production of IL-6, IL12p40, TNF-a, and GM-CSF cytokines in the J774.2 macrophage cell line. At all concentrations where LPS and fluoxetine were used together, IL-6, IL12p40, TNF-α, and GM-CSF production went down to undetectable levels (Figs. (Figs.1,1, ,2,2, ,3,3, and and4).4). In addition, using salicylic acid combined with LPS, which has anti-inflammatory properties, its effects on the level of cytokines were compared with the group given fluoxetine. In addition, Al2O3, which is used as an adjuvant in vaccine formulations, has been used in combination with LPS to support proinflammatory activity. Although fluoxetine had immunostimulatory activity by itself, in the presence of an inflammatory reaction it was suppressing the inflammation. It should be used with caution in the clinical setting by considering these activities. Besides, PI3K and P38 protein percentage values were measured for the intracellular mechanism of action of fluoxetine. According to these results, we found that the PI3K and P38 pathways were not effective in the immunostimulatory effect, while these pathways were downregulated during fluoxetine’s anti-inflammatory action which overlaps with the previous studies’ findings.
Conclusion
More studies should be conducted with different cell types by focusing on different signaling pathways to fully decipher the effects of the antidepressant drug molecules on the immune system of the patients. This information will be imperative for their effective and safe usage.
Author contribution
The manuscript was written through the contributions of all authors. All authors have approved for the final version of the manuscript. All authors contributed equally.
Declarations
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12026-022-09350-4
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s12026-022-09350-4.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s12026-022-09350-4
Article citations
Pharmacological investigation of taxifolin for its therapeutic potential in depression.
Heliyon, 10(9):e30467, 27 Apr 2024
Cited by: 0 articles | PMID: 38694040 | PMCID: PMC11061746
Anti-inflammatory activity of benidipine hydrochloride in LPS-activated mammalian macrophages.
Naunyn Schmiedebergs Arch Pharmacol, 397(8):5757-5763, 05 Feb 2024
Cited by: 1 article | PMID: 38315186 | PMCID: PMC11329695
Psilocybin and Eugenol Reduce Inflammation in Human 3D EpiIntestinal Tissue.
Life (Basel), 13(12):2345, 15 Dec 2023
Cited by: 2 articles | PMID: 38137946 | PMCID: PMC10744792
Paroxetine's effect on the proinflammatory cytokine stimulation and intracellular signaling pathways in J774.2 cells.
Naunyn Schmiedebergs Arch Pharmacol, 396(11):3327-3335, 17 Aug 2023
Cited by: 1 article | PMID: 37589738
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells.
Immunobiology, 218(12):1452-1467, 09 May 2013
Cited by: 129 articles | PMID: 23735482
Granulocyte-macrophage colony-stimulating factor modulates lipopolysaccharide (LPS)-binding and LPS-response of human macrophages: inverse regulation of tumour necrosis factor-alpha and interleukin-10.
Immunology, 98(4):491-496, 01 Dec 1999
Cited by: 11 articles | PMID: 10594679
Paroxetine's effect on the proinflammatory cytokine stimulation and intracellular signaling pathways in J774.2 cells.
Naunyn Schmiedebergs Arch Pharmacol, 396(11):3327-3335, 17 Aug 2023
Cited by: 1 article | PMID: 37589738
Cytokine-specific activation of distinct mitogen-activated protein kinase subtype cascades in human neutrophils stimulated by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-alpha.
Blood, 93(1):341-349, 01 Jan 1999
Cited by: 100 articles | PMID: 9864179

 1
1 


