Abstract
Free full text

Elevated VCAM-1, MCP-1 and ADMA serum levels related to pulmonary fibrosis of interstitial lung disease associated with rheumatoid arthritis
Abstract
Introduction: Early diagnosis of interstitial lung disease (ILD) associated with rheumatoid arthritis (RA) constitutes a challenge for the clinicians. Pulmonary vasculopathy is relevant in the development of interstitial lung disease. Accordingly, we aimed to explore the role of vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein-1 (MCP-1) and asymmetric dimethylarginine (ADMA), key molecules in the vasculopathy, as potential biomarkers of pulmonary fibrosis in RA-ILD+.
Methods: We included 21 RA-ILD+ patients and two comparative groups: 25 RA-ILD- patients and 21 idiopathic pulmonary fibrosis (IPF) patients. Serum levels of the molecules were determined by ELISA, and mRNA expression was quantified by qPCR.
Results: VCAM-1, MCP-1 and ADMA serum levels were increased in RA-ILD+ patients in relation to RA-ILD- and IPF patients. Additionally, RA-ILD+ patients exhibited increased CCL2 (gene encoding MCP-1) and decreased PRMT1 (gene related to ADMA synthesis) mRNA expression in relation to RA-ILD- patients. A lower expression of VCAM1, CCL2, and PRMT1 was observed in RA-ILD+ patients when compared with those with IPF. Furthermore, MCP-1 serum levels and PRMT1 mRNA expression were positively correlated with RA duration, and ADMA serum levels were positively associated with C-reactive protein in RA-ILD+ patients.
Conclusion: Our study suggests that VCAM-1, MCP-1 and ADMA could be considered as useful biomarkers to identify ILD in RA patients, as well as to discriminate RA-ILD+ from IPF, contributing to the early diagnosis of RA-ILD+.
1 Introduction
Endothelial dysfunction is characterized by an imbalance in the bioavailability of active substances of endothelial origin, being critical in the development of several autoimmune and inflammatory diseases such as rheumatoid arthritis (RA) (Sattar et al., 2003; González-Gay et al., 2008; Murdaca et al., 2012; Steyers and Miller, 2014; Yang et al., 2016). In this sense, key factors in the vasculopathy and inflammatory response include vascular cell adhesion molecule-1 (VCAM-1), one of the main responsibles of the firm adhesion of leukocytes to the endothelium (Dessein et al., 2005; Navarro-Hernández et al., 2009; Murdaca et al., 2012; Steyers and Miller, 2014; Wang et al., 2015; Kong et al., 2018), and the monocyte chemoattractant protein-1 (MCP-1), a chemokine that regulates the endothelial transmigration of monocyte (Hayashida et al., 2001; Rantapää-Dahlqvist et al., 2007; Deshmane et al., 2009; Dessein et al., 2013; Yang et al., 2016). Besides, asymmetric dimethylarginine (ADMA) is also involved in these disorders, regulating the vascular tone and inhibiting the nitric oxide synthesis (Dimitroulas et al., 2012; Murdaca et al., 2012; Steyers and Miller, 2014; Dimitroulas and Kitas, 2019; Zhao et al., 2019).
Interestingly, pulmonary endothelium dysfunction has also been described as a key stage for the development of lung damage and the subsequent onset and progression of interstitial lung disease (ILD). ILD represents one of the main causes of mortality in patients with RA, being the early detection of pulmonary involvement crucial to avoid an irreversible lung damage in these patients (Gómez-Carrera and Bonilla-Hernan, 2013; Bacha et al., 2017; Hyldgaard et al., 2017; Zamora-legoff et al., 2017; Bendstrup et al., 2019; Manfredi et al., 2021). However, the early diagnosis of RA-ILD+ constitutes a challenge for the clinicians because of the absence of symptoms in early or mild disease in some patients as well as the similarity of clinical, pathological, and epidemiological features with idiopathic pulmonary fibrosis (IPF), the most severe ILD (Paulin et al., 2015; Bendstrup et al., 2019; Atienza-Mateo et al., 2020; Manfredi et al., 2021).
In this context, we recently described that endothelial progenitor cells and angiogenic T cells, key cells in the vascular endothelium repair, are involved in the pathogenesis of ILD linked to autoimmune diseases (Pulito-Cueto et al., 2020; Pulito-Cueto et al., 2021; Pulito-Cueto et al., 2022). Hence, it is conceivable to think that other endothelial dysfunction-related markers could also contribute to the early identification of ILD in patients with RA. Nevertheless, this issue is far from being fully elucidated yet.
Accordingly, the aim of this study was to explore the functional role of VCAM-1, MCP-1 and ADMA, assessing both protein and mRNA, as potential biomarkers of pulmonary fibrosis in RA-ILD+.
2 Materials and methods
2.1 Study population
Peripheral venous blood was collected from 21 patients with RA-ILD+ and from patients of two comparative groups: 25 patients with RA-ILD- and 21 patients with IPF. All patients were recruited from the Pneumology and Rheumatology departments of Hospital Universitario Marqués de Valdecilla (Santander, Spain).
Patients with RA fulfilled the 2010 American College of Rheumatology criteria for RA (Aletaha et al., 2010). Pulmonary involvement was assessed in all the patients by high-resolution computed tomography (HRCT) images of the chest and pulmonary function tests (PFTs). RA-ILD- patients lacked lung involvement, whereas those with RA-ILD+ fulfilled the American Thoracic and European Respiratory Society’s (ATS/ERS) criteria for ILD (Travis et al., 2013). IPF patients fulfilled the ATS/ERS criteria (Travis et al., 2013). HRCT patterns of ILD patients were stratified according to the criteria for usual interstitial pneumonia (UIP) pattern of the Fleischner Society (Lynch et al., 2018).
All procedures involving humans and human blood samples were carried out in accordance with the approved guidelines and regulations, according to the Declaration of Helsinki. All experimental protocols were approved by the Ethics Committee of clinical research of Cantabria, Spain (2016.092). All subjects gave written informed consent to participate in this study before their inclusion.
2.2 Serum levels determination
VCAM-1, MCP-1 and ADMA serum levels were determined by a commercial Enzyme-Linked ImmunoSorbent Assay kit (VCAM-1: BMS232, Invitrogen, Vienna, Austria; MCP-1: BMS281, Invitrogen, Vienna, Austria; ADMA: K7860, Immundiagnostik AG, Bensheim, Germany), in accordance with the manufacturer’s instructions. All the samples were analyzed in duplicate and quantified relative to a standard curve, using 5-parameter logistic regression for VCAM-1 and MCP-1 and 4-parameter logistic regression for ADMA through MyAssays® online software as recommended by the manufacturer.
2.3 mRNA expression studies
Total RNA was isolated from peripheral blood using the NucleoSpin RNA Blood Kit (Macherey-Nagel, Germany), according to the manufacturer’s instructions. RNA was reverse transcribed into complementary DNA (cDNA) using iScript™ Advanced cDNA Synthesis Kit for reverse transcription-quantitative real-time polymerase chain reaction (qPCR) (Bio-Rad,United States). cDNA was amplified by qPCR in the thermocycler QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, United States) using SsoAdvanced™ universal SYBR® Green Supermix (Bio-Rad, United States). All samples were assayed in duplicate and experimental control assays were included. The relative VCAM1, CCL2 (gene enconding MCP-1) and PRMT1 (gene enconding the enzyme that catalyzes the ADMA synthesis reaction) mRNA expression was analyzed by the comparative Ct method using GAPDH as housekeeping gene.
2.4 Statistical analyses
In the first place, outliers were identified using the formula mean ±2 × standard deviation (SD) and excluded from the analyses. Data were expressed as mean ± SD for continuous variables, and number of individuals (n) and percentage (%) for categorical variables.
Differences related to demographic and clinical features between RA-ILD+ and RA-ILD- patients as well as between RA-ILD+ and IPF patients were analysed. In particular, sex, smoking ever and antibody status were assessed by chi-squared test, whereas continuous variables were evaluated by Student’s t-test.
Comparisons of protein levels or mRNA expression between two study groups were performed through linear regression adjusting for the following potential confounding factors: sex, age at the time of the study, and smoking history. Relationship of protein levels or mRNA expression with continuous and categorical variables was carried out via estimation of the Pearson’s partial correlation coefficient (r) and linear regression, respectively, adjusting for the potential confounding factors above mentioned.
The usefulness of serum VCAM-1, MCP-1, and ADMA as diagnostic biomarkers for RA-ILD+ was assessed by receiver operating characteristic (ROC) analysis. The area under the curve (AUC) with a 95% confidence interval (CI) was calculated. The optimal cutoff values for discriminating RA-ILD+ from RA-ILD- and IPF were calculated by the Youden index, with the maximum value obtained corresponding to the optimal cutoff point.
Statistically significant differences were considered as p ≤ 0.05. Statistical analysis was performed using STATA statistical software 12/SE (Stata Corp., College Station, TX, United States).
3 Results
3.1 Differences in the demographic and clinical features between the study groups
Demographic and clinical features including sex, age, smoking history, PFTs and HRCT patterns were collected from all the patients (Table 1).
TABLE 1
Demographic and clinical characteristics of all the patients included in the study.
| RA-ILD+ n = 21 | RA-ILD− n = 25 | IPF n = 21 | p RA-ILD+ vs. RA-ILD−/RA-ILD+ vs. IPF | |
|---|---|---|---|---|
| Sex (women), n (%) | 9 (45.9) | 15 (60.0) | 7 (33.3) | 0.84/0.53 |
| Age at study (years), mean ± SD | 66.5 ± 10.1 | 60.1 ± 11.8 | 69.2 ± 10.0 | 0.05/0.38 |
| Smoking ever, n (%) | 13 (65.0) | 13 (52.0) | 16 (76.2) | 0.50/0.32 |
| RA duration (years), mean ± SD | 9.2 ± 10.2 | 4.1 ± 7.4 | — | 0.06/— |
| CRP (mg/dl), mean ± SD | 1.1 ± 1.1 | 0.5 ± 0.5 | — | 0.04/— |
| ESR (mm/1st hour), mean ± SD | 22.8 ± 27.2 | 14.4 ± 12.4 | — | 0.24/— |
| Disease activity | — | |||
 DAS28-CRP, mean ± SD DAS28-CRP, mean ± SD | 3.4 ± 1.4 | 2.2 ± 1.1 | — | <0.01/— |
 DAS28-ESR, mean ± SD DAS28-ESR, mean ± SD | 3.4 ± 1.8 | 2.6 ± 1.6 | — | 0.12/— |
| Antibody status | — | |||
 RF+, n (%) RF+, n (%) | 17 (81.0) | 11 (44.0) | — | 0.01/— |
 ACPA+, n (%) ACPA+, n (%) | 19 (90.4) | 15 (60.0) | — | 0.02/— |
| Pulmonary function tests | ||||
 FVC (% predicted), mean ± SD FVC (% predicted), mean ± SD | 95.2 ± 24.1 | 99.2 ± 16.0 | 84.9 ± 14.7 | 0.58/0.10 |
 FEV1 (% predicted), mean ± SD FEV1 (% predicted), mean ± SD | 92.2 ± 21.0 | 94.9 ± 22.0 | 87.3 ± 19.6 | 0.67/0.43 |
 FEV1/FVC (% predicted), mean ± SD FEV1/FVC (% predicted), mean ± SD | 77.8 ± 9.1 | 93.6 ± 12.3 | 79.7 ± 7.8 | <0.01/0.47 |
 DLCO (% predicted), mean ± SD DLCO (% predicted), mean ± SD | 43.3 ± 15.9 | 79.9 ± 20.0 | 43.6 ± 18.4 | <0.01/0.97 |
| HRCT | ||||
 Pulmonary involvement Pulmonary involvement | 21 (100.0) | 0 (0.0) | 21 (100.0) | — |
 UIP pattern, n (%) UIP pattern, n (%) | 11 (52.4) | — | 21 (100.0) | — |
 Probable UIP pattern, n (%) Probable UIP pattern, n (%) | 2 (9.5) | — | 0 (0.0) | — |
 NSIP pattern, n (%) NSIP pattern, n (%) | 7 (33.3) | — | 0 (0.0) | — |
 Non-NSIP pattern, n (%) Non-NSIP pattern, n (%) | 1 (4.8) | — | 0 (0.0) | — |
RA, rheumatoid arthritis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; SD, standard deviation; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; DAS, disease activity score; RF, rheumatoid factor; ACPA, anti-cyclic citrullinated peptide antibodies; FVC, forced vital capacity; FEV1, forced expiratory volume at first second; DLCO, diffusing capacity of the lung for carbon monoxide; HRCT, high resolution computed tomography; UIP, usual interstitial pneumonia; NSIP, non-specific interstitial pneumonia. Significant results are highlighted in bold.
Statistically significant differences were found between patients with RA-ILD+ and RA-ILD- regarding the following demographic and clinical features: age, C-reactive protein (CRP) levels, disease activity score 28-CRP, rheumatoid factor and anti-cyclic citrullinated peptide antibodies status (Table 1). Likewise, we also observed differences in forced expiratory volume at first second/forced vital capacity and diffusing capacity of the lung for carbon monoxide (Table 1). However, no differences were disclosed between RA-ILD+ and IPF patients (Table 1).
3.2 VCAM-1, MCP-1 and ADMA associated with RA-ILD+
On the one hand, VCAM-1, MCP-1 and ADMA levels were increased in patients with RA-ILD+ in relation to RA-ILD- patients (p < 0.01 in all cases, Figures 1A,C,E, Supplementary Table S1). Moreover, patients with RA-ILD+ exhibited a higher CCL2 mRNA expression than RA-ILD- patients (p < 0.01, Figure 1D, Supplementary Table S1). Furthermore, a lower PRMT1 mRNA expression was found in patients with RA-ILD+ in comparison with their negative counterpants (p = 0.04, Figure 1F, Supplementary Table S1).
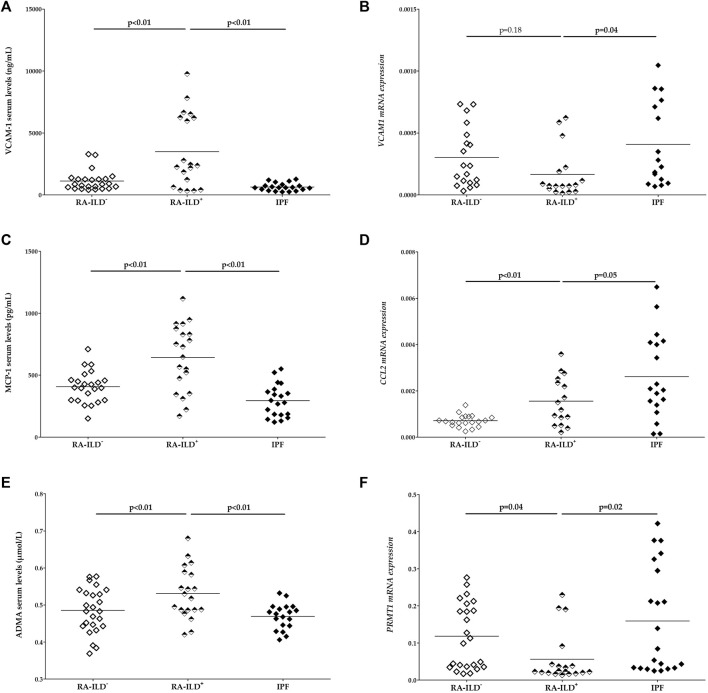
Differences in serum levels of VCAM-1 (A), MCP-1 (C) and ADMA (E) as well as mRNA expression of VCAM1 (B), CCL2 (D) and PRMT1 (F) between RA-ILD+ patients and the comparative groups. RA: rheumatoid arthritis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; VCAM-1, vascular cell adhesion molecule 1; MCP-1, monocyte chemoattractant protein-1; ADMA, asymmetric dimethylarginine. Significant results are highlighted in bold.
On the other hand, patients with RA-ILD+ showed significantly higher serum levels of VCAM-1, MCP-1 and ADMA compared to those with IPF (p < 0.01 in all cases, Figures 1A, C, E, Supplementary Table S1). Additionally, a lower expression of VCAM1, CCL2, and PRMT1 was observed in patients with RA-ILD+ when compared with those with IPF (p = 0.04, p = 0.05, p = 0.02, respectively, Figures 1B, D, F, Supplementary Table S1).
3.3 VCAM-1, MCP-1 and ADMA as diagnostic biomarkers for RA-ILD+
To evaluate the ability of serum VCAM-1, MCP-1 and ADMA levels for discriminating patients with RA-ILD+ from those with RA-ILD-, ROC curves were drawn for each biomarker (Figure 2A). Interestingly, the AUC was 0.704, 0.765 and 0.695, respectively (Table 2). The optimal cutoff value for VCAM-1, MCP-1 and ADMA showing the best sensitivity and specificity was 1680.0 ng/ml, 468.2 pg/ml and 0.5795 μmol/L, respectively (Table 2).
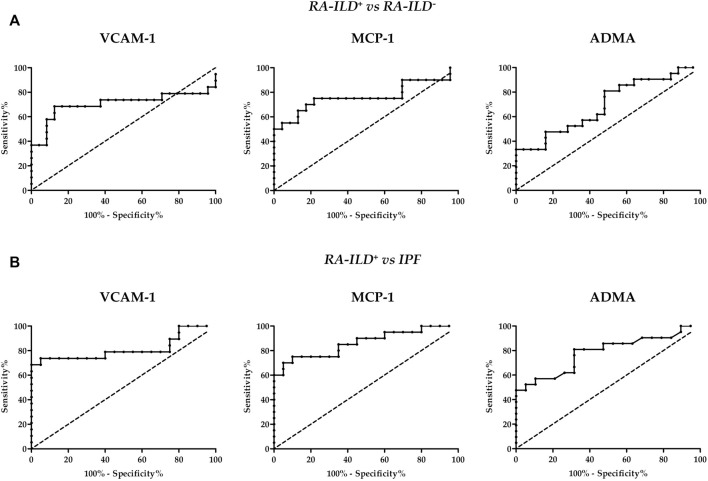
ROC curves of VCAM-1, MCP-1 and ADMA for the screening of RA-ILD+ and RA-ILD− (A) and RA-ILD+ and IPF (B). RA, rheumatoid arthritis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; VCAM-1, vascular cell adhesion molecule 1; MCP-1, monocyte chemoattractant protein-1; ADMA, asymmetric dimethylarginine.
TABLE 2
Receiver operating characteristic curves analysis for the discrimination of RA-ILD+ from RA-ILD− and IPF.
| AUC (95% CI) | Optimal cutoff value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| RA-ILD+ vs. RA-ILD− | ||||
 VCAM-1 VCAM-1 | 0.704 (0.520–0.888) | 1680.0 ng/ml | 68.4 | 87.5 |
 MCP-1 MCP-1 | 0.765 (0.607–0.923) | 468.2 pg/ml | 75.0 | 78.3 |
 ADMA ADMA | 0.695 (0.542–0.849) | 0.5795 μmol/L | 33.3 | 100.0 |
| RA-ILD+ vs. IPF | ||||
 VCAM-1 VCAM-1 | 0.813 (0.663–0.964) | 1220.0 ng/ml | 73.7 | 95.0 |
 MCP-1 MCP-1 | 0.863 (0.746–0.979) | 458.4 pg/ml | 75.0 | 90.0 |
 ADMA ADMA | 0.783 (0.638–0.927) | 0.4855 μmol/L | 81.0 | 68.4 |
RA, rheumatoid arthritis; ILD: interstitial lung disease; IPF, idiopathic pulmonary fibrosis; AUC, area under the curve; CI, confidence interval; VCAM-1, vascular cell adhesion molecule 1; MCP-1, monocyte chemoattractant protein-1; ADMA, asymmetric dimethylarginine.
In addition, ROC curves were used for the differentiation of patients with RA-ILD+ from those with IPF (Figure 2B). Of note, the AUC was 0.813 for VCAM-1, 0.863 for MCP-1 and 0.783 for ADMA (Table 2). The optimal cutoff value was 1220.0 ng/ml, 458.4 pg/ml and 0.4855 μmol/L, respectively (Table 2).
3.4 Lack of association of VCAM-1, MCP-1 and ADMA with clinical characteristics related to pulmonary involvement of RA-ILD+ patients
No relationship between VCAM-1, MCP-1 and ADMA protein levels and PFTs as well as HRCT patterns of patients with RA-ILD+ was found (Supplementary Table S2). Likewise, no significant results were obtained regarding VCAM1, CCL2 and PRMT1 mRNA expression (Supplementary Table S2).
3.5 Relationship of VCAM-1, MCP-1 and ADMA with clinical characteristics intrinsic of the rheumatic disease in RA-ILD+ and RA-ILD− patients
Regarding RA-ILD+ patients, a positive partial correlation was found between MCP-1 levels and RA duration (r = 0.542, p = 0.037, Figure 3A, Supplementary Table S3). This was also the case when PRMT1 mRNA expression was assessed (r = 0.645, p = 0.013, Figure 3B, Supplementary Table S3). In addition, ADMA serum levels were positively partially correlated with CRP (r = 0.585, p = 0.022, Figure 3C, Supplementary Table S3). No relationship between VCAM-1, MCP-1 and ADMA protein levels and other clinical characteristics of patients with RA-ILD+ were found (Supplementary Table S3). Likewise, no other significant results were obtained regarding VCAM1, CCL2 and PRMT1 mRNA expression (Supplementary Table S3).
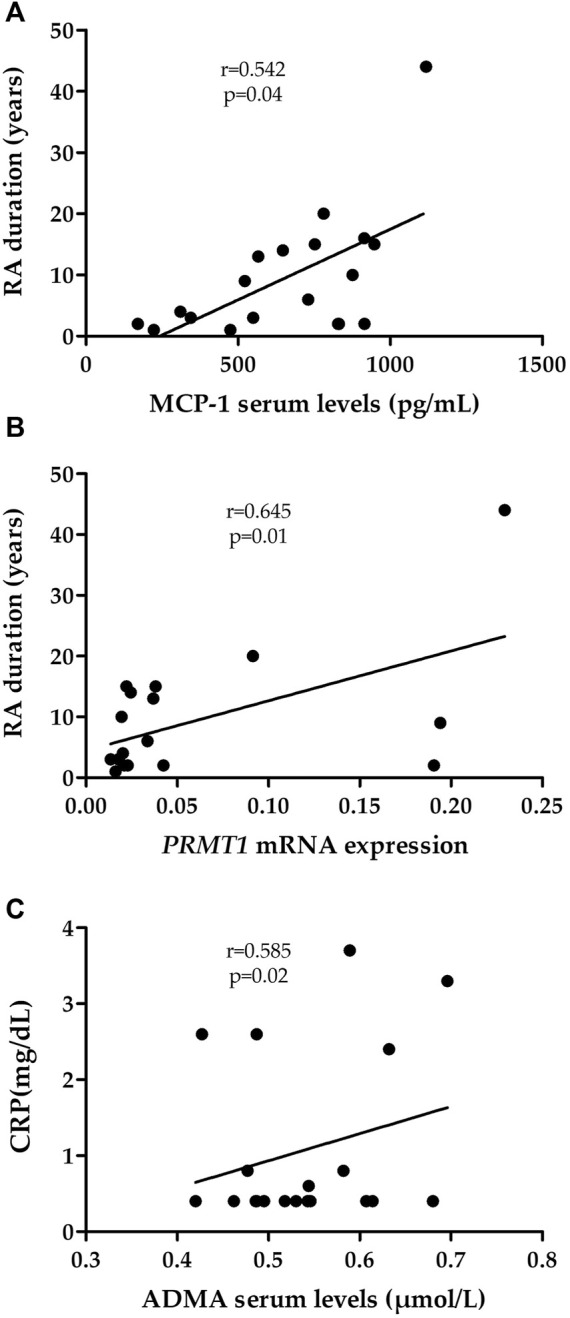
Relationship of MCP-1 (A), PRMT1 mRNA expression (B) and ADMA (C) with clinical characteristics of RA-ILD+ patients. RA, rheumatoid arthritis; MCP-1, monocyte chemoattractant protein-1; CRP, C-reactive protein; ADMA, asymmetric dimethylarginine.
With respect to patients with RA-ILD-, no association was found between VCAM-1, MCP-1 and ADMA and clinical characteristics intrinsic of RA, both when assessing protein levels and mRNA expression (Supplementary Table S4).
4 Discussion
Endothelial dysfunction, an early stage in the vascular damage, is observed in patients with RA (González-Gay et al., 2008) and is also implicated in ILD (Gómez-Carrera and Bonilla-Hernan, 2013; Bacha et al., 2017; Hyldgaard et al., 2017; Zamora-legoff et al., 2017; Bendstrup et al., 2019; Manfredi et al., 2021). RA-ILD+ diagnosis is often delayed due to the heterogeneity and unpredictability of ILD, constituting a major concern for the clinicians (Paulin et al., 2015; Bendstrup et al., 2019; Atienza-Mateo et al., 2020; Manfredi et al., 2021).
Given that pulmonary vasculopathy is important for the development of ILD, endothelial dysfunction-related markers could help to the early identification of RA-ILD+. Interestingly, this study provides evidence, for the first time, of an association of VCAM-1, MCP-1 and ADMA with pulmonary fibrosis in RA-ILD+.
First, we found that VCAM-1 serum levels were increased in patients with RA-ILD+ in relation to those with RA-ILD- and IPF. In this context, it has been described that VCAM-1 is induced by both inflammatory cytokines and hypoxia (Peter et al., 1999; Dessein et al., 2005; Navarro-Hernández et al., 2009; Cook-Mills et al., 2011; Murdaca et al., 2012; Wang et al., 2015; Kong et al., 2018; McGroder et al., 2019), which may explain the increase of this protein observed in our RA-ILD+ patients. Likewise, its ability to stimulate the fibroblast proliferation may be related to the fibrotic properties of this disease (Agassandian et al., 2015; McGroder et al., 2019). Moreover, our findings revealed a decrease of VCAM1 expression in RA-ILD+ patients with respect to the comparative groups, suggesting the involvement of post-transcriptional regulatory mechanisms (Greenbaum et al., 2003; Stevens and Brown, 2013; Li et al., 2020).
In the second place, we also disclosed a relationship of MCP-1 with lung involvement in RA since patients with RA-ILD+ presented higher levels of MCP-1 than those with RA-ILD− and IPF. Of note, we noticed that information on the role of MCP-1 in RA-ILD+ was limited to a single study in Asian population that also showed the same differences between RA-ILD+ and RA-ILD− patients (Ling et al., 2010). In this regard, a profibrotic role of MCP-1 has been previously described in other autoimmune diseases such as systemic sclerosis (Hasegawa et al., 1999; Schmidt et al., 2009; Yalçinkaya et al., 2016). The higher levels of MCP-1 in RA-ILD+ may also be caused by the chronic systemic exposure to proinflammatory cytokines characteristic of RA (Hayashida et al., 2001; Rantapää-Dahlqvist et al., 2007; Deshmane et al., 2009; Lin et al., 2014). It is noteworthy that an increase of CCL2 expression was observed in our patients with RA-ILD+ compared to those with RA-ILD−, whereas IPF patients showed the highest CCL2 expression. These results could indicate that CCL2 expression increases in response to ILD severity, supporting its known profibrotic role (Hasegawa et al., 1999; Schmidt et al., 2009; Ling et al., 2010). Moreover, we found a positive correlation between MCP-1 serum levels and RA duration in RA-ILD+ patients, which is in line with its key endothelial function reported in RA (Hayashida et al., 2001; Rantapää-Dahlqvist et al., 2007; Deshmane et al., 2009; Lin et al., 2014).
Finally, RA-ILD+ patients showed an increase of ADMA serum levels compared to both RA-ILD− and IPF patients, pointing out ADMA as a biomarker of RA-ILD+. The highest ADMA levels in RA-ILD+ patients make sense considering that ADMA has been previously described as a contributor of lung fibrosis and that its degradation may be inhibited by hypoxia and inflammatory cytokines, common processes of this disease (Ito et al., 1999; Millatt et al., 2003; Sattar et al., 2003; Sandoo et al., 2012; Janssen et al., 2013). Indeed, a positive correlation of ADMA and CRP was found in our cohort of RA-ILD+ patients. It should also be mentioned that RA-ILD+ patients presented the lowest PRMT1 expression, probably as a result of post-transcriptional regulatory mechanisms (Greenbaum et al., 2003; Stevens and Brown, 2013; Li et al., 2020). Furthermore, PRMT1 expression was positively correlated with RA duration in RA-ILD+ patients, confirming its previously described relationship with RA (Sandoo et al., 2012; Dimitroulas and Kitas, 2019; Zhao et al., 2019).
Considering all these findings, we can hypothesize that an additive effect combining the vascular damage caused by chronic inflammation and other features of RA as well as that associated with the fibrotic processes typical of ILD is related to increased serum concentrations of VCAM-1, MCP-1 and ADMA in RA-ILD+. In addition, serum levels of VCAM-1, MCP-1 and ADMA higher than 1680.0 ng/ml, 468.2 pg/ml and 0.5795 μmol/L may differentiate RA-ILD+ from RA-ILD-. Likewise, serum levels higher than 1220.0 ng/ml, 458.4 pg/ml and 0.4855 μmol/L, respectively, may discriminate RA-ILD+ from IPF. These results could be relevant in the clinical practice for the diagnosis of RA-ILD+.
In conclusion, our study suggests that VCAM-1, MCP-1 and ADMA can be useful biomarkers to identify the presence of ILD in patients with RA, as well as to discriminate between RA-ILD+ and IPF, contributing to the early diagnosis of RA-ILD+.
Acknowledgments
We thank all the subjects that participated in this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of clinical research of Cantabria, Spain (2016.092). The patients/participants provided their written informed consent to participate in this study.
Author contributions
VP-C, SR-M and FG contributed to the conceptualization, formal analysis, methodology, visualization, writing—original draft and writing-review and editing. VP-C, SR-M, FG, BA-M, VMM-C, DI-F, LL-G, MSM-G, DP-P, VP, RB, AC, JGO-V, OG, IF-A and JMC performed data curation and investigation. RL-M and MAG-G contributed to the project administration, supervision and writing—review and editing. All authors have contributed significantly to the article. All authors have read and approved the submitted version of the manuscript.
Funding
VP-C is supported by funds of PI18/00042 from Instituto de Salud Carlos III (ISCIII), co-funded by European Regional Development Fund (ERDF). SR-M is supported by funds of RETICS Program (RD16/0012/0009) from ISCIII, co-funded by ERDF; FG is supported by funds of the RICORS Program (RD21/0002/0025) from ISCIII, co-funded by the European Union; OG is staff personnel of Xunta de Galicia (Servizo Galego de Saude (SERGAS) through a research-staff stabilization contract (ISCIII/SERGAS) and his work is funded by ISCIII and ERDF [RD16/0012/0014 (RIER) and PI17/00409]. He is beneficiary of project funds from the Research Executive Agency of the European Union in the framework of MSCA-RISE Action of the H2020 Programme, project 734899—Olive-Net. RL-M is a recipient of a Miguel Servet type II Program fellowship from ISCIII, co-funded by the European Social Fund, ‘Investing in your future’ (CPII21/00004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.1056121/full#supplementary-material
Abbreviations
ACPA, anti-cyclic citrullinated peptide antibodies; ADMA, asymmetric dimethylarginine; ATS/ERS, American Thoracic and European Respiratory Society’s; CRP, C-reactive protein; DAS, diasease activity score; DLCO, diffusing capacity of the lung for carbon monoxide; ESR, erythrocyte sedimentation rate; FEV1, forced expiratory volume at first second; FVC, forced vital capacity; HRCT, high resolution computed tomography; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MCP-1, monocyte chemoattractant protein-1; NSIP, non-specific interstitial pneumonia; PFTs, pulmonary function tests; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation; UIP, usual interstitial pneumonia; VCAM-1, vascular cell adhesion molecule-1.
References
- Agassandian M., Tedrow J. R., Sembrat J., Kass D. J., Zhang Y., Goncharova E. A., et al. (2015). VCAM-1 is a TGF-β1 inducible gene upregulated in idiopathic pulmonary fibrosis. Cell. Signal. 27 (12), 2467–2473. 10.1016/j.cellsig.2015.09.003 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., et al. (2010). 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 62 (9), 2569–2581. 10.1002/art.27584 [Abstract] [CrossRef] [Google Scholar]
- Atienza-Mateo B., Remuzgo-Martínez S., Mora Cuesta V. M., Iturbe-Fernández D., Fernández-Rozas S., Prieto-Peña D., et al. (2020). The spectrum of interstitial lung disease associated with autoimmune diseases: data of a 3.6-year prospective study from a referral center of interstitial lung disease and lung transplantation. J. Clin. Med. 9 (6), 1606. 10.3390/jcm9061606 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bacha N. C., Blandinieres A., Rossi E., Gendron N., Nevo N., Lecourt S., et al. (2017). Endothelial microparticles are associated to pathogenesis of idiopathic pulmonary fibrosis. Stem Cell Rev. Rep. 14 (2), 223–235. 10.1007/s12015-017-9778-5 [Abstract] [CrossRef] [Google Scholar]
- Bendstrup E., Møller J., Kronborg-white S., Prior T. S., Hyldgaard C. (2019). Interstitial lung disease in rheumatoid arthritis remains a challenge for clinicians. J. Clin. Med. 8 (12), 2038. 10.3390/jcm8122038 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cook-Mills J. M., Marchese M. E., Abdala-Valencia H. (2011). Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 15 (6), 1607–1638. 10.1089/ars.2010.3522 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deshmane S. L., Kremlev S., Amini S., Sawaya B. E. (2009). Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29 (6), 313–326. 10.1089/jir.2008.0027 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dessein P. H., Joffe B. I., Singh S. (2005). Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res. Ther. 7 (3), R634–R643. 10.1186/ar1717 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dessein P. H., Solomon A., Woodiwiss A. J., Norton G. R., Tsang L., Gonzalez-Gay M. A. (2013). Marked independent relationship between circulating interleukin-6 concentrations and endothelial activation in rheumatoid arthritis. Mediat. Inflamm. 2013, 510243. 10.1155/2013/510243 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dimitroulas T., Kitas G. D. (2019). Genetic regulation of dimethylarginines and endothelial dysfunction in rheumatoid arthritis. Amino Acids 51 (7), 983–990. 10.1007/s00726-019-02740-3 [Abstract] [CrossRef] [Google Scholar]
- Dimitroulas T., Sandoo A., Kitas G. D. (2012). Asymmetric dimethylarginine as a surrogate marker of endothelial dysfunction and cardiovascular risk in patients with systemic rheumatic diseases. Int. J. Mol. Sci. 13 (10), 12315–12335. 10.3390/ijms131012315 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gómez-Carrera L., Bonilla-Hernan G. (2013). Pulmonary manifestations of collagen diseases. Arch. Bronconeumol. 49 (6), 249–260. 10.1016/j.arbres.2012.11.005 [Abstract] [CrossRef] [Google Scholar]
- González-Gay M. A., Gonzalez-Juanatey C., Vazquez-Rodriguez T. R., Martin J., Llorca J. (2008). Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin. Arthritis Rheum. 38 (2), 67–70. 10.1016/j.semarthrit.2008.02.001 [Abstract] [CrossRef] [Google Scholar]
- Greenbaum D., Colangelo C., Williams K., Gerstein M. (2003). Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 4 (9), 117. 10.1186/gb-2003-4-9-117 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hasegawa M., Sato S., Takehara K. (1999). Augmented production of chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1alpha) and MIP-1beta) in patients with systemic sclerosis: MCP-1 and MIP-1alpha may be involved in the development of pulmonary fibrosis. Clin. Exp. Immunol. 117 (1), 159–165. 10.1046/j.1365-2249.1999.00929.x [Abstract] [CrossRef] [Google Scholar]
- Hayashida K., Nanki T., Girschick H., Yavuz S., Ochi T., Lipsky P. E. (2001). Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 3 (2), 118–126. 10.1186/ar149 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hyldgaard C., Hilberg O., Pedersen A. B., Ulrichsen S. P., Løkke A., Bendstrup E., et al. (2017). A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann. Rheum. Dis. 76 (10), 1700–1706. 10.1136/annrheumdis-2017-211138 [Abstract] [CrossRef] [Google Scholar]
- Ito A., Tsao P. S., Adimoolam S., Kimoto M., Ogawa T., Cooke J. P. (1999). Novel mechanism for endothelial dysfunction dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 99 (24), 3092–3095. 10.1161/01.cir.99.24.3092 [Abstract] [CrossRef] [Google Scholar]
- Janssen W., Pullamsetti S. S., Cooke J., Weissmann N., Guenther A., Schermuly R. T. (2013). The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. J. Pathol. 229 (2), 242–249. 10.1002/path.4127 [Abstract] [CrossRef] [Google Scholar]
- Kong D. H., Kim Y. K., Kim M. R., Jang J. H., Lee S. (2018). Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 19 (4), 1057. 10.3390/ijms19041057 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li J., Zhang Y., Yang C., Rong R. (2020). Discrepant mRNA and protein expression in immune cells. Curr. Genomics 21 (8), 560–563. 10.2174/1389202921999200716103758 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lin J., Kakkar V., Lu X. (2014). Impact of MCP -1 in atherosclerosis. Curr. Pharm. Des. 20 (28), 4580–4588. 10.2174/1381612820666140522115801 [Abstract] [CrossRef] [Google Scholar]
- Ling T., Lv H-Z., Ma Y-X., Shang Q-H., Zhang J-T. (2010). Relationship between the level of MCP-1 expression in sera of RA patients and ILD. Xi bao yu fen zi mian yi xue za zhi. 26 (1), 59–60. [Abstract] [Google Scholar]
- Lynch D. A., Sverzellati N., Travis W. D., Brown K. K., Colby T. V., Galvin J. R., et al. (2018). Diagnostic criteria for idiopathic pulmonary fibrosis: a fleischner society white paper. Lancet. Respir. Med. 6 (2), 138–153. 10.1016/S2213-2600(17)30433-2 [Abstract] [CrossRef] [Google Scholar]
- Manfredi A., Cassone G., Luppi F., Atienza-mateo B., Cavazza A., Sverzellati N., et al. (2021). Rheumatoid arthritis related interstitial lung disease. Expert Rev. Clin. Immunol. 17 (5), 485–497. 10.1080/1744666X.2021.1905524 [Abstract] [CrossRef] [Google Scholar]
- McGroder C. F., Aaron C. P., Bielinski S. J., Kawut S. M., Tracy R. P., Raghu G., et al. (2019). Circulating adhesion molecules and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis. Eur. Respir. J. 54 (3), 1900295. 10.1183/13993003.00295-2019 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Millatt L. J., Whitley G. S. J., Li D., Leiper J. M., Siragy H. M., Carey R. M., et al. (2003). Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation 108 (12), 1493–1498. 10.1161/01.CIR.0000089087.25930.FF [Abstract] [CrossRef] [Google Scholar]
- Murdaca G., Colombo B. M., Cagnati P., Gulli R., Spanò F., Puppo F. (2012). Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224 (2), 309–317. 10.1016/j.atherosclerosis.2012.05.013 [Abstract] [CrossRef] [Google Scholar]
- Navarro-Hernández R. E., Oregon-Romero E., Vázquez-Del Mercado M., Rangel-Villalobos H., Palafox-Sánchez C. A., Muñoz-Valle J. F. (2009). Expression of ICAM1 and VCAM1 serum levels in rheumatoid arthritis clinical activity. Association with genetic polymorphisms. Dis. Markers 26 (3), 119–126. 10.3233/DMA-2009-0621 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Paulin F., Doyle T. J., Fletcher E. A., Ascherman D. P., Rosas I. O. (2015). Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis: shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev. Invest. Clin. 67 (5), 280–286. [Europe PMC free article] [Abstract] [Google Scholar]
- Peter K., Weirich U., Nordt T. K., Ruef J., Bode C. (1999). Soluble vascular cell adhesion molecule-1 (VCAM-1) as potential marker of atherosclerosis. Thromb. Haemost. 82, 38–43. 10.1055/s-0037-1615551 [Abstract] [CrossRef] [Google Scholar]
- Pulito-Cueto V., Remuzgo-Martínez S., Genre F., Mora-Cuesta V. M., Iturbe-Fernández D., Fernández-Rozas S., et al. (2020). Endothelial progenitor cells as a potential biomarker in interstitial lung disease associated with rheumatoid arthritis. J. Clin. Med. 9 (12), 4098. 10.3390/jcm9124098 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pulito-Cueto V., Remuzgo-Martínez S., Genre F., Atienza-mateo B., Mora-cuesta V. M., Iturbe-Fernández D., et al. (2021). Endothelial progenitor cells: relevant players in the vasculopathy and lung fibrosis associated with the presence of interstitial lung disease in systemic sclerosis patients. Biomedicines 9 (7), 847. 10.3390/biomedicines9070847 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pulito-Cueto V., Remuzgo-Martínez S., Genre F., Atienza-Mateo B., Mora-Cuesta V. M., Iturbe-Fernández D., et al. (2022). Angiogenic T cells: potential biomarkers for the early diagnosis of interstitial lung disease in autoimmune diseases? Biomedicines 10 (4), 851. 10.3390/biomedicines10040851 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rantapää-Dahlqvist S., Boman K., Tarkowski A., Hallmans G. (2007). Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann. Rheum. Dis. 66 (1), 121–123. 10.1136/ard.2006.057331 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sandoo A., Dimitroulas T., VeldhuijzenVan Zanten J. J. C. S., Smith J. P., Metsios G. S., Nightingale P., et al. (2012). Lack of association between asymmetric dimethylarginine and in vivo microvascular and macrovascular endothelial function in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 30 (3), 388–396. [Abstract] [Google Scholar]
- Sattar N., Mccarey D. W., Capell H., Mcinnes I. B. (2003). Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108 (24), 2957–2963. 10.1161/01.CIR.0000099844.31524.05 [Abstract] [CrossRef] [Google Scholar]
- Schmidt K., Martinez-Gamboa L., Meier S., Witt C., Meisel C., Hanitsch L. G., et al. (2009). Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res. Ther. 11 (4), R111. 10.1186/ar2766 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stevens S. G., Brown C. M. (2013). In silico estimation of translation efficiency in human cell lines: potential evidence for widespread translational control. PLOS One 8 (2), e57625. 10.1371/journal.pone.0057625 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Steyers C. M., Miller F. J. (2014). Endothelial dysfunction in chronic inflammatory diseases. Int. J. Mol. Sci. 15 (7), 11324–11349. 10.3390/ijms150711324 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Travis W. D., Costabel U., Hansell D. M., King T. E., Lynch D. A., Nicholson A. G., et al. (2013). An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188 (6), 733–748. 10.1164/rccm.201308-1483ST [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang L., Ding Y., Guo X., Zhao Q. (2015). Role and mechanism of vascular cell adhesion molecule-1 in the development of rheumatoid arthritis. Exp. Ther. Med. 10 (3), 1229–1233. 10.3892/etm.2015.2635 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yalçinkaya Y., Çinar S., Artim-Esen B., Kamali S., Öcal L., Deniz G., et al. (2016). The relationship between vascular biomarkers and disease characteristics in systemic sclerosis: elevated MCP-1 is predominantly associated with fibrotic manifestations. Clin. Exp. Rheumatol. 34, 110–114. [Abstract] [Google Scholar]
- Yang X., Chang Y., Wei W. (2016). Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediat. Inflamm. 2016, 6813016. 10.1155/2016/6813016 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zamora-legoff J. A., Krause M. L., Crowson C. S., Ryu J. H., Matteson E. L. (2017). Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology 56 (3), 344–350. 10.1093/rheumatology/kew391 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhao C. N., Wu Q., Mao Y. M., Liu L. N., Dan Y. L., Li X. M., et al. (2019). Elevated circulating asymmetric dimethylarginine levels in rheumatoid arthritis: a systematic review and meta-analysis. Amino Acids 51 (5), 773–782. 10.1007/s00726-019-02714-5 [Abstract] [CrossRef] [Google Scholar]
Articles from Frontiers in Molecular Biosciences are provided here courtesy of Frontiers Media SA
Full text links
Read article at publisher's site: https://doi.org/10.3389/fmolb.2022.1056121
Read article for free, from open access legal sources, via Unpaywall:
https://www.frontiersin.org/articles/10.3389/fmolb.2022.1056121/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/141042770
Article citations
Biomarkers of rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis.
Front Immunol, 15:1455346, 29 Oct 2024
Cited by: 0 articles | PMID: 39534599 | PMCID: PMC11554464
Review Free full text in Europe PMC
Rheumatoid Arthritis-Associated Interstitial Lung Disease (RA-ILD): Update on Prevalence, Risk Factors, Pathogenesis, and Therapy.
Curr Rheumatol Rep, 26(12):431-449, 25 Sep 2024
Cited by: 0 articles | PMID: 39320427
Review
Extensive study of CCN4, VCAM-1, MMP-3, and GM-CSF as reliable markers for disease activity in rheumatoid arthritis.
J Taibah Univ Med Sci, 19(4):885-900, 13 Aug 2024
Cited by: 0 articles | PMID: 39262670 | PMCID: PMC11387248
A Systematic Review on Biomarkers: Are There Reliable Molecular Biomarkers in Patients With Rheumatoid Arthritis-Associated Interstitial Lung Disease?
Cureus, 16(8):e66422, 08 Aug 2024
Cited by: 0 articles | PMID: 39247043 | PMCID: PMC11380652
Review Free full text in Europe PMC
Pharmacological mechanisms of Taohe Chengqi decoction in diabetic cardiovascular complications: A systematic review, network pharmacology and molecular docking.
Heliyon, 10(13):e33308, 25 Jun 2024
Cited by: 0 articles | PMID: 39044965 | PMCID: PMC11263673
Go to all (11) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases.
Int J Mol Sci, 24(15):12518, 07 Aug 2023
Cited by: 2 articles | PMID: 37569893 | PMCID: PMC10420063
Plasma Wnt7b protein in rheumatoid arthritis: Detection of interstitial lung disease.
Immunobiology, 228(4):152397, 29 May 2023
Cited by: 0 articles | PMID: 37290224
A Systematic Review on Biomarkers: Are There Reliable Molecular Biomarkers in Patients With Rheumatoid Arthritis-Associated Interstitial Lung Disease?
Cureus, 16(8):e66422, 08 Aug 2024
Cited by: 0 articles | PMID: 39247043 | PMCID: PMC11380652
Review Free full text in Europe PMC
Comparative Profiling of Serum Protein Biomarkers in Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis.
Arthritis Rheumatol, 72(3):409-419, 05 Feb 2020
Cited by: 30 articles | PMID: 31532072

 1
,*
†
1
,*
†



