Abstract
Background
Acute undifferentiated fever (AUF) ranges from self-limiting illness to life-threatening infections, such as sepsis, malaria, dengue, leptospirosis and rickettsioses. Similar clinical presentation challenges the clinical management. This study describes risk factors for death in patients hospitalized with AUF in India.Methods
Patients aged ≥5 y admitted with fever for 2-14 d without localizing signs were included in a prospective observational study at seven hospitals in India during 2011-2012. Predictors identified by univariate analysis were analyzed by multivariate logistic regression for survival analysis.Results
Mortality was 2.4% (37/1521) and 46.9% (15/32) died within 2 d. History of heart disease (p=0.013), steroid use (p=0.011), altered consciousness (p<0.0001), bleeding (p<0.0001), oliguria (p=0.020) and breathlessness (p=0.015) were predictors of death, as were reduced Glasgow coma score (p=0.005), low urinary output (p=0.004), abnormal breathing (p=0.006), abdominal tenderness (p=0.023), leucocytosis (p<0.0001) and thrombocytopenia (p=0.001) at admission. Etiology was identified in 48.6% (18/37) of fatal cases.Conclusions
Bleeding, cerebral dysfunction, respiratory failure and oliguria at admission, suggestive of severe organ failure secondary to systemic infection, were predictors of death. Almost half of the patients who died, died shortly after admission, which, together with organ failure, suggests that delay in hospitalization and, consequently, delayed treatment, contribute to death from AUF.Free full text

Clinical features and risk factors for death in acute undifferentiated fever: A prospective observational study in rural community hospitals in six states of India
Abstract
Background
Acute undifferentiated fever (AUF) ranges from self-limiting illness to life-threatening infections, such as sepsis, malaria, dengue, leptospirosis and rickettsioses. Similar clinical presentation challenges the clinical management. This study describes risk factors for death in patients hospitalized with AUF in India.
Methods
Patients aged ≥5 y admitted with fever for 2–14 d without localizing signs were included in a prospective observational study at seven hospitals in India during 2011–2012. Predictors identified by univariate analysis were analyzed by multivariate logistic regression for survival analysis.
Results
Mortality was 2.4% (37/1521) and 46.9% (15/32) died within 2 d. History of heart disease (p=0.013), steroid use (p=0.011), altered consciousness (p<0.0001), bleeding (p<0.0001), oliguria (p=0.020) and breathlessness (p=0.015) were predictors of death, as were reduced Glasgow coma score (p=0.005), low urinary output (p=0.004), abnormal breathing (p=0.006), abdominal tenderness (p=0.023), leucocytosis (p<0.0001) and thrombocytopenia (p=0.001) at admission. Etiology was identified in 48.6% (18/37) of fatal cases.
Conclusions
Bleeding, cerebral dysfunction, respiratory failure and oliguria at admission, suggestive of severe organ failure secondary to systemic infection, were predictors of death. Almost half of the patients who died, died shortly after admission, which, together with organ failure, suggests that delay in hospitalization and, consequently, delayed treatment, contribute to death from AUF.
Introduction
Infectious diseases are leading causes of morbidity and death in India, accounting for half of the deaths among children aged 5–14 y.1 Common infections presenting as acute undifferentiated fever (AUF) characterized by fever without localizing signs and symptoms may be mild and self-limiting, while others can be rapidly fatal if left untreated.2 Malaria, leptospirosis, scrub typhus, dengue and bacterial blood stream infections including enteric fever are the most common causes of AUF in hospitalized patients in India.2–7 In South East Asia, the reported case-fatality rates in hospitalized patients presenting with AUF are 5–12% in bacterial bloodstream infections,7,8 2–22% in malaria,2,4,6,7 4–12% in scrub typhus,2,4,9 8–22% in leptospirosis4,6 and 0–25% in dengue.2,4,10,11 In cases of infections with drug-resistant bacteria, mortality in resource-constrained settings can reach 18–70%.12,13
Unfortunately, due to the similar clinical presentation and optimal confirmatory tests being inaccessible and unaffordable,14 the etiology of AUF frequently remains uncertain.3,7 Consequently, there is considerable uncertainty around estimates of the morbidity and mortality that can be attributed to each pathogen.
Focus on clinical evaluation and identification of risk factors for severe prognosis of bacterial, viral and parasitic infections is particularly important to prioritize the right patients for treatment in hospital and to triage the right patients for intensive care treatment.
The objective of this study was to investigate the clinical features, treatment and risk factors for death among patients admitted with AUF in rural hospitals in India.
Materials and methods
Study sites and participants
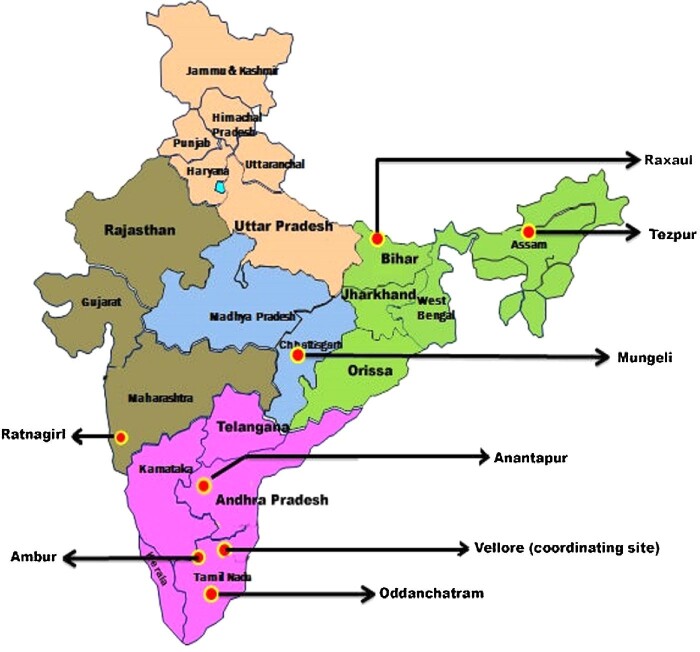
Patients aged ≥5 y with AUF were consecutively included at admission to hospital during April 2011–November 2012. Patients were admitted to the following seven secondary community 100–500-bed hospitals in six states: Baptist Christian hospital in Tezpur (Assam, North East India), Duncan hospital in Raxaul (Bihar, North India), Christian hospital in Mungeli (Chhattisgarh, Central India), B.K.L. Walawalkar hospital in Ratnagiri (Maharashtra, Western India), Rural Development Trust hospital in Anantapur (Andhra Pradesh, South India), Christian Fellowship hospital in Oddanchatram (Tamil Nadu, South India) and Bethesda hospital in Ambur (Tamil Nadu, South India) (Figure 1).
The study monitoring center and reference laboratory was the Infectious Diseases Training and Research Centre and the Dr Benjamin M. Pulimood Laboratories for Infection Immunity and Inflammation, Department of Medicine and Infectious diseases, Christian Medical College (CMC), Vellore, India. Details of the climate variation among the study sites have been published previously.15 AUF was defined as a temperature ≥38ºC of 2–14 d duration before admission, with no localized infection as judged by the treating physician at the time of evaluation for inclusion. Patients with abdominal pain, diarrhea, hematochezia, nausea or vomiting, rhinorrhea, dyspnea, ocular pain, altered sensorium, headache, stiff neck, rash, arthralgia, myalgia, petechiae, ecchymosis, epistaxis, gingival bleeding or jaundice were not excluded. Patients with presentations suggestive of pneumonia, urinary tract infections, soft tissue infections and other localized causes were excluded.
Study procedures
The treating physicians prospectively recorded predefined clinical data. Reason for visit, fever duration, 13 specified symptoms, 12 specified prehospital comorbidities, travel history, animal exposure, alcohol and smoking habits, clinical findings upon admission, radiological, biochemical and microbiological test results, tentative differential diagnoses, treatment including 11 specified antibiotics and seven specified antimalarials, outcome and condition at discharge, were recorded in a standardized case report form at all sites. One site (Tezpur) included information on all WHO-defined criteria for severe malaria.
This was a prospective observational study, and clinical work up and treatment were performed according to the hospital's routines. Point of care tests were performed in the hospitals, while microbiological case definitions based on analyses performed later in the reference laboratory were not available at the hospitals while treating the patients.
The following investigations were performed at the study hospitals on all patients: malaria blood smears, scrub typhus IgM ELISA (In Bios, USA), Leptospira IgM ELISA (Alere, Australia), chikungunya IgM ELISA (NIV, India), dengue rapid NS1 antigen and IgM Combo test (SD bioline, USA) and blood cultures. Blood cultures were collected before starting antibiotics and were cultured with the conventional method or by Bactec (Becton Dickinson, MD, USA); if growth was identified at the site it was frozen, then sent by cold chain to the study reference laboratory for new identification.
The following investigations were performed at the reference laboratory: scrub typhus IgM ELISA, Leptospira IgM ELISA, chikungunya IgM ELISA and dengue NS1/IgM Combo test if not performed at site. Dengue IgM capture ELISA (MAC-ELISA) on all samples. Scrub typhus immunofluorescence (IFA) on IgM ELISA positive samples, Leptospira microscopic agglutination test (MAT) on IgM ELISA positive samples. A genus-specific mitochondrial malaria PCR method, and the immunochromatographic rapid diagnostic test (RDT) Parahit Total (Span Diagnostics Ltd, Surat, India), were performed on all samples. A species-specific 18S PCR method, or sequencing, was performed on malaria genus PCR positive samples.
Case definitions were defined as follows: Leptospirosis, positive ELISA and positive MAT; scrub typhus, positive ELISA and positive IFA; dengue, positive RDT and/or positive MAC-ELISA; chikungunya, positive ELISA; bacteremia, growth of bacteria not considered to be contaminants in blood culture; malaria, positive malaria genus-specific PCR.
Microbiological methods and findings in this study have been reported previously.3,15,16
The study was reported in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) checklist.
Statistical analysis
In descriptive analysis, dichotomous variables were given as proportions and continuous variables as medians with IQR. Univariate and multivariate analysis of risk factors for death included demographic variables, comorbidity, signs, symptoms, clinical and biochemical findings, laboratory-confirmed diagnosis and duration of hospitalization. Risk factors were analyzed group-wise for (i) demographic characteristics and comorbidities, (ii) history of illness, (iii) clinical findings on admission and (iv) microbiological and biochemical test results. Missing values were analyzed as missing values, except for imputation in merged variables, where missing values were coded as negative. In univariable analyses we calculated unadjusted OR, 95% CI and p-values, using logistic regression for both dichotomous variables and continuous explanatory factors. Age, gender and significant risk factors with p<0.05 in the univariable analyses were included in multivariable analyses. For multivariable analysis, we used logistic regression and presented results as adjusted OR (aOR), 95% CI and p-values. Statistical analyses were performed using SPSS Statistics version 26 (IBM, Armonk, NY, USA).
Results
Characteristics and outcome
A total of 1564 patients were included in the study. In-hospital mortality was 2% (37/1521), and additionally, 2% (25/1525) were discharged in a state of deteriorating health with unknown outcome. Discrepancies from the total (n=1564) in the numbers of patients included in each analysis are due to missing values and are shown in the tables. Characteristics and outcome at each study site are shown in Table 1.
Table 1.
Characteristics and outcome among patients with AUF in seven secondary hospitals in India (N=1564)
| Characteristics | Na | Oddanchatram (N=330) N (%) | Ambur (N=316) N (%) | Tezpur (N=336) N (%) | Mungeli (N=62) N (%) | Anantapur (N=160) N (%) | Ratnagiri (N=251) N (%) | Raxaul (N=109) N (%) | Total (N=1564) N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gender | 1527 | ||||||||
 Male Male | 895 | 176 (53) | 170 (55) | 195 (59) | 27 (52) | 108 (72) | 154 (62) | 65 (61) | 895 (59) |
 Female Female | 632 | 154 (47) | 139 (45) | 135 (41) | 25 (48) | 42 (28) | 96 (38) | 41 (39) | 632 (41) |
| Age, median (IQR), y | 1422 | 31 (17–45) | 32 (20–50) | 30 (20–46) | 27 (18–53) | 30 (20–40) | 36 (24–49) | 26 (17–38) | 31 (20–45) |
 ≤14 ≤14 | 71 (22) | 33 (16) | 41 (12) | 10 (19) | 22 (15) | 10 (4) | 12 (11) | 199 (14) | |
 >14 >14 | 259 (78) | 176 (84) | 287 (88) | 42 (81) | 126 (85) | 239 (96) | 94 (89) | 1223 (86) | |
| Residency | 1495 | ||||||||
 Urban Urban | 57 (17) | 107 (37) | 25 (8) | 8 (15) | 35 (24) | 39 (16) | 5 (5) | 276 (18) | |
 Rural Rural | 271 (83) | 186 (64) | 294 (92) | 44 (85) | 113 (76) | 209 (84) | 102 (95) | 1219 (82) | |
| Characteristics | |||||||||
 Antibiotic before admission Antibiotic before admission | 1486 | 0 | 28 (10) | 111 (34) | 7 (14) | 17 (11) | 111 (45) | 61 (62) | 335 (23) |
 Antimalarial before admission Antimalarial before admission | 1476 | 0 | 3 (1) | 25 (8) | 5 (10) | 11 (7) | 63 (26) | 8 (9) | 115 (8) |
 Use of bed nets Use of bed nets | 1509 | 2 (1) | 15 (5) | 291 (90) | 0 | 25 (17) | 6 (2) | 53 (56) | 392 (26) |
 Animal exposure Animal exposure | 1509 | 30 (9) | 50 (16) | 74 (23) | 4 (8) | 34 (23) | 55 (22) | 24 (26) | 271 (18) |
 Alcohol use Alcohol use | 1510 | 52 (16) | 19 (6) | 61 (19) | 4 (8) | 34 (23) | 28 (11) | 8 (9) | 206 (14) |
 Smoker Smoker | 1507 | 69 (21) | 14 (5) | 43 (13) | 8 (15) | 38 (26) | 7 (3) | 7 (8) | 186 (12) |
| Comorbidity | |||||||||
 HIV known from before HIV known from before | 1512 | 0 | 0 | 0 | 0 | 75 (47) | 0 | 0 | 75 (5) |
 Diabetes Diabetes | 1511 | 0 | 32 (10) | 13 (4) | 4 (8) | 0 | 23 (9) | 4 (4) | 76 (5) |
 Cardiac disorder Cardiac disorder | 1514 | 0 | 3 (1) | 0 | 0 | 1 (1) | 13 (5) | 2 (2) | 19 (1) |
| Outcome | |||||||||
 Death in hospital Death in hospital | 1521 | 0 | 1 (0.3) | 15 (5) | 6 (12) | 0 | 13 (5) | 2 (2) | 37 (2.4) |
The majority (82%, 1219/1495) of the population lived in rural areas. Median age was 31 y and 14% (199/1422) were aged ≤14 y. Fatal cases were not significantly older (median age 34 y) than survivors (31 y, p=0.197) (Table 2).
Table 2.
Characteristics and comorbidity among survivors and non-survivors (N=1521)
| Univariableb | Multivariablec | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Survival N (%) | Death N (%) | OR | 95% CI | p | aOR | 95% CI | p | |
| Total, N | 1521 | 1484 | 37 | ||||||
| Gender | 1517 | 0.35 to 1.28 | NS | 1.546 | 0.797 to 2.996 | NS | |||
 Men Men | 887 | 869 (59) | 18 (49) | 0.67 | |||||
 Women Women | 630 | 611 (41) | 19 (51) | 1.00 | |||||
| Age, median (IQR), y | 1412 | 31 (20–45) | 34 (22–55) | 1.01 | 0.99 to 1.03 | NS | 0.995 | 0.977 to 1.014 | NS |
 ≤14 ≤14 | 198 | 195 (14) | 3 (8) | ||||||
 >14 >14 | 1214 | 1180 (86) | 34 (92) | ||||||
| Characteristics | |||||||||
 Bed nets use Bed nets use | 1499 | 375 (26) | 14 (38) | 1.76 | 0.90 to 3.46 | NS | |||
 Animal exposure Animal exposure | 1499 | 260 (18) | 11 (30) | 1.96 | 0.95 to 4.01 | NS | |||
 Alcohol use Alcohol use | 1500 | 198 (14) | 7 (19) | 1.49 | 0.65 to 3.44 | NS | |||
 Antibiotics prior Antibiotics prior | 1477 | 320 (22) | 12 (33) | 1.75 | 0.87 to 3.54 | NS | |||
 Antimalarial prior Antimalarial prior | 1467 | 111 (8) | 3 (8) | 1.08 | 0.33 to 3.58 | NS | |||
 Smoking Smoking | 1497 | 182 (13) | 2 (5) | 0.40 | 0.10 to 1.68 | NS | |||
 Recent intake raw milk Recent intake raw milk | 1505 | 23 (2) | 1 (3) | 0.57 | 0.08 to 4.36 | NS | |||
 Recent travel outside district Recent travel outside district | 1505 | 134 (9) | 1 (3) | 0.28 | 0.04 to 2.03 | NS | |||
| Comorbidity | |||||||||
 Heart disorder Heart disorder | 1504 | 16 (1) | 3 (8) | 8.00 | 2.23 to 28.8 | 0.001 | 5.85 | 1.46 to 23.48 | 0.013 |
 Condition with steroid treatment Condition with steroid treatment | 1503 | 6 (0.4) | 2 (5) | 13.9 | 2.71 to 71.3 | 0.002 | 9.25 | 1.65 to 51.82 | 0.011 |
 Diabetes Diabetes | 1501 | 74 (5) | 2 (5) | 1.07 | 0.25 to 4.55 | NS | |||
 Hypertension Hypertension | 1502 | 88 (6) | 4 (11) | 1.90 | 0.66 to 5.47 | NS | |||
 Seizure disorder Seizure disorder | 1503 | 9 (1) | 1 (3) | 4.50 | 0.56 to 36.4 | NS | |||
 Respiratory disease Respiratory disease | 1503 | 32 (2) | 2 (6) | 2.64 | 0.60 to 11.46 | NS | |||
 Kidney disease Kidney disease | 1505 | 12 (1) | 1 (3) | 3.37 | 0.48 to 26.6 | NS | |||
 Liver disease Liver disease | 1500 | 15 (1) | 1 (3) | 2.68 | 0.35 to 20.85 | NS | |||
 Stroke Stroke | 1504 | 5 (0.3) | 0 | - | |||||
 HIV HIV | 1502 | 74 (5) | 0 | - | |||||
 TB TB | 1434 | 11 (1) | 0 | - | |||||
 Cancer Cancer | 1504 | 4 | 0 | - | |||||
 Splenectomized Splenectomized | 1504 | 0 | 0 | - | |||||
 Chemotherapy Chemotherapy | 1504 | 1 | 0 | - | |||||
Abbreviations: aOR, adjusted OR; NS, not significant.
Fifty-nine percent (895/1527) were men and 41% (632/1527) women. There was no significant difference in case-fatality rates between male (2%, 18/887) and female patients (3%, 19/630) (p=0.223).
Antibiotics had been used before admission by 23% (335/1486); 33% (12/1477) among those who died and by 22% (320/1477) of survivors, but the difference was not a significant risk factor (OR 1.75, 95% CI 0.87 to 3.54) for death (Table 2). Eight percent (115/1476) had used antimalarial treatment before admission, and this was not associated with risk of death (p=0.900).
Among comorbid conditions, heart disease (aOR 5.85, 95% CI 1.46 to 23.48) and steroid use before admission (aOR 9.25, 95% CI 1.65 to 51.82) were significant risk factors for death (Table 2). One study site found 47% (75/160) of patients to have positive HIV status upon admission; none of these patients died.
Symptoms before admission
A history of altered consciousness (aOR 21.68, 95% CI 8.72 to 53.92), bleeding (aOR 14.08, 95% CI 4.21 to 47.13), oliguria (aOR 3.41, 95% CI 1.21 to 9.61) and breathlessness (aOR 2.91, 95% CI 1.24 to 6.87) were significant risk factors for death in both univariable and multivariable analyses (Table 3).
Table 3.
History of signs and symptoms associated with death and survival (N=1521)
| Univariableb | Multivariablec | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Survival N (%) | Death N (%) | OR | 95% CI | p | aOR | 95% CI | p | |
| Total | 1521 | 1484 | 37 | ||||||
| Fever duration, median (IQR), d | 1509 | 5 (3–7) | 5 (4–7) | 0.99 | 0.91 to 1.07 | NS | |||
| Symptom duration, median (IQR), d | 1453 | 4 (3–4) | 4 (3–4) | 1.19 | 0.84 to 1.68 | NS | |||
| History of signs and symptoms | |||||||||
 Altered consciousness Altered consciousness | 1515 | 62 (4) | 21 (57) | 30.00 | 14.91 to 60.26 | <0.0001 | 21.68 | 8.72 to 53.92 | <0.0001 |
 Bleedingd Bleedingd | 1517 | 32 (2) | 7 (19) | 10.59 | 4.33 to 25.89 | <0.0001 | 14.08 | 4.21 to 47.13 | <0.0001 |
 Oliguria Oliguria | 1515 | 67 (5) | 12 (32) | 10.11 | 4.87 to 21.00 | <0.0001 | 3.41 | 1.21 to 9.61 | 0.020 |
 Breathlessness Breathlessness | 1484 | 190 (13) | 17 (47) | 5.92 | 3.03 to 11.60 | <0.0001 | 2.91 | 1.24 to 6.87 | 0.015 |
 Seizures Seizures | 1514 | 26 (2) | 6 (17) | 11.17 | 4.28 to 29.13 | <0.0001 | 2.13 | 0.62 to 7.38 | NS |
 Jaundice Jaundice | 1488 | 91 (6) | 9 (24) | 4.80 | 2.20 to 10.48 | <0.0001 | 1.69 | 0.61 to 4.70 | NS |
 Prostration Prostration | 1507 | 343 (23) | 16 (46) | 2.78 | 1.41 to 5.45 | 0.003 | 0.63 | 0.26 to 1.55 | NS |
 Vomiting Vomiting | 1492 | 494 (34) | 19 (51) | 2.05 | 1.07 to 3.95 | 0.031 | 1.82 | 0.80 to 4.14 | NS |
 Nausea Nausea | 1485 | 514 (36) | 18 (49) | 1.72 | 0.90 to 3.31 | NS | |||
 Myalgia Myalgia | 1516 | 493 (33) | 18 (49) | 1.90 | 0.99 to 3.64 | NS | |||
 Cough Cough | 1518 | 489 (33) | 10 (27) | 0.75 | 0.36 to 1.57 | NS | |||
 Sputum Sputum | 1516 | 181 (12) | 5 (14) | 0.82 | 0.34 to 2.32 | NS | |||
 Headache Headache | 1517 | 816 (55) | 21 (57) | 1.07 | 0.55 to 2.06 | NS | |||
 Retro orbital pain Retro orbital pain | 1514 | 151 (10) | 2 (6) | 0.52 | 0.12 to 2.17 | NS | |||
 Abdominal pain Abdominal pain | 1485 | 292 (20) | 6 (17) | 0.79 | 0.33 to 1.92 | NS | |||
 Diarrhea Diarrhea | 1486 | 225 (16) | 5 (14) | 0.88 | 0.34 to 2.28 | NS | |||
 Arthralgia Arthralgia | 1515 | 210 (14) | 6 (16) | 1.17 | 0.48 to 2.84 | NS | |||
 Rash Rash | 1515 | 33 (2) | 1 (3) | 1.22 | 0.16 to 9.14 | NS | |||
Abbreviations: aOR, adjusted odds ratio; NS, not significant.
Missing coded as negative in merged variables for the sake of the analyses.
History of seizures, jaundice, prostration and vomiting were significant risk factors for death in univariable analyses but were not significant when included in the multivariable analysis (Table 3). Median duration of fever was 5 d among both survivors and those who died.
Clinical and laboratory findings
The following clinical signs at admission were predictors of death in the multivariable analysis: Glasgow Coma Scale (GCS) score (aOR 0.76, 95% CI 0.63 to 0.92), decreased urinary output (aOR 2.55, 95% CI 1.34 to 4.85), abnormal breathing (aOR 6.81, 95% CI 1.76 to 26.40) and abdominal tenderness (aOR 5.76, 95% CI 1.27 to 26.10) (Table 4).
Table 4.
Clinical signs at admittance associated with death and survival (N=1521)
| Univariableb | Multivariablec | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Na | Survival | Death | OR | 95% CI | p | aOR | 95% CI | p | |
| Total, N | 1521 | 1484 | 37 | ||||||
| Clinical signs at admittance | |||||||||
 GCS score, median (IQR) GCS score, median (IQR) | 1232 | 15 (0) | 14 (7–15) | 0.72 | 0.65 to 0.78 | <0.0001 | 0.76 | 0.63 to 0.92 | 0.005 |
 Abnormal breathingd Abnormal breathingd | 1513 | 123 (8) | 18 (49) | 10.48 | 5.36 to 20.50 | <0.0001 | 6.81 | 1.76 to 26.40 | 0.006 |
 Low urinary output (<750 ml) Low urinary output (<750 ml) | 1196 | 3.32 | 2.35 to 4.69 | <0.0001 | 2.55 | 1.34 to 4.85 | 0.004 | ||
 >750 ml >750 ml | 988 (85) | 13 (48) | |||||||
 500–750 ml 500–750 ml | 114 (12) | 4 (15) | |||||||
 400–500 ml 400–500 ml | 23 (2) | 3 (11) | |||||||
 < 400 ml < 400 ml | 14 (1) | 7 (26) | |||||||
 Abdominal tendernesse Abdominal tendernesse | 1516 | 108 (7) | 8 (22) | 3.52 | 1.57 to 7.88 | 0.002 | 5.76 | 1.27 to 26.10 | 0.023 |
 Saturation <95% Saturation <95% | 1257 | 264 (22) | 15 (50) | 3.65 | 1.76 to 7.56 | <0.0001 | 3.52 | 0.97 to 12.72 | NS |
 Pulse rate, median (IQR) Pulse rate, median (IQR) | 1497 | 92 (84–100) | 105 (87–119) | 1.02 | 1.00 to 1.04 | 0.014 | 0.98 | 0.95 to 1.01 | NS |
 Spontaneous bleedingf Spontaneous bleedingf | 1508 | 13 (1) | 5 (14) | 17.68 | 5.95 to 52.55 | <0.0001 | 0.47 | 0.02 to 14.12 | NS |
 Icterus Icterus | 1514 | 106 (7) | 13 (35) | 7.01 | 3.47 to 14.15 | <0.0001 | 3.63 | 0.72 to 18.25 | NS |
 Neck stiffnessg Neck stiffnessg | 1514 | 26 (2) | 3 (8) | 4.92 | 1.42 to 17.06 | 0.012 | NA | NA | NA |
 Edemah Edemah | 1515 | 89 (6) | 11 (30) | 6.63 | 3.17 to 13.86 | <0.0001 | 0.94 | 0.16 to 5.45 | NS |
 Pallor Pallor | 1515 | 320 (22) | 18 (50) | 3.62 | 1.86 to 7.04 | <0.0001 | 3.75 | 0.79 to 17.74 | NS |
 Conjunctival congestion Conjunctival congestion | 1500 | 71 (5) | 6 (17) | 4.06 | 1.63 to 10.10 | 0.003 | 0.36 | 0.02 to 6.17 | NS |
 Eschar Eschar | 1476 | 38 (3) | 3 (8) | 3.35 | 0.99 to 11.41 | NS | |||
 Temp F, median (IQR) Temp F, median (IQR) | 1425 | 101 (99–102) | 99 (99–100) | 1.00 | 0.98 to 1.02 | NS | |||
 BP systolic, median (IQR) BP systolic, median (IQR) | 1470 | 110 (100–120) | 110 (94–120) | 1.00 | 0.98 to 1.02 | NS | |||
 Respiratory rate, median (IQR) Respiratory rate, median (IQR) | 1487 | 24 (22–26) | 24 (20–30) | 1.04 | 0.99 to 1.09 | NS | |||
 Joint tenderness Joint tenderness | 1520 | 46 (3) | 3 (8) | 2.76 | 0.82 to 9.30 | NS | |||
 Joint swelling Joint swelling | 1519 | 23 (2) | 1 (3) | 1.75 | 0.23 to 13.4 | NS | |||
 Rash Rash | 1502 | 40 (3) | 1 (3) | 0.99 | 0.13 to 7.40 | NS | |||
 Coated tongue Coated tongue | 1504 | 126 (9) | 4 (11) | 1.38 | 0.48 to 3.96 | NS | |||
 Lymphadenopathy Lymphadenopathy | 1499 | 85 (6) | 2 (6) | 0.02 | 0.24 to 4.31 | NS | |||
 Spleen palpable Spleen palpable | 1490 | 110 (8) | 2 (6) | 0.72 | 0.17 to 3.03 | NS | |||
 Liver palpable Liver palpable | 1487 | 149 (10) | 5 (14) | 1.41| | 0.54 to 3.68 | NS | |||
 Liver tenderness Liver tenderness | 1321 | 39 | 1 | 1.07 | 0.14 to 8.04 | NS | |||
 Renal angel tenderness Renal angel tenderness | 1509 | 5 | 0 | NS | |||||
 Ascites Ascites | 1514 | 10 (1) | 0 | NS | |||||
 Cardiac murmur Cardiac murmur | 1514 | 5 (0.3) | 0 | NS | |||||
Abbreviations: aOR, adjusted odds ratio; GCS, Glasgow coma scale, score 1–15 and 15 optimal cerebral function; NA, not applicable.
Missing coded as negative in merged variables for the sake of the analyses.
Reduced oxygen saturation, increased pulse rate, icterus, neck stiffness, edema, spontaneous bleeding, pallor and conjunctival congestion at admission were significant risk factors for death in the univariable analyses (p<0.0001), but were not significant when included in the multivariable analysis. An eschar was found in 8% (3/37) of those who died compared with 3% (38/1473) of survivors, but the difference was not statistically significant. Leucocytosis and thrombocytopenia were associated with risk of death in the multivariable analysis (Table 5).
Table 5.
Microbiological and biochemical findings at admission associated with death and survival (n=1521)
| Univariableb | Multivariablec | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases Na | Survival N (%) | Death N (%) | OR | 95% CI | p | aOR | 95% CI | p | |
| Total | 1521 | 1484 | 37 | ||||||
| Biochemical tests | |||||||||
 Leucocytes, median (IQR) Leucocytes, median (IQR) | 1265 | 7,6 (5,7–11.0) | 15,4 (8.3–22.7) | 1.06 | 1.03 to 1.09 | <0.0001 | 1.07 | 1.04 to 1.10 | <0.0001 |
 Neutrophils, median (IQR) Neutrophils, median (IQR) | 1307 | 71 (60–80) | 84 (76–89) | 1.07 | 1.04 to 1.11 | <0.0001 | |||
 Platelets, median (IQR) Platelets, median (IQR) | 1367 | 160 (93–242) | 100 (27–182) | 0.992 | 0.988 to 0.997 | 0.001 | 0.992 | 0.987 to 0.997 | 0.001 |
 Bilirubin total, median (IQR) Bilirubin total, median (IQR) | 476 | 1.0 (0.6–2.2) | 2.3 (0.83–11.3) | 1.03 | 1.00 to 1.06 | 0.047 | |||
 Blood urea (median (IQR) Blood urea (median (IQR) | 267 | 25.0 (17.6–39.5) | 64.1 (2.7–139.8) | 1.01 | 1.00 to 1.02 | 0.005 | |||
 Hemoglobin, median (IQR) Hemoglobin, median (IQR) | 1413 | 11.4 (9.9–12.8) | 10.4 (8.5–12.1) | 0.91 | 0.80 to 1.00 | NS | |||
 ALT, median (IQR) ALT, median (IQR) | 707 | 44 (24–71) | 86 (50–167) | 1.00 | 0.99 to 1.00 | NS | |||
 Creatinine, median (IQR) Creatinine, median (IQR) | 873 | 1.0 (0.8–1.3) | 1.8 (1.2–3.1) | 1.01 | 0.97 to 1.06 | NS | |||
| Microbiological diagnosesd | |||||||||
 Malaria Malaria | 1394 | 258e (17) | 7f (19) | 1.12 | 0.48 to 2.55 | NS | |||
 Scrub typhus Scrub typhus | 1410 | 151 (10) | 5 (14) | 1.38 | 0.53 to 3.59 | NS | |||
 Dengue Dengue | 1501 | 236 (16) | 4 (11) | 0.64 | 0.23 to 1.83 | NS | |||
 Leptospirosis Leptospirosis | 1410 | 109 (7) | 4 (11) | 1.53 | 0.53 to 4.40 | NS | |||
 Bacteremia Bacteremia | 1113 | 122 (8) | 2g (5) | 0.64 | 0.15 to 2.68 | NS | |||
 Chikungunya Chikungunya | 1460 | 94 (6) | 2 (5) | 0.85 | 0.20 to 3.57 | NS | |||
Abbreviations: ALT, alanine transaminase; aOR, adjusted odds ratio; NS, not significant.
Bilirubin and urea were not included in the multivariable analysis due to high number of missing values, and neutrophils was not included due to likely confounding factor.
Increased total bilirubin (p=0.047) and blood urea (p=0.005) at admission were risk factors in the univariable analyses, but were not included in the multivariable analysis due to a high number of missing values.
A diagnosis of malaria (19%, 265/1394), scrub typhus (11%, 156/1410), dengue (16%, 240/1501), leptospirosis (8%, 113/1410), bacteremia (11%, 124/1113) or chikungunya (7%, 96/1460) was not associated with death, either in univariable or multivariable analyses (Table 5).
The etiology of AUF was confirmed in 49% (18/37) of those who died: Plasmodium falciparum mono-infection (n=2), P. falciparum and leptospirosis (n=2), Plasmodium vivax (N = 3), scrub typhus mono-infection (n=3), scrub typhus and dengue (n = 2), scrub typhus and leptospirosis (n=1), bacteremia with Escherichia coli or Staphylococcus aureus (n=2), leptospirosis mono-infection (n = 1) and dengue mono-infection (n=2) (Table 6).
Table 6.
Duration of hospitalization and antimicrobial treatment associated with microbiological diagnoses among patients who died (n=37)
| Died | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Na | Survived | Total died | P . f. | P . v. | P. f + lepto-spirosis | Lepto-spirosis | Bacteremiab | Scrub typhus | Scrub and lepto-spirosis | Scrub and dengue | Dengue | No microbiol. diagnosis | |
| Total, N | 1521 | 1484 | 37 | 2 | 3 | 2 | 1 | 2 | 3c | 1 | 2 | 2 | 19 |
| Duration in hospital, median (IQR), dd | 1270 | 4 (3–5) | 3 (1–4) | ||||||||||
| Antimalarials, n (%) | |||||||||||||
 Artemisinine and/or quinine Artemisinine and/or quinine | 2 | 3 | 2 | ||||||||||
 Artesunate Artesunate | 233 | 215 (92) | 18 (8) | 3 | 1 | 1 | 1 | 1 | 11 | ||||
 Artemether Artemether | 31 | 26 (84) | 5 (16) | 2 | 1 | 2 | |||||||
 Quinine Quinine | 21 | 15 (71) | 6 (29) | 2 | 1 | 3 | |||||||
 Chloroquine Chloroquine | 93 | 87 (94) | 6 (7) | 2 | 1 | 3 | |||||||
 Primaquine Primaquine | 50 | 49 (98) | 1 (2) | 1 | |||||||||
 Mefloquine Mefloquine | 6 | 4 (67) | 2 (33) | 2 | |||||||||
 Sulphadox/pyrimeth Sulphadox/pyrimeth | 4 | 3 (75) | 1 (25) | 1 | 1 | 1 | 1 | ||||||
| Antibiotics, n (%) | |||||||||||||
 3. gen cephalosporin 3. gen cephalosporin | 971 | 944 (97) | 27 (3) | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 17 | ||
 2. gen cephalosporin 2. gen cephalosporin | 38 | 37 (97) | 1 (3) | 1 | |||||||||
 1. gen cephalosporin 1. gen cephalosporin | 27 | 26 (96) | 1 (4) | 1 | |||||||||
 Carbapenem Carbapenem | 6 | 6 (100) | 0 | ||||||||||
 Penicillin Penicillin | 117 | 105 (90) | 12 (10) | 2 | 1 | 1 | 1 | 7 | |||||
 Aminoglycoside Aminoglycoside | 71 | 63 (89) | 8 (11) | 2 | 1 | 1 | 4 | ||||||
 Piperacillin/tazobactam Piperacillin/tazobactam | 54 | 49 (91) | 5 (9) | 1 | 2 | 2 | |||||||
 Quinolone Quinolone | 62 | 60 (97) | 2 (3) | 1 | 1 | ||||||||
 Macrolide Macrolide | 123 | 122 (99) | 1 (1) | 1 | |||||||||
 Tetracycline Tetracycline | 609 | 586 (92) | 23 (4) | 2 | 1 | 1 | 1 | 3 | 1 | 2 | 12 | ||
 Clindamycin Clindamycin | 3 | 2 | 1 | 1 | |||||||||
Patients who died had a significantly shorter duration of hospitalization compared with patients who survived (p=0.001). As many as 28% (9/32) died during the first day and 19% (6/32) during the second day of hospitalization (Table 6).
Treatment
All patients with malaria who died (n=7) had received treatment with intravenous artesunate or quinine, or intramuscular artemether (Table 6). Among all patients, 16% (245/1564) received antimalarials; 76% (73/96) of malaria smear positive cases and 15% (172/1167) of smear negative cases.
All patients with scrub typhus who died (n=6) had received doxycycline. Among all patients, 40% (609/1521) received doxycycline; 39% (586/1484) of those who survived and 62% (23/37) of those who died.
All patients with leptospirosis (n=4) who died had received beta-lactam antibiotics.
Among patients with bacteremia who died (n=2), one patient with E. coli bacteremia had not received antibiotics.
The majority of patients who died had received broad-spectrum antimicrobial treatment. A third-generation cephalosporin was given to 64% (971/1521) overall, to 73% (27/37) of those who died and to 64% (944/1484) of survivors. The combination of penicillin and aminoglycoside was given to 5% (71/1521) overall and to 22% (8/32) of those who died, while piperacillin-tazobactam was given to 4% (54/1521) overall and to 14% (5/37) of those who died. A carbapenem was given to six patients only, none of whom died (Table 6). Antimicrobial resistance data were not available in this study.
Discussion
This study showed that as many as 98% of patients with AUF potentially caused by bacterial, viral or parasitic infections survived when treated in hospital.
Fatal cases more often had symptoms and findings at admission that were consistent with systemic infection with severe organ failure, such as altered consciousness, thrombocytopenia, bleeding, respiratory failure and oliguria. Leucocytosis and neutrophilia consistent with severe systemic inflammation were also associated with death. This emphasizes that a delay in seeking healthcare is the main reason for death from AUF, because patients frequently have advanced incurable disease at the time of hospitalization (Tables 3 and 4).
As many as 47% (15/32) died on day 1 or 2 after hospitalization, consistent with advanced disease at admission and consequent delay in antimicrobial and supportive treatment. Malaria, bacterial sepsis, dengue, leptospirosis or scrub typhus were diagnosed in 49% among patients who died, and these infections may all present with organ failure in severe cases if left untreated. In a study of patients admitted with sepsis in the USA, mortality increased by 14% in those who received antibiotics within 3–12 h compared with within 3 h.17 Duration of fever was <1 wk in a study of patients with severe malaria with a mortality of 35% in Orissa, India.18 Scrub typhus is still often under-recognized and at high risk of delayed treatment and thereby severe disease and death.2,4,9,19,20 The risk of secondary severe dengue presenting as AUF in need for urgent supportive treatment is high in India, where dengue seroprevalence in the healthy population is >50%.11 In leptospirosis, duration of fever before treatment is associated with an increased risk of death in patients who develop icterus, bleedings and proteinuria (Weil's disease) with high mortality.21
The etiology of AUF was not associated with increased risk of death (Table 5). The majority of patients who died had received antimicrobials likely to be effective against malaria, scrub typhus, leptospirosis and bacteremia (Table 6).
Both mono and double infections were identified among those who died. However, a large overlap between diagnoses in this study has been reported previously, and the prevalence of cross reactivity or background positivity of serological tests, and subclinical malaria, is unknown.3
A broad-spectrum antibiotic given to patients with suspected severe bacterial infection may have contributed to the low death rate from bacteremia. A third-generation cephalosporin was given to 64% (971/1521), and among these, 97% (944/971) survived (Table 6).
The use of empiric cephalosporin rather than penicillin in combination with aminoglycoside may reflect a clinical suspicion of typhoid fever taking the high prevalence in India into account. However, the prevalence of extensively drug-resistant bacteria in India, particularly extended-spectrum beta-lactamase and carbapenemase-producing Enterobacteriaceae, is associated with increased mortality,13,22,23 and we cannot rule out that failure of cephalosporin treatment in unidentified bacterial bloodstream infections could be the cause of death in some of the 13 patients who died without a definitive etiological diagnosis. Regarding leptospirosis, both streptomycin, gentamicin, penicillin, cephalosporins and macrolides are effective,24 and most patients in the study received effective antibiotics against leptospirosis. Empirical treatment against scrub typhus in the form of doxycycline was given to as many as 40% (609/1521) of all patients, and to all who died from scrub typhus (n=6). Doxycycline was given to 62% (23/37) among patients who died, indicating a high level of suspicion against scrub typhus in severely ill patients.
Fewer patients (19%, 245/1263) overall, but 76% (73/96) of those with laboratory-confirmed malaria, received empirical treatment against malaria. However, all who died from malaria (n=7) had received effective antimalarial treatment, indicating that they already were critically ill at admission. Three patients who died had P. vivax malaria, in line with findings from other studies supporting that the mortality of vivax-malaria is higher than previously thought.25–28 Only 15% (172/1167) of smear negative cases had received antimalarial treatment compared with 76% of smear positive cases. This reflects that targeted antimicrobial treatment depends on available diagnostic facilities such as malaria microscopy, blood culture and resistance testing facilities and rapid diagnostic tests, and underlines the obvious need for accurate point of care tests in order to save lives and avoid overuse of broad-spectrum antimicrobials.29 A recent study of antibiotic use in AUF from India reported a significant association between testing for malaria and dengue, and faster antibiotic discontinuation.30
The study adds to the sparse literature on AUF in rural India, providing data on morbidity, mortality and attributable causes, as well as differences in the burden of AUF across the regions. The data can guide treatment guidelines and facilitate the clinical work of doctors in the most resource-limited settings. The strength of this study is a prospective observational design, thorough clinical investigations and etiology data with gold standard diagnostic methods from a large cohort in rural settings across six different states of India. An etiological diagnosis was not confirmed in half of the patients, which reflects that many infectious and non-infectious causes of AUF remain undiagnosed because a broad range of diagnostic tests are not routinely available in rural hospitals in India.
The time since recruitment is a limitation, but it is not likely, unfortunately, that presentation and outcome of severe AUF has changed much during the last decade in resource-poor settings. Immunosuppression is a risk factor for severe infection, and steroid use was a significant risk factor for death (Table 2), while HIV infection was not. Data to explore level of immunosuppression, CD4 count or HIV treatment in these patients were not available in this study. HIV infections were reported only from Anantapur in Andhra Pradesh, which probably reflects that this center had an active anti-retroviral therapy program during the period, and the fact that no one died could be due to effective HIV treatment.
This study provides knowledge about the fatal consequences of delay in the hospitalization and treatment of AUF in India, and calls for strengthening of microbiological diagnostic facilities in order to provide targeted antimicrobial treatment.
Conclusion
In this study, we investigated clinical features and treatment associated with death from AUF in rural community hospitals in six states in India. Overall mortality from AUF was 2%. Although most patients received appropriate empirical treatment, death was associated with signs of advanced disease at admission with altered consciousness, oliguria, bleeding, abnormal breathing, thrombocytopenia and leucocytosis. The majority of patients received broad-spectrum antibiotics, while antimalarials were given mainly to malaria microscopy positive cases. The study indicates that early hospitalization and timely treatment could improve survival from AUF in rural hospitals in India.
Acknowledgements
We sincerely thank the clinicians and personnel who contributed to this study by work up of the patients, laboratory testing, logistics and handling of data.
Contributor Information
Kristine Mørch, Norwegian National Advisory Unit on Tropical Infectious Diseases, Department of Medicine, Haukeland University Hospital, 5021, Bergen, Norway. Department of Clinical Science, University of Bergen, 5021, Bergen, Norway.
Anand Manoharan, Infectious Diseases Training and Research Centre, Department of Medicine, Christian Medical College, 632004, Vellore, India.
Sara Chandy, Infectious Diseases Training and Research Centre, Department of Medicine, Christian Medical College, 632004, Vellore, India.
Ashita Singh, Baptist Christian Hospital, 784001, Tezpur, Assam, India.
Cijoy Kuriakose, Christian Fellowship Hospital, 624619, Oddanchatram, Tamil Nadu, India.
Suvarna Patil, B.K.L. Walawalkar Hospital, 415612, Ratnagiri, Maharashtra, India.
Anil Henry, Christian Hospital, Mungeli, 495001, Chhattisgarh, India.
Novin Chacko, Duncan Hospital, Raxaul, 803101, Bihar, India.
Gerardo Alvarez-Uria, Rural Development Trust Hospital, 510051, Anantapur, Andhra Pradesh, India.
Joel Nesaraj, Bethesda Hospital, 635802, Ambur, Tamil Nadu, India.
Bjørn Blomberg, Norwegian National Advisory Unit on Tropical Infectious Diseases, Department of Medicine, Haukeland University Hospital, 5021, Bergen, Norway. Department of Clinical Science, University of Bergen, 5021, Bergen, Norway.
Siby Kurian, Infectious Diseases Training and Research Centre, Department of Medicine, Christian Medical College, 632004, Vellore, India.
Christel Gill Haanshuus, Norwegian National Advisory Unit on Tropical Infectious Diseases, Department of Medicine, Haukeland University Hospital, 5021, Bergen, Norway.
George Vasanthan Antony, Infectious Diseases Training and Research Centre, Department of Medicine, Christian Medical College, 632004, Vellore, India.
Nina Langeland, Norwegian National Advisory Unit on Tropical Infectious Diseases, Department of Medicine, Haukeland University Hospital, 5021, Bergen, Norway. Department of Clinical Science, University of Bergen, 5021, Bergen, Norway.
Dilip Mathai, Infectious Diseases Training and Research Centre, Department of Medicine, Christian Medical College, 632004, Vellore, India.
Authors’ contributions
DM, KM, AM, NL and BB conceived the study; DM, KM, AM, NL, BB, AS and GVA designed the study protocol; AS, CK, SP, AH, NC, GAU and JN carried out the clinical assessment and data collection; AM, SC, CGH, GVA and SK carried out the microbiological investigations; KM and BB carried out the analysis and interpretation of the data. KM drafted the manuscript; KM, BB, AM, SC, AS, CK, SP, AH, NC, GAU, JN, CGH, SK, GVA, NL and DM critically revised the manuscript for intellectual content. All the authors read and approved the final manuscript. KM and DM are guarantors of the paper.
Funding
The institutions where the study was performed funded the study: Infectious Diseases Training and Research Centre, Department of Medicine and Infectious Diseases, CMC, Vellore, India and Norwegian National Advisory Unit for Tropical Infectious Diseases, Haukeland University Hospital, Bergen, Norway. The authors received no external funding for this work.
Ethical approval
The study was approved by the Institutional Research Board at CMC, Vellore, in Tamil Nadu, India (No. 7242 dated 11 August 2010) and by the Regional Ethics Committee of Norway (2010/2271–5). The participating sites, B.K.L. Walawalkar Hospital, Ratnagiri, Maharashtra, India and Rural Development Trust Hospital, Anantapur, Andhra Pradesh, India, had the study approved through their ethics committees. The other participating sites were secondary mission hospitals affiliated to CMC, Vellore, and therefore the ethics committee of CMC Vellore approved the study at these sites. Written informed consent was obtained from the patients or a legally acceptable representative; in cases of children from the parent or guardian. All methods were carried out in accordance with the relevant guidelines and regulations.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Articles from Transactions of the Royal Society of Tropical Medicine and Hygiene are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/trstmh/trac091
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/trstmh/article-pdf/117/2/91/49016952/trac091.pdf
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/136236286
Article citations
Clinical Manifestations of Dengue in Children and Adults in a Hyperendemic Region of Colombia.
Am J Trop Med Hyg, 110(5):971-978, 19 Mar 2024
Cited by: 0 articles | PMID: 38507814 | PMCID: PMC11066339
From fever to action: diagnosis, treatment, and prevention of acute undifferentiated febrile illnesses.
Pathog Dis, 82:ftae006, 01 Feb 2024
Cited by: 1 article | PMID: 38614961 | PMCID: PMC11067964
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Coinfections as an aetiology of acute undifferentiated febrile illness among adult patients in the sub-Himalayan region of north India.
J Vector Borne Dis, 55(2):130-136, 01 Apr 2018
Cited by: 10 articles | PMID: 30280711
Acute undifferentiated fever in India: a multicentre study of aetiology and diagnostic accuracy.
BMC Infect Dis, 17(1):665, 04 Oct 2017
Cited by: 46 articles | PMID: 28978319 | PMCID: PMC5628453
Fever in the tropics: aetiology and case-fatality - a prospective observational study in a tertiary care hospital in South India.
BMC Infect Dis, 13:355, 30 Jul 2013
Cited by: 15 articles | PMID: 23899336 | PMCID: PMC3750507
Identifying the Probable Etiology of Acute Undifferentiated Fever through Inflammatory Markers.
J Assoc Physicians India, 72(5):13-16, 01 May 2024
Cited by: 0 articles | PMID: 38881103
Funding
Funders who supported this work.